Highlights
-
•
First description of delayed spinal adhesive arachnoiditis after spine trauma surgery.
-
•
Titanium instrumentation produces less artifacts make MRI a useful to evaluate operated lesions.
-
•
Gabapentin can be the treatment of good option for delayed adhesive arachnoiditis.
Keywords: Flexion-distraction injury, Incomplete spinal cord injury, Adhesive arachnoiditis, Gabapentin-cauda equina syndrome
Abstract
Background
Adhesive arachnoiditis is an uncommon lesion caused by an inflammatory reaction in spinal nerves. Reports of substantial symptomatic thoracolumbar (TL) adhesive arachnoiditis after spinal surgery are rare. To the best of our knowledge, this is the first presentation of delayed adhesive arachnoiditis with cauda equina syndrome after decompression and fusion for a traumatic TL flexion-distraction injury.
Presentation of case
A 51-year-old man presented to the emergency room with absence of lower extremity muscle power and partial sensation preservation below T12 after slipping. Magnetic resonance imaging (MRI) and computed tomography demonstrated a flexion-distraction injury at T12-L1 and unstable burst fracture at L1 with posterior fragment displacement and cauda equina compression. Emergency decompression, fracture reduction, and posterior fusion with pedicle screw instrumentation (T11-L2) were performed. After the surgical wound completely healed, the patient was transferred to the rehabilitation department. Three months after surgery, the patient complained of severe pain around the anal and testis area and had absent anal sensation and sphincter tone. We re-evaluated the spine MRI and diagnosed the patient with adhesive arachnoiditis in the previous injury site. After gabapentin was administered, the symptoms dramatically subsided.
Conclusion
To the best of our knowledge, this is the first description of delayed spinal adhesive arachnoiditis after TL spinal surgery due to trauma. Developments in technology and resolution and the fact that titanium instrumentation produces less artifacts make MRI a useful tool to evaluate previously operated lesions. Gabapentin may be a good option in the treatment for delayed-onset postoperative adhesive arachnoiditis.
1. Introduction
Spinal adhesive arachnoiditis of the spinal nerves is caused by nonspecific inflammation of intrathecal neural elements, and spinal leptomeninges and also features vascular and circulatory involvement [[1], [2], [3]]. The inflamed arachnoid lining the nerve roots may have an inflammatory reaction and block cerebrospinal fluid (CSF) flow by capturing additional adjacent nerve roots. Several etiologies have been proposed, such as iatrogenic, infectious, degenerative, traumatic, and idiopathic [4]. In the past, myelography using oil-based or ionic water-soluble contrast media was a major contributing factor. Currently, the most frequent traumatic causes are probably paraspinal injections and herniated disc disease compressing the nerve roots [1,5]. Following lumbar disc surgery, 4.6 % of patients have lumbar adhesive arachnoiditis visible in magnetic resonance imaging (MRI) evaluation [6]. However, the injury location frequently does not correlate with the clinical presentation [7]. We report the first presentation of delayed adhesive arachnoiditis with cauda equina syndrome after operation for a traumatic spine flexion-distraction injury. This article has been written according to SCARE criteria as described by Agha et al. for the SCARE group. ‘The SCARE Steatement: Consensus-based surgical case report guidelines. International Journal of Sugery 2018′ [8].
2. Presentation of case
A 51-year-old man presented to the emergency room with no lower extremity muscle power and partial sensation preservation below T12 after slipping. The American Spinal Injury Association impairment score was grade B. A thoracolumbar (TL) flexion-distraction injury at T12-L1 and unstable L1 burst fracture with posterior fragment displacement and conus medullaris and cauda equina compression were seen on computed tomography (CT) and MRI. Emergency decompression, reduction, and posterior fusion with pedicle screw instrumentation (T11-L2) and bone graft were performed. Fracture reduction was performed at the site through the implanted rod and by applying compression. There was no CSF leakage during decompression. (Fig. 1, Fig. 2) One week after surgery, the patient began to sit with a thoraco-lumbo-sacral orthosis. Rehabilitation started two weeks after surgery. After the surgical wound fully healed, the patient was transferred to the rehabilitation department. One month after surgery, distal motor power was partially recovered, with knee extension and hip flexion manual muscle testing showing grades 1-2/5. Sensory recovery was at 80 % according to the patient’s assessment, the anal sphincter tone was partially recovered, and perianal sensation was nearly fully recovered. Seventy-six days after surgery, during rehabilitation, the patient suddenly complained of severe pain with hypoesthesia in the perianal area, absent sphincter tone, and no glans and testis sensation, which were suggestive of cauda equina syndrome (CES) with sensory dissociation. Inflammatory markers were not elevated and the lower extremity muscle power showed no change. Foley indwelling catheterization was performed at admission. Postoperative MRI was performed, which showed that the conus medullaris and cauda equina presented novel characteristics of adhesive arachnoiditis (matted, clumped, empty thecal sac) at the L1 level [9,10] (Fig. 3). We decided not to perform further decompression in previous injury sites and to administer gabapentin (600 mg once before sleep and 400 mg three times per day), after which pain dramatically subsided from 10 to 1 in the visual analogue scale. Three months after the sudden pain onset, the patient completely recovered from pain, hypoesthesia, and sphincter tone abnormality and there was a gradual improvement in other residual symptoms. The patient’s return to daily ambulatory activities with a single cane was confirmed at 1 year and 2 months postoperatively in a visit to the outpatient clinic. Both knee extension and ankle dorsiflexion manual muscle testing improved to grade 3-4/5 compared with 0 before surgery.
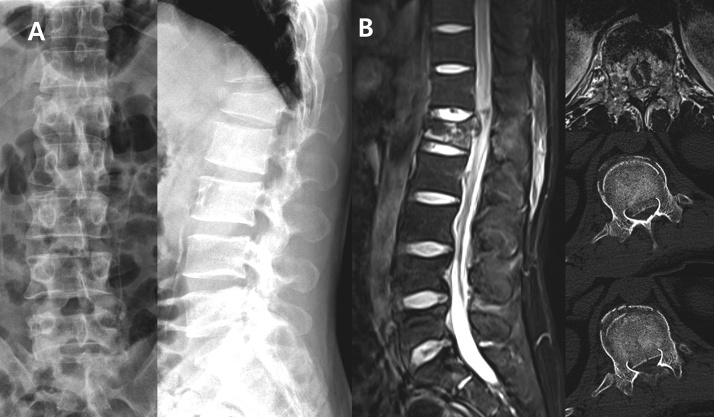
Fig. 1.
A. Anteroposterior and lateral radiographs showing a flexion-distraction injury at T12-L1 and L1 unstable burst fracture.
B. Posterior displacement of L1 body fracture fragment with compression of conus medullaris in MRI and CT.
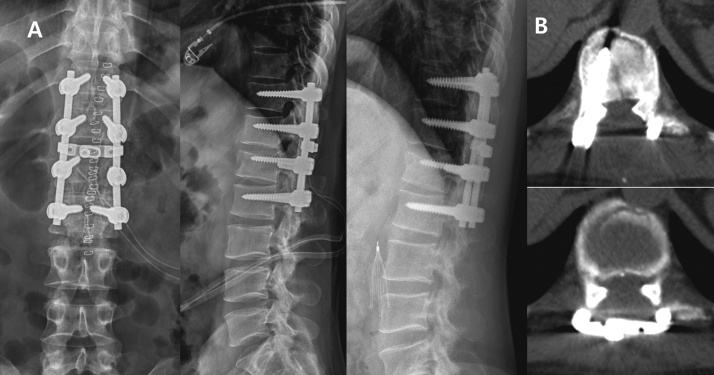
Fig. 2.
A. Decompression, reduction, and posterior fusion with pedicle screw instrumentation (T11-L2) and bone graft; last follow-up radiograph.
B. Decompression and fracture reduction was performed at the injury site.
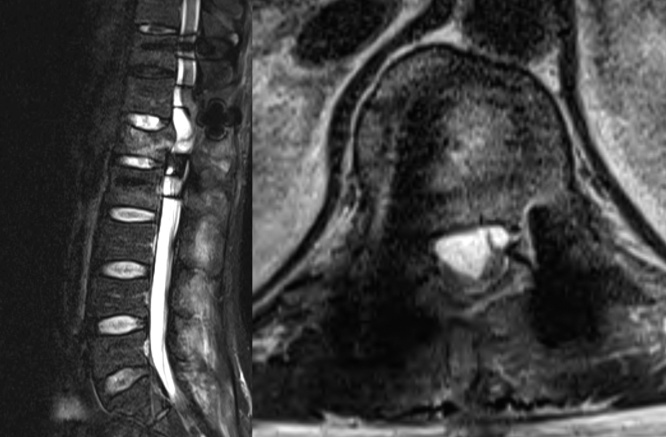
Fig. 3.
Conus medullaris and cauda equina presented characteristics of adhesive arachnoiditis (matted, clumped, empty thecal sac).
This retrospective study was approved by the Institutional Review Board of the Sanggye Paik Hospital and informed written consent was obtained from the patient for publication of this case report and accompanying images.
3. Discussion
Patients with adhesive arachnoiditis patients may develop spinal roots adhesions, a debilitating neurologic disorder with a complex and poorly understood pathophysiology [11]. The resulting inflammatory response eventually proliferates to other intrathecal neural elements, leading to fibrosis, adhesions, and vascular structure abnormalities which involve the nerve roots, the arachnoid, the spinal cord, and the dura mater [3]. Studies from the 2000s show contradictory findings regarding the association between spinal arachnoiditis and surgery or epidural injections [1,7]. In several studies, arachnoiditis was found to be a delayed postoperative complication [[12], [13], [14]]. In our study, after TL spinal surgery, there was an improvement in motor and sensory symptoms and a delayed onset of CES symptoms. We decompressed the lamina to relieve dural compression in the first surgery, while laminectomy was performed to increase the vascular permeability of the cauda equina. This increased permeability has been associated with cauda equina adhesion in rats [15]. In other study, foreign material retention accelerated arachnoiditis and peridural fibrosis post-laminectomy [16]. The delayed occurrence of spinal arachnoiditis is rare. Nevertheless, previous studies have reported cases of spinal arachnoiditis with a delayed symptom onset following spine procedures or diseases. Most of the associated spinal procedures were caudal blocks, epidural injections, and spinal anesthesia [13,14]. In our case, the patient had no history related to known causes of arachnoiditis like paraspinal injection or previous multiple operations other than a history of TL spinal cord injury and surgery.
Three-dimensional CT and MRI are cross-sectional imaging techniques that are more sensitive than radiographs and play an important role in the evaluation of patients after spine surgery. Nuclear medicine, CT, and MRI have a crucial role in the diagnosis and evaluation of infection and symptomatic new or recurrent disc herniation, peri- or epidural fibrosis, arachnoiditis, and radicular causes after spinal surgery [12]. Arachnoiditis has typical MRI findings such as variable enhancement of mass-like filling of the thecal sac, clumped nerve roots, and empty thecal sac with neural adhesions to the dural walls [9,10,12]. In our case, characteristic dural tissue, spinal cord, and cauda equina findings were seen in MRI. A previous study tested the correlation between anatomical changes and pain-related behavior in rats. Animals that underwent laminectomy were tested with systemic or intrathecal drugs to reduce straight leg raising positive test evaluated through pain vocalizations. Cauda equina clumping was related to pain severity [17]. However, specific analysis of myelographic patterns did not show any correlation with the clinical presentation [7,11]. Disturbance of CSF flow around the spinal cord is thought to produce syringomyelia but this patient did not present with this condition. Hardware material composition and size affect MRI artifacts. Titanium alloy is both less magnetic and dense than stainless steel, resulting in less streak artifacts from beam hardening and less distortion on MRI. Metal artifact reduction techniques have been developed, which can significantly improve image resolution, enabling early and precise detection of postoperative complications [12].
Treating arachnoiditis is difficult and complex; the cases are rare and hard to diagnose. There are multiple trials of treatment modalities for adhesive arachnoiditis. Surgery is a treatment option but is not always recommended. In a Japanese study, 36 surgeries were performed over 30 years; fusion and microlysis were found to be superior to microlysis only for symptomatic adhesive arachnoiditis. In a three-case series, expansive duraplasty was performed using a Gore-Tex surgical membrane and no postoperative neurological abnormalities were found [18]. However, around the spinal cord area, this experimental procedure may lead to a worse disease course.
There are various pain management modalities for adhesive arachnoiditis, such as nonsteroidal anti-inflammatory drugs, corticosteroids, antispasmodics, anticonvulsants, and opioids. However, there is no clear evidence about which is more effective. In an Ethiopian study, 507 patients with spinal cord syndrome and nontraumatic adhesive arachnoiditis showed no improvement after treatment with antibiotics and steroids [19]. In this case, after gabapentin administration, anal and penile pain dramatically improved. Gabapentin is a gamma-aminobutyric acid agonist previously used in an arachnoiditis trial which showed that patients with neuropathic pain related to arachnoiditis present improvement after gabapentin administration.Gabapentin normalizes the modulation of afferent stimuli in patients with dysautonomic symptoms by increasing the inhibitory drive within the spinal cord, preventing the onset of the efferent input in neuropathic pain [20]. Additionally, an experimental animal study about intrathecal gabapentin administration found decreased pain-related vocalizations in rats [17].
4. Conclusion
To the best of our knowledge, the association between spinal adhesive arachnoiditis suggestive of CES after incomplete cord injury due to traumatic TL spinal injury has never been reported. MRI is indispensable for the accurate identification of the affected spinal structures. Many surgeons and anesthetists are unaware that these sequelae exist because they appear after the patient has been discharged by the surgeon. We found that accidental spine trauma can cause delayed arachnoiditis; spine surgeons should, therefore, consider it as a possible cause. Gabapentin was found to relieve symptoms in this patient with arachnoiditis.
Declaration of Competing Interest
The authors report no declarations of interest.
Sources of funding
The authors have no conflict of interests to declare.
Ethical approval
This case report has been approved by institutional review board (IRB) of Sanggyepaik Hospital, Inje University with waived informed consent (SGPAIK2020-04-010).
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Author contribution
Dong-Ju, Lim- study concept, design, writing the paper and data interpretation.
Jong-min,Sohn- data collection, data analysis.
Registration of research studies
Institutional review board of Sanggye paik Hospital, Inje University with waived informed consent (SGPAIK2020-04-010).
I will send the documents if you want.
Guarantor
Dong-Ju, Lim.
Provenance and peer review
Not commissioned, externally peer-reviewed.
References
- 1.Rice I., Wee M., Thomson K. Obstetric epidurals and chronic adhesive arachnoiditis. Br. J. Anaesth. 2004;92:109–120. doi: 10.1093/bja/aeh009. [DOI] [PubMed] [Google Scholar]
- 2.Bourne I. Lumbo-sacral adhesive arachnoiditis: a review. J. R. Soc. Med. 1990;83:262–265. doi: 10.1177/014107689008300418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heary R.F., Northrup B.E., Barolat G. Arachnoiditis. In: Benzel E.C., editor. Spine Surgery. 2nd ed. Elsevier; Philadelphia, PA: 2005. pp. 2004–2012. [Google Scholar]
- 4.Long D.M. Chronic adhesive spinal arachnoiditis: pathogenesis, prognosis, and treatment. Neurosurg. Q. 1992;2:296–320. [Google Scholar]
- 5.Eisenberg E., Goldman R., Schlag-Eisenberg D., Grinfeld A. Adhesive arachnoiditis following lumbar epidural steroid injections: a report of two cases and review of the literature. J. Pain Res. 2019;12:513–518. doi: 10.2147/JPR.S192706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitt G.J., Stevens J.M. Postoperative arachnoiditis diagnosed by high resolution fast spin-echo MRI of the lumbar spine. Neuroradiology. 1995;37:139–145. doi: 10.1007/bf00588631. [DOI] [PubMed] [Google Scholar]
- 7.Benner B., Ehni G. Spinal arachnoiditis. The postoperative variety in particular. Spine (Phila Pa 1976) 1978;3:40–44. [PubMed] [Google Scholar]
- 8.Agha R.A., Borrelli M.R., Farwana R., Koshy K., Fowler A., Orgill D.P., For the SCARE Group The SCARE 2018 statement: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2018;60:132–136. doi: 10.1016/j.ijsu.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 9.Ross J.S., Masaryk T.J., Modic M.T., Delamater R., Bohlman H., Wilbur G. MR imaging of lumbar arachnoiditis. AJR Am. J. Roentgenol. 1987;149:1025–1032. doi: 10.2214/ajr.149.5.1025. [DOI] [PubMed] [Google Scholar]
- 10.Delamarter R.B., Ross J.S., Masaryk T.J., Modic M.T., Bohlman H.H. Diagnosis of lumbar arachnoiditis by magnetic resonance imaging. Spine (Phila Pa 1976) 1990;15:304–310. doi: 10.1097/00007632-199004000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Parenti V., Huda F., Richardson P.K., Brown D., Aulakh M., Taheri M.R. Lumbar arachnoiditis: does imaging associate with clinical features? Clin. Neurol. Neurosurg. 2020;192 doi: 10.1016/j.clineuro.2020.105717. [DOI] [PubMed] [Google Scholar]
- 12.Malhotra A., Kalra V.B., Wu X., Grant R., Bronen R.A., Abbed K.M. Imaging of lumbar spinal surgery complications. Insights Imaging. 2015;6:579–590. doi: 10.1007/s13244-015-0435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sghirlanzoni A., Marazzi R., Pareyson D., Olivieri A., Bracchi M. Epidural anaesthesia and spinal arachnoiditis. Anaesthesia. 1989;44:317–321. doi: 10.1111/j.1365-2044.1989.tb11285.x. [DOI] [PubMed] [Google Scholar]
- 14.Ploteau S., de Kersaint-Gilly A., Boog G. Medullar adhesive arachnoiditis: A. late complication after obstetrical epidural analgesia. Gynecol. Obstet. Fertil. 2004;32:961–964. doi: 10.1016/j.gyobfe.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Nakano M., Matsui H., Miaki K., Yamagami T., Tsuji H. Postlaminectomy adhesion of the cauda equina. Changes of postoperative vascular permeability of the equina in rats. Spine (Phila Pa 1976) 1997;22:1105–1114. doi: 10.1097/00007632-199705150-00010. [DOI] [PubMed] [Google Scholar]
- 16.Hoyland J.A., Freemont A.J., Denton J., Thomas A., McMillan J.J., Jayson M.I. Retained surgical swab debris in post-laminectomy arachnoiditis and peridural fibrosis. J. Bone Joint Surg. Br. 1988;70:659–662. doi: 10.1302/0301-620X.70B4.3403620. [DOI] [PubMed] [Google Scholar]
- 17.Kroin J.S., Buvanendran A., Cochran E., Tuman K.J. Characterization of pain and pharmacologic responses in an animal model of lumbar adhesive arachnoiditis. Spine (Phila Pa 1976) 2005;30:1828–1831. doi: 10.1097/01.brs.0000174276.73908.f0. [DOI] [PubMed] [Google Scholar]
- 18.Seki T., Hida K., Yano S., Iwasaki Y. Expansive duralplasty and subarachnoid reconstruction for spinal adhesive arachnoiditis using gore-tex surgical membrane. No Shinkei Geka. 2004;32:1247–1251. [PubMed] [Google Scholar]
- 19.Jenik F., Tekle-Haimanot R., Hamory B.H. Non-traumatic adhesive arachnoiditis as a cause of spinal cord syndromes. Investigation on 507 patients. Spinal Cord. 1981;19:140–154. doi: 10.1038/sc.1981.31. [DOI] [PubMed] [Google Scholar]
- 20.Aldrete J.A., Aldrete V.T., Vascello L.A. Gabapentin reduces neuropathic pain in arachnoiditis. Reg. Anesth. Pain Med. 1999;24:66. [Google Scholar]