Abstract
Background
To determine whether abnormal coagulation parameters are associated with disease severity and poor prognosis in patients with 2019 Corona Virus Disease (COVID-19).
Methods
A systematic literature search was conducted using the databases PubMed, Embase, and Web of sciences until April 25, 2020. We included a total of 15 studies with 2277 patients. Platelet count (PLT), prothrombin time (PT), activated partial thromboplastin time (APTT), D-dimer (D-D), and fibrinogen (FIB) were collected and analyzed. The statistical results were expressed as the effect measured by mean difference (MD) with the related 95% confidence interval (CI).
Results
The PLT level of severe cases was lower than that of mild cases, while the levels of PT, D-D, and FIB were higher than those of mild cases (P < 0.05). The level of APTT had no statistical difference between two groups (P > 0.05). PT of ICU patients was significantly longer (P < 0.05) than that of non-ICU patients. In non-survivors, PT and D-D were higher, yet PLT was lower than that of survivors (P < 0.05). There was no significant difference in APTT between survivors and non-survivors (P > 0.05). The funnel plot and Egger's regression test demonstrated that there was no publication bias.
Conclusions
Our data support the notion that coagulopathy could be considered as a risk factor for disease severity and mortality of COVID-19, which may help clinicians to identify the incidence of poor outcomes in COVID-19 patients.
Keywords: COVID-19, Coagulation parameters, Coagulopathy, Laboratory, Prognosis
Introduction
In early December 2019, a new coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) caused a catastrophic international phenomenon of the respiratory disease COVID-19 (Chan et al., 2020). This is the third serious coronavirus outbreak in less than 20 years, following severe acute respiratory syndrome (SARS) in 2002 and Middle East respiratory syndrome (MERS) in 2012 (Yang et al., 2020). Since the outbreak, this innovative type of pneumonia, far more contagious than SARS, has spread rapidly around the world, posing a serious threat to human life and health (Ungaro et al., 2020). Confirmed cases have been reported in 216 countries, areas, or territories. As of July, 23 2020, a total of 15,012,731 cases, including 619,150 deaths, have been reported worldwide (World Health Organization, 2020). Although about 15% of cases caused by human coronavirus strains are the common cold, SARS-COV-2 infection can have a variety of manifestations ranging in severity from influenza to death (Yang et al., 2020). Therefore, the identification of certain laboratory parameters that could distinguish between severe and non-severe cases, or between high and low risk of death, will help to improve the understanding of the clinical situation (Henry et al., 2020).
The most common manifestations of COVID-19 infection are fever, cough, and progressive dyspnea caused by respiratory infection (Wang et al., 2020a). Emerging evidence suggested that severe COVID-19 may be complicated with coagulopathy, and even severe cases may cause disseminated intravascular coagulation (DIC) (Kollias et al., 2020). Research report by Tang et al. (2020a) showed that 71.4% of patients who died of coronavirus met international society of thrombosis and hemostasis (ISTH) criteria for DIC. However, a recent study suggested that the characteristics of COVID-19-associated coagulopathy (CAC) are different from clotting disorders caused by bacterial infections and other diseases. CAC usually presents with elevated D-dimer (D-D) and fibrinogen (FIB) levels, but there are few abnormalities in the prothrombin time (PT) and platelet count (PLT) during the initial course of the disease (Iba et al., 2020a). In order to explore the relationship between coagulopathy and the severity and prognosis of the disease, we conducted this meta-analysis to compare the difference in blood coagulation parameters among COVID-19 patients.
Methods
Information sources and search strategy
We conducted a systematic review using PubMed, Embase, and Web of Science databases with the keywords “laboratory” in all fields AND “COVID-19” OR “2019 novel coronavirus disease” OR “COVID-19 pandemic” OR “2019 novel coronavirus infection” OR “2019-nCoV infection” OR “2019-nCoV disease” OR “COVID-19 virus infection” OR “wuhan coronavirus”, between 2019 and present time (i.e., April 25, 2020), with no language restrictions. The reference lists of selected studies were also checked for identifying additional eligible studies. All included studies were managed using the EndNoteX9.2 software and duplicates were removed.
Study selection
Our inclusion criteria included (1) study population: adult patients (>18 years of age) who were laboratory-confirmed or clinically diagnosed as infected with COVID-19; (2) study design: cross-sectional study, prospective/retrospective cohort study, case–control study, and randomized controlled trials. Our exclusion criteria included (1) asymptomatic patients; (2) studies without reporting coagulation parameters; (3) systematic reviews, meta-analyses, editorials, and other forms not presenting original data. The results of the initial search strategy were first screened by title and abstract to exclude apparently irrelevant articles. Remainings were delivered the full text to further screen based on inclusion and exclusion criteria. Two reviewers independently examined the literature, and when there was any disagreement, the opinion of a third researcher was sought to resolve it through discussion.
Data extraction and analysis
Two reviewers independently extracted the following data from the included references: patient basic characteristics (age and sex), clinical classification or clinical outcome and coagulation parameters. There were five coagulation parameters included: PLT, PT, activated partial thromboplastin time (APTT), D-D, and FIB. A third researcher checked the data extraction to ensure compliance with our inclusion criteria and the accuracy of the data. Continuous variables were presented as mean ± standard deviation (SD). If variables were represented by median and interquartile range (IQR), we used the Excel software to convert them to the form of mean ± SD. The data were meta-analyzed using Revman5.3 software provided by the Cochrane collaboration. Statistical results were expressed as the effect measured by mean difference (MD) with the related 95% confidence interval (CI). Heterogeneity analysis of the included studies was carried out by I2, an indicator in percentages used to determine whether the fixed-effect model or random-effect model was applied. “I2 > 50%” considered the heterogeneity to be statistically significant that the random-effect model was adopted for analysis; otherwise, the fixed-effect model was selected. The level of meta-analysis was equal to 0.05.
Assessment of methodological quality and risk of bias
The AXIS tool (Downes et al., 2016) was used to score the methodological quality of included studies, which is a critical appraisal tool to assess study design, reporting quality, and the risk of bias in cross-sectional studies (Ma et al., 2020). The components of the AXIS tool consist of 20 questions, each of which could be answered “yes” (1 point) or “no or don't know/comment” (0 point). A funnel plot was developed using Stata 12.0 software to assess publication bias. Meanwhile, Egger’s regression test was applied to make a quantitative analysis of publication bias.
Results
Study selection and characteristics
The initial search identified 1209 potentially relevant citations through PubMed database and 43 through other sources (Figure 1 ). After eliminating the duplicated literature as well as reading titles and abstracts, 39 articles were screened out for full-text assessment. Of these, 28 were excluded for reasons listed in Figure 1. Four additional studies were identified by reading the reference lists of the selected documents, thus the pooled analysis finally included 15 studies (Tang et al. (2020a); Gao et al. (2020); Liu et al., 2020a, Liu et al., 2020b; Wan et al., 2020; Xu et al., 2020; Zhang et al., 2020a, Zhang et al., 2020b; Huang et al., 2020; Lei et al., 2020; Peng et al., 2020; Wang et al., 2020b; Tang et al., 2020b; Jin et al., 2020; Zhou et al., 2020). We listed the basic characteristics and quality score of each study included in Table 1 . All the studies were cross-sectional studies conducted in China, involving a total of 2277 patients with sample sizes ranging from 30 to 449. Among them, seven studies were included to evaluate differences in coagulation function between mild and severe cases, four between ICU and non-ICU patients and five between survivors and non-survivors. All the statistical results are presented in Table 2 , as well as visually displayed through the forest plots.
Figure 1.
Study selection and characteristics.
Table 1.
Characteristics of the included studies on COVID-19, 2020.
| Author | N | Males [N (%)] | Mean age | Quality score | Country |
|---|---|---|---|---|---|
| Gao Y et al. | 43 | 26(60.5) | – | 17 | China |
| Liu M et al. | 30 | – | – | 14 | China |
| Liu Y et al. | 76 | 49(64.5) | 45 | 17 | China |
| Wan S et al. | 135 | 72(53.3) | 46 | 18 | China |
| Xu B et al. | 145 | 76(52.4) | – | 18 | China |
| Zhang G et al. | 221 | 108(48.9) | 53 | 16 | China |
| Zhang J et al. | 140 | 71(50.7) | 56 | 16 | China |
| Huang C et al. | 41 | 30(73.2) | 49 | 19 | China |
| Lei S et al. | 34 | 14(41.2) | 54 | 18 | China |
| Peng Y et al. | 112 | 53(47.3) | 61 | 16 | China |
| Wang D et al. | 138 | 75(54.3) | 55 | 19 | China |
| Li D et al. | 183 | 98(53.6) | 54 | 17 | China |
| Tang N et al. | 449 | 268(59.7) | 65 | 18 | China |
| Wang L et al. | 339 | 166(49.0) | 70 | 19 | China |
| Zhou F et al. | 191 | 119(62.3) | 56 | 16 | China |
–, Not available, not reported.
Table 2.
Results of meta-analysis comparing coagulopathy in COVID-19 patients with and without severe illness or mortality.
| Coagulation parameters | Mild vs severe |
Non-ICU vs ICU |
Survival vs Non-survival |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #of studies (#of ptsa) | MD (95%CI) | I2 | Cochran’s Q P-value | #of studies (# of ptsa) | MD (95%CI) | I2 | Cochran’s Q P-value | #of studies (# of ptsa) | MD (95%CI) | I2 | Cochran’s Q P-value | |
| PLT(×109/L) | 2 (356) | 16.63 (0.39,32.86) | 0% | 0.04 | 3 (213) | −0.19 (−20.22,19.85) | 0% | 0.99 | 3 (979) | 51.47 (38.41,64.54) | 0% | <0.001 |
| PT(s) | 5 (620) | −0.50 (−0.97,−0.03) | 82% | 0.04 | 4 (325) | 0.54 (0.13,0.95) | 0% | 0.01 | 5 (1307) | −1.10 (−1.37,−0.83) | 38% | <0.001 |
| APTT(s) | 4 (475) | −1.15 (−3.59,1.30) | 75% | 0.36 | 4 (325) | −0.59 (−1.84,0.67) | 0% | 0.36 | 2 (522) | −2.42 (−5.84,1.01) | 70% | 0.17 |
| D-D(mg/L) | 7 (790) | −0.83 (−1.31,−0.34) | 84% | <0.001 | 3 (213) | 3.51 (−7.40,14.41) | 85% | 0.53 | 5 (1307) | −6.01 (−8.99,−3.03) | 81% | <0.001 |
| FIB(g/L) | 2 (119) | −0.76 (−1.20,−0.32) | 0% | <0.001 | – | – | – | – | – | – | – | – |
–, Not available, not reported.
pts: patients.
Meta-analysis results
Coagulation parameters between mild and severe cases
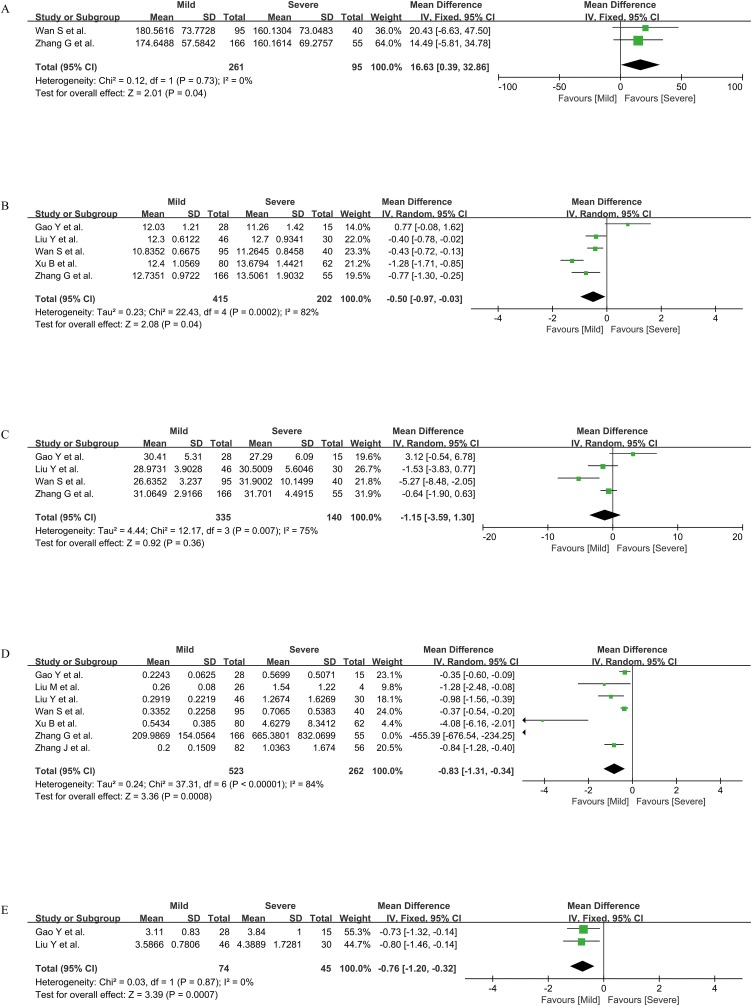
Five indicators of PLT, PT, APTT, D-D, and FIB were compared between mild and severe cases, including two, five, four, seven, and two studies respectively. According to the I2 value, the fixed-effect model was adopted for the statistical analysis of PLT and FIB (both I2 = 0), and the random-effect model was adopted for the statistical analysis of PT, APTT, and D-D (I2 = 82%, 75%, and 84%, respectively). The results showed that the PLT level of mild cases was higher than that of severe cases [MD = 16.63, 95%CI = (0.39, 32.86), P < 0.05]. PT, D-D, and FIB of mild cases were all lower than those of severe cases [MD = −0.50, 95%CI = (−0.97, −0.03), P < 0.05; MD = −0.83, 95%CI = (−1.31, −0.34), P < 0.05; MD = −0.76, 95%CI = (−1.20, −0.32), P <0.01; separately]. There was no significant difference in APTT between the two groups [MD = −1.15, 95%CI = (−3.59, 1.30), P > 0.05] (Figure 2 ).
Figure 2.
Coagulation parameters PLT (A), PT (B), APTT (C), D-D (D), and FIB (E) between mild and severe cases.
Coagulation parameters between ICU and non-ICU patients
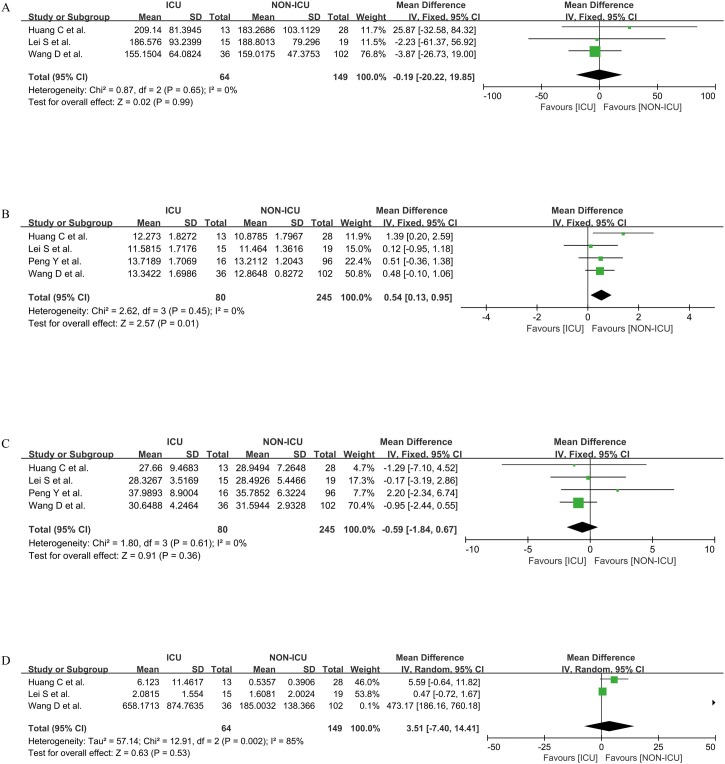
Four parameters of PLT, PT, APTT, and D-D were carried out by quantitative synthesis between ICU and non-ICU patients, which separately involved three, four, four, and three research studies. We used the fixed-effect model to calculate the differences in PLT, PT, and APTT between the two groups (all I2 = 0) and the random-effect model to calculate the D-D difference between the two groups (I = 85%). We found that PLT, APTT, and D-D between ICU and non-ICU patients had no statistical difference [MD = −0.19, 95%CI = (−20.22, 19.85), P > 0.05; MD = −0.59, 95%CI = (−1.84, 0.67), P > 0.05; MD = 3.51, 95%CI = (−7.40, 14.41), P > 0.05; respectively]. The PT of ICU patients was higher than that of non-ICU patients [MD = 0.54, 95%CI = (0.13, 0.95), P < 0.05] (Figure 3 ).
Figure 3.
Coagulation parameters PLT (A), PT (B), APTT (C), and D-D (D) between ICU and non-ICU patients.
Coagulation parameters between survivors and non-survivors
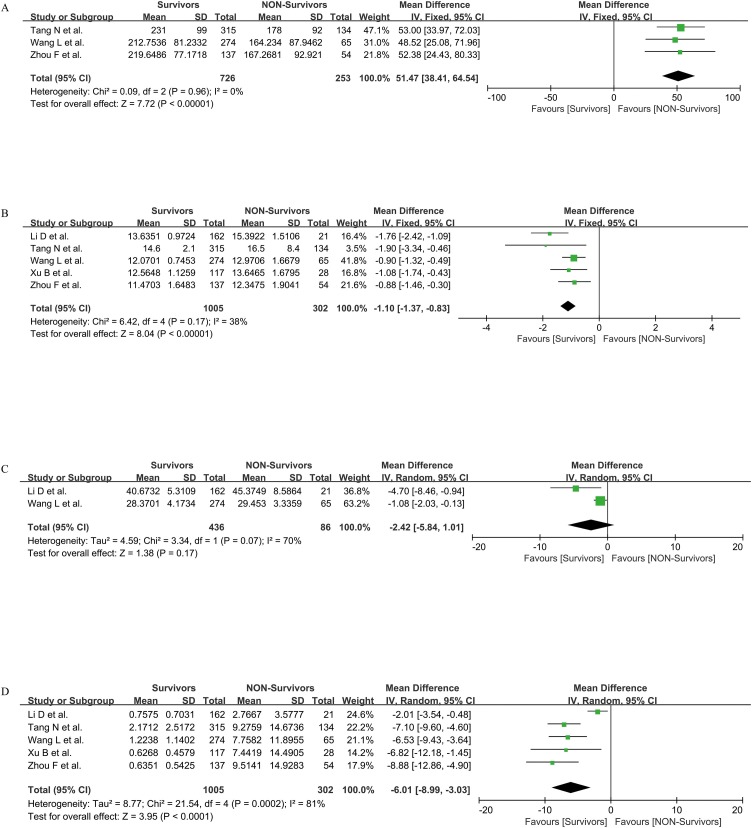
We evaluated four indicators of PLT, PT, APTT, and D-D to investigate the coagulation function between survivors and non-survivors, including three, five, two, and five studies respectively. Referred to the I2 value, we used the fixed-effect model to compare the differences in PLT and PT between the two groups (I2 = 0, 38%, separately) and the random-effect model to compare APTT and D-D between the two groups (I = 70%, 81%, separately). The statistics showed that the PLT level of survivors was higher than that of non-survivors [MD = 51.47, 95%CI = (38.41, 64.54), P < 0.001]. PT and D-D of survivors were both lower than those of non-survivors [MD = −1.10, 95%CI = (−1.37, −0.83), P < 0.001; MD = −6.01, 95%CI = (−8.99, −3.03), P < 0.001; separately]. There was no significant difference in APTT between survivors and non-survivors [MD = −2.42, 95%CI = (−5.84, 1.01), P > 0.05] (Figure 4 ).
Figure 4.
Coagulation parameters PLT (A), PT (B), APTT (C), and D-D (D) between survivors and non-survivors.
Publication bias
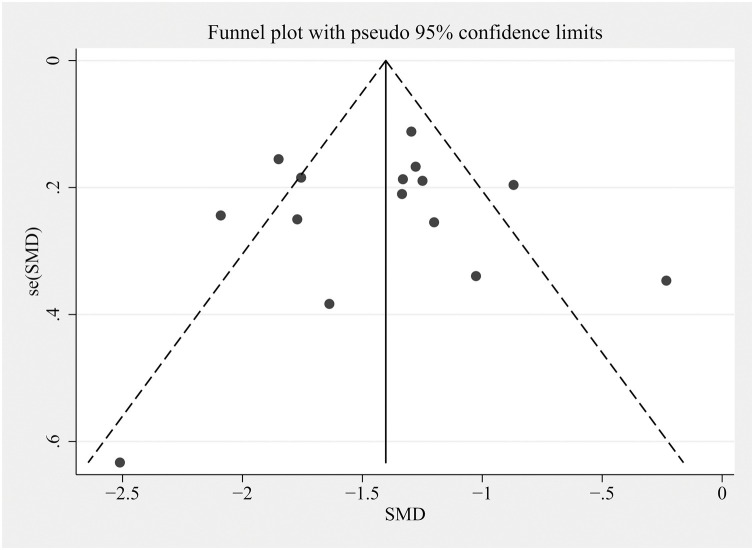
Studies comparing the D-D indicator were used to draw a funnel plot (Figure 5 ) for the analysis of publication bias. The selected research studies were distributed in the plot in a basically symmetrical way, indicating that the possible bias was small. For further quantitative analysis, we conducted Egger’s regression test (P = 0.923) and confirmed that there was no statistically significant evidence of publication bias (Table 3 ).
Figure 5.
Funnel plot comparing the level of D-D indicators among patients.
Table 3.
Egger’s test.
| Std-Eff | Coef. | Std. Err. | t | P>| t | | [95% Conf. | Interval] |
|---|---|---|---|---|---|---|
| Slope | −1.376091 | 0.2850025 | −4.83 | 0.000 | −1.991801 | −0.7603804 |
| Bias | −0.138335 | 1.408603 | −0.10 | 0.923 | −3.181437 | 2.904767 |
Discussion
Although the mortality rate of this novel coronary pneumonia is lower than that of SARS and MERS, the risk of severe and critically ill patients progressing to acute respiratory distress syndrome (ARDS) and being admitted to ICU still remains fairly high (Han et al., 2020). There is an urgent need to identify a few indicators for the early diagnosis of disease progression and prognosis in order to provide more appropriate treatment options. Studies have shown that the cytokines IL-6 and procalcitonin can be used to predict the severity of COVID-19 (Iba et al., 2020a, Lippi and Plebani, 2020). Currently, emerging research studies from Wuhan, China, suggest that severe or critically ill COVID-19 patients may develop coagulation disorders and increase the risk of thromboembolic events (Patel and Sengupta, 2020, Chen et al., 2020, Yin et al., 2020a). Professor Taisheng Li (Li et al., 2020) pointed out that COVID-19 patients showed obvious abnormal coagulation functions. The study of Zhai et al. (2020) similarly demonstrated that nearly 20% of patients with COVID-19 had severe coagulation abnormalities, for which the clinical types were all severe or critical.
Some researchers concluded that COVID-19 can activate the coagulation cascade through a variety of mechanisms, resulting in a severe hypercoagulable state and secondary DIC (Lin et al., 2020). The current view is that SARS-COV-2 enters host cells through the cell surface receptor, ACE2. This process leads to local inflammation, endothelial activation, tissue damage, and cytokine release changes that lead to coagulation activation (Connors and Levy, 2020, Leisman et al., 2020). Another perspective is that the virus interferes, directly or indirectly, with the clotting pathways. The susceptibility of these two pathways to coagulation disorders is mainly related to host factors such as age, comorbidities, and degree of lung injury (Marchandot et al., 2020). However, when the coagulation function is excessively activated and a large amount of coagulation factors are over-consumed, it will stimulate secondarily severe DIC, cause a fatal threat to the body, and have a significant negative impact on prognosis (Kawano et al., 2020). Recently, it has been found that with the aggravation of clinical symptoms and chest imaging, PLT gradually decreased, D-D gradually increased, and PT gradually prolonged. These changes are consistent with the pathological process of DIC (Li et al., 2020). Most of the current studies only use D-D as an indicator of disease progression (Tang et al., 2020a, Zhou et al., 2020, Pryzdial et al., 2020). Yet our quantitative synthesis showed that the coagulation status of critically ill or dead patients was worse than that of lightly infected patients, including increased D-D, decreased PLT, and prolonged PT. This suggests that attention should be paid to anticoagulation therapy for COVID-19 patients.
COVID‐19 usually does not cause PT to be prolonged for more than 3 seconds, PLT to <100 × 109/L or FIB to <1 gm/L. Therefore, COVID-19 coagulopathy does not lead to a bleeding state, but to a thrombotic state (Pryzdial et al., 2020). Although the role of anticoagulant therapy in inhibiting progression of disease and reducing death is not yet clear, most hospitals have begun to use preventive medium doses or even therapeutic doses of anticoagulant therapy for specific patients to prevent potential thrombosis complications (Patel and Sengupta, 2020, Proctor et al., 2020, Yin et al., 2020b). The study by Liu et al. (2020c) demonstrated that when treated with heparin molecules, not only did heparin act as an anticoagulant, but it also bound to the SARS-COV-2 spike protein, preventing the virus from binding to the receptor cells. A notable problem is that patients with severe COVID-19 are more likely to show gastrointestinal abnormalities (Jin et al., 2020, Iba et al., 2020b). Anticoagulant therapy increases the risk of gastrointestinal bleeding and exacerbates the underlying bleeding condition in COVID-19 patients (Schelleman et al., 2011, Hansen et al., 2010). Therefore, we recommend screening for coagulation tests at the time when patients were admitted to hospitals or early in the course of the disease. At the same time, the dynamic changes in coagulation parameters should always be paid attention to during the treatment process, so as to adjust the patients’ anticoagulation treatment plan in real time. If PT, D-D as well as other indicators are increased, it may warn patients to get worse and have a poor prognosis.
Our research has several limitations including the following: first, the articles are biased in the nature of observation during statistical analysis. Second, few studies on SARS-CoV-2 infection have divided the cohort into ICU and non-ICU patients, as well as survivors and non-survivors, which limited the number of studies and related patients included in our meta-analysis. Third, we could not exclude patients with basic coagulopathy from the study.
In conclusion, this study demonstrated the benefits of screening abnormal coagulation parameters such as decreased PLT levels, prolonged PT, and elevated D-D and FIB levels for predicting the severity and prognosis of COVID-19. We suggest clinicians to pay attention to changes in blood coagulation parameters of COVID-19 patients and explore their potential guidance for therapy.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics
The study does not require ethical approval because the meta-analysis are based on published research and the original data are anonymous.
Conflict of interest
The authors declared that they have no conflicts of interest to this work.
CRediT authorship contribution statement
Aining Zhang: Writing - original draft, Data curation, Formal analysis. Yan Leng: Writing - original draft, Data curation, Formal analysis. Yi Zhang: Data curation, Software. Kefan Wu: Data curation, Software. Yelong Ji: Data curation, Software. Shaoqing Lei: Methodology. Zhongyuan Xia: Writing - review & editing.
References
- Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes M.J., Brennan M.L., Williams H.C., Dean R.S. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS) BMJ Open. 2016;6(12) doi: 10.1136/bmjopen-2016-011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Li T., Han M., Li X., Wu D., Xu Y., et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020;92(7):791–796. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., Yang L., Liu R., Liu F., Wu K.L., Li J., et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58(7):1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- Hansen M.L., Sørensen R., Clausen M.T., Fog-Petersen M.L., Raunsø J., Gadsbøll N., et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;170(16):1433–1441. doi: 10.1001/archinternmed.2010.271. [DOI] [PubMed] [Google Scholar]
- Henry B., M.; de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58(7):1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba T., Levy J.H., Connors J.M., Warkentin T.E., Thachil J., Levi M. The unique characteristics of COVID-19 coagulopathy. Crit Care. 2020;24(1):360. doi: 10.1186/s13054-020-03077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba T., Levy J.H., Levi M., Thachil J. Coagulopathy in COVID-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Lian J.S., Hu J.H., Gao J., Zheng L., Zhang Y.M., et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano N., Wada H., Uchiyama T., Kawasugi K., Madoiwa S., Takezako N., et al. Analysis of the association between resolution of disseminated intravascular coagulation (DIC) and treatment outcomes in post-marketing surveillance of thrombomodulin alpha for DIC with infectious disease and with hematological malignancy by organ failure. Thromb J. 2020;18:2. doi: 10.1186/s12959-020-0216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollias A., Kyriakoulis K.G., Dimakakos E., Poulakou G., Stergiou G.S., Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: Emerging evidence and call for action. Br J Haematol. 2020;189(5):846–847. doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S., Jiang F., Su W., Chen C., Chen J., Mei W., et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020 doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisman D.E., Deutschman C.S., Legrand M. Facing COVID-19 in the ICU: vascular dysfunction, thrombosis, and dysregulated inflammation. Intensive Care Med. 2020;46(6):1105–1108. doi: 10.1007/s00134-020-06059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Lu H., Zhang W. Clinical observation and management of COVID-19 patients. Emerg Microbes Infect. 2020;9(1):687–690. doi: 10.1080/22221751.2020.1741327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Lu L., Cao W., Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9(1):727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G., Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505 doi: 10.1016/j.cca.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., He P., Liu H.G., Wang X.J., Li F.J., Chen S., et al. Clinical characteristics of 30 medical workers infected with new coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(3):209–214. doi: 10.3760/cma.j.issn.1001-0939.2020.03.014. [DOI] [PubMed] [Google Scholar]
- Liu Y., Liao W., Wan L., Xiang T., Zhang W. Correlation between relative nasopharyngeal virus RNA load and lymphocyte count disease severity in patients with COVID-19. Viral Immunol. 2020 doi: 10.1089/vim.2020.0062. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Liu J., Li J., Arnold K., Pawlinski R., Key N.S. Using heparin molecules to manage COVID-2019. Res Pract Thromb Haemost. 2020;4(4):518–523. doi: 10.1002/rth2.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L.L., Wang Y.Y., Yang Z.H., Huang D., Weng H., Zeng X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. 2020;7(1):7. doi: 10.1186/s40779-020-00238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchandot B., Trimaille A., Curtiaud A., Matsushita K., Jesel L., Morel O. Thromboprophylaxis: balancing evidence and experience during the COVID-19 pandemic. J Thromb Thrombolysis. 2020:1–10. doi: 10.1007/s11239-020-02231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P., Sengupta N. PPIs and beyond: a framework for managing anticoagulation-related gastrointestinal bleeding in the era of COVID-19. Dig Dis Sci. 2020;65(8):2181–2186. doi: 10.1007/s10620-020-06408-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y.D., Meng K., Guan H.Q., Leng L., Zhu R.R., Wang B.Y., et al. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48 doi: 10.3760/cma.j.cn112148-20200220-00105. [DOI] [PubMed] [Google Scholar]
- Proctor E.A., Dineen S.M., Van Nostrand S.C., Kuhn M.K., Barrett C.D., Brubaker D.K., et al. Coagulopathy signature precedes and predicts severity of end-organ heat stroke pathology in a mouse model. J Thromb Haemost. 2020;18(8):1900–1910. doi: 10.1111/jth.14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryzdial E.L.G., Sutherland M.R., Lin B.H., Horwitz M. Antiviral anticoagulation. Res Pract Thromb Haemost. 2020;4(5):774–788. doi: 10.1002/rth2.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelleman H., Brensinger C.M., Bilker W.B., Hennessy S. Antidepressant-warfarin interaction and associated gastrointestinal bleeding risk in a case-control study. PLoS One. 2011;6(6) doi: 10.1371/journal.pone.0021447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungaro R.C., Sullivan T., Colombel J.F., Patel G. What should gastroenterologists and patients know about COVID-19? Clin Gastroenterol Hepatol. 2020;18(7):1409–1411. doi: 10.1016/j.cgh.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S., Xiang Y., Fang W., Zheng Y., Li B., Hu Y., et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92(7):797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020. Coronavirus disease (COVID-19) outbreak situation.https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [Cited 25 July 2020]. Available at: [Google Scholar]
- Xu B., Fan C.Y., Wang A.L., Zou Y.L., Yu Y.H., He C., et al. Suppressed T cell-mediated immunity in patients with COVID-19: a clinical retrospective study in Wuhan, China. J Infect. 2020;81(1):e51–e60. doi: 10.1016/j.jinf.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Peng F., Wang R., Yange M., Guan K., Jiang T., et al. The deadly coronaviruses: the 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. 2020:1–4. doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. 2020:1–4. doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Z., Li C., Chen Y., Gerotziafas G., Zhang Z., Wan J., et al. Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: a consensus statement before guidelines. Thromb Haemost. 2020;120(6):937–948. doi: 10.1055/s-0040-1710019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Hu C., Luo L., Fang F., Chen Y., Li J., et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]