Abstract
Self-inactivating lentiviral vectors (LVVs) are used regularly for genetic modification of cells, including T cells and hematopoietic stem cells for cellular gene therapy. As vector demand grows, scalable and controllable methods are needed for production. LVVs are typically produced in HEK293T cells in suspension bioreactors using serum-free media or adherent cultures with serum. The iCELLis® is a packed-bed bioreactor for adherent or entrained cells with surface areas from 0.53 to 500 m2. Media are pumped through the fixed bed and overflows, creating a thin film that is replenished with oxygen and depleted of CO2 as media return to the reservoir. We describe the optimization and scale-up of the production of GPRTG-EF1α-hγc-OPT LVV using a stable packaging cell line in the iCELLis Nano 2-cm to the 10-cm bed height low compaction bioreactors (0.53 and 2.6 m2 surface area) and compare to the productivity and efficacy of GPRTG-EF1α-hγc-OPT LVV manufactured under current Good Manufacturing Practice (cGMP) using 10-layer cell factories for the treatment of X-linked severe combined immunodeficiency. By optimizing fetal bovine serum (FBS) concentration, pH post-induction, and day of induction, we attain viral yields of more than 2 × 107 transducing units/mL. We compared transduction efficiency between LVVs produced from the iCELLis Nano and cell factories on healthy, purified CD34+ cells and found similar results.
Keywords: lentivirus, vector, bioreactor, packed bed, fixed bed, gene therapy, iCELLis, XSCID
Graphical Abstract

This study compares cell factories to the iCELLis Nano to produce a lentiviral vector from an adherent stable packaging cell line for the treatment of X-linked SCID by hematopoietic stem cell gene therapy. The iCELLis yielded 2 × 107 transducing units/mL with similar CD34+ transduction efficiency as vector from cell factories.
Introduction
Lentiviral vectors (LVVs) transduce both dividing and non-dividing cells and, unlike gammaretroviruses, do not preferentially integrate near proto-oncogenes, making these vectors a promising option for gene therapy.1 Clinical trials have successfully used LVVs to treat Wiskott-Aldrich syndrome,2 X-linked severe combined immunodeficiency (XSCID),3,4 X-linked adrenoleukodystrophy,5 β-thalassemia,6 metachromatic leukodystrophy,7 and leukemia,8 and the lentivirus CART-19 vector for treating acute lymphoblastic leukemia became the first US Food and Drug Administration (FDA)-approved gene therapy in 2017.9 As the number of therapeutic LVVs increases, more efficient, reliable, and larger scale methods of LVV manufacturing will be needed.
LVVs have typically been generated using transient transfection. Initially, calcium phosphate was used as the transfection reagent, but transfection by this method was sensitive to pH variations and required serum.10 Toxicity of calcium phosphate to the cells requires that a media change be performed after transfection. Polyethylenimine (PEI) now offers a more versatile alternative to calcium phosphate. PEI functions over a wider range of pH values and lacks the toxicity of calcium phosphate and so does not require a media change after transfection. It can be used with or without serum and in adherent or suspension cells.10,11 Transient transfection is faster than producing a stable cell line but is not ideal for large-scale manufacturing due to variability between batches, the cost of plasmid, and potential plasmid contamination in the final product.12 While they require a longer time to generate, lentiviral stable producing cell lines have been created for X-SCID gene therapy13 and have the potential to create particles with higher transfection efficiency than what is observed from transient transfection.14
While suspension systems for LVV production are being developed,14, 15, 16 production of LVVs frequently occurs in adherent cells requiring an adherent cell system and serum for scale-up.17 An adherent cell system facilitates media changes and the harvest of secreted products. At a medium scale, adherent cells can be grown in roller bottles (0.085–0.43 m2 each, Corning Life Sciences) or 0.63-m2 10-layer cell factory (CF) and HYPERStacks (1.8 m2 for a 36 stack), but these methods scale linearly, are labor-intensive, and lack control of pH or dissolved oxygen (DO).18 Microcarriers in suspension culture offer a more scalable and controllable option for adherent cells but can expose cells to high shear stress and turbulence.18
The iCELLis packed-bed disposable bioreactor provides a high-density, closed, controlled environment for adherent cell growth (Pall Corporation). The iCELLis Nano provides a surface area ranging from 0.53 to 4 m2, and the iCELLis 500 ranges from 66 to 500 m2 of surface area and requires less manual manipulation than do roller bottles or cell factories. Vector production in a bioreactor allows for more consistent control of process parameters such as pH and DO, as compared to a flask-based process such as the one described in Figure 4 of Bauler et al.16 The use of a bioreactor also allows for scaling up from the iCELLis Nano in process development to the iCELLis 500 in production, which reduces labor as compared to a flask-based process, which must be scaled out rather than scaled up. Immobilization in polyester macrocarriers protects cells from stresses associated with bubble sparging or an impeller while media circulates up through the fixed bed and is replenished with gases as it falls back to the reservoir as a thin film.19 The high surface area-to-volume ratio of the fixed bed allows for a low inoculation density per surface area with the option to increase media volume with perfusion or recirculation as the cells grow.20 Cell growth can be measured using a permittivity-based biomass probe.21 Diverse biologicals, including monoclonal antibodies,22 vaccines,19,23 gammaretroviral vectors,24 adenoviral vectors,25 adeno-associated viral vectors,26, 27, 28 and LVVs29,30 have been produced in the iCELLis reactor, but often with little or no optimization. With this wide array of applications, much need exists to investigate the effects of culture parameters in the iCELLis reactor. Valkama et al.29 have varied parameters in the iCELLis such as low versus high compaction, transfection media, perfusion rates, and two pH levels after transfection and examined the effect of these parameters on viral titer in a lentiviral transient transfection process for GFP. We further this analysis of the impact of pH as well as examining the effects of fetal bovine serum (FBS) concentration and day of induction in the iCELLis reactor on viral yield.
In this study, we describe the optimization and scale-up of GPRTG-EF1α-hγc-OPT LVV production in the iCELLis Nano from a 2-cm (0.53-m2) to a 10-cm (2.6-m2) bed-height low compaction bioreactor and compare the results to GPRTG-EF1α-hγc-OPT LVV manufactured in 10-layer cell factories under cGMP in support of a phase I clinical study for the treatment of X-SCID in newly diagnosed patients by cellular gene therapy (IND 14570).
Results and Discussion
The Effect of pH and Glucose Consumption on Yield
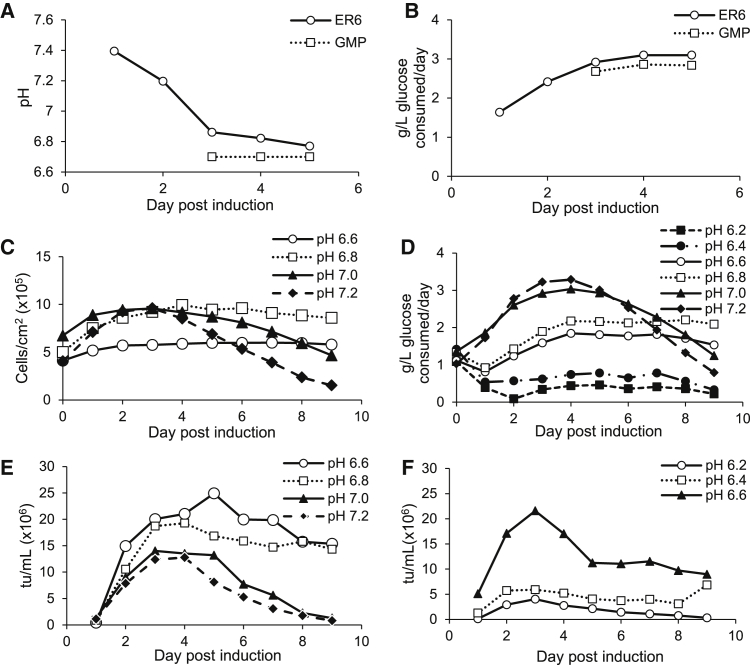
The GPRTG-EF1α-hγc-OPT LV vector was previously manufactured under cGMP in 50 ten-layer cell factories. The 0.63-m2 cell factories were inoculated with 1.4 × 108 cells in 1,121 mL of Dulbecco’s modified Eagle’s medium (DMEM) with 10% FBS and 1% GlutaMAX (D10 media) with 1 ng/mL doxycycline and induced 3 days after inoculation. Spent media were removed, and 1,121 mL of fresh D10 media was added each day after induction. Supernatant was collected on the third, fourth, and fifth day post-induction. The change in pH of the daily spent media post-induction was monitored for a single-cell factory engineering run (ER6) and the combined supernatant from 50 cell factories from the GMP run (Figure 1A). In both runs, the pH dropped from 7.4 to 6.7 to 6.85 during 24 h on peak glucose consumption days of vector production, i.e., days 3, 4, and 5 (Figure 1B).
Figure 1.
Comparison of pH and Glucose Consumption after Induction
(A) pH after induction in engineering run 6 (ER6) and production run (GMP batch 17-135). Both runs were performed in 10-layer cell factories. (B) Glucose consumption post-induction in ER6 and production run (GMP batch 17-135). (C) Cell density post-induction in iCELLis bioreactors at pH 6.6, 6.8, 7.0, and 7.2. Bioreactors were inoculated with 8 × 107 cells and induced 5 days later in DMEM with 10% FBS and 2 mM GlutaMAX. (D) Glucose consumption per day after induction at each pH value in iCELLis bioreactors. (E) Average viral yield on each day post-induction in iCELLis bioreactors at pH 6.6, 6.8, 7.0, and 7.2 (n = 2). (F) Viral yield on each day post-induction in bioreactors at pH 6.2, 6.4, and 6.6. Bioreactors were inoculated with 8 × 107 cells and induced 5 days later in DMEM with 10% FBS and 2 mM GlutaMAX.
Based on the drop in pH post-induction for the GPRTG-EF1α-hγc-OPT stable cell line in cell factories and studies that have shown transient LVV production to increase at lower pH values,29,31 the effect of maintaining a range of pH set points throughout the vector production phase was investigated. Reactors were inoculated at 15,000 cells/cm2 (8 × 107 cells total) and maintained at pH 7.2 for 5 days. At day 5 after inoculation, the reactors were induced by changing the media, and the pH set point was adjusted to 6.6–7.2 for an additional 9 days. Two sets of four reactors were run with a reactor at pH 6.6, 6.8, 7.0, and 7.2 in each set. Because cells adhere or become entrapped in the macrocarriers of the iCELLis, direct cell counts are not possible. Nuclei counts can be performed by removing carriers from the iCELLis and lysing the cells, but cell numbers vary between carriers, the nuclei can be difficult to count, and this sampling method introduces a potential contamination opportunity. Therefore, we used Aber Futura capacitance probes to measure cell density in some of our iCELLis reactors. Capacitance values are correlated linearly to cell density based on 25 nuclei counts (data not shown). Figure 1C shows cell density over time as a function of pH. Cell density remained steady for 9 days in bioreactors run at pH 6.6 or 6.8 post-induction, while cell density decreased beginning at 4 days post-induction in iCELLis reactors run at pH 7.0 or 7.2. Glucose consumption rates mirrored cell density. Cultures at pH 7.0 or 7.2 reached the highest glucose consumption and declined more rapidly as compared to cultures at pH 6.6 and 6.8, which experienced more sustained glucose consumption rates (Figure 1D).
Titers in the iCELLis were measured by transduction of ED7R cells. The reactors at set points of pH 6.6 and 6.8 yielded the highest overall average transducing units (tu) of 1.1 × 1011 (pH 6.6, standard deviation of 2.4 × 1010 tu) and 1.0 × 1011 (pH 6.8, standard deviation 3.8 × 1010 tu) during 9 days with maximum production on days 3, 4, and 5 post-induction (Figure 1E). Viral yields were lower at pH set points 7.0 (4.5 × 1010 tu, standard deviation of 1.33x1010) and 7.2 (3.6 × 1010 tu, standard deviation of 9.46 × 109 tu). These results suggest that a higher viral titer per cell is obtained at pH values of 6.6 or 6.8 during the first 4 days post-induction and that the maintained health of these cells allows for viral production to continue for 9 days after induction. It is unknown what role viral stability at pH 6.6 and 6.8 plays in the increased titers at these pH values.
Because pH values of 6.6 and 6.8 yielded high viral titers, we further decreased the post-induction pH to 6.2 and 6.4. Reactors were inoculated at 15,000 cells/cm2 (8 × 107 cells total) and maintained at pH 7.2 for 5 days. At day 5 after inoculation, the reactors were induced by changing the media, and the pH set point was adjusted to 6.2, 6.4, or 6.6 for an additional 9 days. Cell density information is not available for this experiment. Below pH 6.6, viral yield decreased to 3.2 × 1010 tu (pH 6.4) and 1.2 × 1010 tu (pH 6.2), and glucose consumption remained less than 1 g/L per day after the pH adjustments to 6.2 or 6.4 (Figure 1F). These results support that a pH of 6.6–6.8 is preferred for LVV production from a stable producer cell line, similar to previous results reported for LVVs produced by transient transfection. We decided to proceed with pH 6.8 for future experiments due to the rapid decrease in viral titer and glucose consumption between pH 6.4 and 6.6.
Day of Induction after Inoculation
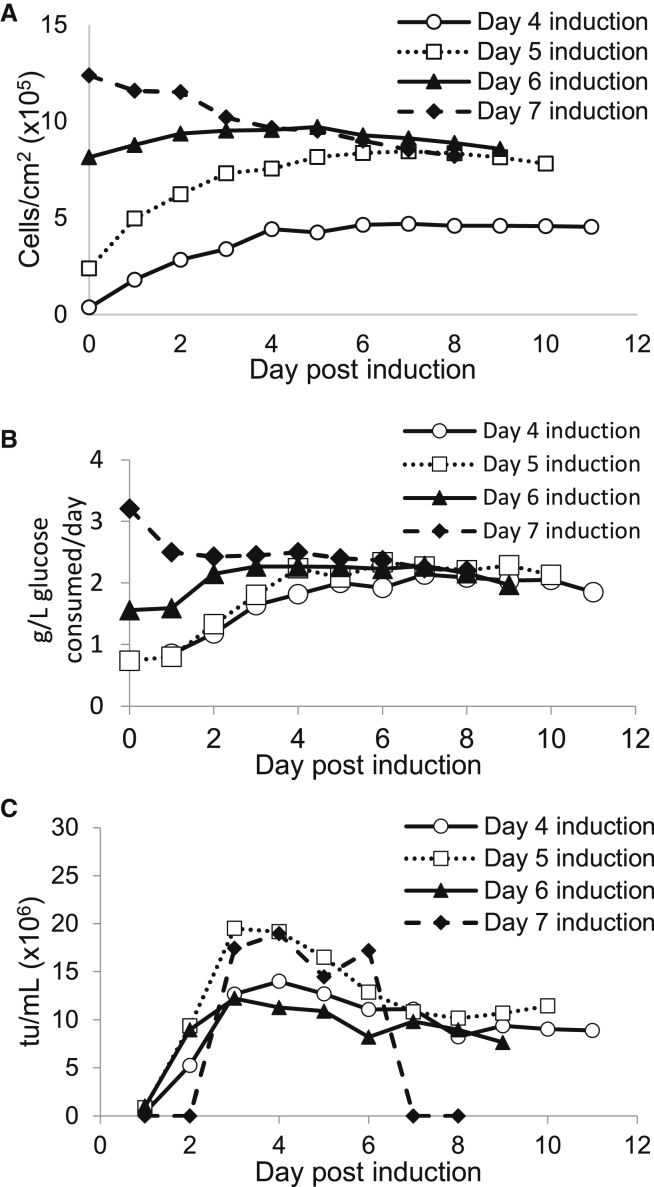
Vector production was evaluated as a function of the day of induction post-inoculation. Reactors with a 2-cm bed height were inoculated at 15,000 cells/cm2 (8 × 107 cells total). The pH set point was changed to 6.8 at induction, and all bioreactors maintained the pH between 6.8 and 6.9 after induction. The reactors ran for a total of 15 days. Cell density at induction increased with every additional day the cells were in the reactor before induction (Figure 2A). Glucose consumption continued to increase after induction in cultures induced at 4 or 5 days after inoculation while glucose consumption increased and then decreased in the culture induced on day 6 and decreased after induction in the culture induced on day 7 (Figure 2B). Induction 5 days after inoculation produced the highest overall yield of 9.7 × 1010 tu (days 1–10 post-induction included, Figure 2C). Lower viral titers were obtained when reactors were induced on day 4 (8.21 × 1010 tu, days 1–11 post-induction included), day 6 (6.32 × 1010 tu, days 1–9 post-induction included), or day 7 (5.44 × 1010 tu, days 1–8 post-induction included). While induction 7 days after inoculation initially produced comparable or higher viral titers to the other cultures, viral production ceased 7 days after induction, limiting the overall viral yield. This effect may have been caused by an over-confluence of cells and cells that were exiting the exponential growth phase at the time of induction that inhibited virus production. The decreasing glucose consumption in cultures induced on days 6 and 7 also supports that cells may have been exiting the exponential growth phase by the time of induction.
Figure 2.
Day of Induction
Bioreactors were inoculated with 8 × 107 cells and induced 4–7 days later in DMEM with 10% FBS and 2 mM GlutaMAX. The pH set point was adjusted to 6.8 after induction in all reactors. Culture continued for 11 days post-induction in the reactor induced 4 days after seeding, for 10 days in the reactor induced 5 days after seeding, for 9 days in the reactor induced 6 days after seeding, and for 8 days in the reactor induced 7 days after seeding. (A) Cell density on each day post-induction in reactors induced 4–7 days after inoculation. (B) Glucose consumption per day post-induction for reactors induced 4–7 days after inoculation. (C) Viral yield on each day post-induction for reactors induced 4–7 days after inoculation.
FBS Optimization
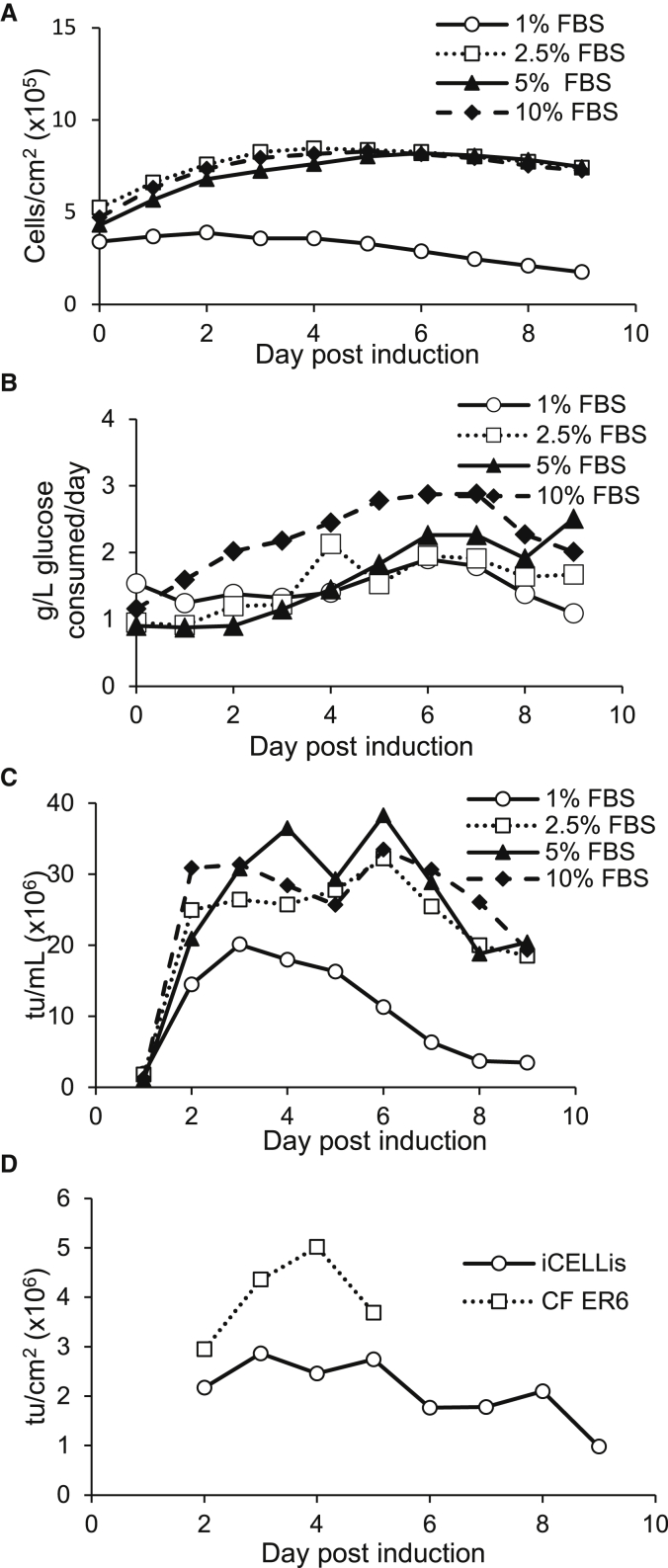
Significant work has been performed to find alternatives to FBS, including either supplements32 or adapting cells to serum-free suspension culture,16 but many producer cell lines require serum for production. Minimizing the FBS concentration during cell culture can reduce the amount of serum proteins that must be removed during purification. Given the cost and availability of FBS, reducing the amount of FBS would provide a supply cost and supply chain benefit as well as reduce variation between batches. We examined viral yield in cultures with FBS levels of 1%, 2.5%, 5%, or 10% of media volume (v/v) post-induction with media containing 10% FBS pre-induction. Reactors with a 2-cm bed height were inoculated with 15,000 cells/cm2 (8 × 107 cells total), induced 5 days after inoculation, and the pH decreased to 6.8 at induction. Cell density after induction was the same for cultures at 2.5%–10% FBS, but the culture at 1% FBS remained at a much lower cell density (Figure 3A). Glucose consumption was highest in the reactor with 10% FBS and decreased as FBS concentration decreased (Figure 3B). Cultures with 5% or 10% FBS gave the highest overall yields with 1.8 × 1011 total tu in each culture with a maximum daily titer of 3.3 × 107 tu/mL on day 6 post-induction in the 10% FBS culture and 3.8 × 107 tu/mL on day 6 post-induction in the 5% FBS culture. The culture with 2.5% FBS had an overall yield of 1.6 × 1011 total tu with a maximum daily titer of 3.2 × 107 tu/mL on day 6 post-induction and the culture with 1% FBS had an overall yield of 7.6 × 1010 total tu with a maximum titer on day 3 post-induction of 2 × 107 tu/mL (Figure 3C). Because 5% FBS gave a slightly higher yield than did 2.5% FBS, we chose to proceed with 5% FBS. Note that viral yields in this experiment are higher than those obtained in previous reactors. The ED7R assay has a 13% interassay variation, and unidentified cell conditions in this particular experiment may have contributed to a higher viral yield since this experiment was only performed once.
Figure 3.
FBS Concentration
Bioreactors were inoculated with 8 × 107 cells and induced 5 days later in DMEM with 1%–10% FBS and 2 mM GlutaMAX. The pH set point was adjusted to 6.8 after induction in all reactors. (A) Cell density on each day post-induction in each reactor at FBS concentrations from 1% to 10%. (B) Glucose consumption per day post-induction at FBS concentrations from 1% to 10%. (C) Viral yield on each day post-induction at FBS concentrations from 1% to 10%. (D) Scale-up. A 10-cm iCELLis bioreactor was inoculated with 4 × 108 cells and induced 5 days later. At induction, the pH set point was adjusted to 6.8 and the FBS concentration was reduced to 5%. A cell factory was inoculated with 1.4 × 108 cells and induced 3 days later. The FBS concentration remained at 10%.
Scale-Up
The iCELLis system has fixed bed heights from 2 to 10 cm with low and high compaction beds. Earlier studies comparing the 2-cm high and low compaction beds using the iCELLis Nano with HEK293T cells showed that low compaction reactors facilitated a more even distribution of cells and higher productivity as compared to high compaction reactors,29,30 so we limited our study to the low compaction iCELLis Nano.
The 10-cm reactor (2.65 m2) was evaluated using the optimum conditions established with the 2-cm reactor. Two 10-cm reactors were inoculated with 15,000 cells/cm2 (4 × 108 cells total) and induced 5 days after inoculation. Hold-up volume in the 10-cm reactor is 800 mL, the same volume used for the 2-cm reactor studies. To accommodate the additional 3,200 mL of media volume required by the 10-cm reactor (5-fold increase over the 2-cm reactor), an external 4,000-mL reservoir was connected to the iCELLis Nano reactor. Tubing exiting the external reservoir was connected to a downcomer on the head plate of the reactor and a return line was connected to a port on the head plate that was set at a height to ensure that the reactor volume was maintained at 800 mL. The 4,000 mL of media was recirculated using a peristaltic pump at 13.5 mL/min, generating a μ of 1.01 h−1. Based on previous experiments, it was estimated that the reactor contained 1.1 × 1010 cells at the time of induction and 1.8 × 1010 cells at the time of harvest (days 2–9 post-induction). After induction, the FBS concentration was reduced from 10% to 5% and the pH of the media was adjusted to 6.8. The daily titers peaked at an average of 1.9 × 107 tu/mL (2.87 × 106 tu/cm2), or 7.6 × 1010 total tu, on day 3 post-induction. Overall vector yield in our process was 4.5 × 1011 tu, from days 2 through 9 after induction, or 1.69 × 107 tu/cm2. In the 10-layer cell factory run ER6, the overall titer was 1.60 × 107 tu/cm2 during days 2–5 (Figure 3D). This daily titer obtained in the iCELLis compares with the work by Valkama et al.,29 who reported 1 × 1010 total tu from a single GFP LVV harvest in a low compaction 10-cm iCELLis Nano, especially when differences in titering methods (HeLa cells versus ED7R cells) were considered. Manceur et al.15 demonstrated that LVV production of GFP in a bioreactor in perfusion mode can yield 5–8 × 107 tu/mL. However, GFP is simpler than most clinical vectors and often generates higher titers than can be obtained with more complex clinical vectors such as shown in Figure 2 of Bauler et al.16 The volumetric titers obtained in the iCELLis were lower than the volumetric titers obtained in suspension culture by Bauler et al. (8 × 107 to 3.7 × 108 tu/mL), but the iCELLis offers the advantage of multiple days of harvest from one reactor as compared to a single day of harvest for transiently transfected suspension cells.16
An 800-mL portion of supernatant from each day 3–6 of one run of the iCELLis Nano 10-cm reactor (3,230 mL total) was purified using a Mustang Q XT5 membrane, eluted into phosphate buffer, and diafiltrated into X-VIVO 10 media. Table 1 shows purification yields. The pooled supernatant sample was taken just before purification after the supernatant had spent 3 days in the refrigerator, which could explain the difference between daily titers and the pooled supernatant titer. This decrease in titer appears less substantial in virus obtained from cell factories (data not shown) and may be attributable to the filtering capacity of the carriers in the iCELLis that remove virus-stabilizing components from the media. One option to overcome this difficulty would be to immediately purify the iCELLis supernatant after harvest, although it would add complexity to the manufacturing process to perform multiple purifications. The Mustang Q load sample was taken after salt- and pH-adjusting the sample, and the elute dilute sample was the eluted virus diluted 5-fold into phosphate buffer to decrease the salt content. The purified sample was from virus that had been diafiltrated into X-VIVO 10, its final formulation buffer, and filtered with a 0.22-μm filter. The overall recovery for purification of the iCELLis material was 49% as compared to 38% in the cell factory ER6 run. If the 10-cm iCELLis Nano directly scales up to the iCELLis 500, an iCELLis 500 low compaction 10-cm reactor is capable of a yield of 8.44 × 1012 tu of vector in one reactor run. The average vector requirement per newly diagnosed patient is 1.3 x 109 tu with a single iCELLis 500 run producing enough purified LVV to treat 6,490 patients.
Table 1.
Purification Yields in 10-cm iCELLis Nano with Estimated 1.8 × 1010 Cells
| tu/mL | Volume (mL) | Total tu | Yield (%) | |
|---|---|---|---|---|
| Pooled supernatant | 8.47 × 106 | 3,230 | 2.74 × 1010 | 100 |
| Mustang Q load | 6.24 × 106 | 3,427 | 2.14 × 1010 | 78 |
| Elute dilute | 2.54 × 108 | 75 | 1.91 × 1010 | 70 |
| Purified | 2.30 × 108 | 58.4 | 1.34 × 1010 | 49 |
Comparison to cGMP Manufacturing of GPRTG-EF1α-hγc-OPT LVVs Using Cell Factories
We compared the product from the iCELLis Nano 10-cm reactor to that from a cGMP fifty 10-layer cell factory run. The release specifications for residual host cell protein, residual DNA, bovine serum albumin, and Benzonase were based on the average from three small-scale engineering runs plus three times the standard deviation. Virus obtained during cGMP manufacturing was titered by transduction of both HOS cells and ED7R cells. The HOS titer release specification ensures that less than 70% of vector volume is required for transduction of CD34+ cells, and the ED7R titer confirms CD132 surface expression. All titer-based release criteria other than the HOS titer were based on ED7R titer values. The SV40T antigen DNA sequence transfer assay (into HeLa cells) is a safety assay and confirms that no SV40T antigen RNA is present in the GPRTG-EF1α-hγc-OPT LVVs. Release tests on the pooled unprocessed bulk and limit for in vitro cell age for production, including sterility, bacteriostasis and fungistasis, mycoplasmastasis, mycoplasma testing, in vitro assay for the presence of viral contaminants, 28 day (MRC-5, Vero, and HEK293T cells), in vitro assay for the presence of bovine viruses (9CFR [code of federal regulations] requirements), and a replication-competent lentivirus (RCL) assay (co-culture and supernatant), were negative and confirmed that GPRTG-EF1α-hγc-OPT LVVs were free of adventitious agents. The LVVs were formulated in X-VIVO 10, filled into 5-mL glass borosilicate-1 vials at a fill volume of 1.7 mL, and stored at −80°C. A real-time stability study, currently at 2 years, has shown that the LVVs are stable based on HOS titer and CD132 expression in ED7R cells. We compared results from one run in the 10-cm iCELLis Nano to release testing results from the clinical vector (Table 2). The iCELLis Nano had a purified ED7R titer of 2.3 × 108 tu/mL compared to a final purified titer of 3.32 × 108 tu/mL of the clinical vector, which meets release specifications of 1 × 108 tu/mL. Other release specifications, including sterility, residual host cell protein for HEK293T cells, and residual Benzonase, were also within release specifications for the iCELLis run. Endotoxin levels in the iCELLis met specification but were significantly higher than those obtained in the manufacturing run. This difference likely resulted from the use of the ÄKTA Avant during purification, which in our experience is known to contribute significant endotoxin to the process. The larger scale ÄKTA Ready was used for the GMP manufacturing run and because of its disposable flow path would not have contributed the level of endotoxin present in the ÄKTA Avant. Residual host cell DNA and residual bovine serum albumin levels in the purified iCELLis product were above release specifications. For the production cell factory run, a 260-mL Mustang Q capsule was used to purify 165 L of product, while for the iCELLis run, a 5-mL Mustang Q capsule was used to purify 3.32 L, leading to a loading volume of 0.63–0.66 L of supernatant per mL of Mustang Q in both processes, so column loading was unlikely to be a contributing factor in contaminant levels. It is possible that more cell lysis occurred during the iCELLis run with continuous recirculation of the media as compared to a static cell factory process, leading to higher contaminating DNA. The filtered supernatant from the iCELLis contained 625 ng of DNA/107 tu, while the filtered supernatant from a cell factory contained 279 ng of DNA/107 tu. The concentration of Benzonase (2.5 U/mL) was optimized for the cell factory process, but other processes in our facility have used up to 25 U/mL Benzonase, which could significantly decrease final host cell DNA concentration.
Table 2.
Release Specification and Assay Results of cGMP GPRTG-EF1α-hγc-OPT LVVs
| Parameter | Specification (tu/mL Based on ED7R Titer Unless Otherwise Indicated) | Result in Clinical Vector | Result in iCELLis Nano-Produced Vector |
|---|---|---|---|
| Purified GRPTG-EF1α-hγc-OPT LVVs | |||
| Container integrity | intact vial, no particulates, solution clear and colorless | intact vial, no particulates, solution clear and colorless | not performed |
| Sterility | sterile | sterile | sterile |
| Bacteriostasis and fungistasis | no bacteriostatic and fungistatic activity | no bacteriostatic and fungistatic activity | not performed |
| Endotoxin | <5.0 EU/mL | <0.05 EU/mL | 1.77 EU/mL |
| Infectious titer (HOS) | ≥2 × 108 tu/mL | 5.2 × 108 tu/mL | not performed |
| γ Chain expression/infectious titer (ED7R) | ≥1 × 108 tu/mL | 3.32 × 108 tu/mL | 2.3 × 108 tu/mL |
| Residual host cell protein HEK293T | <7 ng/107 tu | 7 ng/107 tu | 2.3 ng/107 tu |
| Residual host cell DNA | ≤26 ng/107 tu | 13 ng/107 tu | 120 ng/107 tu |
| Residual bovine serum albumin | ≤39 ng /107 tu | 17 ng/107 tu | 54.1 ng/107 tu |
| Residual Benzonase | ≤0.026 ng/107 tu | <0.006 ng/107 tu | <0.009 ng/107 tu |
| Vector insert integrity by sequence | consistent with expected results | consistent with expected results | not performed |
| SV40 large T antigen DNA sequence transfer | not detected | not detected | not performed |
| Limit for In Vitro Cell Age for Production | |||
| In vivo assay for the presence of inapparent viruses | negative | negative | not performed |
| Vector insert integrity by DNA sequence in the producer cell line | consistent with expected result | consistent with expected result | not performed |
| TEM of cultured cells | no identifiable viral particles other than expected lentivirus-like particles | no identifiable viral particles other than expected lentivirus-like particles | not performed |
| Cell line identity by CO1 barcode | consistent with human origin | consistent with human origin | not performed |
EU, endotoxin units.
Transduction Efficiency
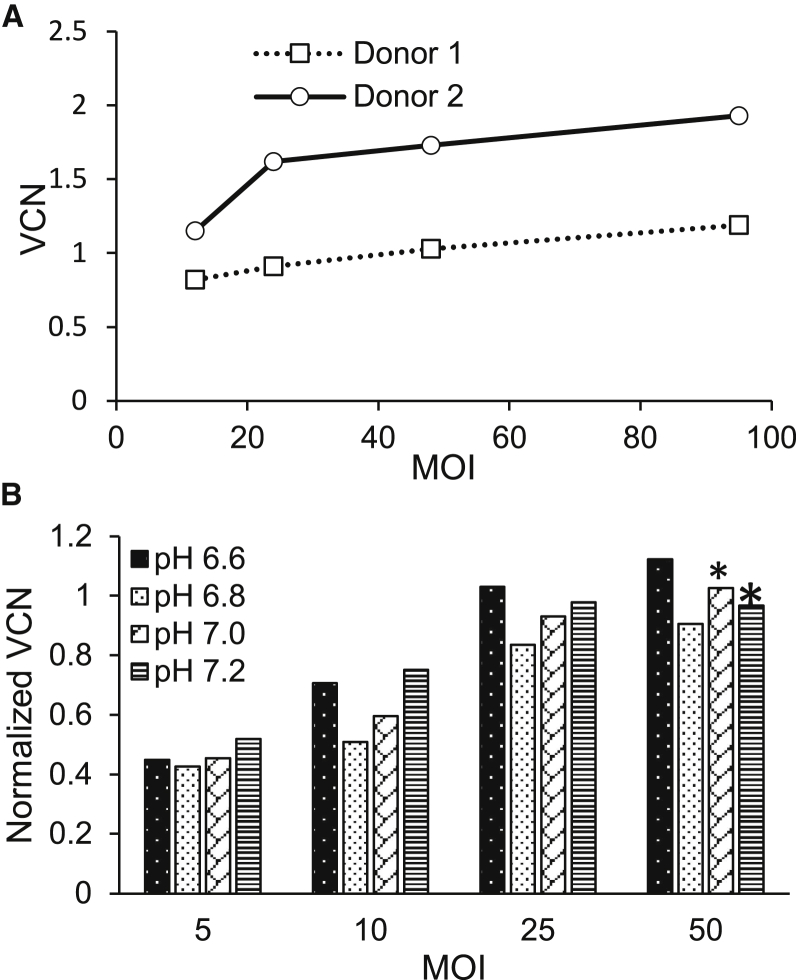
In addition to viral titer, the ability of the LVV to transduce patient CD34+ cells affects its clinical efficacy. For clinical vectors produced in 10-layer cell factories, the transduction efficacy of the GPRTG-EF1α-hγc-OPT LVVs on purified CD34+ cells from two different mobilized healthy peripheral blood donors was determined with a multiplicity of infection (MOI) of 12–98 and a cell concentration of 2 × 106 CD34+ cells/mL. The chromosomally integrated vector copy number (VCN) ranged from 0.8 to 1.7 (Figure 4A).
Figure 4.
Transduction Efficiency in CD34+ Cells
After transduction, genomic DNA was extracted from these cells and analyzed by qPCR to measure vector copy number. (A) Transduction as a function of MOI for two different donors. 2 × 106 cells/mL from each donor were transfected with viral vector produced in the 50-cell factory production run. (B) Cells were transduced with viral vector produced in iCELLis reactors run at pH 6.6, 6.8, 7.0, and 7.2 after induction or in cell factories (clinical vector). Values are normalized by clinical vector (MOI of 16.6) VCN value (ranges from 0.7 to 0.92) for each transduction run. ∗Maximum MOI value tested at pH 7.0 was 40 and maximum MOI value tested at pH 7.2 was 36 because of the available vector concentration.
We examined the transduction efficiency of the GPRTG-EF1α-hγc-OPT LVVs produced in the iCELLis as compared to vectors produced in cell factories for clinical production. Harvested supernatants from reactors induced 5 days after inoculation in D10 media at pH 6.6, 6.8, 7.0, and 7.2 were purified, and the purified virus was used to infect CD34+ cells. The cells were infected at MOIs beginning at 5 and ranging up to 36–50 for each pH value. After culturing these cells for 6 days in MethoCult, the cells were harvested, and the genomes were extracted for qPCR. The acquired VCN was normalized by the VCN of the clinical vector produced in cell factories and included in each assay at an MOI of 16.6. The VCN of the clinical vector ranged from 0.70 to 0.92 in each assay. As shown in Figure 4B, the normalized VCN detected in each cell increased as the MOI increased for all pH values. Cells transduced at an MOI of 5 demonstrated a normalized VCN ranging from 0.44 to 0.52, while cells transduced at an MOI of 25 demonstrated VCN values ranging from 0.93 to 1.03. These results show that vector produced in the iCELLis reactor has a similar transduction efficiency as did vector produced with the more traditional 10-layer cell factory method and that production of vector at pH values ranging from 6.6 to 7.2 does not impact VCN values of that vector.
Conclusions
Gene therapy and the use of LVVs in gene therapy continue to increase, but methods to produce LVVs in a commercially viable way are lacking. While cell factories can be used at a limited scale for virus production, they are labor-intensive and must be scaled out rather than scaled up. They also lack the controlled environment of bioreactors to achieve consistent quality across different production lots. This work has demonstrated that the iCELLis bioreactor can produce LVVs from the stable producer cell line GPRTG-EF1α-hγc that is comparable in both titer and transduction efficiency to vector produced in cell factories. It was shown that lowering the pH below the traditional value of 7.2 increases viral yield in the iCELLis and that the iCELLis environment is amenable to a decreased concentration of FBS, which decreases costs and facilitates downstream processing. Volumetric viral titer remained consistent when the iCELLis Nano culture was scaled up from a 2-cm to a 10-cm packed bed, suggesting that the iCELLis 500 will be a practical option for large-scale manufacture of LVVs. Further investigation into the mechanisms of the pH effect and optimization of media composition may provide additional gains in viral titer and ease of downstream processing. Additionally, optimization of the downstream process through additional Benzonase and membrane sizing may further increase viral yields.
Materials and Methods
Construction of the HEK293T Lentiviral Stable Producer Cell Line
Throm et al.33 described the construction of HEK293T self-inactivating (SIN) LVV stable producer cell lines that are transiently transfected with a concatemer array of the target gene, i.e., interleukin (IL)-2 receptor for XSCID, generating a LVV-specific producer cell line. There were two parent cell lines, i.e., the GPRG cell line, which has the HIV gagpol, rev, and vesicular stomatitis virus G protein (VSV-G) genes, and the GPRTG cell line, which includes the HIV tat gene. A brief description of the construction of the GPRTG cell line is presented below. The same approach was used to generate the GPRG cell line by excluding the tat gene transduction.
The first step was to incorporate the gagpol genes into HEK293T/17 cells using a gammaretrovirus to generate GP cells that constitutively express the gagpol genes under a cytomegalovirus (CMV) promotor with puromycin selection. The best GP clone (and sequential clones) was selected by transiently transfecting the remaining LV genes, including a GFP reporter gene, generating LV supernatant, transducing HeLa cells with the LV supernatant, and selecting the best clone based on titer using flow cytometry.
The second step involves the transduction with three different gammaretroviral vectors during 2–3 days depending on the desired parent cell line. Starting with GP cells, the first transduction was with a gammaretroviral vector SFG-tc-revco, which expresses a codon-optimized HIV rev gene under the control of a tetracycline-regulated promotor. On day 2, the media was exchanged, and cells were transduced with gammaretroviral vector SFG-tc-tatco, which is a codon-optimized HIV tat gene under the control of a tetracycline-regulated promotor. On day 3, the media was exchanged, and the cells were transduced with gammaretroviral vector SFG-tTA, which is a chimeric transcriptional activator under the control of a retroviral long terminal repeat (LTR). The best growing clone that produces LVV able to express GFP protein into HeLa cells after being transiently transfected with VSV-G plasmid and GFP plasmid was expanded and frozen down as a GPRT research cell bank.
The third step included the transduction of the GPRT cells with the gammaretroviral vector SFG-tc-VSV-G, which is under the control of a tetracycline regulated promotor. The best growing cell that produces LVV able to transfer GFP to HeLa cells after being transiently transfected with GFP plasmid was expanded and frozen down as the parent GPRTG cell line.
The fourth step was the production of multimeric concatemer with a tetracycline-regulated, LVV codon-optimized IL-2 receptor (IL2R) gene expression cassette under the control of EF1α promotor ligated with a zeocin-resistant expression cassette at a molar ratio of 25:1, respectively. The gel-purified concatemer was transiently transfected into the GPRTG cell line, selected with zeocin, and clones were isolated by limiting dilution. The induced supernatant was titered for expression of IL-2R in ED7R cells. The best producing clone was confirmed in small-scale manufacturing studies, and a cGMP master cell bank (MCB) was manufactured by expanding the clone in DMEM + 10% FBS + 100 ng/mL doxycycline (Clontech Laboratories, Mountain View, CA, USA) + 2 μg/mL puromycin + 50 μg/mL Zeocin (Invitrogen, Carlsbad, CA, USA) D10 and formulated in Recovery cell culture freezing medium (Thermo Fisher Scientific) with 100 ng/mL doxycycline at 1 × 107 cells/mL and stored in vapor phase liquid nitrogen, and designated as the GPRTG-EF1α-hγc-OPT MCB.
Description of iCELLis Bioreactor
The iCELLis bioreactor (Pall Corporation, Port Washington, NY, USA) is a disposable bioreactor containing a fixed bed of polyester microfiber macrocarriers on which cells attach or become entrained and grow. The fixed bed at the center of the bioreactor was surrounded by culture media. Stirring at the bottom of the reactor was set at a speed to ensure that medium flows over the top of the fixed bed at 1–2 cm/s and is replenished with oxygen as it returns to the surrounding reservoir of medium. The fixed bed may be from 2 to 10 cm in height and either a low or high compaction. Available surface area for cell growth ranges from 0.53 m2 (low compaction, 2-cm bed height) to 4 m2 (high compaction, 10-cm height) for the iCELLis Nano. All experiments in this study were performed with the low compaction iCELLis Nano reactors. The bioreactor has a reusable metal lid that contains ports for pH and DO probes, sampling, media addition and removal, and air flow, as well as a thermowell for a temperature probe.
Operation of iCELLis Bioreactor
An autoclaved iCELLis vessel containing a 2-cm packed bed was filled with 800 mL of DMEM (Gibco, Thermo Fisher Scientific), 2 mM GlutaMAX, and 10% (v/v) FBS (HyClone) (D10 media) and equilibrated overnight at the set points of 37°C, pH of 7.20, initial stir speed of 1 cm/s, and DO at 50%. The vessel was inoculated with 8 × 107 GPRTG-EF1α-hγc cells, corresponding to 15,000 cells/cm2 bed area, and the stir speed was increased to 3 cm/s for 1 h before being decreased back to 1 cm/s. Doxycycline was added to the reactor at 1 ng/mL during inoculation and an additional 1 ng/mL of doxycycline was added on the third day after inoculation. A metabolic analyte measurement was taken the day before induction. An Aber Futura biomass probe was used to record cell density in some reactors.
Induction of GPRTG-EF1-α-hγc Cells in iCELLis
On day 5 after inoculation with GPRTG-EF1α-hγc cells, the iCELLis vessel was induced. Cell density was on average 4.5 × 105 cells/cm2 at this point in the culture, and no additional media changes occurred before induction. Media were pumped from the reactor and the reactor was washed twice for 5 min at a media recirculation speed of 1 cm/s with 600 mL of DMEM. After the wash, DMEM was removed from the reactor and 800 mL of D10 media without doxycycline was added to the reactor. For 9 days after induction, media were pumped out of the reactor each day, and 800 mL of fresh D10 medium was added to the reactor. Beginning on the second day post-induction, 2.5 U/mL of Benzonase (Millipore) was added to the reactor daily, and the supernatant was collected daily for purification beginning on the third day post-induction. Aliquots were taken each day for titer analysis and metabolic analysis. When the pH set point in the reactor was below 7.2, the addition of 0.5 N HCl to fresh D10 was used to decrease the pH of the culture medium before pumping it into the iCELLis reactor.
Lentiviral Purification
Supernatants collected from an iCELLis on days 3–6 post-induction were filtered through an Opticap XL2 capsule Durapore 0.45-μm filter (EMD Millipore, Darmstadt, Germany) and pooled together into a 5-L Flexboy bag (Sartorius, Goettingen, Germany). The concentration of NaCl in the sample was increased by 250 mM with the addition of AccuGene 5 M NaCl (Lonza, Basel, Switzerland). The pH of the sample was adjusted with 1 M Tris (pH 8.0) to a final concentration of 50 mM Tris (pH 8.0).
After NaCl and pH adjustment, the sample was loaded on a Mustang Q XT5 (Pall Corporation) at 50 mL/min and equilibrated with 400 mM NaCl and 50 mM Tris (pH 8.0). Lentivirus was eluted from the column using 1.5 M NaCl/50 mM Tris (pH 8.0) in 15 mL. Eluted fractions were diluted 5-fold into 6.6 mM phosphate buffer (pH 7.4). The diluted fraction was concentrated and diafiltrated into X-VIVO 10 (Lonza) by tangential-flow filtration at 50 mL/min using a 0.005-m2 Pellicon XL ultrafiltration module with a 500-kDa cutoff (EMD Millipore, USA). No pressure gauges were used, and the retentate line remained fully open. Purified supernatant was filtered through a 0.22-μm polyvinylidene fluoride (PVDF) Millex syringe filter (Millipore).
Vector Titering
ED7R cells, which do not express CD132, were cultured in RPMI 1640 (Life Technologies) with 10% FBS and 2 mM glutamine (R10 media) and plated at 2 × 105 cells/well in a 12-well tissue culture plate. Purified LVVs and 8 μg/mL Polybrene (EMD Millipore) were added to each well for a final volume of 500 μL/well. The plates were centrifuged at 1,000 × g for 1 h and incubated at 37°C for 3 h before the addition of 1 mL of fresh R10 medium. After 72 h in culture, cells were harvested into a 96-well deep well plate. Cells were washed with phosphate-buffered saline (PBS) (Lonza) and incubated briefly on ice with rat immunoglobulin G (IgG) in PBS with 2% FBS. Biotin-labeled rat anti-human CD132 primary antibody (BD Biosciences, 10 μg/mL) in PBS with 2% FBS was added to the cells for 30 min on ice. The cells were washed with PBS, and 0.5 μg/mL of streptavidin phycoerythrin (PE)-conjugated secondary antibody (BD Biosciences) prepared in PBS with 2% FBS was added for 20 min on ice. Cells were washed with PBS, filtered through 30- to 40-μm 96-well AcroPrep Advance filter plates (Pall Corporation) and suspended in 150 μL of DAPI + PBS with 2% FBS for sorting by using the BD FACSLyric system. Cells that stably express CD132 were used as a positive control, and data were analyzed by linear regression of known titer values of an internal standard.
VCN Analysis
CD34+ cells were separated from apheresis-mobilized peripheral healthy donors using CD34 Microbeads and a CliniMACS Plus (program 2.1, CD34+ cell selection) and formulated at 1 × 107 cells/mL in Plasma-Lyte-A with 5% by volume dimethyl sulfoxide (DMSO), 6% pentastarch, and 3.75% human serum albumin (HSA) (cryopreservation medium).
Transduced CD34+ cells were thawed, centrifuged at 300 × g for 10 min, supernatant was discarded, and the cells were resuspended at a concentration of 1 × 106 cells/mL and prestimulated with 100 ng/mL each of recombinant human stem cell factor (CellGenix, Portsmouth, NH, USA), recombinant human Flt3 ligand (CellGenix), and human recombinant thrombopoietin (CellGenix) in X-VIVO 10 (without gentamicin or phenol red; Lonza) media with 1% HSA (Grifols). After 18–24 h, cells were counted, centrifuged for 15 min at 200 × g, and resuspended in X-VIVO 10 with 1% HSA and cytokines before adding to wells of a 24-well plate at 1 × 106 cells/well. Protamine sulfate (Fresnius Kabi, USA) was added to each well at a concentration of 8 μg/mL, and purified vector produced in the iCELLis was added to the wells at specified MOIs for a final volume of 500 μL/well. After 16–20 h, cells were harvested from each well, and the wells were washed with 1 mL of PlasmaLyte (Baxter) with 4% HSA. Harvested cells and wash solution were centrifuged at 200 × g and resuspended in 500 μL of PlasmaLyte with 4% HSA. The CD34+-transduced cells were adjusted to 100,000 cells/mL in X-VIVO 10 and 40,000 cells were added to 4 mL of MethoCult (STEMCELL Technologies, Canada). The suspension was vortexed for 5 s and allowed to sit for 3–5 min to remove air bubbles. 1.1 mL of MethoCult-containing cells was placed into three 35-mm tissue culture dishes with grids (Sarstedt) and incubated for 6 days at 37°C, 5% CO2, and 95% relative humidity. The 35-mm dishes were placed in one 150-mm dish along with a 35-mm dish containing sterile water to provide humidity. The cells were harvested on day 6 of culture by gently resuspending them with 4 mL of PBS (Corning Cellgro). The suspension was transferred to a 15-mL tube, rinsed with 2 mL of PBS, and centrifuged at 400 × g for 5 min. Additional PBS was added and cells were resuspended and washed a second time. Cells were resuspended in 200 μL of PBS and transferred to a microcentrifuge tube. Genomic DNA was extracted using the DNeasy blood and tissue kit (QIAGEN) for Digital Droplet PCR (ddPCR) analysis.
ddPCR Analysis
The QX200 ddPCR system (Bio-Rad, Hercules, CA, USA) was used to determine the VCN of the common γ chain gene of IL2RG using DNA isolated from day 6 colony-forming unit cell (CFU-C) colonies of CD34+ LV-XSCID-transduced and mock-transduced cells. After determining the DNA concentration, each sample was adjusted to 10 ng/μL. Primers and probe for the target, hγc-OPT labeled with 6-carboxyfluorescein (FAM), and the RPP30 housekeeping gene labeled with HEK were purchased from Integrated DNA Technologies (IDT; Coralville, IA, USA). For one reaction of 95 μL (samples were run in triplicate at 25 μL/well), reagents were added as follows: 2.5 μL of 40× hγc-OPT primer/probe mixture, 50 μL of ddPCR supermix for probes (Bio-Rad), 900 nM RPP30 forward (Fwd) and reverse (Rvs) primers, and 250 nM probe. Water (Molecular Probes, Eugene, OR, USA) was added to adjust the volume to 95 μL (approximately 40.45 μL). 5 μL of DNA at 10 ng/μL was added to the master mix and mixed well. The PCR was performed using a C1000 Touch thermal cycler (Bio-Rad) at 95°C for 10 min, 94°C for 30 s, followed by 60°C for 1 min with 40 cycles and 98°C for 10 min. Droplets were read using the droplet reader of the QX200 ddPCR system. Results were analyzed using QuantaSoft Analysis Pro software version 1.0 to determine the VCN.
cGMP Manufacturing of GPRTG-EF1α-hγc-OPT LVV Using Cell Factories
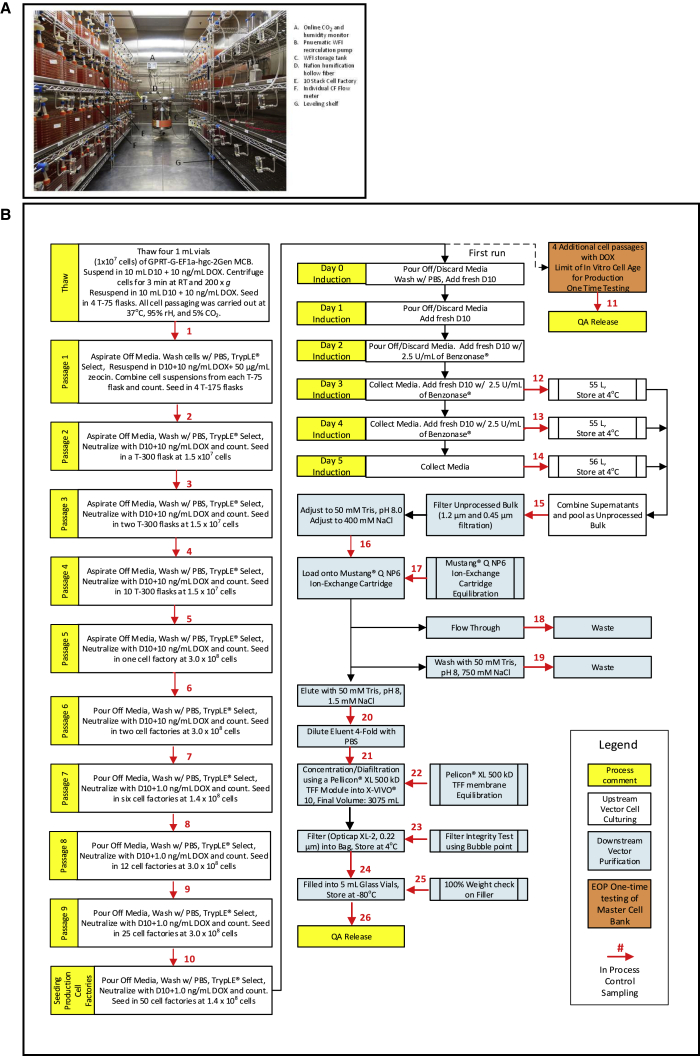
Children’s GMP, LLC (CGL), which is solely owned by St. Jude, is located on the St. Jude campus in the GMP Building (64,000 ft2) and is responsible for all cGMP manufacturing activities in support of phase I and II clinical trials at St. Jude. CGL’s platform for cGMP manufacturing of vectors (adeno-associated virus and lentivirus) from adherent HEK293T/17 cells is 10-layer cell factories. The GMP building has an environmental growth chamber modified to actively gas (100–500 mL/min) eighty 10-layer cell factories with 95%/5% air/CO2 and 95% humidity with online monitoring of %CO2 and humidity (Figure 5A).
Figure 5.
Manufacturing of GMP Vector
(A) Environmental growth chamber modified for active gassing of cell factory. (B) Process flow diagram of the cGMP manufacturing of GPRTG-EF1α-hγc-OPT LVVs.
The cGMP manufacturing of GPRTG-EF1α-hγc-OPT LVV was performed at the 50-cell factory scale using D10 media. A process flow diagram that describes each manufacturing step and the in-process assays are presented in Figure 5B and Table 3, respectively. Manufacturing was initiated from four identical 1-mL vials of the MCB, and cells were passaged when reaching 50%–70% confluency. Zeocin was added at a concentration of 50 μg/mL at the first passage following thaw, and nine passages were required to generate enough cells to seed 50-cell factories at 1.4 × 108 cells/cell factory. The doxycycline concentration was maintained at 10 ng/mL for passages 1–6 and reduced to 1 ng/mL for passages 7–9 and seeding of the 50-cell factories. The medium was not changed for the first 3 days after seeding. On day 3 after seeding, i.e., day 0 of induction, the media were changed in all 50-cell factories with D10 (no doxycycline) media, and the media were exchanged daily for the following 4 days. On day 2 through day 4 of induction, 2.5 U/mL of Benzonase was added to the D10 media. Supernatants from days 0, 1, and 2 were discarded. On days 3, 4, and 5 post-induction, supernatants were collected individually and stored at 2°C–8°C. The following day (day 6 post-induction), days 3–5 supernatants were combined, filtered, and the pH and NaCl concentrations were adjusted to 8.0 and 400 mM, respectively, loaded onto a Mustang Q NP6 ion exchange cartridge, washed with 50 mM Tris (pH 8.0) and 750 mM NaCl, and the LVVs were eluted with 50 mM Tris (pH 8.0) and 1,500 mM NaCl using an ÄKTA Ready (GE Healthcare). The product was immediately diluted 4-fold with PBS, concentrated with a Pellicon XL 500-kDa ultrafiltration membrane (Millipore) to 3,075 mL, and diafiltered with 3 vol X-VIVO 10, filtered through a 0.22-μm Opticap XL-2 filter (Sartorius Stedim), and immediately filled into one thousand two hundred 5-mL borosilicate-1 vials (1.7-mL fill volume) with a Chase-Logeman FSAS-2205 vial filler and stored at −80°C.
Table 3.
Description of Sample and the Corresponding In-Process Assay
| Sample No. | Sample Description | In-Process Assay |
|---|---|---|
| 1–10 | passages 1–10 | pH, glucose, lactate |
| 11 | limit of in vitro cell age for production | N/A |
| 12 | unprocessed bulk day 3 PI | sterility, pH, glucose, lactate, ED7R titer |
| 13 | unprocessed bulk day 4 PI | sterility, pH, glucose, lactate, ED7R titer |
| 14 | unprocessed bulk day 5 PI | sterility, pH, glucose, lactate, ED7R titer |
| 15 | pooled unprocessed bulk | N/A |
| 16 | Mustang Q load | ED7R titer |
| 17 | equilibration of Mustang Q | pH and endotoxin |
| 18 | Mustang Q flowthrough | ED7R titer |
| 19 | Mustang Q wash | ED7R titer |
| 20 | Mustang Q elution fraction | ED7R titer |
| 21 | diluted Mustang Q elution fraction | ED7R titer |
| 22 | TFF membrane equilibration | pH and endotoxin |
| 23 | Opticap XL-2, 0.22-μm filter | bubble point test |
| 24 | drug substance | ED7R titer |
| 25 | drug product | 100% weight check |
TFF, tangential flow filtration; PI, post induction; N/A, not applicable.
Detection of Residual Host Cell Protein
The Cygnus HEK293T host cell protein ELISA kit was used to detect host cell protein. Samples were reacted with a horseradish peroxidase (HRP) enzyme-labeled anti-HEK293T antibody and washed to remove any unbound reactants. The substrate tetramethylbenzidine (TMB) was then reacted. The amount of hydrolyzed substrate was read on a microplate reader and was directly proportional to the concentration of HEK293T host cell proteins present.
Detection of Residual DNA
The Molecular Probes PicoGreen double-stranded DNA (dsDNA) quantitation reagent and kit was used to detect residual DNA. Samples were diluted in Tris-EDTA buffer and PicoGreen reagent provided with the kit. Samples were incubated for 2–5 min protected from light and read at excitation 490 nm, emission 525 nm.
Detection of Residual BSA
This assay used the Cygnus BSA ELISA kit (catalog no. F030). Samples were reacted in kit-provided microtiter strips coated with an affinity-purified capture antibody. A second anti-BSA antibody labeled with the enzyme HRP was added simultaneously and then incubated for 1 h at room temperature, forming a sandwich complex of a solid phase antibody-BSA-HRP-labeled antibody. The microtiter strips were washed to remove unbound reactants, and the strips were reacted for 30 min at room temperature with the HRP substrate TMB. A stop solution was added to all wells to stop the kinetic reaction, and strips were read within 30 min. The amount of hydrolyzed substrate was read on a microtiter plate reader at 450 nm and was directly proportional to the concentration of BSA present in the sample.
Detection of Residual Benzonase
This assay used the EM Industries Benzonase endonuclease ELISA kit II (catalog no. 1.01681.002). The kit uses a 96-well polystyrene microtiter plate precoated with polyclonal antibodies specific to Benzonase endonuclease. Reference standard and samples were added to the wells in triplicate at room temperature and placed on a rotating shaker at 450 rpm for a minimum of 2 h. After a buffer wash, the secondary HPR antibody was added and plate was incubated for 1 h. The colorimetric reagent was added and plate was incubated for 15 min in the dark. Reaction was stopped by adding 0.2 M H2SO4 and the plates were read within 30 min at 450 nm. The amount of hydrolyzed substrate was directly proportional to the concentration of Benzonase present in the sample.
Author Contributions
Conceptualization, A.D.P., T.D.L., and M.M.M.; Methodology, A.D.P., J.E.D., T.D.L., and M.M.M.; Investigation, A.D.P., J.E.D., and C.F.H.; Writing – Original Draft, A.D.P. and J.E.D.; Writing – Review & Editing, A.D.P., J.E.D., T.D.L., and M.M.M.; Supervision, T.D.L. and M.M.M.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
The authors would like to thank Dr. Rob Throm (St. Jude Children’s Research Hospital) and Dr. Byoung Ryu (St. Jude Children’s Research Hospital) for providing the cell line used in this work; Dr. Soumyasri Das Gupta (St. Jude Children’s Research Hospital) and Dr. Aixia Ren (St. Jude Children’s Research Hospital) for help with the iCELLis reactor; Dr. Aaron Shafer (St. Jude Children’s Research Hospital) and Dr. Shanthi Vadali (Children’s GMP, LLC) for help with the ED7R titering assay; and Dr. Clifford Froelich (Children’s GMP, LLC), Dr. Susan Sleep (Children’s GMP, LLC), Shirley Steward (Children’s GMP, LLC), and Suzette Wingo (St. Jude Children’s Research Hospital) for help with the VCN assay. The authors would also like to thank the Children’s GMP manufacturing team for their work manufacturing GPRTG-EF1α-hγc-OPT LVVs in cell factories. Funding for this project was provide by the American Lebanese Syrian Associated Charities (ALSAC). This research was also supported by NIH grant (5P01HL053749-22). The content is solely the responsibility of the authors and does not necessarily represent the offiical views of the National Institutes of Health.
References
- 1.Kotterman M.A., Chalberg T.W., Schaffer D.V. Viral vectors for gene therapy: translational and clinical outlook. Annu. Rev. Biomed. Eng. 2015;17:63–89. doi: 10.1146/annurev-bioeng-071813-104938. [DOI] [PubMed] [Google Scholar]
- 2.Aiuti A., Biasco L., Scaramuzza S., Ferrua F., Cicalese M.P., Baricordi C., Dionisio F., Calabria A., Giannelli S., Castiello M.C. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013;341:1233151. doi: 10.1126/science.1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mamcarz E., Zhou S., Lockey T., Abdelsamed H., Cross S.J., Kang G., Ma Z., Condori J., Dowdy J., Triplett B. Lentiviral gene therapy combined with low-dose busulfan in infants with SCID-X1. N. Engl. J. Med. 2019;380:1525–1534. doi: 10.1056/NEJMoa1815408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Ravin S.S., Wu X., Moir S., Anaya-O’Brien S., Kwatemaa N., Littel P., Theobald N., Choi U., Su L., Marquesen M. Lentiviral hematopoietic stem cell gene therapy for X-linked severe combined immunodeficiency. Sci. Transl. Med. 2016;8:335ra57. doi: 10.1126/scitranslmed.aad8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cartier N., Hacein-Bey-Abina S., Bartholomae C.C., Bougnères P., Schmidt M., Kalle C.V., Fischer A., Cavazzana-Calvo M., Aubourg P. Lentiviral hematopoietic cell gene therapy for X-linked adrenoleukodystrophy. Methods Enzymol. 2012;507:187–198. doi: 10.1016/B978-0-12-386509-0.00010-7. [DOI] [PubMed] [Google Scholar]
- 6.Cavazzana-Calvo M., Payen E., Negre O., Wang G., Hehir K., Fusil F., Down J., Denaro M., Brady T., Westerman K. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010;467:318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biffi A., Montini E., Lorioli L., Cesani M., Fumagalli F., Plati T., Baldoli C., Martino S., Calabria A., Canale S. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- 8.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J., Chew A., Gonzalez V.E., Zheng Z., Lacey S.F. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruella M., Kenderian S.S. Next-generation chimeric antigen receptor T-cell therapy: going off the shelf. BioDrugs. 2017;31:473–481. doi: 10.1007/s40259-017-0247-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuroda H., Kutner R.H., Bazan N.G., Reiser J. Simplified lentivirus vector production in protein-free media using polyethylenimine-mediated transfection. J. Virol. Methods. 2009;157:113–121. doi: 10.1016/j.jviromet.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 11.Reed S.E., Staley E.M., Mayginnes J.P., Pintel D.J., Tullis G.E. Transfection of mammalian cells using linear polyethylenimine is a simple and effective means of producing recombinant adeno-associated virus vectors. J. Virol. Methods. 2006;138:85–98. doi: 10.1016/j.jviromet.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 12.McCarron A., Donnelley M., McIntyre C., Parsons D. Challenges of up-scaling lentivirus production and processing. J. Biotechnol. 2016;240:23–30. doi: 10.1016/j.jbiotec.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Greene M.R., Lockey T., Mehta P.K., Kim Y.S., Eldridge P.W., Gray J.T., Sorrentino B.P. Transduction of human CD34+ repopulating cells with a self-inactivating lentiviral vector for SCID-X1 produced at clinical scale by a stable cell line. Hum. Gene Ther. Methods. 2012;23:297–308. doi: 10.1089/hgtb.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stornaiuolo A., Piovani B.M., Bossi S., Zucchelli E., Corna S., Salvatori F., Mavilio F., Bordignon C., Rizzardi G.P., Bovolenta C. RD2-MolPack-Chim3, a packaging cell line for stable production of lentiviral vectors for anti-HIV gene therapy. Hum. Gene Ther. Methods. 2013;24:228–240. doi: 10.1089/hgtb.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manceur A.P., Kim H., Misic V., Andreev N., Dorion-Thibaudeau J., Lanthier S., Bernier A., Tremblay S., Gélinas A.M., Broussau S. Scalable lentiviral vector production using stable HEK293SF producer cell lines. Hum. Gene Ther. Methods. 2017;28:330–339. doi: 10.1089/hgtb.2017.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauler M., Roberts J.K., Wu C.C., Fan B., Ferrara F., Yip B.H., Diao S., Kim Y.I., Moore J., Zhou S. Production of lentiviral vectors using suspension cells grown in serum-free media. Mol. Ther. Methods Clin. Dev. 2019;17:58–68. doi: 10.1016/j.omtm.2019.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merten O.W., Hebben M., Bovolenta C. Production of lentiviral vectors. Mol. Ther. Methods Clin. Dev. 2016;3:16017. doi: 10.1038/mtm.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merten O.W. Advances in cell culture: anchorage dependence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015;370:20140040. doi: 10.1098/rstb.2014.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Havelange N., Marigliano M., Sainte-Marie M., Debras F., Tazir N., Castillo J. Poxvirus production on chicken embryo fibroblasts in iCELLis™ disposable fixed-bed bioreactor. In: Jenkins N., Barron A.P., editors. Proceedings of the 21st Annual Meeting of the European Society for Animal Cell Technology (ESACT) 2012. pp. 719–722. Springer. [Google Scholar]
- 20.Hambor J.E. Bioreactor design and bioprocess controls for industrialized cell processing. BioProcess Int. 2012;10:22–33. [Google Scholar]
- 21.Drugmand J.-C., Esteban G., Alaoui N., Jafâr N., Havelange N., Berteau O., Castillo J. On-line monitoring: animal cell cultivation in iCELLis™ fixed-bed reactor using dielectric measurements. In: Jenkins N., Barron A.P., editors. Proceedings of the 21st Annual Meeting of the European Society for Animal Cell Technology (ESACT) 2012. pp. 395–399. Springer. [Google Scholar]
- 22.Drugmand J.-C., Havelange N., Collignon F., Castillo J., Girod P.-A. 4 g/L. day: monoclonal antibody volumetric productivity in the iCELLis™ disposable fixed-bed bioreactor. In: Jenkins N., Barron A.P., editors. Proceedings of the 21st Annual Meeting of the European Society for Animal Cell Technology (ESACT) 2012. pp. 375–378. Springer. [Google Scholar]
- 23.Rajendran R., Lingala R., Vuppu S.K., Bandi B.O., Manickam E., Macherla S.R., Dubois S., Havelange N., Maithal K. Assessment of packed bed bioreactor systems in the production of viral vaccines. AMB Express. 2014;4:25. doi: 10.1186/s13568-014-0025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X., Olszewska M., Qu J., Wasielewska T., Bartido S., Hermetet G., Sadelain M., Rivière I. Large-scale clinical-grade retroviral vector production in a fixed-bed bioreactor. J. Immunother. 2015;38:127–135. doi: 10.1097/CJI.0000000000000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lesch H.P., Heikkilä K.M., Lipponen E.M., Valonen P., Müller A., Räsänen E., Tuunanen T., Hassinen M.M., Parker N., Karhinen M. Process development of adenoviral vector production in fixed bed bioreactor: from bench to commercial scale. Hum. Gene Ther. 2015;26:560–571. doi: 10.1089/hum.2015.081. [DOI] [PubMed] [Google Scholar]
- 26.Lennaertz A., Knowles S., Drugmand J.-C., Castillo J. Viral vector production in the Integrity>® iCELLis>® single-use fixed-bed bioreactor, from bench-scale to industrial scale. BMC Proc. 2013;7:59. [Google Scholar]
- 27.Powers A.D., Piras B.A., Clark R.K., Lockey T.D., Meagher M.M. Development and optimization of AAV hFIX particles by transient transfection in an iCELLis>® fixed-bed bioreactor. Hum. Gene Ther. Methods. 2016;27:112–121. doi: 10.1089/hgtb.2016.021. [DOI] [PubMed] [Google Scholar]
- 28.Emmerling V.V., Pegel A., Milian E.G., Venereo-Sanchez A., Kunz M., Wegele J., Kamen A.A., Kochanek S., Hoerer M. Rational plasmid design and bioprocess optimization to enhance recombinant adeno-associated virus (AAV) productivity in mammalian cells. Biotechnol. J. 2016;11:290–297. doi: 10.1002/biot.201500176. [DOI] [PubMed] [Google Scholar]
- 29.Valkama A.J., Leinonen H.M., Lipponen E.M., Turkki V., Malinen J., Heikura T., Ylä-Herttuala S., Lesch H.P. Optimization of lentiviral vector production for scale-up in fixed-bed bioreactor. Gene Ther. 2018;25:39–46. doi: 10.1038/gt.2017.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leinonen H.M., Lipponen E.M., Valkama A.J., Hynynen H., Oruetxebarria I., Turkki V., Olsson V., Kurkipuro J., Samaranayake H., Määttä A.M. Preclinical proof-of-concept, analytical development, and commercial scale production of lentiviral vector in adherent cells. Mol. Ther. Methods Clin. Dev. 2019;15:63–71. doi: 10.1016/j.omtm.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holic N., Seye A.K., Majdoul S., Martin S., Merten O.W., Galy A., Fenard D. Influence of mildly acidic pH conditions on the production of lentiviral and retroviral vectors. Hum. Gene Ther. Clin. Dev. 2014;25:178–185. doi: 10.1089/humc.2014.027. [DOI] [PubMed] [Google Scholar]
- 32.Chimenti I., Gaetani R., Forte E., Angelini F., De Falco E., Zoccai G.B., Messina E., Frati G., Giacomello A. Serum and supplement optimization for EU GMP-compliance in cardiospheres cell culture. J. Cell. Mol. Med. 2014;18:624–634. doi: 10.1111/jcmm.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Throm R.E., Ouma A.A., Zhou S., Chandrasekaran A., Lockey T., Greene M., De Ravin S.S., Moayeri M., Malech H.L., Sorrentino B.P., Gray J.T. Efficient construction of producer cell lines for a SIN lentiviral vector for SCID-X1 gene therapy by concatemeric array transfection. Blood. 2009;113:5104–5110. doi: 10.1182/blood-2008-11-191049. [DOI] [PMC free article] [PubMed] [Google Scholar]