Abstract
To ensure the high purity and biological activity of the adenovirus vector to be used for clinical applications, a stable and linearly scalable preparation method is highly imperative. During the adenovirus-harvesting process, the Triton X-100-based lysis method possesses the advantages of higher efficiency as well as easier linearization and amplification. Most Triton X-100 can be removed from the adenovirus sample by chromatographic purification. However, there is no report that a small amount of residual Triton X-100, present in adenovirus sample, can affect the particle integrity, infectivity, and structure of adenoviruses. Here, we found that although residual Triton X-100 affected the short-term stability, purity, infectivity, and structure of adenoviruses at 37°C, it did not hamper these properties of adenoviruses at 4°C. This study suggests that although the Triton X-100-based lysis method is a simple, efficient, and easy-to-scale process for lysing host cells to release the adenovirus, the storage conditions of adenovirus products must be taken into consideration.
Keywords: adenovirus, Triton X-100, purification, stability
Graphical Abstract

The Triton X-100-based lysis method is a simple, efficient, and easy-to-scale process for lysing host cells to release the adenovirus. However, Ping Cheng and colleagues reported the residual Triton X-100 in purified adenovirus had different effects on the structural stability and infection activity of adenovirus particles under different temperature storage conditions.
Introduction
Gene therapy refers to the introduction of human normal genes or therapeutic genes into target cells in a certain way to either correct gene defects or exert therapeutic effects, thereby achieving the purpose of treating different diseases.1 The first clinical trial of human gene therapy was approved by the U.S. Food and Drug Administration (FDA) in 1990. Since then, many gene therapy-based clinical trials are carried out for the treatment of different diseases, including cancers, cardiovascular diseases, as well as metabolic diseases.2,3 Gene-therapy vectors can be divided into two types, namely viral vectors and nonviral vectors. Whereas nonviral vectors mainly include naked DNA, liposomes, nano-vectors, etc., viral vectors are majorly adenoviruses, adeno-associated viruses, retroviruses, lentiviruses, and herpes.4, 5, 6 Viral vectors, possessing high gene transduction efficiency and good biocompatibility, are the most common gene therapy vectors used in clinical trials. Among viral vectors, adenovirus can easily infect a wide range of dividing and nondividing host cells, and it does not integrate with the host cell genome, leading to avoidance of the risk of insertion mutations.7 Therefore, adenovirus is frequently used for the gene therapy-based clinical trials to treat several diseases, such as cancers, cardiovascular diseases, central nervous system diseases, infectious diseases, etc.8, 9, 10, 11, 12
One of the bottlenecks of gene therapy is the large-scale production of clinical-grade viral vectors with high purity and biological activity. The preparation process of viral vectors should be highly stable and amenable for scaling up.13 The production of adenoviral vectors consists of two kinds of processes, namely upstream and downstream. Whereas the upstream process focuses on adenovirus amplification and harvesting of viral fluid, the downstream process is involved with purification of adenovirus.14,15 Adenovirus can infect HEK293 or PER.C6 host cells and replicate within the cells, ultimately leading to lyse the infected cells. Adenovirus lysis characteristics allow two different modes of virus production. The first mode is to harvest the virus by lysing host cells employing external factors prior to the cell lysis.16 The second method is to harvest cells after almost complete lysis of virus supernatant. In the case of the latter method, a longer culture time is required for the cells to be completely lysed. The prolonged culture of cells after virus infection can enhance the production of replication-capable adenovirus (RCA). However, the long-term exposure of adenovirus, released in the medium, to the higher temperature can reduce its infectivity. Therefore, in the present study, we have adopted a process of harvesting infected cells before cell lysis and employed external factors to lyse the host cells for the release of virus.
The conventional methods of lysing host cells are a repeated freeze-thaw method, solid shear method, hypotonic solution lysis method, liquid shear method, ultrasonic disruption method, detergent lysis method, and others. The disadvantages of a repeated freeze-thaw method include lengthy time-consuming processing, limited production of small batches of adenovirus, as well as incomplete cell lysis, leading to incomplete release of adenovirus.16,17 Contrastingly, the shearing method needs special equipment, requiring a large amount of investment and causing exogenous contamination of the adenovirus samples. Similarly, the ultrasonic disruption method also requires special equipment and a large investment. Moreover, the adenovirus samples can easily be contaminated during this process. Also, the hypotonic solution-based lysis method has a low efficiency in lysing the cells, and scale-up production cannot be achieved. Contrastingly, the detergent-mediated lysis method is simple, economical, easy to produce on a large scale, and widely employed for the preparation of a variety of viral vectors.18
In the present study, during an upstream process for large-scale production of adenovirus, we have used Triton X-100 to lyse the host cells for complete release of the adenovirus. Most of the Triton X-100 in the cell lysate can be removed by subsequent chromatographic purification processes, but the trace amount of Triton X-100 can remain in the purified adenovirus sample. However, no report demonstrates the effect of this residual Triton X-100 on the properties of adenovirus samples. Herein, we have thoroughly investigated the effect of residual Triton X-100 on the short-term stability, purity, infectivity, and structure of purified adenovirus samples, employing different analytical techniques.
Results
Effect of Triton X-100 on the Purification Process of Adenovirus
To examine the effect of Triton X-100 on the purification process of adenovirus, cell supernatant and cell supernatant along with the cell pellet of adenovirus fermentation broth were treated with or without 0.25% Triton X-100 lysis buffer, followed by their purification using ultrafiltration and ion-exchange methods. After chromatographic purification, the light absorption values of the adenovirus, eluted at 260 nm and 280 nm were found to be higher for samples with residual Triton X-100 (obtained through a Triton X-100-based lysis method) as compared to that of samples without residual Triton X-100 (obtained through a repetitive freeze-thaw method) (Figures 1A and 1B) (∗∗∗p < 0.001). However, the immunohistochemistry study exhibited that the total amount of virus in the cell-culture supernatant was similar in the presence and absence of Triton X-100 lysis buffer before purification, but there was a significant difference in the total amount of virus in the corresponding cell-sediment samples (Figures 1C and 1D) (∗∗p < 0.01). Even after the purification process, the amount of virus in cell-sediment samples in the presence of Triton X-100 lysis buffer was more than that obtained by a repeated freeze-thaw method (Figures 1E and 1F) (∗p < 0.05). Altogether, the results suggest that the Triton X-100 lysis method lysed the host cells more completely and released more viruses as compared to the repeated freeze-thaw method.
Figure 1.
Effect of Triton X-100 on the Purification of Adenovirus
(A) The representative chromatogram of adenovirus fermentation broth. (B) Statistical analysis of adenoviral peak integral area. (C) Corresponding representative immunohistochemistry staining images of adenovirus fermentation broth. (D) Statistical analysis of total infectious virus titers of various adenovirus fermentation broth (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001). (E) Corresponding representative immunohistochemistry staining images of adenovirus elutes. (F) Statistical analysis of total infectious virus titers of various adenovirus elutes (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001).
Determination of Residual Triton X-100 Concentration in Adenovirus Samples
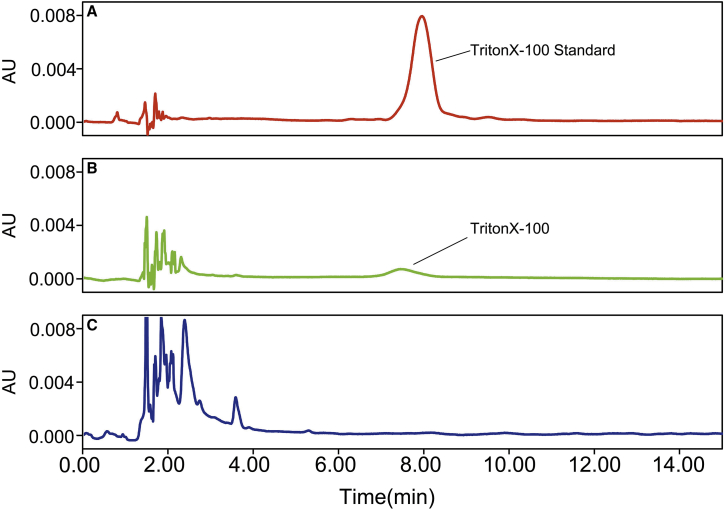
A series of Triton X-100 standards was prepared to determine the residual Triton X-100 concentration in adenovirus samples using high-performance liquid chromatography (HPLC). After analysis on a C18 column, a single Triton X-100 chromatographic peak appeared for a standard sample at 7.0 min (Figure 2A). A single Triton X-100 chromatographic peak also appeared at about 7.0 min for the purified adenovirus sample with residual Triton X-100, and the residual Triton X-100 concentration was 0.17 ± 0.0073 ng/μL (Figure 2B). The concentration of Triton X-100 was obtained by drawing a standard curve with the peak area as the ordinate and the concentration as the abscissa. As per the standard curve, a linear regression equation, y = 25,714x + 20,045 (R2 = 0.9999), was established. The purified adenovirus sample (obtained through the freeze-thaw method) without Triton X-100 did not show a chromatographic peak at 7.0 min (Figure 2C).
Figure 2.
Determination of Residual Triton X-100 in Adenovirus Samples Employing C18 HPLC
(A) The chromatogram of Triton X-100 lysis buffer standard. (B) The chromatogram of pure adenovirus containing residual Triton X-100. (C) The chromatogram of pure adenovirus without residual Triton X-100.
Effect of Residual Triton X-100 on the Absorbance Value and Number of Virus Particles of Adenovirus Samples
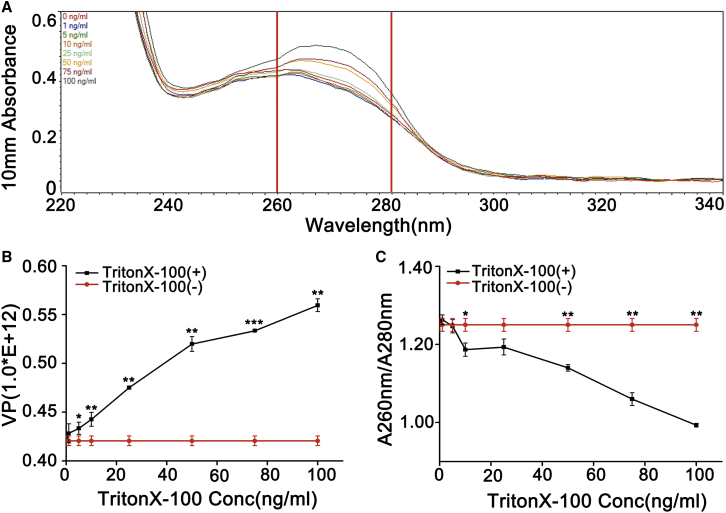
To examine the effect of residual Triton X-100 on absorbance and number of virus particles of pure adenovirus samples, a different concentration of Triton X-100 was added to the pure adenovirus samples without Triton X-100. UV-visible spectroscopy detected the light absorption of samples with equal adenovirus particle content in the wavelength range of 260 nm to 280 nm. The result exhibits that the absorption values were increased with the increasing concentration of addition of Triton X-100 (Figure 3A). According to the detected absorbance value, the number of virus particles was also enhanced significantly with increasing concentration of Triton X-100 added to adenovirus samples as compared to adenovirus samples without Triton X-100 (Figure 3B) (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001). The purity of virus particles (A260 nm/A280 nm) was gradually decreased with increasing concentration of addition of Triton X-100 (Figure 3C) (∗p < 0.05, ∗∗p < 0.01).
Figure 3.
Effect of Triton X-100 on UV Absorbance of Pure Adenovirus
(A) Effect of different concentrations of Triton X-100 on UV absorption value of adenovirus products. (B) Effect of different concentrations of Triton X-100 on the number of virus particles. (C) Effects of different concentrations of Triton X-100 on the ratio of A260 nm/A280 nm in adenovirus preparations.
Effect of Triton X-100 on the Stability (Purity) of Adenovirus Particles
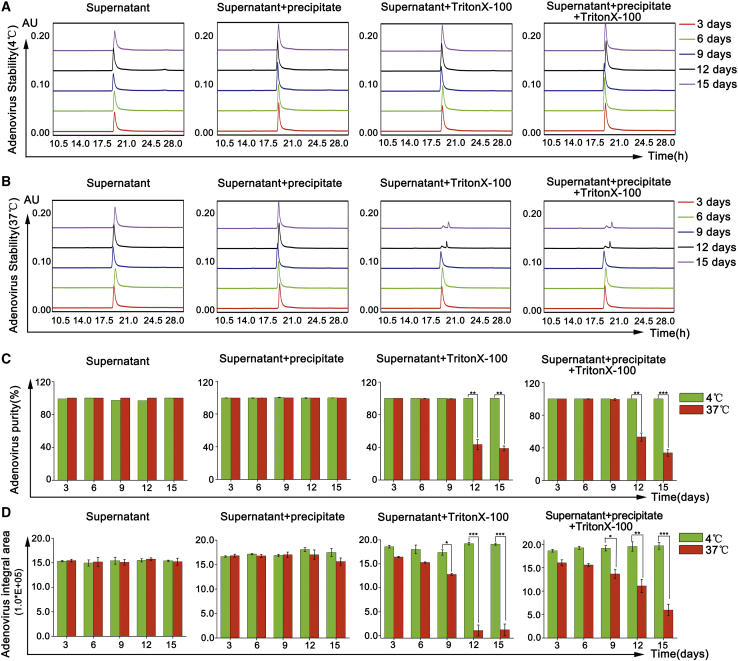
To evaluate the effect of Triton X-100 on the stability of the adenovirus particles, the purified adenoviruses were placed at 4°C and 37°C, and the purity of the adenovirus samples was detected using HPLC. The chromatograms of purified adenovirus samples with and without Triton X-100 residual at 4°C illustrated only a single peak (Figure 4A), suggesting that the presence of residual Triton X-100 had no significant effect on the stability of adenovirus particles in terms of peak purity and peak integrated area (Figures 4C and 4D) at 4°C up to the 15th day. However, for purified adenovirus samples with residual Triton X-100 at 37°C, either the peak integration surface was decreased, or double peaks appeared in chromatograms, particularly on the 9th, 12th, and 15th days (Figure 4B), and the stability of the virus particles (peak purity and integrated area) with and without Triton X-100 residual was found to be significantly different (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001) (Figures 4C and 4D). The results indicate that residual Triton X-100 can affect the stability (purity) of the adenovirus particles in a short time upon storing the adenovirus samples at 37°C.
Figure 4.
Effect of Residual Triton X-100 on the Stability (Purity) of Adenovirus Particles
(A) Representative HPLC chromatograms of adenovirus placed at 4°C. (B) Representative HPLC chromatograms of adenovirus placed at 37°C. (C and D) Statistical analysis of changes in peak purity (C) and integrated area (D) of the adenovirus products (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001).
Effect of Triton X-100 on Adenovirus Infection Activity
To evaluate the infection activity, the purified adenovirus samples were placed at 4°C and 37°C, followed by antibody staining. The results showed that there was no significant change in the infection activity of the adenovirus samples with and without Triton X-100 residual up to 15 days at 4°C (Figures 5A and 5C–5F). However, the adenovirus samples, with and without residual Triton X-100, demonstrated significantly different infection activity at 37°C (Figure 5B). The infection activity of purified adenoviruses with Triton X-100 residual started to decrease from the 3rd day onward at 37°C (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001), and the activity was basically lost on the 12th day (Figures 5B, 5E, and 5F). Interestingly, the infection activity of the purified adenovirus test products without the remnant Triton X-100 did not alter significantly with time at 37°C (p > 0.05) (Figures 5B–5D).
Figure 5.
Effect of Residual Triton X-100 on Infection Activity of Adenovirus
(A) Representative immunohistochemistry staining images of adenoviruses placed at 4°C. (B) Representative immunohistochemistry staining images of adenoviruses placed at 37°C. (C and D) Statistical analysis of changes in infection activity of Triton X-100-free adenovirus (C) and adenovirus with residual Triton X-100 (D) stored at 4°C. (E and F) Statistical analysis of changes in infection activity of Triton X-100-free adenovirus (E) and adenovirus with residual Triton X-100 (F) stored at 37°C (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001).
Effect of Additionally Added Triton X-100 and Residual Triton X-100 on the Stability of Triton X-100-Free Adenovirus Samples and Adenovirus Samples with Remnant Triton X-100
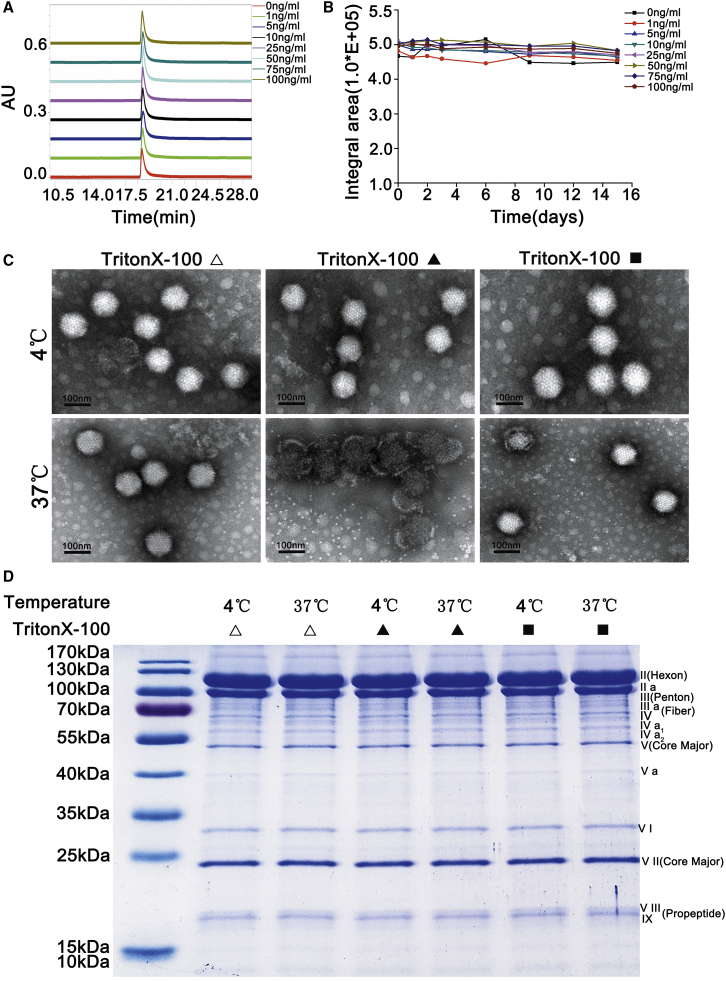
To explore whether Triton X-100 could directly interact with adenovirus components leading to adenovirus instability, Triton X-100 of different concentrations was added to the Triton X-100-free adenovirus, and the samples were placed at 37°C for 15 days. The purity of the samples was detected by HPLC to evaluate the effect of the addition of Triton X-100 on the stability of adenovirus particles. The chromatograms of the test products showed a single peak (Figure 6A), and there were no significant changes in the integrated area (Figure 6B).
Figure 6.
The Difference of the Effect of Residual Triton X-100 and Additionally Added Triton X-100 on the Stability of Adenovirus
(A) The HPLC chromatograms of the adenovirus samples additionally adding different concentrations of Triton X-100 showed a single peak. (B) Statistical analysis of the peak integral area of the virus particles (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001). (C) Transmission electron microscopy images exhibiting the structural changes of Triton X-100 free adenovirus (▵), adenovirus with additionally added Triton X-100 (▪) and adenovirus containing residual Triton X-100 (▴) at 4°C and 37°C. (D) The components of these adenoviruses were analyzed by SDS-PAGE.
Furthermore, the structure and composition of the virus particles were examined using an electron microscope and SDS-PAGE, respectively. The electron microscopy results exhibited that the adenovirus particles without Triton X-100 (▵) or additionally added Triton X-100 (▪) had a complete structure at 4°C and 37°C. Moreover, the crusts and cilia on the surface of the virus were clearly visible. The structure of the virus particles with residual Triton X-100 (▴) was also complete under the storage condition of 4°C. However, these virus particles were disintegrated substantially at 37°C, and a large number of scattered shell particles were also observed, as shown in Figure 6C. In the case of SDS-PAGE analysis, three different samples showed the same electrophoretic bands at 4°C and 37°C, and the protein composition of the virus particles remained almost unchanged (Figure 6D). The experimental results demonstrated that the residual Triton X-100 had a significant effect on the stability (purity, structure, and components) of purified adenovirus samples at 37°C. However, the additionally added Triton X-100 had no significant effect on the stability of adenovirus samples without residual Triton X-100. Overall, these results depict that the instability of adenovirus particles caused by Triton X-100 may not be due to the direct interaction between Triton X-100 and adenovirus components.
Discussion
In the present study, during the upstream process for large-scale preparation of adenovirus, Triton X-100 was added (with a final concentration of 0.25%) to the bioreactor for lysing the cells to release the adenovirus.19 In this method, host cells can be lysed in a closed environment of the bioreactor, leading to reduced probability of contamination of the adenovirus feed, followed by linear scale-up. The cell lysate harvested at the same time can be well connected with the downstream adenovirus purification process. The cell debris in virus fermentation broth can easily be removed by filtration to attain the purpose of clarification, avoiding the use of small-throughput centrifugation to clarify the virus feed. Herein, the cell lysis and virus-release capability of the repeated freeze-thaw method and detergent (Triton X-100)-based lysis method were thoroughly investigated. It was found that the virus released from host cells using the detergent lysis method was more effective as compared to that of the repeated freeze-thaw method, which corroborates with earlier reports. In addition, the repeated freeze-thaw method is only suitable for lysing a small number of samples and cannot be linearly scaled up to industrial scale unlike the detergent-based lysis method.
Detergents are amphiphilic molecules consisting of aliphatic or aromatic nonpolar ends and polar ends with or without charge. Detergents can either be denaturing or nondenaturing in nature. The denaturing detergents destroy the entire cell membrane and denature proteins by disrupting protein-protein interactions, However, the nondenaturing detergents are relatively weak in destroying the protein-lipid interactions, and they do not hamper the natural structure and activity of proteins. Detergents commonly used to lyse the host cells include Triton X-100, nonyl phenoxypolyethoxylethanol (NP-40), Tween 20, Brij-58, etc.20, 21, 22, 23, 24 The removal of these detergents added in biological products is highly imperative in their processing. The detergents can be removed in a variety of ways. For instance, dialysis is a very effective method for removal of detergents in the form of monomers. However, dialysis has a poor removal effect on detergents that can easily aggregate into micelles, because the large micelles cannot pass through the dialysis membrane. The detergents, which are prone to aggregate into colloidal particles, can easily be removed by ion-exchange chromatography technique. Triton X-100 possesses low viscosity as compared to Brij-58, Tween 20, and other detergents and can easily be removed during sample purification using chromatography. The literature demonstrated that Triton X-100 can gently lyse the host cells and release the virus completely.15,25 With the consideration of these facts, we finally selected Triton X-100 in the host cell lysis process for releasing the viruses.
In the present study, it was found that the adenovirus elute, purified from the cell-culture supernatant containing 0.25% Triton X-100 lysis buffer by anion-exchange chromatography, had higher light A260 nm and A280 nm values than the samples purified from the cell-culture supernatant without Triton X-100 lysis buffer. However, the immunohistochemistry study illustrated that the number of infectious virus particles of the two elutes was similar. We speculate that Triton X-100 has an effect on the light A260 nm and A280 nm values of adenoviruses. In order to further explain the effect of Triton X-100 on the light absorption value of adenoviruses, Triton X-100, with different concentrations, was added to the purified adenovirus sample without residual Triton X-100. UV spectroscopy studies showed that with the increase of the concentration of Triton X-100, the light A260 nm and A280 nm values were gradually increased, and the ratio of A260 nm/A280 nm decreased. These results suggest that Triton X-100 and adenovirus have the maximum absorption effect on UV in the wavelength range of 260–280 nm. UV-visible spectroscopy is generally employed for the determination of the number and purity of adenovirus particles, and our research suggests that UV spectroscopy may not be suitable for detecting the number and purity of adenovirus samples containing Triton X-100 residues.26,27
After adding Triton X-100 to the host cells for the lysis process to release the virus, the fermentation broth was purified by ultrafiltration and anion-exchange chromatography. Analysis of the purified adenovirus employing a C18 HPLC column revealed the presence of traces of Triton X-100 in the samples. Further studies revealed that the adenovirus, with or without residual Triton X-100, could maintain good particle stability and infection activity for a short time under a 4°C storage condition. Previous studies demonstrated that nonreplicating and replicating adenoviruses can maintain the stability and infection activity of virus particles at 37°C for a short period of time. We also found that Triton X-100-free pure adenovirus samples and pure adenovirus containing additionally added Triton X-100 kept good particle stability, purity, and infection activity under 37°C storage condition. However, purified adenovirus containing residual Triton X-100 cannot lead to good stability and infectivity under a 37°C storage condition for a short time. We speculate that Triton X-100 may not directly interact with adenovirus components to alter the stability and infection activity of adenovirus particles. During the adenovirus-release process, Triton X-100 was employed for the cell lysis. Triton X-100 might interact with host cell components to indirectly change the stability and infectivity of adenovirus particles. Moreover, we also found that the effect of 0.1% Triton X-100 or milder detergent Tween 20 on structural stability and infection activity of adenovirus particles was similar to that of 0.25% Triton X-100 (Figures S1 and S2). The effect of detergent on structural stability and infection activity of adenovirus particles may have nothing to do with the strength of the detergent. Further investigation is needed to comprehend the specific components of the host cells interacting with the detergent to affect the stability of adenoviruses. Earlier reports depicted that the ability of the adenovirus infectivity majorly relies on the expression of capsid protein, and it is closely related to the cephalic region of the virus ciliated.28,29 The electron microscopy structure of the adenovirus with residual Triton X-100 at 37°C further demonstrated that the presence of residual Triton X-100 can lead to almost complete disintegration of the structure of adenovirus samples upon storage for 15 days, and a large number of scattered virus shells were visible. However, in this study, SDS-PAGE analysis revealed no significant degradation of the capsid protein as well as other core proteins of the adenovirus. The adenovirus is composed of 252 shell particles arranged in an icosahedron. Triton X-100 may lead to the shell particles (virus protein) fall off the adenovirus icosahedron, causing disintegration of the adenovirus structure. However, Triton X-100 may not degrade the viral protein directly.
The major factors affecting the stability of adenoviruses during the large-scale preparation are physical, chemical, and biological factors. The physical factors mainly include temperature and shear force. The literature demonstrated that the stability of adenoviruses gradually decreases with increasing temperature. When the temperature reaches above 47°C, the structure of the adenoviruses can completely be disintegrated, and they lose their infection ability.30 Bedsides temperature, shear forces generated during bioreactor stirring, fermentation broth centrifugation, and ultrafiltration can destroy the structure of adenoviruses, thereby reducing their infectivity.31,32 The chemical factors mainly include pH and concentration of salt ions in buffer, which can affect the solubility of the protein. Excessively high- or low-salt ion concentrations may cause precipitation or denaturation of adenovirus structural proteins.33, 34, 35 High or low pH might also affect the capsid proteins on the surface of adenoviruses, eventually causing inactivation of the adenovirus infectivity.36, 37, 38 On the other hand, the biological factors are majorly derived from various proteases released by host cells, and these proteases can hydrolyze the adenovirus surface proteins, leading to destroyed structure and reduced infectivity of the adenovirus particles.39,40 In the present study, we confirm that Triton X-100 added during cell lysis of the upstream process can reduce the stability and infectivity of adenovirus in subsequent purification processes.
It is well known that the Triton X-100-based lysis method of host cells is a simple, efficient, and easy-to-scale process to release viruses. A trace amount of Triton X-100 can remain in the adenovirus product after purification of the cell lysate. Pure adenoviruses are commonly stored under −20°C. The residual Triton X-100 has no effect on the stability and infectivity of the adenovirus under a low-temperature storage condition. However, further investigation is required to comprehend whether the residual Triton X-100 could affect the long-term stability of cryopreserved adenoviruses. In addition, Triton X-100 may reduce the stability and infectivity of adenoviruses after purification process. Therefore, unpurified adenovirus samples containing Triton X-100 should not be exposed to high temperature for a long time during purification. It was reported that the addition of metal nanoparticles or Polyethylene Glycol 8000 (PEG 8000) to adenovirus preparations can extend the stability of the samples from hours to months.41 The present study provides the idea for further research to improve the stability of residual Triton X-100-containing adenovirus particles.
Materials and Methods
Cell Culture
HEK293 cells were purchased from American Type Culture Collection (ATCC) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) with high sugar (without antibiotics) and 10% fetal bovine serum (FBS) (HyClone). DMEM dry powder was purchased from Gibco.
Adenovirus Type 5 Amplification and Host Cell Lysis
The recovered HEK293 cells were cultured and scaled up to a 10-layer cell factory. When the density of cells in the cell factory became more than 90%, the cells were incubated with adenovirus type 5 seed solution (multiplicity of infection [MOI] = 25) for 48–72 h. When about 80% of the cells became round, the culture supernatant and cell pellet were separated by centrifugation. Host cells were then lysed by either Triton X-100 lysis buffer (10 mM Tris-HCl, 2 mM MgCl2, 0.25% Triton X-100, pH 8.0) or freezing and thawing repeatedly, leading to obtaining cell supernatant and cell supernatant along with cell pellet with or without Triton X-100 lysis buffer, respectively. In the case of the Triton X-100 lysis method, the cell suspension was treated with Triton X-100 lysis buffer for 60 min at room temperature, and the supernatant was collected by centrifugation after the lysing process. Contrastingly, the cell suspension was repeatedly frozen and thawed at −80°C and 37°C, respectively, three times, followed by centrifugation in the case of the freeze-thaw method.
Ultrafiltration and Purification of Adenovirus
The four types of crude adenovirus samples—(1) cell supernatant, (2) cell supernatant containing 0.25% Triton X-100 lysis buffer, (3) cell supernatant and cell pellet lysed with 0.25% Triton X-100 lysis buffer, and (4) cell supernatant and cell pellet repeatedly frozen and thawed for three times, as mentioned above—were centrifuged at 6,000 rpm at 4°C for 30 min (Sorvall LYNX 4000; Thermo Scientific). The cell debris was discarded, and the supernatant was retained. Next, the virus supernatant was filtered employing 1.2 μM and 0.45 μM capsule filters. It was then concentrated and diafiltered using a tangential flow ultrafiltration (TFF) device (Sartorius) with 300 kDa molecular weight cutoff (MWCO) membrane (Sartorius).42 After that, the retentate was separated and purified by a two-step anion-exchange method, namely crude purification of Q Sepharose XL (GE Healthcare) packing and fine purification of SOURCE 30Q packing (GE Healthcare). The purified adenovirus sample was then dialyzed against a 4% sucrose solution overnight, filtered through a 0.22-μM filter, and finally stored at −80°C for subsequent experiments.
Determination of Residual Triton X-100 Concentration in Adenovirus Samples
To determine the residual Triton X-100 concentration in adenovirus samples, a series of standard Triton X-100 solutions (10 ng/mL, 25 ng/mL, 50 ng/mL, 75 ng/mL, 100 ng/mL, 250 ng/mL, and 500 ng/mL) was prepared. The purified adenovirus samples were used as test samples, and the concentration of Triton X-100 in those samples was measured using HPLC at detection wavelength 225 nm. The stationary phase for HPLC analysis was a C18 column, whereas the mobile phase was a methanol-water solution (volume ratio 85:15). The standard curve was drawn with the standard peak area as the ordinate and the concentration as the abscissa. Triton X-100 concentration in adenovirus samples was calculated from the standard curve.
Short-Term Stability Testing of Adenovirus Particles
The adenovirus test products purified by different processing methods stated above were placed at 4°C and 37°C up to 15 days, and the short-term stability of the adenovirus samples was tested on the 3rd, 6th, 9th, 12th, and 15th day. The stability of adenovirus samples was evaluated in terms of their purity and infection activity. The purity of the recombinant adenovirus samples was determined by HPLC. The conditions for HPLC analysis were as follows: column, SOURCE 15Q (4.6 mm i.d. × 100 mm); mobile phase A liquid, 50 mM Tris-HCl, 2 mM MgCl2, pH 8.0; liquid B, 50 mM Tris-HCl, 2 mM MgCl2, 1 M NaCl, pH 8.0; and gradient elution, liquid A to 100% liquid B. The time from equilibrium, loading, and elution was 30 min, with flow rate 1.0 mL/min, and detection wavelength was 260 nm. 2.5 × 1010 virus particles (VP)/100 μL sample was injected into the liquid chromatograph, followed by the recording of the chromatogram and evaluation of the purity, according to the area-normalization method. The determination of infection activity of the adenovirus particle was performed according to the instructions of the TaKaRa adenovirus rapid detection kit. 100 μL of the filtered sample was taken, and a 10-fold gradient dilution was made. The appropriate five dilutions were selected. 50 μL of each dilution was poured into a 24-well tissue-culture plate seeded with HEK293 cells at a concentration of 5.0 × 105 cells/well in FBS containing DMEM for 48 h. The medium was then discarded, and the cells were treated with pre-cold methanol for 10 min, followed by washing with buffer (PBS + 1% bovine serum albumin), three times, and incubation with mouse anti-hexon antibody (1:1,000) for 1 h and rat anti-mouse antibody (1:500) for another 1 h. 3,3′-diaminobenzidine (DAB) staining was then employed to develop the color, and the cells were observed and counted under a microscope.26
SDS-PAGE Analysis
The SDS-PAGE method was used to analyze the composition of adenovirus samples purified through ultrafiltration and ion-exchange methods. In brief, an 80-μL sample was mixed with 20 μL, 5 × loading buffer, and the mixture was boiled at 100°C for 10 min, followed by centrifugation at 13,000 rpm for 10 min to collect the supernatant. The samples were then electrophoresed on a 12.5% polyacrylamide gel at 120 V for 90 min. After the electrophoresis, the gels were analyzed by staining with Coomassie brilliant blue R250.
Investigation of the Effect of Triton X-100 on the Structure of Adenovirus Using Transmission Electron Microscope
To evaluate the effect of Triton X-100 on the structure of adenovirus, 20 μL of adenovirus samples was placed onto the copper net of the transmission electron microscope for 1 min, and the excess residual sample was soaked using a filter paper. A drop of 1% (w/v) phosphotungstic acid was then added on the sample for 5 min. After that, the copper mesh was mounted on a transmission electron microscope to observe the morphological characteristics, as well as uniform distribution of the adenovirus.
Statistical Analysis
The results were expressed as mean ± SD. All statistical tests were conducted through the SPSS version 15.0 software. One-way ANOVA analysis was employed for the statistical comparisons, and p value < 0.05 was considered statistically significant.
Author Contributions
J.M. and C.S. designed and conducted the experiments and wrote the paper. S.H., D.Y., and Y.K. conducted the experiments. Y.S., B.Z., Z.Q., Y.C., S.L., and X.W. performed the data analysis. P.C. designed the experiments and supervised the research. All authors discussed the results and commented on the manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported by the National Science and Technology Major Projects of New Drugs (2018ZX09201018-013), National Science and Technology Major Project for Infectious Diseases Control (2017ZX10203206-004), National Natural Science Foundation of China (81101728), Sichuan Regional Innovation Cooperation Project (20QYCX0100), and Innovation Spark Project of Sichuan University (2018SCUH0084).
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtm.2020.08.013.
Supplemental Information
References
- 1.Verma I.M., Weitzman M.D. Gene therapy: twenty-first century medicine. Annu. Rev. Biochem. 2005;74:711–738. doi: 10.1146/annurev.biochem.74.050304.091637. [DOI] [PubMed] [Google Scholar]
- 2.Miesbach W., Meijer K., Coppens M., Kampmann P., Klamroth R., Schutgens R., Tangelder M., Castaman G., Schwäble J., Bonig H. Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood. 2018;131:1022–1031. doi: 10.1182/blood-2017-09-804419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naldini L. Gene therapy returns to centre stage. Nature. 2015;526:351–360. doi: 10.1038/nature15818. [DOI] [PubMed] [Google Scholar]
- 4.Xing L., Tikoo S.K. Promoter activity of left inverted terminal repeat and downstream sequences of porcine adenovirus type 3. Virus Res. 2005;109:51–58. doi: 10.1016/j.virusres.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y., Tikoo S.K. Analysis of early region 1 of porcine adenovirus type 3. Virology. 2001;291:68–76. doi: 10.1006/viro.2001.1219. [DOI] [PubMed] [Google Scholar]
- 6.Li X., Babiuk L.A., Tikoo S.K. Analysis of early region 4 of porcine adenovirus type 3. Virus Res. 2004;104:181–190. doi: 10.1016/j.virusres.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Alonso-Padilla J., Papp T., Kaján G.L., Benkő M., Havenga M., Lemckert A., Harrach B., Baker A.H. Development of Novel Adenoviral Vectors to Overcome Challenges Observed With HAdV-5-based Constructs. Mol. Ther. 2016;24:6–16. doi: 10.1038/mt.2015.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanley D.A., Honko A.N., Asiedu C., Trefry J.C., Lau-Kilby A.W., Johnson J.C., Hensley L., Ammendola V., Abbate A., Grazioli F. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat. Med. 2014;20:1126–1129. doi: 10.1038/nm.3702. [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez E.S., Pose A.G., Moltó M.P., Espinoza A.S., Zamora P.A., Pedroso M.S. Biosafety evaluation of recombinant protein production in goat mammary gland using adenoviral vectors: preliminary study. Biotechnol. J. 2012;7:1049–1053. doi: 10.1002/biot.201100455. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z.H., Yu Y.P., Zuo Z.H., Nelson J.B., Michalopoulos G.K., Monga S., Liu S., Tseng G., Luo J.H. Targeting genomic rearrangements in tumor cells through Cas9-mediated insertion of a suicide gene. Nat. Biotechnol. 2017;35:543–550. doi: 10.1038/nbt.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartikainen J., Hassinen I., Hedman A., Kivelä A., Saraste A., Knuuti J., Husso M., Mussalo H., Hedman M., Rissanen T.T. Adenoviral intramyocardial VEGF-DΔNΔC gene transfer increases myocardial perfusion reserve in refractory angina patients: a phase I/IIa study with 1-year follow-up. Eur. Heart J. 2017;38:2547–2555. doi: 10.1093/eurheartj/ehx352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz K.L., Richardson S.E., MacGregor D., Mahant S., Raghuram K., Bitnun A. Adenovirus-Associated Central Nervous System Disease in Children. J. Pediatr. 2019;205:130–137. doi: 10.1016/j.jpeds.2018.09.036. [DOI] [PubMed] [Google Scholar]
- 13.Gaete-Argel A., Márquez C.L., Barriga G.P., Soto-Rifo R., Valiente-Echeverría F. Strategies for Success. Viral Infections and Membraneless Organelles. Front. Cell. Infect. Microbiol. 2019;9:336. doi: 10.3389/fcimb.2019.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altaras N.E., Aunins J.G., Evans R.K., Kamen A., Konz J.O., Wolf J.J. Production and formulation of adenovirus vectors. Adv. Biochem. Eng. Biotechnol. 2005;99:193–260. doi: 10.1007/10_008. [DOI] [PubMed] [Google Scholar]
- 15.Silva A.C., Peixoto C., Lucas T., Küppers C., Cruz P.E., Alves P.M., Kochanek S. Adenovirus vector production and purification. Curr. Gene Ther. 2010;10:437–455. doi: 10.2174/156652310793797694. [DOI] [PubMed] [Google Scholar]
- 16.Schoofs G., Monica T.J., Ayala J., Horwitz J., Montgomery T., Roth G., Castillo F.J. A high-yielding serum-free, suspension cell culture process to manufacture recombinant adenoviral vectors for gene therapy. Cytotechnology. 1998;28:81–89. doi: 10.1023/A:1008021428969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konz J.O., Lee A.L., Lewis J.A., Sagar S.L. Development of a purification process for adenovirus: controlling virus aggregation to improve the clearance of host cell DNA. Biotechnol. Prog. 2005;21:466–472. doi: 10.1021/bp049644r. [DOI] [PubMed] [Google Scholar]
- 18.Berg M., Difatta J., Hoiczyk E., Schlegel R., Ketner G. Viable adenovirus vaccine prototypes: high-level production of a papillomavirus capsid antigen from the major late transcriptional unit. Proc. Natl. Acad. Sci. USA. 2005;102:4590–4595. doi: 10.1073/pnas.0500933102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma J., Su C., Wang X., Shu Y., Hu S., Zhao C., Kuang Y., Chen Y., Li Y., Wei Y., Cheng P. A novel method to purify adenovirus based on increasing salt concentrations in buffer. Eur. J. Pharm. Sci. 2020;141:105090. doi: 10.1016/j.ejps.2019.105090. [DOI] [PubMed] [Google Scholar]
- 20.Konz J.O., Pitts L.R., Sagar S.L. Scaleable purification of adenovirus vectors. Methods Mol. Biol. 2008;434:13–23. doi: 10.1007/978-1-60327-248-3_2. [DOI] [PubMed] [Google Scholar]
- 21.Oshima T., Sasaki M., Kataoka H., Miwa H., Takeuchi T., Joh T. Wip1 protects hydrogen peroxide-induced colonic epithelial barrier dysfunction. Cell. Mol. Life Sci. 2007;64:3139–3147. doi: 10.1007/s00018-007-7268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Everitt E., de Luca A., Blixt Y. Antibody-mediated uncoating of adenovirus in vitro. FEMS Microbiol. Lett. 1992;77:21–27. doi: 10.1016/0378-1097(92)90126-9. [DOI] [PubMed] [Google Scholar]
- 23.Gautschi M., Reinhard P. Synthesis of a mammalian parvovirus in Brij-58-lysed cells. J. Virol. 1978;27:453–456. doi: 10.1128/jvi.27.2.453-456.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen T., Gong X., Chen H., Zhang Y., Qu H. Chromatographic elution process design space development for the purification of saponins in Panax notoginseng extract using a probability-based approach. J. Sep. Sci. 2016;39:306–315. doi: 10.1002/jssc.201500976. [DOI] [PubMed] [Google Scholar]
- 25.Peixoto C., Ferreira T.B., Carrondo M.J., Cruz P.E., Alves P.M. Purification of adenoviral vectors using expanded bed chromatography. J. Virol. Methods. 2006;132:121–126. doi: 10.1016/j.jviromet.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Pavlović B., Cvijetić N., Dragačević L., Ivković B., Vujić Z., Kuntić V. Direct UV Spectrophotometry and HPLC Determination of Triton X-100 in Split Virus Influenza Vaccine. J. AOAC Int. 2016;99:396–400. doi: 10.5740/jaoacint.15-0201. [DOI] [PubMed] [Google Scholar]
- 27.Garcia L.A., Nascimento M.A., Barardi C.R. Effect of UV light on the inactivation of recombinant human adenovirus and murine norovirus seeded in seawater in shellfish depuration tanks. Food Environ. Virol. 2015;7:67–75. doi: 10.1007/s12560-014-9177-x. [DOI] [PubMed] [Google Scholar]
- 28.Cheng L., Huang X., Li X., Xiong W., Sun W., Yang C., Zhang K., Wang Y., Liu H., Huang X. Cryo-EM structures of two bovine adenovirus type 3 intermediates. Virology. 2014;450-451:174–181. doi: 10.1016/j.virol.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Hackenbrack N., Rogers M.B., Ashley R.E., Keel M.K., Kubiski S.V., Bryan J.A., Ghedin E., Holmes E.C., Hafenstein S.L., Allison A.B. Evolution and Cryo-electron Microscopy Capsid Structure of a North American Bat Adenovirus and Its Relationship to Other Mastadenoviruses. J. Virol. 2017;91:e01504-16. doi: 10.1128/JVI.01504-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ihnat P.M., Vellekamp G., Obenauer-Kutner L.J., Duan J., Han M.A., Witchey-Lakshmanan L.C., Grace M.J. Comparative thermal stabilities of recombinant adenoviruses and hexon protein. Biochim. Biophys. Acta. 2005;1726:138–151. doi: 10.1016/j.bbagen.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Sluzky V., Tamada J.A., Klibanov A.M., Langer R. Kinetics of insulin aggregation in aqueous solutions upon agitation in the presence of hydrophobic surfaces. Proc. Natl. Acad. Sci. USA. 1991;88:9377–9381. doi: 10.1073/pnas.88.21.9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coventry V.K., Conn G.L. Analysis of adenovirus VA RNAI structure and stability using compensatory base pair modifications. Nucleic Acids Res. 2008;36:1645–1653. doi: 10.1093/nar/gkn020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye L., Van Eps N., Zimmer M., Ernst O.P., Prosser R.S. Activation of the A2A adenosine G-protein-coupled receptor by conformational selection. Nature. 2016;533:265–268. doi: 10.1038/nature17668. [DOI] [PubMed] [Google Scholar]
- 34.Brogan A.P., Sessions R.B., Perriman A.W., Mann S. Molecular dynamics simulations reveal a dielectric-responsive coronal structure in protein-polymer surfactant hybrid nanoconstructs. J. Am. Chem. Soc. 2014;136:16824–16831. doi: 10.1021/ja507592b. [DOI] [PubMed] [Google Scholar]
- 35.An L., Wang Y., Zhang N., Yan S., Bax A., Yao L. Protein apparent dielectric constant and its temperature dependence from remote chemical shift effects. J. Am. Chem. Soc. 2014;136:12816–12819. doi: 10.1021/ja505852b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto T. The effect of ion concentration and pH on the thermal stability of a canine adenovirus. Can. J. Microbiol. 1967;13:1139–1149. doi: 10.1139/m67-158. [DOI] [PubMed] [Google Scholar]
- 37.Rexroad J., Martin T.T., McNeilly D., Godwin S., Middaugh C.R. Thermal stability of adenovirus type 2 as a function of pH. J. Pharm. Sci. 2006;95:1469–1479. doi: 10.1002/jps.20617. [DOI] [PubMed] [Google Scholar]
- 38.Rexroad J., Evans R.K., Middaugh C.R. Effect of pH and ionic strength on the physical stability of adenovirus type 5. J. Pharm. Sci. 2006;95:237–247. doi: 10.1002/jps.20496. [DOI] [PubMed] [Google Scholar]
- 39.Shah G.A., O’Shea C.C. Viral and Cellular Genomes Activate Distinct DNA Damage Responses. Cell. 2015;162:987–1002. doi: 10.1016/j.cell.2015.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mul Y.M., Van der Vliet P.C. Nuclear factor I enhances adenovirus DNA replication by increasing the stability of a preinitiation complex. EMBO J. 1992;11:751–760. doi: 10.1002/j.1460-2075.1992.tb05108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pelliccia M., Andreozzi P., Paulose J., D’Alicarnasso M., Cagno V., Donalisio M., Civra A., Broeckel R.M., Haese N., Jacob Silva P. Additives for vaccine storage to improve thermal stability of adenoviruses from hours to months. Nat. Commun. 2016;7:13520. doi: 10.1038/ncomms13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chalmers N.I., Palmer R.J., Jr., Du-Thumm L., Sullivan R., Shi W., Kolenbrander P.E. Use of quantum dot luminescent probes to achieve single-cell resolution of human oral bacteria in biofilms. Appl. Environ. Microbiol. 2007;73:630–636. doi: 10.1128/AEM.02164-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.