Dear Editor,
Identifying the significant parameters for early progression toward worse prognosis is fundamental for the management of COVID-19 patients.
In this Journal, Zhi Lin and colleagues1 recently reported that Chinese patients with severe Sars-CoV2 disease showed higher levels of serum ferritin than patients with not severe one, confirming data from other authors on Chinese2 , 3 and Caucasian populations.4 , 5
Here we aimed to establish the most suitable panel for routine prognostic serum laboratory testing in COVID-19 patients upon first admission to the Emergency Department.
We thus enrolled 141 patients (59 females and 82 males, aging 64,48 ± 16,58 years) diagnosed as COVID-19 by means of real-time polymerase chain reaction testing and admitted to the isolation ward of Emergency Department at Policlinico Umberto I Hospital in Rome, Italy, between March 2020 and June 2020. Serum samples were collected from patients upon admission before starting any treatment and tested by Laboratory Department.
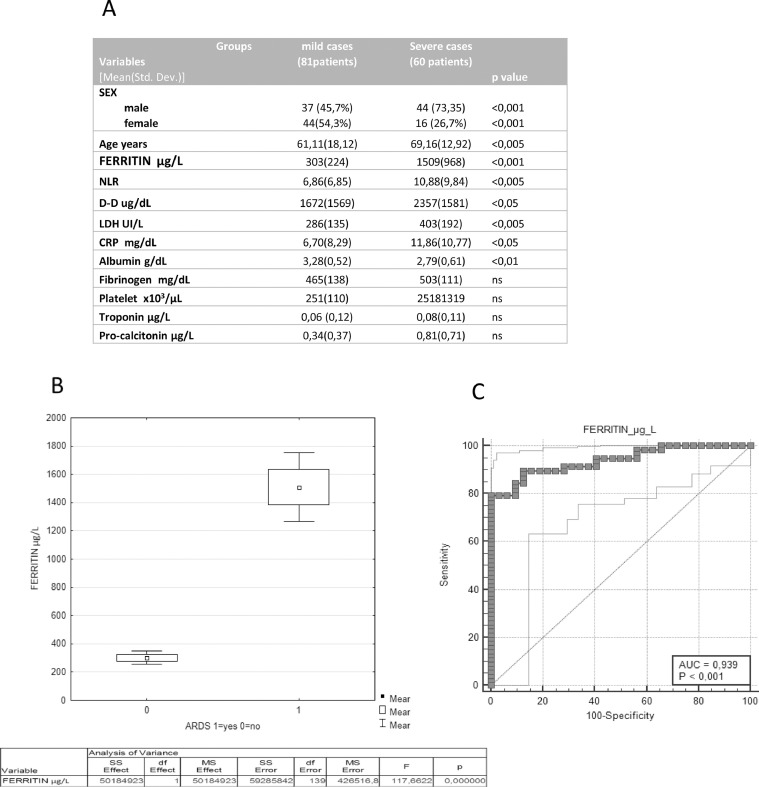
Of all patients included, 81 patients (57%) showed mild disease (control group) and 60 (43%) showed acute respiratory distress syndrome (ARDS) and systemic inflammation (severe group). Fig. 1 A shows the differences in the baseline characteristics between severe and non-severe COVID-19 patients. The severe patients were older and more frequently males and showed significant higher levels of C Reactive Protein (CRP), d-Dimer (D-D), Lactate Dehydrogenase (LDH), Neutrophil to Lymphocyte ratio (NLR) and Ferritin.
Fig. 1.
1A) Characteristics of the study population; 1B) Analysis of Variance of serum ferritin between severe (60) and non severe COVID-19 patients (81); 1C) ROC curve analysis of serum ferritin levels for the severity of COVID-19.
Serum ferritin levels were positively correlated with severity of COVID-19 (Fig. 1B) and hyperferritinemia (ferritin level > 500 µg/L), was observed in all patients with severe disease on admission. Moreover, ROC curve analysis confirmed the excellent prognostic accuracies of serum Ferritin in discriminate patients with severe clinical conditions. (AUC 0.939, CI: 0,894 to 0,985 p < 0.001) (Fig. 1C).
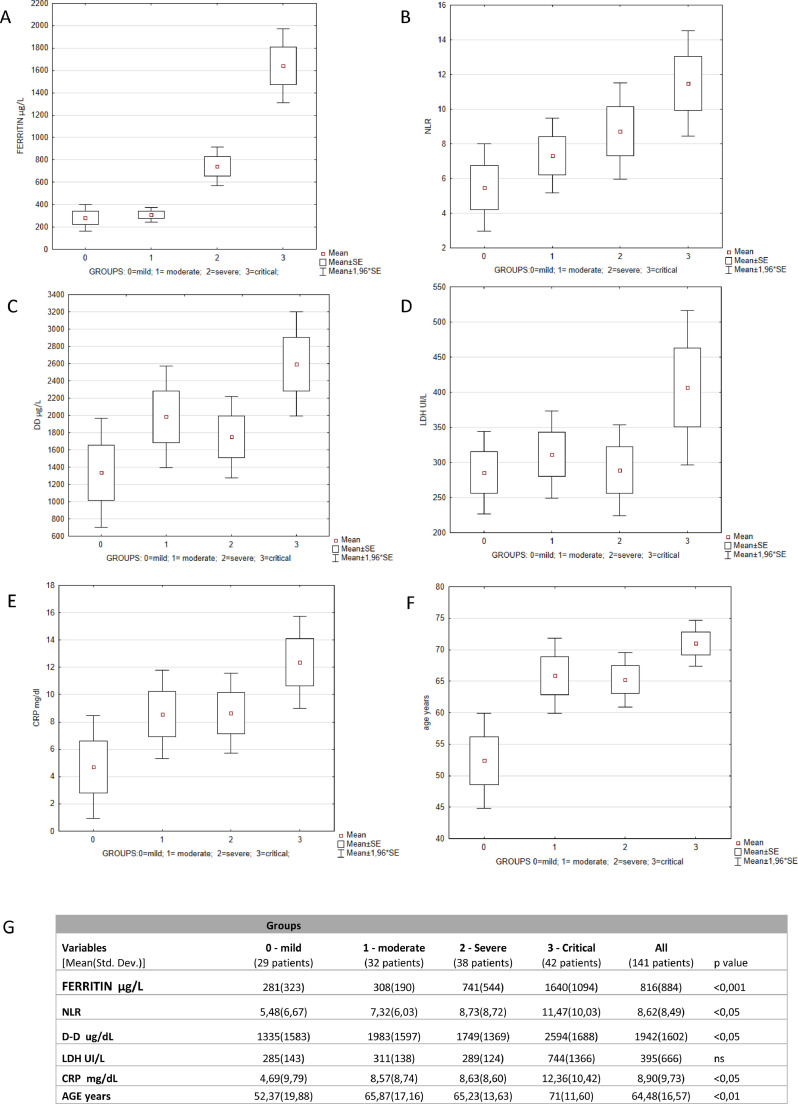
The triaging of COVID-19 patients is based on a combination of clinical, laboratory and instrumental parameters, mainly represented by Computed Tomography (CT). Thus, based on the severity of pulmonary impairment in CT scan and respiratory failure in need of mechanical ventilation, patients were further divided in 4 groups according to the WHO guidelines updated in May 20206: 29 patients with no CT alterations (Group 0-mild); 32 patients with changes in CT scan no oxygen (Group 1-moderate); 38 patients with CT scan plus oxygen (Group 2-severe) and 42 patients with CT abnormalities plus intensive care unit (ICU) admission (critical-Group 3).
Our data strongly confirm that increased levels of ferritin were directly related with the disease severity (Fig 2 A). Particularly, not only severe group showed 2.6 times higher ferritin levels than the mild group, but patients who needed admission to the ICU showed 5.8 times higher ferritin compared to patients with mild COVID-19. Among all parameters considered, we also noted that the NLR was statistically correlated with the severity of disease. (Fig. 2B). Conversely, D-D, LDH and CRP increased only in the group of critical patients (group 3), being substantially stable in the other groups characterized by mild, moderate and severe disease (Fig. 2, panel C, D, E).
Fig. 2.
2A–F) Analysis of Variance – Categorized box and whisker plot of ferritin, NLR, DD, LDH, CRP and age according to COVID-19 severity; 2 G) Analysis of Variance and concentrations of ferritin, NLR, D-D, LDH, CRP and age according to COVID-19 severity.
Multivariate logistic regression model adjusted for several disease-related risk factors at admission, including age, sex, NLR, D-D, LDH, ferritin and CRP, demonstrated that serum ferritin resulted as an independent predictor of disease severity in COVID-19 patients (OR = 1,0048, 95% CI, 1,0029 to 1,0083, p < 0,001.).
If patients were grouped according to the serum ferritin level with a cut off of 500 µg/ml derived from the HLH-20047 criterion, hyperferritinemia accounted for 48,22% (68/141) of patients and the hyperferritinemia group had a higher proportion of severe cases (77,94% vs 10,30%, p < 0,001) than patients without hyperferritinemia.
This is the first Italian report about the prognostic value of laboratory biomarkers considering 4 groups of mild, moderate, severe and critical patients with COVID-19. We clearly demonstrated that serum levels of ferritin progressively increased with the severity of disease and correlate with poor prognosis in COVID-19 patients.
Increased ferritin levels could be indicative of a strong inflammatory reaction in COVID-19 and recent studies suggest that increased levels of circulating ferritin levels play a critical role by contributing to the development of a cytokine storm8 , 9 resembling macrophage activating syndrome.10 Timely control of the cytokine storm in its early stage through immunomodulators and cytokine antagonists, as well as the reduction of lung inflammatory cell infiltration, is the key to improving the treatment success rate and reducing the mortality rate of patients with COVID-19. In this regard, ferritin evaluation could be an early, available and easy to use screening tool to assess the disease severity at the first admission in the emergency department. This test might be of crucial importance for the timely identification of patients at higher risk of an adverse outcome.
References
- 1.Lin Z., Long F., Yang Y. Serum ferritin as an independent risk factor for severity in COVID-19 patients. J Infect. 2020 Jun 24 doi: 10.1016/j.jinf.2020.06.053. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk Factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henry B.M., de Oliveira M., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020 doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 4.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020 doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu X.W., Wu X.X., Jiang X.G., Xu K.J., Ying L.J., Ma C.L. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical management of COVID-19 - interim guidance (May 2020)-WHO - https://reliefweb.int/sites/reliefweb.int/files/resources/2005_clinical_management_of_covid-19-v7.pdf
- 7.Henter J.I., Horne A., Aricó M. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 8.Kernan K.F., Carcillo JA</b> Hyperferritinemia and inflammation. Int Immunol. 2017;29(9):401–409. doi: 10.1093/intimm/dxx031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wessling-Resnick M. Crossing the iron gate: why and how transferrin receptors mediate viral entry. Annu Rev Nutr. 2018;38:431–458. doi: 10.1146/annurev-nutr-082117-051749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoenfeld Y. Corona (COVID-19) time musings: our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmun Rev. 2020 doi: 10.1016/j.autrev.2020.102538. [DOI] [PMC free article] [PubMed] [Google Scholar]