Abstract
Background
Endocrine treatment is one of the most effective therapies for estrogen receptor-positive breast cancer. However, most tumors will develop resistance to endocrine therapy as the cancer progresses. This review focuses on the mechanisms and markers of endocrine-resistant breast cancer. In addition, current and future strategies to overcome endocrine resistance are discussed.
Summary
Several molecular mechanisms of endocrine resistance have been identified, including alterations in the ESR1 gene or in the PIK3CA/mTOR pathway. Meanwhile, CDK4/6, mTOR, and PI3K inhibition have shown to improve the efficacy of endocrine treatment and new promising approaches are being developed.
Key Message
Overcoming primary or acquired resistance to endocrine treatment remains a major challenge. Since the molecular mechanisms of endocrine resistance are manifold, optimal combination and sequencing strategies will have to be developed in the future.
Keywords: Breast cancer, Endocrine resistance, Endocrine treatment, Metastases
Introduction
Breast cancer is the most common cancer disease among women. Approximately 70% of all tumors express the estrogen receptor (ER), a transcription factor that is activated by estrogen binding and that regulates the expression of various genes involved in tumor formation [1, 2, 3]. Two isoforms exist: ERα, which is predominantly expressed in breast cancer and the main target of endocrine therapies, and ERβ, which seems to have an opposite effect to ERα and inhibits estrogen-dependent cell proliferation [3, 4]. Since the roles of ERα are well recognized in breast cancer, in this review, it will be referred as ER.
Endocrine therapy is one of the most effective treatment options for ER-positive breast cancer. In the metastatic setting, it is the therapy of choice, except in cases of immediately life-threatening disease, i.e., a visceral crisis [5, 6]. Classical endocrine therapies modulate the estrogen effect by blocking the ER (selective ER modulators, SERMs) or aim to reduce estrogen levels (e.g., the aromatase inhibitors [AIs] letrozole, anastrozole, and exemestane) [7]. Whereas SERMs, like tamoxifen, are effective in both premenopausal and postmenopausal women, AIs should only be used in the postmenopausal situation. For the use of AIs in premenopausal women, ovarian suppression must be performed, which today is usually induced by the administration of GnRH analogues.
A major challenge in treating ER-positive breast cancer is to overcome endocrine resistance [8]. Primary endocrine resistance is defined as a relapse within 2 years of adjuvant endocrine treatment or disease progression during the first 6 months of first-line endocrine therapy for advanced or metastatic breast cancer (MBC). Secondary resistance is defined in early breast cancer as a relapse that occurs after at least 2 years of endocrine therapy and during or within the first year of completing adjuvant endocrine therapy. In advanced breast cancer or MBC, secondary resistance is defined as disease progression after more than 6 months of endocrine therapy [6].
This review aims to focus on mechanisms of and markers for endocrine resistance. We will moreover discuss current and future strategies to overcome endocrine resistance.
Mechanisms of Endocrine Resistance
Mechanisms of endocrine resistance include aberrations in the ER/PgR pathway (deregulation of ER expression, co-activators and co-repressors), genomic and epigenetic alterations of ERS1, expression of truncated ER-isoforms, post-translational modification, increased receptor tyrosine kinase signaling, and altered cell cycle regulation [9]. Moreover, genetic and/or epigenetic deregulations affect uptake, metabolism, and cellular responses of endocrine agents [10]. As efficacy of endocrine therapy relies on breast cancer cells that are dependent on ER activation for proliferation and differentiation, loss of the ER is one principal cause of de novo resistance to endocrine treatment [11, 12]. Several studies have shown that receptor expression may change during disease progression, and conversion from ER-positive to ER-negative breast cancer might occur in approximately 10–20% of the cases. This is why the ER status should be reevaluated from metastatic tissue whenever possible [6].
ESR1 Mutations
Mutations of the ESR1 gene, encoding for ER, are rare in primary breast cancer but have high prevalence (20–40%) in patients with MBC who have previously received endocrine treatment [13, 14, 15, 16]. Most mutations (D538G, Y537S, Y537N, Y537C, and E380) occur at hot spots in the ligand-binding domain of ERα resulting in constitutive ER activity [17, 18]. Analysis of circulating tumor DNA (ctDNA) from MBC patients of the BOLERO-2 trial (exemestane versus exemestane plus everolimus) revealed that the Y537S and D538G mutations are associated with a more aggressive disease biology [14]. Moreover, while the D538G mutation was associated with shorter progression-free survival (PFS) in patients treated with exemestane only, both the wild-type and mutant groups benefited from the addition of everolimus. Similarly, ctDNA analysis of plasma samples from MBC patients of the SoFEA (fulvestrant plus anastrozole versus fulvestrant versus exemestane) trial have shown that the detection of ESR1 mutations predicts resistance to exemestane while sensitivity to the selective estrogen receptor degrader (SERD) fulvestrant is partially maintained [19]. It must, however, be noted that ESR1 mutations also inhibit fulvestrant binding and that patients with ESR1 mutant cancers also had reduced PFS rates when treated with fulvestrant as compared to patients with wild-type tumors. Findings of these retrospective ctDNA investigations from prospective clinical trials indicate that ESR1 mutations can be acquired during endocrine therapy and may be used to monitor endocrine-resistant tumor cell clones and to guide endocrine treatment decisions.
Receptor Tyrosine Kinases
Receptor tyrosine kinases (RTKs) are a family of cell membrane-bound receptors whose intracellular domain contains a tyrosine kinase that phosphorylates tyrosine residues of target proteins [20]. RTKs (e.g., epidermal growth factor receptor [EGFR/ErbB family], insulin-like growth factor receptors [IGFR], vascular endothelial growth factor receptors [VEGFR], and fibroblast growth factor receptors [FGFR]) are activated upon ligand binding, which are mainly growth factors, cytokines, or hormones, and their activation/overexpression is associated with endocrine resistance [21, 22, 23, 24]. Upon binding, intracellular signal transduction pathways are initiated, which include the mitogen activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K)/AKT pathways (Fig. 1) [2]. These pathways can activate the transcriptional activity of ER in the absence of estrogen signaling [25]. Additionally, RTKs can reduce estrogen dependence by decreasing the expression of ER [26].
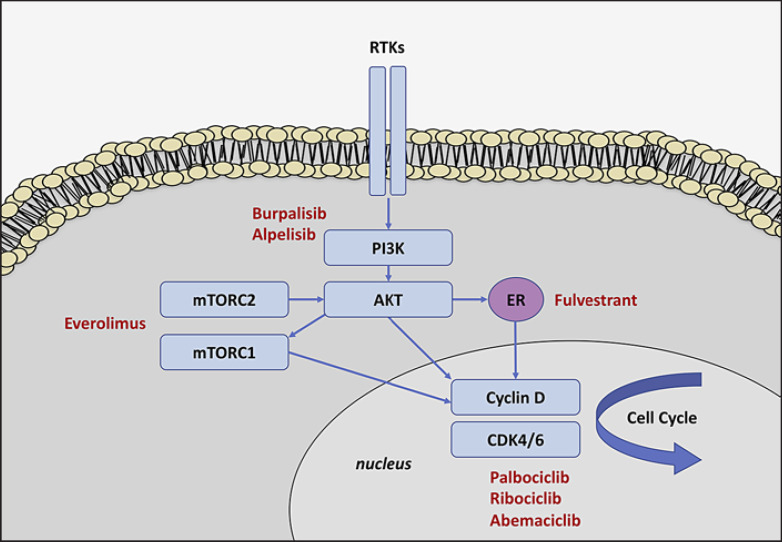
Fig. 1.
Simplified presentation of phosphoinositide 3-kinase (PI3K)/AKT/mTOR signaling in endocrine-resistant breast cancer. The PI3K/AKT pathway is activated by various receptor tyrosine kinases (RTKs). The mammalian target of rapamycin complex (mTORC) is an important downstream effector of PIK3/AKT. Activation of the PI3K/AKT/mTOR pathway promotes cell growth and proliferation. The estrogen receptor (ER) is a transcription factor that is activated by estrogen binding and that regulates various genes involved in tumor formation. Accumulation and binding of cyclin D to the cyclin-dependent kinases (CDK) 4 and 6 leads to cell cycle progression. Drugs to overcome endocrine resistance are displayed in red.
PI3K/AKT signaling plays an important role in many cellular processes that regulate growth, survival, motility, and metabolism [27]. PI3Ks are a family of kinases necessary for normal cell growth and proliferation [28]. Mammalian class 1A PI3Ks are heterodimeric protein complexes and consist of a regulatory subunit (p85α) and a catalytic subunit (p110) of which three isoforms exist: p110α (encoded by PIK3CA), p110β (encoded by PIK3CB) and p110δ (encoded by PIK3CD). [29]. Somatic mutations in genes encoding for components of the PI3K/AKT occur in up to 70% of breast cancers and include mutations or amplification of the catalytic (p110) subunits of PI3K as well as mutations of PI3K modulators, such as the phosphatase and tensin homolog (PTEN), AKT, and mTOR [29, 30]. PIK3CA is mutated in up to 40% of human breast cancers and most alterations occur within the kinase (H1047R) and helical domains (E542K and E545K) of p110α resulting in hyperactivation of PI3K [31, 32]. The prognostic impact of PI3CA mutations is still unclear: while in early ER-positive/HER2-negative breast cancer, an association with an increased disease-free survival has been reported, they seem to decrease prognosis in the advanced setting [33, 34, 35]. Importantly, the rate of PIK3CA mutations is not increased in MBC as compared to primary breast cancer and the concordance between matched primary and metastatic tissue samples is high [36]. An important downstream effector of the PI3K/AKT pathway is the mammalian target of rapamycin (mTOR) complex. [37]. The mTOR complex comprises two interdepended factors, mTORC1 and mTORC2, which are part of a positive feedback loop to the PIK3K/AKT pathway. PI3K with subsequent AKT activation leads to increased mTORC1 kinase activity and promotes cell growth and proliferation. The tumor suppressor PTEN is a negative regulator of the mTOR loop and germline mutations of PTEN cause the PTEN hamartoma tumor syndrome, including the Cowden syndrome, which is associated with an increased risk of breast cancer [2, 38].
Cell Cycle Regulators
The cyclin D/cyclin-dependent kinases (CDK) 4/6/retinoblastoma (Rb) pathway controls cell cycle progression by regulating the G1-S checkpoint [39]. An important reason for the non-response or decreasing efficacy of endocrine therapy is the sustained activation of CDK4/6 [39]. The Rb is a tumor suppressor protein that prevents the cell from passing through the cell cycle from G1- to S-phase [40, 41]. Phosphorylation of Rb by the cyclin D1-CDK4/6 complex leads to its inactivation and allows the cell to enter into the cell cycle. Cyclin D1 amplification has been linked to tamoxifen resistance and occurs in 58% of luminal B cancers and 29% of luminal A breast cancers [13, 40, 42]. Additionally, in vitro data have shown activity of the CDK4/6 inhibitor palbociclib especially in ER-positive human breast cancer cell lines including those with estrogen resistance [43].
Strategies to Overcome Endocrine Resistance
Selective Estrogen Receptor Degraders
Many breast cancers being resistant to AI or tamoxifen treatment still depend on ER signaling [44]. SERDs destabilize the ER and, in contrast to SERMs, act as pure antagonists without any tissue tropism. Fulvestrant binds to the ER to block its dimerisation and nuclear localization. Moreover, as the fulvestrant-ER complex is unstable, its degradation is accelerated [45, 46]. Fulvestrant can bind to the ER even in the context of ESR1 mutations; however, higher doses are required to maintain clinical efficacy [19, 47].
In a preplanned combined analysis of two phase III trials, fulvestrant 250 mg every 4 weeks was compared to anastrozole (1 mg/day) in postmenopausal women who experienced progression after prior endocrine therapy [48]. No differences with respect to the median time to progression were found, which was 5.5 months in the fulvestrant group and 4.1 months in the anastrozole group. The same dose of fulvestrant was used in the phase III SoFEA trial that compared fulvestrant + AI versus fulvestrant versus AI. Again, no differences with respect to PFS, which was 4.4 months, 4.7 months, and 3.4 months, respectively, were found [49]. Interestingly, a retrospective analysis of the ESR1 mutation status in archival plasma ctDNA revealed that PFS was improved in patients with ESR1 mutant tumors receiving fulvestrant as compared to AI [19].
The phase III CONFIRM trial compared an increased dose of 500 mg fulvestrant (every 4 weeks) to 250 mg in patients who had previously progressed after receiving endocrine treatment. The higher dose was associated with an improved PFS (hazard ratio [HR]: 0.80; 95% confidence interval [CI], 0.68–0.94; p = 0.006) and overall survival (OS) of 26.4 versus 22.3 months (HR: 0.81; 95% CI: 0.69–0.96; p = 0.02) [50, 51]. The phase II FIRST study confirmed these findings by comparing the higher 500 mg dose of fulvestrant with anastrozole. In this trial, however, the study population comprised of patients that had not received any endocrine treatment for MBC [52]. Patients treated with fulvestrant, had a significantly prolonged PFS (23.4 months vs. 13.1 months; HR: 0.66; 95% CI: 0.47–0.92; p = 0.01) and also seemed to have an increased OS. [53]. However, analysis of OS was not preplanned and not all patients participated in the additional follow-up [54].
The phase III FALCON trial randomly assigned patients to receive either anastrozole or fulvestrant (500 mg) as first-line treatment for hormone receptor (HR)-positive/HER2-negative MBC. Fulvestrant significantly improved PFS from 13.8 to 16.6 months (HR: 0.80; 95% CI: 0.64–1.00; p = 0.486) [55]. Due to the encouraging OS results of the FIRST study, long-term results of FALCON are eagerly awaited [56].
Inhibitors of the PI3K/AKT/mTOR Pathway
Several clinical trials have evaluated the combination of endocrine therapies with inhibitors of the PIK3k/AKT/mTOR pathway to overcome endocrine resistance. In a neoadjuvant trial, adding the mTOR inhibitor RAD001 (everolimus) to letrozole resulted in reduced tumor cell proliferation and enhanced clinical efficacy to endocrine treatment [57]. The phase II TAMRAD trial investigated the efficacy of everolimus in combination with tamoxifen in patients with endocrine-resistant MBC. Everolimus increased PFS from 4.5 to 8.6 months (HR: 0.54; 95% CI: 0.36–0.81) and also prolonged OS (HR: 0.45; 95% CI: 0.24–0.81); however, the trial was not powered for OS analysis. The efficacy of everolimus was especially pronounced in patients with secondary as compared to primary endocrine resistance.
The phase III BOLERO-2 study compared everolimus and exemestane versus exemestane and placebo in patients with advanced HR-positive/HER2-negative breast cancer who suffered from disease recurrence or progression after/while receiving a nonsteroidal AI [58]. PFS was significantly longer with everolimus plus exemestane (7.8 versus 3.2 months; HR: 0.45; 95% CI: 0.38–0.54; p < 0.001) [59]. There was no statistically significant improvement in OS; however, the trial was not powered for this endpoint [59, 60]. Analysis of archival tumor tissue revealed that the efficacy of everolimus was independent from the PI3K mutational status while a potential lack of benefit was shown in patients with a Y537S mutation in the ESR1 gene as detected by ctDNA analysis [14, 61].
Buparlisib is a pan-PI3K inhibitor that targets each of the four catalytic isoforms of class I PI3Ks. The phase III BELLE-2 trial randomized HR-positive/HER2-negative MBC patients who had previously progressed under treatment with an AI to receive either fulvestrant alone or in combination with buparlisib. Buparlisib significantly increased PFS in the overall population (5.0 versus 6.9 months; HR: 0.78; 95% CI: 0.67–0.89; p < 0.001). In patients with PI3KCA mutations, as detected by ctDNA analysis, PFS was 3.2 versus 7.0 months (HR: 0.56; 95% CI: 0.39–0.80; p < 0.001). In the subsequent phase III BELLE-3 trial, which included HR-positive/HER2-negative MBC patients who had previously received endocrine therapy and an mTOR inhibitor, the addition of buparlisib to fulvestrant increased PFS from 1.8 to 3.9 months (HR: 0.67; 95% CI: 0.53–0.84; p < 0.001). Again, the PIK3CA mutational status, as assessed by analysis archival tumor tissue or plasma ctDNA, was predictive of efficacy.
Due to inactivation of different PI3K isoforms, various side effects can occur (e.g., hyperglycemia, rash, elevated liver enzymes, and mood disorders), resulting in low tolerability of buparlisib. The more specific PIK3K inhibitor alpelisib, which selectively targets the p110α isoform (encoded by PIK3CA), was evaluated in the clinical phase III SOLAR-1 study. HR-positive/HER2-negative patients with MBC who had been pretreated with endocrine therapy either received fulvestrant or a combination of fulvestrant and alpelisib. Again, hyperglycemia and rash were the two main adverse events that led to treatment discontinuation; however, safety profile was more favorable than with buparlisib. In patients with a PIK3CA mutated tumor, median PFS was 11.0 months in the alpelisib arm versus 5.7 months in the placebo group (HR: 0.65; 95% CI: 0.50–0.85; p < 0.001). In patients with wild-type PIK3CA tumors, alpelisib was not effective. Interestingly, patients with a PI3K mutation identified by ctDNA analysis seemed to have an even greater benefit of alpelisib as compared to the analysis of tumor tissue, which is in line with the results of a combined analysis from the BELLE-2 and BELLE-3 trials. Other PI3K inhibitors are talesilib, which was evaluated in the SANDPIPER trial, and pictilisib, assessed in the PEGGY and FERGY trials [62, 63, 64]. Because of modest PFS improvement at the cost of higher toxicities as compared to alpelisib, these compounds as well as buparlisib are currently not further developed for metastatic HR-positive/HER2-negative breast cancer.
CDK4/6 Inhibitors
Currently, three structurally similar selective CDK4/6 inhibitors are clinically used for the treatment of advanced ER-positive breast cancer: palbociclib (PD0332991), ribociclib (LEE011), and abemaciclib (LY2835219) [65]. All of these drugs show a similar impressive activity in endocrine-naïve and endocrine-resistant breast cancer and are well tolerated. The most common side effect is neutropenia; nevertheless, febrile neutropenia is rare as compared to that induced by chemotherapy. As the risk of neutropenia is lower with abemaciclib, this is the only CDK4/6 inhibitor that is administered continuously, while palbociclib and ribociclib are used 3 weeks on, 1 week off.
In the phase II trial PALOMA-1, addition of palbociclib to letrozole increased median PF of HR-positive/HER2-negative MBC patients in the first-line setting from 10.2 to 20.2 months (HR: 0.49; 95% CI: 0.319–0.748; p < 0.001) [66]. These results were confirmed by the larger phase III trial, PALOMA-2 that yielded a median PFS of 14.5 versus 27.6 months (HR: 0.56; 95% CI: 0.46–0.72; p < 0.001) [67, 68]. Similarly, the phase III trial MONALEESA-2 revealed a median PFS of 16.0 months for letrozole alone as compared to 25.3 months for letrozole plus ribociclib (HR: 0.57; 95% CI 0.46–0.69; p < 0.001) [69, 70]. In MONALEESA-7, only premenopausal HR-positive/HER2-negative patients with MBC were included. All patients received the GnRH antagonist goserelin to induce ovarian function suppression. As alternative to treatment with an AI (letrozole or anastrozole) tamoxifen could be used. The addition of ribociclib to endocrine therapy yielded an increased PFS (13.0 vs. 28.8 months; HR: 0.55; 95% CI: 0.44–0.69; p < 0.001) and OS (HR: 0.71; 95% CI; 0.54–0.95; p = 0.010) [71, 72]. MONARCH-3 is a phase III trial that evaluated the combination of abemaciclib with anastrozole or letrozole in HR-positive/HER2-negative MBC patients who had no prior systemic therapy for MBC. Abemaciclib significantly enhanced median PFS from 14.8 to 28.18 months (HR: 0.54; 95% CI: 0.42–0.70; p < 0.001) [73, 74].
For the treatment of endocrine-resistant breast cancer, all three CDK4/6 inhibitors have been combined with fulvestrant (at the 500-mg dose). The addition of palbociclib to fulvestrant yielded a PFS benefit of 4.6 versus 9.5 months (PALOMA-3 trial; HR: 0.46; 95% CI: 9.2–11.0; p < 0.001), the addition of ribociclib a PFS benefit of 12.8 versus 20.5 months (MONALEESA-3 trial; HR: 0.59; 95% CI: 0.48–0,73; p < 0.001), and the addition of abemaciclib prolonged PFS from 9.3 to 16.4 months (MONARCH-2 trial; HR: 0.55; 95% CI: 0.45–0.68; p < 0.001) [75, 76, 77]. Although the HRs were similar, the absolute benefit differed in these trials, which is due to the different pretreatments of the study populations: whereas in MONALEESA-3, MBC patients were included in the first and second line, MONARCH-3 only included second-line patients, while in PALOMA, second- and higher-line patients could participate. Importantly, an OS benefit was observed in MONALEESA-3 (HR: 0.72; 95% CI: 0.57–0.92; p = 0.005) and MONARCH-2 (HR: 0.76; 95% CI: 0.61–0.95; p = 0.01) [78, 79].
Despite detailed knowledge about cell cycle regulation and the CDK4/6 pathway, no biomarker has yet been identified to predict efficacy of CDK4/6 inhibition. Although loss of RB, amplification of CCND, or low expression of p16 should theoretically result in a reduced probability of responding to CDK4/6 inhibitor treatment, none of these markers is clearly associated with treatment response [80, 81, 82]. The reason for these findings remains elusive but might be due to inadequate assays to reflect protein function as well as to tumor heterogeneity and the fact that most analyses have been done with archival tissue from primary tumors. Additionally, neither ESR1 mutations nor PI3CA mutations were found to be associated with efficacy of CDK4/6 treatment [83, 84, 85].
Perspectives
Even in endocrine-resistant breast cancer, the ER remains a promising target. This is why next-generation more selective SERDs with enhanced bioavailability are under investigation. AZD9496 is an orally available small molecule that downregulates ER with D538G, Y537S, Y537N, or Y537C alterations [86]. AZD9496 was found to block growth of ESR1-mutant breast tumors in preclinical models and was well tolerated in phase I clinical trials [87, 88]. Other SERDs currently being assessed are elacestrant (RAD1901) and GDC-0927 [88, 89].
Next to PI3K inhibitors, other strategies are under investigation to target the PI3K/AKT pathway. The AKT inhibitor capivasertib (AZD5363) leads to an increased dependency of tumor cells on ligand-dependent ER activation. This is why there is a strong rationale to combine AKT inhibition with endocrine therapy. The phase II FAKTION trial found that the combination of capivasertib with fulvestrant in ER-positive/Her2-negative MBC doubles PFS (4.8 vs. 10.3 months; HR: 0.58; 95% CI: 0.39–0.84; p = 0.004). Promisingly, there was a strong trend towards improved OS [90].
Crosstalk between PIK3K/AKT/mTOR pathway provides a strong rationale to combine CDK4/6 with PI3K inhibitors. PI3K activation is a potential mechanism of resistance to CDK4/6 inhibitors [91]. Several trials that combine CDK4/6 inhibitors with various PI3K/AKT/mTOR inhibitors are underway (NCT03128619, NCT03006172, NCT02684032, NCT02732119, NCT02871791, NCT02599714).
Conclusion
Endocrine therapy is the therapy of choice in ER-positive breast cancer. It is of high clinical relevance to maintain endocrine-based treatment as long as possible and to overcome or delay endocrine resistance. CDK4/6, mTOR, and PI3K inhibition have shown to enhance efficacy of endocrine treatment, and novel promising drugs that target further components of key pathways like the PI3K/AKT/mTOR pathway are currently being developed. As the molecular mechanism of endocrine resistance are manifold, optimal combination and sequencing strategies need to be developed. For this purpose, (circulating) biomarkers are promising to guide individual therapy decisions. Prospective cancer registries that prospectively collect various biomarkers at multiple timepoints during cancer progression and combine these with clinical data on prognosis, tolerability, and efficacy of different endocrine-based treatments are vital to face these challenges [92].
Conflict of Interest Statement
A.D.H. received honoraria and consulting fees from Teva, GenomicHealth, Celgene, AstraZeneca, Novartis, Pfizer, Lilly, MSD, Eisai, and Roche. E.-M.G. received honoraria from Lilly, Novartis, and AstraZeneca. S.Y.B. received honoraria from Pfizer, Roche, Novartis, MSD, and Teva. No funding was received to support this study.
Funding Sources
None.
Author Contributions
A.D.H., E.-M.G., and S.Y.B. wrote the manuscript.
References
- 1.Anderson WF, Chatterjee N, Ershler WB, Brawley OW. Estrogen receptor breast cancer phenotypes in the Surveillance, Epidemiology, and End Results database. Breast Cancer Res Treat. 2002 Nov;76((1)):27–36. doi: 10.1023/a:1020299707510. [DOI] [PubMed] [Google Scholar]
- 2.Rani A, Stebbing J, Giamas G, Murphy J. Endocrine Resistance in Hormone Receptor Positive Breast Cancer-From Mechanism to Therapy. Front Endocrinol (Lausanne) 2019 May;10:245. doi: 10.3389/fendo.2019.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walter P, Green S, Greene G, Krust A, Bornert JM, Jeltsch JM, et al. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci USA. 1985 Dec;82((23)):7889–93. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mann S, Laucirica R, Carlson N, Younes PS, Ali N, Younes A, et al. Estrogen receptor beta expression in invasive breast cancer. Hum Pathol. 2001 Jan;32((1)):113–8. doi: 10.1053/hupa.2001.21506. [DOI] [PubMed] [Google Scholar]
- 5.Rugo HS, Rumble RB, Macrae E, Barton DL, Connolly HK, Dickler MN, et al. Endocrine Therapy for Hormone Receptor-Positive Metastatic Breast Cancer: American Society of Clinical Oncology Guideline. J Clin Oncol. 2016 Sep;34((25)):3069–103. doi: 10.1200/JCO.2016.67.1487. [DOI] [PubMed] [Google Scholar]
- 6.Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, André F, et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4) Ann Oncol. 2018 Aug;29((8)):1634–57. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, et al. Breast cancer. Nat Rev Dis Primers. 2019 Sep;5((1)):66. doi: 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- 8.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62((1)):233–47. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy CG, Dickler MN. Endocrine resistance in hormone-responsive breast cancer: mechanisms and therapeutic strategies. Endocr Relat Cancer. 2016 Aug;23((8)):R337–52. doi: 10.1530/ERC-16-0121. [DOI] [PubMed] [Google Scholar]
- 10.Mürdter TE, Schroth W, Bacchus-Gerybadze L, Winter S, Heinkele G, Simon W, et al. German Tamoxifen and AI Clinicians Group Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin Pharmacol Ther. 2011 May;89((5)):708–17. doi: 10.1038/clpt.2011.27. [DOI] [PubMed] [Google Scholar]
- 11.Gutierrez MC, Detre S, Johnston S, Mohsin SK, Shou J, Allred DC, et al. Molecular changes in tamoxifen-resistant breast cancer: relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J Clin Oncol. 2005 Apr;23((11)):2469–76. doi: 10.1200/JCO.2005.01.172. [DOI] [PubMed] [Google Scholar]
- 12.Lindström LS, Karlsson E, Wilking UM, Johansson U, Hartman J, Lidbrink EK, et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol. 2012 Jul;30((21)):2601–8. doi: 10.1200/JCO.2011.37.2482. [DOI] [PubMed] [Google Scholar]
- 13.Cancer Genome Atlas N, Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012 Oct;490((7418)):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandarlapaty S, Chen D, He W, Sung P, Samoila A, You D, et al. Prevalence of ESR1 Mutations in Cell-Free DNA and Outcomes in Metastatic Breast Cancer: A Secondary Analysis of the BOLERO-2 Clinical Trial. JAMA Oncol. 2016 Oct;2((10)):1310–5. doi: 10.1001/jamaoncol.2016.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kingston K, Bye H, Hubank M, et al. The genomic landscape of breast cancer based on ctDNA analysis: Data from the plasmaMATCH trial San Antonio Breast Cancer Symposium. 2019 GS3-06.
- 16.Andre F, Filleron T, Ng C, et al. Genomic characterisation of metastatic breast cancer. San Antonio Breast Cancer Symposium. 2018:GS1–08. [Google Scholar]
- 17.Robinson DR, Wu YM, Vats P, Su F, Lonigro RJ, Cao X, et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet. 2013 Dec;45((12)):1446–51. doi: 10.1038/ng.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toy W, Shen Y, Won H, Green B, Sakr RA, Will M, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 2013 Dec;45((12)):1439–45. doi: 10.1038/ng.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fribbens C, O'Leary B, Kilburn L, Hrebien S, Garcia-Murillas I, Beaney M, et al. Plasma ESR1 Mutations and the Treatment of Estrogen Receptor-Positive Advanced Breast Cancer. J Clin Oncol. 2016 Sep;34((25)):2961–8. doi: 10.1200/JCO.2016.67.3061. [DOI] [PubMed] [Google Scholar]
- 20.Presti D, Quaquarini E. The PI3K/AKT/mTOR and CDK4/6 Pathways in Endocrine Resistant HR+/HER2- Metastatic Breast Cancer: Biological Mechanisms and New Treatments. Cancers (Basel) 2019 Aug;11((9)):11. doi: 10.3390/cancers11091242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox EM, Miller TW, Balko JM, Kuba MG, Sánchez V, Smith RA, et al. A kinome-wide screen identifies the insulin/IGF-I receptor pathway as a mechanism of escape from hormone dependence in breast cancer. Cancer Res. 2011 Nov;71((21)):6773–84. doi: 10.1158/0008-5472.CAN-11-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner N, Pearson A, Sharpe R, Lambros M, Geyer F, Lopez-Garcia MA, et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res. 2010 Mar;70((5)):2085–94. doi: 10.1158/0008-5472.CAN-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frogne T, Benjaminsen RV, Sonne-Hansen K, Sorensen BS, Nexo E, Laenkholm AV, et al. Activation of ErbB3, EGFR and Erk is essential for growth of human breast cancer cell lines with acquired resistance to fulvestrant. Breast Cancer Res Treat. 2009 Mar;114((2)):263–75. doi: 10.1007/s10549-008-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellis MJ, Tao Y, Young O, White S, Proia AD, Murray J, et al. Estrogen-independent proliferation is present in estrogen-receptor HER2-positive primary breast cancer after neoadjuvant letrozole. J Clin Oncol. 2006 Jul;24((19)):3019–25. doi: 10.1200/JCO.2005.04.3034. [DOI] [PubMed] [Google Scholar]
- 25.Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem. 2001 Mar;276((13)):9817–24. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- 26.Fox EM, Arteaga CL, Miller TW. Abrogating endocrine resistance by targeting ERα and PI3K in breast cancer. Front Oncol. 2012 Oct;2:145. doi: 10.3389/fonc.2012.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller TW, Rexer BN, Garrett JT, Arteaga CL. Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res. 2011;13((6)):224. doi: 10.1186/bcr3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006 Aug;7((8)):606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 29.Whitman M, Downes CP, Keeler M, Keller T, Cantley L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature. 1988 Apr;332((6165)):644–6. doi: 10.1038/332644a0. [DOI] [PubMed] [Google Scholar]
- 30.Vasan N, Toska E, Scaltriti M. Overview of the relevance of PI3K pathway in HR-positive breast cancer. Ann Oncol. 2019;30(Suppl 10):x3–11. doi: 10.1093/annonc/mdz281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell IG, Russell SE, Choong DY, Montgomery KG, Ciavarella ML, Hooi CS, et al. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 2004 Nov;64((21)):7678–81. doi: 10.1158/0008-5472.CAN-04-2933. [DOI] [PubMed] [Google Scholar]
- 32.Mollon L, Aguilar A, Anderson E, et al. Abstract 1207: A systematic literature review of the prevalence of PIK3CA mutations and mutation hotspots in HR+/HER2- metastatic breast cancer. Cancer Res. 2018;78:1207. [Google Scholar]
- 33.Pang B, Cheng S, Sun SP, An C, Liu ZY, Feng X, et al. Prognostic role of PIK3CA mutations and their association with hormone receptor expression in breast cancer: a meta-analysis. Sci Rep. 2014 Sep;4((1)):6255. doi: 10.1038/srep06255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu YR, Jiang YZ, Zuo WJ, Yu KD, Shao ZM. PIK3CA mutations define favorable prognostic biomarkers in operable breast cancer: a systematic review and meta-analysis. Onco Targets Ther. 2014 Apr;7:543–52. doi: 10.2147/OTT.S60115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosele MF, Lusque A, Tran Dien A, Droin N, Le Tourneau C, Lacroix L, et al. Outcome and mutational landscape of patients with PIK3CA-mutated metastatic breast cancer (mBC) Ann Oncol. 2019;30:iii47. doi: 10.1016/j.annonc.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Meric-Bernstam F, Frampton GM, Ferrer-Lozano J, Yelensky R, Pérez-Fidalgo JA, Wang Y, et al. Concordance of genomic alterations between primary and recurrent breast cancer. Mol Cancer Ther. 2014 May;13((5)):1382–9. doi: 10.1158/1535-7163.MCT-13-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lauring J, Park BH, Wolff AC. The phosphoinositide-3-kinase-Akt-mTOR pathway as a therapeutic target in breast cancer. J Natl Compr Canc Netw. 2013 Jun;11((6)):670–8. doi: 10.6004/jnccn.2013.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eng C. Will the real Cowden syndrome please stand up: revised diagnostic criteria. J Med Genet. 2000 Nov;37((11)):828–30. doi: 10.1136/jmg.37.11.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knudsen ES, Pruitt SC, Hershberger PA, Witkiewicz AK, Goodrich DW. Cell Cycle and Beyond: Exploiting New RB1 Controlled Mechanisms for Cancer Therapy. Trends Cancer. 2019 May;5((5)):308–24. doi: 10.1016/j.trecan.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Casimiro MC, Wang C, Li Z, Di Sante G, Willmart NE, Addya S, et al. Cyclin D1 determines estrogen signaling in the mammary gland in vivo. Mol Endocrinol. 2013 Sep;27((9)):1415–28. doi: 10.1210/me.2013-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zwijsen RM, Wientjens E, Klompmaker R, van der Sman J, Bernards R, Michalides RJ. CDK-independent activation of estrogen receptor by cyclin D1. Cell. 1997 Feb;88((3)):405–15. doi: 10.1016/s0092-8674(00)81879-6. [DOI] [PubMed] [Google Scholar]
- 42.Stendahl M, Kronblad A, Rydén L, Emdin S, Bengtsson NO, Landberg G. Cyclin D1 overexpression is a negative predictive factor for tamoxifen response in postmenopausal breast cancer patients. Br J Cancer. 2004 May;90((10)):1942–8. doi: 10.1038/sj.bjc.6601831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11((5)):R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel HK, Bihani T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol Ther. 2018 Jun;186:1–24. doi: 10.1016/j.pharmthera.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 45.Dodwell D, Wardley A, Johnston S. Postmenopausal advanced breast cancer: options for therapy after tamoxifen and aromatase inhibitors. Breast. 2006 Oct;15((5)):584–94. doi: 10.1016/j.breast.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Fawell SE, White R, Hoare S, Sydenham M, Page M, Parker MG. Inhibition of estrogen receptor-DNA binding by the “pure” antiestrogen ICI 164,384 appears to be mediated by impaired receptor dimerization. Proc Natl Acad Sci USA. 1990 Sep;87((17)):6883–7. doi: 10.1073/pnas.87.17.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Santo I, McCartney A, Migliaccio I, Di Leo A, Malorni L. The Emerging Role of ESR1 Mutations in Luminal Breast Cancer as a Prognostic and Predictive Biomarker of Response to Endocrine Therapy. Cancers (Basel) 2019 Nov;11((12)):11. doi: 10.3390/cancers11121894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robertson JF, Osborne CK, Howell A, Jones SE, Mauriac L, Ellis M, et al. Fulvestrant versus anastrozole for the treatment of advanced breast carcinoma in postmenopausal women: a prospective combined analysis of two multicenter trials. Cancer. 2003 Jul;98((2)):229–38. doi: 10.1002/cncr.11468. [DOI] [PubMed] [Google Scholar]
- 49.Johnston SR, Kilburn LS, Ellis P, Dodwell D, Cameron D, Hayward L, et al. SoFEA investigators Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol. 2013 Sep;14((10)):989–98. doi: 10.1016/S1470-2045(13)70322-X. [DOI] [PubMed] [Google Scholar]
- 50.Di Leo A, Jerusalem G, Petruzelka L, Torres R, Bondarenko IN, Khasanov R, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010 Oct;28((30)):4594–600. doi: 10.1200/JCO.2010.28.8415. [DOI] [PubMed] [Google Scholar]
- 51.Di Leo A, Jerusalem G, Petruzelka L, Torres R, Bondarenko IN, Khasanov R, et al. Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst. 2014 Jan;106((1)):djt337. doi: 10.1093/jnci/djt337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robertson JF, Llombart-Cussac A, Rolski J, Feltl D, Dewar J, Macpherson E, et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: results from the FIRST study. J Clin Oncol. 2009 Sep;27((27)):4530–5. doi: 10.1200/JCO.2008.21.1136. [DOI] [PubMed] [Google Scholar]
- 53.Robertson JF, Lindemann JP, Llombart-Cussac A, Rolski J, Feltl D, Dewar J, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomized ‘FIRST’ study. Breast Cancer Res Treat. 2012 Nov;136((2)):503–11. doi: 10.1007/s10549-012-2192-4. [DOI] [PubMed] [Google Scholar]
- 54.Ellis MJ, Llombart-Cussac A, Feltl D, Dewar JA, Jasiówka M, Hewson N, et al. Fulvestrant 500 mg Versus Anastrozole 1 mg for the First-Line Treatment of Advanced Breast Cancer: Overall Survival Analysis From the Phase II FIRST Study. J Clin Oncol. 2015 Nov;33((32)):3781–7. doi: 10.1200/JCO.2015.61.5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robertson JF, Bondarenko IM, Trishkina E, Dvorkin M, Panasci L, Manikhas A, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet. 2016 Dec;388((10063)):2997–3005. doi: 10.1016/S0140-6736(16)32389-3. [DOI] [PubMed] [Google Scholar]
- 56.Mehta RS, Barlow WE, Albain KS, Vandenberg TA, Dakhil SR, Tirumali NR, et al. Overall Survival with Fulvestrant plus Anastrozole in Metastatic Breast Cancer. N Engl J Med. 2019 Mar;380((13)):1226–34. doi: 10.1056/NEJMoa1811714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baselga J, Semiglazov V, van Dam P, Manikhas A, Bellet M, Mayordomo J, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009 Jun;27((16)):2630–7. doi: 10.1200/JCO.2008.18.8391. [DOI] [PubMed] [Google Scholar]
- 58.Baselga J, Campone M, Piccart M, Burris HA, 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012 Feb;366((6)):520–9. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yardley DA, Noguchi S, Pritchard KI, Burris HA, 3rd, Baselga J, Gnant M, et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther. 2013 Oct;30((10)):870–84. doi: 10.1007/s12325-013-0060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piccart M, Hortobagyi GN, Campone M, Pritchard KI, Lebrun F, Ito Y, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2. Ann Oncol. 2014 Dec;25((12)):2357–62. doi: 10.1093/annonc/mdu456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hortobagyi GN, Chen D, Piccart M, Rugo HS, Burris HA, 3rd, Pritchard KI, et al. Correlative Analysis of Genetic Alterations and Everolimus Benefit in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: results From BOLERO-2. J Clin Oncol. 2016 Feb;34((5)):419–26. doi: 10.1200/JCO.2014.60.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baselga J, Dent SF, Cortés J, et al. Phase III study of taselisib (GDC-0032)+ fulvestrant (FULV) v FULV in patients (pts) with estrogen receptor (ER)-positive, PIK3CA-mutant (MUT), locally advanced or metastatic breast cancer (MBC): Primary analysis from SANDPIPER. American Society of Clinical Oncology. 2018 [Google Scholar]
- 63.Vuylsteke P, Huizing M, Petrakova K, Roylance R, Laing R, Chan S, et al. Pictilisib PI3Kinase inhibitor (a phosphatidylinositol 3-kinase [PI3K] inhibitor) plus paclitaxel for the treatment of hormone receptor-positive, HER2-negative, locally recurrent, or metastatic breast cancer: interim analysis of the multicentre, placebo-controlled, phase II randomised PEGGY study. Ann Oncol. 2016 Nov;27((11)):2059–66. doi: 10.1093/annonc/mdw320. [DOI] [PubMed] [Google Scholar]
- 64.Krop IE, Mayer IA, Ganju V, Dickler M, Johnston S, Morales S, et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016 Jun;17((6)):811–21. doi: 10.1016/S1470-2045(16)00106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pernas S, Tolaney SM, Winer EP, Goel S. CDK4/6 inhibition in breast cancer: current practice and future directions. Ther Adv Med Oncol. 2018 Jul;10:1758835918786451. doi: 10.1177/1758835918786451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015 Jan;16((1)):25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 67.Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016 Nov;375((20)):1925–36. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 68.Rugo HS, Finn RS, Diéras V, Ettl J, Lipatov O, Joy AA, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019 Apr;174((3)):719–29. doi: 10.1007/s10549-018-05125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018 Jul;29((7)):1541–7. doi: 10.1093/annonc/mdy155. [DOI] [PubMed] [Google Scholar]
- 70.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N Engl J Med. 2016 Nov;375((18)):1738–48. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 71.Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N Engl J Med. 2019 Jul;381((4)):307–16. doi: 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 72.Tripathy D, Im SA, Colleoni M, Franke F, Bardia A, Harbeck N, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018 Jul;19((7)):904–15. doi: 10.1016/S1470-2045(18)30292-4. [DOI] [PubMed] [Google Scholar]
- 73.Johnston S, Martin M, Di Leo A, Im SA, Awada A, Forrester T, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019 Jan;5((1)):5. doi: 10.1038/s41523-018-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J Clin Oncol. 2017 Nov;35((32)):3638–46. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 75.Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J Clin Oncol. 2018 Aug;36((24)):2465–72. doi: 10.1200/JCO.2018.78.9909. [DOI] [PubMed] [Google Scholar]
- 76.Sledge GW, Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J Clin Oncol. 2017 Sep;35((25)):2875–84. doi: 10.1200/JCO.2017.73.7585. [DOI] [PubMed] [Google Scholar]
- 77.Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016 Apr;17((4)):425–39. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 78.Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Overall Survival with Ribociclib plus Fulvestrant in Advanced Breast Cancer. N Engl J Med. 2020 Feb;382((6)):514–24. doi: 10.1056/NEJMoa1911149. [DOI] [PubMed] [Google Scholar]
- 79.Sledge GW, Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor-Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy-MONARCH 2: A Randomized Clinical Trial. JAMA Oncol. 2019 Sep; doi: 10.1001/jamaoncol.2019.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Finn R, Jiang Y, Rugo H, Moulder SL, Im SA, Gelmon KA, et al. Biomarker analyses from the phase 3 PALOMA-2 trial of palbociclib (P) with letrozole (L) compared with placebo (PLB) plus L in postmenopausal women with ER+/HER2–advanced breast cancer (ABC) Ann Oncol. 2016;27:27. [Google Scholar]
- 81.Finn RS, Liu Y, Martin M, et al., editors. San Antonio Breast Cancer Symposium. 2017. Comprehensive gene expression biomarker analysis of cyclin-dependent kinases 4/6 and endocrine pathways from the PALOMA-2 study. [Google Scholar]
- 82.Turner NC, Liu Y, Zhu Z, et al. Abstract CT039: Cyclin E1 (CCNE1) expression associates with benefit from palbociclib in metastatic breast cancer (MBC) in the PALOMA3 trial. AACR; 2018. [Google Scholar]
- 83.Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016 Apr;17((4)):425–39. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 84.Hortobagyi G, Stemmer S, Campone M, et al. Abstract PD4-06: First-line ribociclib+ letrozole in hormone receptor-positive, HER2-negative advanced breast cancer: Efficacy by baseline circulating tumor DNA alterations in MONALEESA-2. AACR; 2018. [Google Scholar]
- 85.Tolaney S, Toi M, Neven P, et al. Clinical significance of PIK3CA and ESR1 mutations in ctDNA and FFPE samples from the MONARCH 2 study of abemaciclib plus fulvestrant. Deutscher Krebskongress. DKK; 2020. p. p. 167. [Google Scholar]
- 86.Robertson JF, Kirwan CC, Dixon JM, Lindemann JP, Roudier MP. A randomized, open-label, pre-surgical, window of opportunity study comparing the pharmacodynamic effects of the novel oral SERD AZD9496 with fulvestrant in patients with newly diagnosed ER+ HER2-primary breast cancer. Clin Cancer Res. 2020 doi: 10.1158/1078-0432.CCR-19-3387. [DOI] [PubMed] [Google Scholar]
- 87.Hamilton EP, Patel MR, Armstrong AC, Baird RD, Jhaveri K, Hoch M, et al. A First-in-Human Study of the New Oral Selective Estrogen Receptor Degrader AZD9496 for ER+/HER2 Advanced Breast Cancer. Clin Cancer Res. 2018 Aug;24((15)):3510–8. doi: 10.1158/1078-0432.CCR-17-3102. [DOI] [PubMed] [Google Scholar]
- 88.Weir HM, Bradbury RH, Lawson M, Rabow AA, Buttar D, Callis RJ, et al. AZD9496: An Oral Estrogen Receptor Inhibitor That Blocks the Growth of ER-Positive and ESR1-Mutant Breast Tumors in Preclinical Models. Cancer Res. 2016 Jun;76((11)):3307–18. doi: 10.1158/0008-5472.CAN-15-2357. [DOI] [PubMed] [Google Scholar]
- 89.Bardia A, Aftimos P, Bihani T, Anderson-Villaluz AT, Jung J, Conlan MG, et al. EMERALD: phase III trial of elacestrant (RAD1901) vs endocrine therapy for previously treated ER+ advanced breast cancer. Future Oncol. 2019 Oct;15((28)):3209–18. doi: 10.2217/fon-2019-0370. [DOI] [PubMed] [Google Scholar]
- 90.Jones RH, Carucci M, Casbard AC, et al. Capivasertib (AZD5363) plus fulvestrant versus placebo plus fulvestrant after relapse or progression on an aromatase inhibitor in metastatic ER-positive breast cancer (FAKTION): A randomized, double-blind, placebo-controlled, phase II trial. American Society of Clinical Oncology; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brandão M, Caparica R, Eiger D, de Azambuja E. Biomarkers of response and resistance to PI3K inhibitors in estrogen receptor-positive breast cancer patients and combination therapies involving PI3K inhibitors. Ann Oncol. 2019;30:x27–42. doi: 10.1093/annonc/mdz280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fasching PA, Brucker SY, Fehm TN, Overkamp F, Janni W, Wallwiener M, et al. Biomarkers in Patients with Metastatic Breast Cancer and the PRAEGNANT Study Network. Geburtshilfe Frauenheilkd. 2015 Jan;75((1)):41–50. doi: 10.1055/s-0034-1396215. [DOI] [PMC free article] [PubMed] [Google Scholar]