Abstract
COVID-19 pandemic presents an unprecedented challenge to identify effective drugs for treatment. Despite multiple clinical trials using different agents, there is still a lack of specific treatment for COVID-19. Having the potential role in suppressing inflammation, immune modulation, antiviral and improving respiratory symptoms, this review discusses the potential role of methylxanthine drugs like pentoxifylline and caffeine in the management of COVID-19 patients. COVID-19 pathogenesis for clinical features like severe pneumonia, acute lung injury (ALI) / acute respiratory distress syndrome (ARDS), and multi-organ failures are excessive inflammation, oxidation, and cytokine storm by the exaggerated immune response. Drugs like pentoxifylline have already shown improvement of the symptoms of ARDS and caffeine has been in clinical use for decades to treat apnea of prematurity (AOP) in preterm infants and improve respiratory function. Pentoxifylline is well-known anti-inflammatory and anti-oxidative molecules that have already shown to suppress Tumor Necrosis Factor (TNF-α) as well as other inflammatory cytokines in pulmonary diseases, and this may be beneficial for better clinical outcomes in COVID-19 patients. Pentoxifylline enhances blood flow, improves microcirculation and tissue oxygenation, and caffeine also efficiently improves tissue oxygenation, asthma, decreases pulmonary hypertension and an effective analgesic. There are significant shreds of evidence that proved the properties of pentoxifylline and caffeine against virus-related diseases as well. Along with the aforementioned evidences and high safety profiles, both pentoxifylline and caffeine offer a glimpse of considerations for future use as a potential adjuvant to COVID-19 treatment. However, additional clinical studies are required to confirm this speculation.
Keywords: COVID-19, Methylxanthines, Pentoxifylline and caffeine
Graphical abstract
1. Introduction
The severe acute respiratory syndrome coronavirus (SARS-CoV) are single-stranded positive-sense RNA viruses that cause severe respiratory diseases to the affected individuals (Cheng et al., 2007). In December 2019, a cluster of pneumonia cases emerged in Wuhan, China (Huang et al., 2020). This disease is now known as coronavirus disease 2019 (COVID-19) caused by the novel coronavirus now known as SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) that has spread globally, affecting a large portion of the human population across the world (Cohen and Normile, 2020; Zhu et al., 2020).
The full range of symptoms for COVID-19 includes self-limiting respiratory tract illness to severe pneumonia, acute respiratory distress syndrome (ARDS), multi-organ failure, and death (Huang et al., 2020; Ruan et al., 2020; Wang et al., 2020a). However, with time as the number of COVID-19 positive patients increase across the world, few neurological symptoms such as headache, paresthesia, and consciousness disorders were reported as well (Wu et al., 2020). More recently, unusual manifestations of COVID-19, including encephalitis, (Ye et al., 2020), acute necrotizing hemorrhagic encephalopathy (Poyiadji et al., 2020), and myocarditis (Doyen et al., 2020) have been documented. Besides, it has been noted that thrombotic complications in COVID-19 intensive care unit (ICU) patients increased remarkably (Klok et al., 2020). Lastly, skin manifestations like erythematous rash, urticaria, or chickenpox-like vesicles mainly in the body trunk in COVID-19 patients were reported in multiple studies (Joob and Wiwanitkit, 2020; Recalcati, 2020).
Therapeutic options to contain the COVID-19 pandemic is urgently needed. Favipiravir (T-705) (Wang et al., 2020b) and ribavirin have been evaluated on COVID-19 patients (ChiCTR2000029387), but ribavirin reported side effects (Zumla et al., 2016). Remdesivir (GS-5734) has been suggested (Al-Tawfiq et al., 2020; Cao et al., 2020a) and a compassionate-use remdesivir study showed 68% clinical improvement in COVID-19 patients (Grein et al., 2020). However, very recently, WHO reported controversy to the aforementioned data, and a full COVID-19 clinical trial of remdesivir was terminated due to the adverse side effects (unpublished report from WHO website). In addition, lopinavir–ritonavir treatment on COVID-19 patients did not show any improvement (Cao et al., 2020b). However, chloroquine, hydroxychloroquine (Gao et al., 2020; Gautret et al., 2020) and azithromycin with hydroxychloroquine showed potential clinical benefits but only in a limited number of COVID-19 patients (Gautret et al., 2020). Tocilizumab (Xu et al., 2020), as well as convalescent plasma therapy (Duan et al., 2020) in severely ill COVID-19 patients, also improved clinical outcomes, but inadequate clinical data to justify the observed effect. Although a range of the aforementioned therapies can be a near-term strategy to tackle COVID-19, there is still an evident lack of specific treatment for COVID-19 (Huang et al., 2020).
Methylxanthines are heterocyclic compounds that are methylated derivatives of xanthine comprising of coupled pyrimidinedione and imidazole rings (Talik et al., 2012). Methylxanthines have been widely used for therapeutic purposes for decades, with proven therapeutic benefits in different medical scopes. For example, the naturally occurring methylxanthines like caffeine, theophylline, and theobromine have been used in the treatment of respiratory diseases (Lam and Newhouse, 1990), cardiovascular diseases, cancer (Hayashi et al., 2005; Kimura et al., 2009) and the commercially produced xanthine derivative drug like pentoxifylline has been widely documented to have immunomodulatory properties including the downregulation of Tumour Necrosis Factor (TNF) α to treat the injurious effects due to immune activation in the syndrome of chronic heart failure (CHF) (Shaw et al., 2009). Pentoxifylline and its active metabolites enhance blood flow by decreasing blood viscosity and ameliorating erythrocyte flexibility. Administration of pentoxifylline produced hemorheological activity in a dose-dependent manner. Based on the aforementioned mode of action, pentoxifylline has been approved to treat intermittent claudication due to chronic occlusive arterial disease of the limbs (Dettelbach and Aviado, 1985). In these patients, pentoxifylline improves microcirculation and tissue oxygenation (Harris et al., 2017; Hsu et al., 1988). Pentoxifylline is also used for the management of alcoholic hepatitis (severe) (Whitfield et al., 2009) and venous leg ulcer off-label (Coccheri and Bignamini, 2016; Zito and Murgia III, 2018). Moreover, the effect of pentoxifylline has been demonstrated to treat fibrotic lesions by immunomodulation and by reducing inflammation (Wen et al., 2017).
Previous research has extensively established the effects of caffeine in the treatment of respiratory disease, its bronchodilatory effect via phosphodiesterase inhibition, and adenosine receptor antagonism (Sullivan et al., 1994; Tilley, 2011). Furthermore, caffeine is widely used to treat apnea of prematurity (AOP) in preterm infants by improving minute ventilation, carbon dioxide sensitivity, respiratory muscle function, and neural respiratory drive. Caffeine administration also improved microcirculation in humans (Okuno et al., 2002), and moderate caffeine consumption was related to reduced coronary heart disease and stroke (Bohn et al., 2012). Therapeutic indications of caffeine also include its role as the central nervous system stimulant to maintain seizure control during epilepsy (van Koert et al., 2018) as well as its role in treating headaches. As an adjuvant to analgesics, it enhances the efficacy of analgesics to treat headache (Lipton et al., 2017). Besides, the over the counter labeling of caffeine is to restore mental alertness or wakefulness during fatigue (Childs and de Wit, 2008). Despite the numerous benefits of caffeine, high doses of caffeine may lead to anxiety disorder (Lara, 2010), and patients with an anxiety disorder are more sensitive to caffeine (Bruce et al., 1992).
Herein, we review with extensive evidence that widely used methylxanthines like pentoxifylline and caffeine may be used as an adjuvant therapy to treat COVID-19 induced respiratory symptoms by exploiting their reported immunomodulatory and anti-inflammatory potentials. Tissue oxygen levels have also been shown to be significantly increased by therapeutic doses of pentoxifylline in patients with peripheral arterial disease.
1.1. COVID-19 pathogenesis and potential of methylxanthines use for therapeutic purpose
Existing research recognizes the critical role played by “cytokine storm” in pathology associated with coronaviruses. It is now well established from a variety of studies; this condition is one of the primary underlying mechanism of the disease aggravation (Mehta et al., 2020). One of the very early publications about COVID-19 reported a suppressed immune system followed by lymphopenia, neutropenia, hypo-albuminemia, as well as a decrease in Cluster of Differentiation 8+ (CD8+) T cells (Chen et al., 2020). Further analysis of blood from COVID-19 patients showed high levels of inflammatory factors, including interleukin 1β (IL-1β), interferon γ (IFN-γ), interferon-inducible protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1), and also IL-4, IL-7, IL-8, IL-9, IL-10 and Tumour Necrosis Factor (TNF) α and overproduction of these inflammatory cytokines and chemokines may contribute to the progression of the disease (Huang et al., 2020). In SARS disease models, the cytokine storm associated disease pathology in Acute Lung Injury (ALI) was accompanied by increased expression of inflammatory genes (Channappanavar et al., 2016). Furthermore, decreasing the inflammatory monocytes/macrophages or ablation of the IFN-α/β receptor resulted in increased survival of the coronavirus host (Channappanavar et al., 2016; Smits et al., 2010). In both cases, a potential amplifying of the inflammation is involved underlying the CoV induced lung diseases. Hence, it could conceivably be hypothesized that cytokine storm, inflammation, and repressed immune function seemed to be a major feature in all COVID-19 patients, and mitigation of disease progression may potentially be achieved by focusing the therapies on these major disease features.
Methylxanthines are well known as respiratory stimulants and used as one of the commonly used therapies for bronchial asthma. Methylxanthines are a unique class of drugs prescribed for asthmatic lung in humans because of their role in reversing the airflow obstruction and reducing airway hyperresponsiveness and airway inflammation. Methylxanthines also exert their effect via additional mechanisms, which include inhibition of immune cell activation, reduction of proinflammatory gene expression via induction of the histone deacetylase activity, and also via its effect on mucociliary transport (Tilley, 2011). Methylxanthines have shown to lower allergic inflammations in several species like rats, rabbits, and guinea pigs (Ali et al., 1992; Manzini et al., 1993; Pauwels, 1987). The anti-inflammatory properties of methylxanthines were eventually established in a series of clinical studies that showed a significant decrease in EG2+ (a monoclonal antibody to eosinophil cationic protein) eosinophils (which correlates to decreased airway inflammation during asthma), reduction of CD4+ lymphocytes in the bronchial wall (Sullivan et al., 1994). Ever since, methylxanthines have been efficiently used therapeutically for respiratory diseases.
2. Pentoxifylline
2.1. Pentoxifylline as a potential anti-inflammatory and antioxidant agent for COVID-19
Viral infections, in general, are associated with the constant generation of oxidized products. Previously it has been shown that viral lung pathogens can trigger the oxidative stress pathways resulting in the generation of reactive oxygen species (ROS) as well as local production of oxidized phospholipid (OxPL) (Imai et al., 2008). Analysis of humans died in SARS-CoV infections showed the massive formation of OxPLs in all the severe cases of ALI (Imai et al., 2008). In a disease model, it was shown that ALI was caused by the overproduction of IL-6 in alveolar macrophages via Toll-like receptor 4 (TLR4)/ nuclear factor kappa- B (NF-kB) signaling and it was a result of the activated innate immune response due to the SARS-CoV induced production of OxPLs (Imai et al., 2008). In addition to the challenge of ALI due to SARS-CoV, severe ARDS treatment is an ongoing challenge for COVID-19 patients infected with SARS-CoV-2 (Matthay et al., 2020). Patients with severe cases of ALI/ARDS are treated in ICUs and have severe inflammation. Several factors contribute to the inflammation, including hypoxia, due to inflammatory mediators like cytokines and viral infection (Sarma and Ward, 2011). Accordingly, we speculate that similar excessive oxidation is likely to be involved in COVID-19 patients. This speculation was further supported by the severe inflammatory response observed in COVID-19 patients with heightened levels of the proinflammatory cytokines like IL-2, IL-4, IL-7, IL-8, IL-9 and also high amounts of IL-1β, IFNγ, IP-10, and MCP-1, which probably points towards an activated T-helper-1 (Th1) cell responses (Huang et al., 2020).
Prior studies have reported that alveolar macrophages release inflammatory cytokines such as TNF-α that plays an important role in the prognosis of inflammatory pulmonary diseases (Kelley, 1990; Sibille and Reynolds, 1990). Consistent with the literature, it has been shown that pentoxifylline elicited a significant reduction in the production of TNF-α in cultured cells of lipopolysaccharide (LPS)-stimulated alveolar macrophages and peripheral blood monocytes isolated from patients with an indication for bronchoalveolar lavage (Poulakis et al., 1999). This finding was also reported by Tong et al. (2003). The results of this study indicate that pentoxifylline suppressed TNF-α production in a dose-dependent manner in alveolar macrophages in sarcoidosis, which is mainly driven by proinflammatory and anti-inflammatory mediators. Data from another study in rat model demonstrated that pentoxifylline suppressed cytokine-induced neutrophil chemoattractant, NF-κB, Matrix metalloproteinase-2, and -9 (MMP) and myeloperoxidase content (Deree et al., 2007).
It was reported that, MMPs are well-known inflammatory mediators that contribute to the aggravation of ALI and ARDS by accelerating the secretion of neutrophils into the lung (Corbel et al., 2000; Torii et al., 1997). This action is correlated with the proteolytic activity of MMPs. In addition to the in vitro and in vivo data, a randomized controlled clinical trial showed that pentoxifylline treatment significantly reduced the circulating levels of pro-inflammatory TNF- α (Brie et al., 2016) indicating its clinical anti-inflammatory role.
The efficacy of pentoxifylline in improving the survival rate during hyperoxia has been shown in neonatal rats (Almario et al., 2012). Shreds of evidence suggested that pentoxifylline enhanced the pulmonary antioxidant activity in enzymes such as glutathione peroxidase, catalase, and superoxide dismutase. Another important finding was that pentoxifylline treatment enhanced vascularization through increasing the vascular endothelial growth factor (VEGF) protein expression (Almario et al., 2012). It is well established that VEGF involved in alveolar structures (Thébaud and Abman, 2007). Subsequently, it has been shown that pentoxifylline not only suppresses proinflammatory macrophages but also enhances both wound healing and anti-inflammatory macrophage in nitrogen mustard -induced lung injury and inflammation (Sunil et al., 2014). Clinical trial in patients with acute coronary syndromes has been shown the meaningful reduction in pro-inflammatory and elevation of the anti-inflammatory response following administration of pentoxifylline 400 mg three times a day for 6 months. These results suggest the potential therapeutic efficacy of pentoxifylline in cardiovascular events (Fernandes et al., 2008).
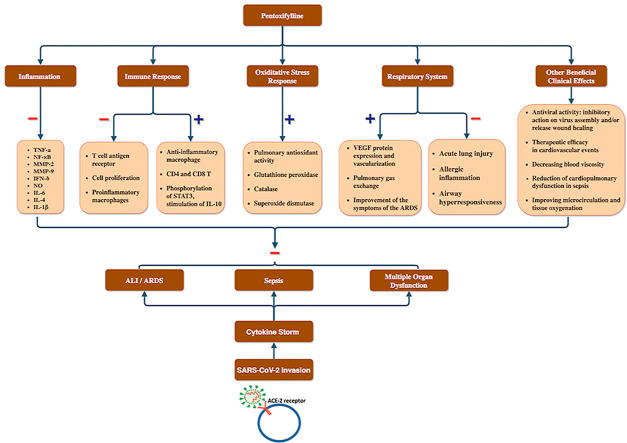
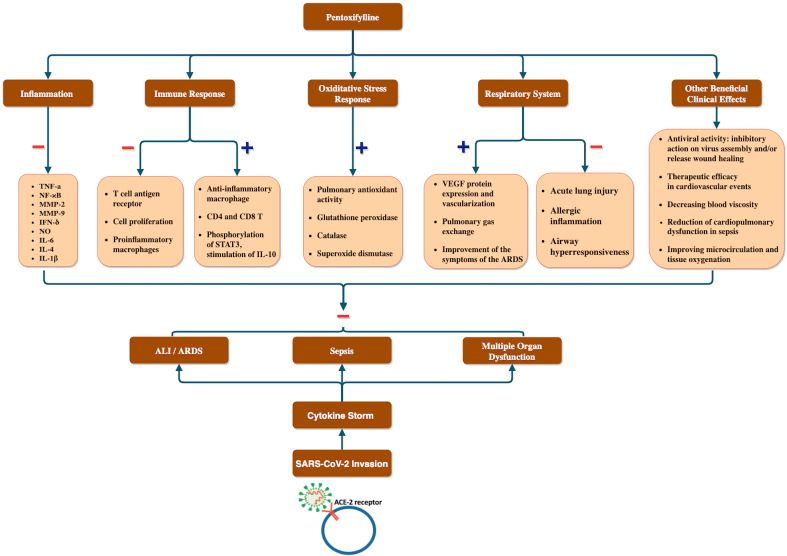
Considering the extensive evidence on anti-inflammatory and antioxidant properties of pentoxifylline ( Fig. 1 ), this molecule may have beneficial clinical effects in COVID-19 patients suffering from the severe inflammatory response.
Fig. 1.
Overview of the potential adjuvant use of pentoxifylline in relation to COVID-19 clinical manifestation. We postulated that cytokine storm results after the SARS-CoV-2 invasion resulting in COVID-19 symptoms like ALI/ARDS, sepsis and multiple organ disorders which can potentially be prevented by pentoxifylline via its inhibitory effect on inflammatory cytokines, proinflammatory immune cells, and ALI as well as its stimulatory effect on anti-inflammatory macrophages, IL-10, pulmonary anti-oxidant enzymes and gas exchange as well as efficacy in cardiovascular events and against viruses. ALI: Acute lung injury; ARDS: Acute respiratory distress syndrome, IL-10: Interleukin-10.
2.2. Pentoxifylline as a potential immunomodulatory agent for COVID-19 treatment
It is well established that following the entrance of respiratory viruses into the epithelial cell of the lung, the viral antigen will be presented on the cell surface to the cytotoxic CD8+ T cells. These cells are capable of killing infected cells by releasing the proinflammatory cytokines, including IFNγ (Rogers and Williams, 2018). Although this process is vital for clearing viral infections, complications can occur as it interferes with uninfected cells as well as lung function. In severe cases, cytotoxic CD8+ T cells and high concentration of the cytokines may cause serious injury to the lung (Bauer et al., 2006).
Amplification of the inflammatory signaling cascade can affect vascular permeability through an increasing influx of more phagocytes such as neutrophils and macrophages, leading to vascular endothelium dysfunction (Sharp et al., 2015). Due to this damage, the capacity of ventilation and gas exchange can be reduced drastically. Consequently, the patient may develop acute respiratory failure and require critical care support (Yang et al., 2018).
Moreover, it was also reported that patients with severe COVID-19 cases in ICU showed high levels of IP10, MCP1, Granulocyte Colony Stimulating Factor (GCSF), and TNFα than non-severe COVID-19 patients, suggesting a possible cytokine storm behind the severity of COVID-19 (Huang et al., 2020). Methylxanthines are known to have immune-modulatory effects at low serum concentration and, therefore can be potentially exploited as immunomodulators (Tilley, 2011).
There is a growing body of literature that recognizes various activities of pentoxifylline on immune cells ( Fig. 1 ). The immunomodulatory effect of this molecule has been studied extensively in both animal models and human clinical trials.
The anti-apoptotic activity of pentoxifylline has been demonstrated in human cell lines (Gupta et al., 1999). Pentoxifylline increases immune memory in CD4 and CD8 T cells by suppressing the activation of mediated T cell apoptosis, which is attributed to the Cyclic adenosine 3′,5′-monophosphate (cAMP) - Protein Kinase A (PKA) -mediated pathway (Suresh et al., 2002). Besides, pentoxifylline was able to attenuate the apoptosis induced by TNF-α, IFN-δ, and nitric oxide (NO) (Mensah-Brown et al., 2002). In a randomized, double-blind, controlled clinical trial, pentoxifylline significantly decreased the serum concentrations of TNFα and IL-6 (Gonzalez-Espinoza et al., 2012). IL-6 is a mortality predictor of COVID-19 patients (Ruan et al., 2020), and in severely affected COVID-19 patients, IL-6 levels are increased (Qin et al., 2020). Therefore, the idea of pentoxifylline to significantly bring down IL-6 levels to dampen the cytokine storm in COVID -19 patients is captivating. As mentioned earlier, due to the high level of cytokines and risks of apoptosis of epithelial and endothelial cells along with the infected cells, pentoxifylline may propose a safer therapeutic option compared to other anti-inflammatory therapies in COVID-19 patients.
Furthermore, pentoxifylline suppressed the expression of surface T cell antigen, including CD25, CD69, and CD98. In line with this study, it has been shown that pentoxifylline interferes with T cell proliferation via the CD3/T-cell receptor complex (Gonzalez-Amaro et al., 1998). Further immunomodulatory activity of pentoxifylline has been shown in animal models. In a murine model, pentoxifylline administration reduced the airway hyperresponsiveness due to Th1 cytokine IFN δ (Fleming et al., 2001). In line with aforementioned study, the reversal of arthritic changes and attenuation of the Th1 (IFN- δ) and Th2 (IL-4) cytokine have been also observed in a rheumatoid arthritis rat model following administration of pentoxifylline (Pal et al., 2016).
Elevated levels of Th1 and Th2 cytokines have been found in SARS-CoV patients (Josset et al., 2013), and Th1, as well as Th17, was reported to contribute to the cytokine storm in SARS-CoV-2 induced pulmonary viral infection (Wu and Yang, 2020). Therefore, the attenuating impact of pentoxifylline on Th1 and Th2 cytokine levels can be utilized for its potential role in COVID-19 patients.
There is emerging evidence showing the beneficial effects of pentoxifylline in chronic heart failure due to its immunomodulatory effects and suppression of TNFα (Shaw et al., 2009). In addition to that, it has been described that pentoxifylline contributes to the suppression of TNFα and IL-1β induced by Toll-like receptors (TLRs). This suppression role of TNFα and IL-1β by pentoxifylline can be exploited as a therapeutic potential because in COVID-19 patients, as SARS-CoV-2 also induces lung inflammation, fever, and fibrosis by inducing active production of TNFα and IL-1β (Conti et al., 2020). TNFα has always been involved in SARS-CoV induced severe immune-based pulmonary injury, which suggests that TNFα inhibitors could be a potential treatment for the respiratory symptoms caused by the coronavirus (Bishop et al., 2005). Collecting the aforementioned evidence regarding the immunomodulatory activity of pentoxifylline suggests a potential therapeutic value of this agent in COVID-19 patients.
2.3. Pentoxifylline as a potential adjuvant therapy for COVID-19 related respiratory symptoms
The respiratory clinical manifestations of patients with SARS-CoV-2 range from pneumonia, dyspnea, rhinorrhea, upper airway congestion, cough, and pharyngalgia (Lai et al., 2020). In severe cases, death may result due to colossal alveolar damage and progressive respiratory failure (Chan et al., 2020). The main feature of COVID-19 patients with severe disease is the acute onset of hypoxemic respiratory failure with bilateral infiltrates known more commonly as Acute Respiratory Distress Syndrome (ARDS) (Murthy et al., 2020). For the COVID-19 patients with ARDS, extracorporeal membrane oxygenation (ECMO) is recommended (Matthay et al., 2020). However, this process of ECMO is invasive and comes with practical constraints that accompany intubation and mechanical ventilation. For the less severe COVID-19 related respiratory symptoms where mechanical ventilation is not required, alternative evidence-based treatment options can be considered.
Preliminary randomized clinical trial on pentoxifylline (400 mg, TDS for 12 weeks) demonstrated the enhancement of pulmonary gas exchange in COPD patients. A possible mechanism for these results could have been by the increase in the cardiac output and mixed venous oxygen partial pressure (Haas et al., 1990). A subsequent trial on severe ARDS has shown that administration of pentoxifylline with a dose of 100 mg IV twice a day for seven days, followed by 400 mg orally, three times a day, led to the improvement of the symptoms of the ARDS, suppression of mean TNF levels and increasing the survival rate (Montravers et al., 1993). Therefore, not surprisingly pentoxifylline for the treatment of ARDS associated with SARS infection was suggested in 2003 (Martín et al., 2003)
Furthermore, the efficacy of pentoxifylline in attenuation of acute lung injury has been shown in animal models such as guinea pigs (Lilly et al., 1989). It is also well established that the administration of pentoxifylline at the time of allergen sensitization airway hyperresponsiveness suppresses the allergic inflammation and airway hyperresponsiveness in vivo (Fleming et al., 2001). The observed bronchodilatory, anti-inflammatory, and immunomodulatory properties provide further support for suggesting the pentoxifylline as a promising medicine in SARS (Martín et al., 2003).
In a more recent study, pentoxifylline treatment improved ALI-induced by Infrarenal aortic cross-clamping in the Wistar rat model. The proposed mechanism involved the phosphorylation of Signal transducer and activator of transcription 3 (STAT3), leading to the stimulation of IL-10 production (Li et al., 2016). The beneficial effect of pentoxifylline on lung has been demonstrated in lung cancer patients. A combination of pentoxifylline and vitamin E decreased radiation-induced lung toxicity frequency in lung cancer patients receiving concurrent chemo-radiotherapy (Misirlioglu et al., 2007). These results are in line with those that found the preventive efficacy of the combination, as mentioned earlier in the alleviation of some radiation-induced side-effects in patients with either breast (Magnusson et al., 2009) or head and neck cancer (Sayed et al., 2020). Since there are no clinical guidelines for radiation induced pulmonary fibrosis management, pentoxifylline is currently recommended for the prevention and treatment of this condition (Hanania et al., 2019). In addition, in a randomized controlled clinical trial in patients with the chronic pulmonary inflammatory disease (pulmonary sarcoidosis), pentoxifylline treatment improved arterial blood oxygen pressure as well as pulmonary diffusion of carbon monoxide (Park et al., 2009). With the massive evidence of lung involvement in COVID-19 patients, pentoxifylline may offer a safe and well-tolerated option to treat the respiratory symptoms in these patients potentially ( Fig. 1).
2.4. Potential antiviral activity of pentoxifylline COVID-19 treatment
The spike (S) protein of coronaviruses allows viral entry to the target cells (Hoffmann et al., 2020). Coronavirus S protein is an essential component in determining the virulence of the virus, tissue tropism, and host range. The SARS-S protein uses angiotensin-converting enzyme 2 (ACE 2) as the entry receptor (Li et al., 2003), and the cellular serine protease TMPRSS2 carries out the S-protein priming and activation (Shulla et al., 2011). SARS-S protein of SARS-CoV and SARS-2-S protein of SARS-CoV-2 share about 76% amino acid identity. Following the previous results, a recent study showed compelling evidence that the entry of SARS-CoV-2 also depends on the ACE 2 receptor, and this entry can be blocked by serine protease TMPRSS2 inhibitor (Hoffmann et al., 2020). Similarly, another study also reported SARS-CoV-2-S protein entry on 293/hACE2 cells (Human embryonic kidney 293 cells stably expressing human ACE 2) is mainly mediated by endocytosis (Ou et al., 2020). Following its entry, the virus expresses the genes encoding all structural and accessory proteins by adopting the genome of their host. The viral nucleocapsids are assembled in the cytoplasm, enter into the lumen of the endoplasmic reticulum (Graham and Baric, 2010). Subsequently, virions will be released through the process of exocytosis. They can infect various cells, including T lymphocytes, as well as organs like the liver, kidney, and the lower respiratory tract (Tynell et al., 2016). Therefore, they can provide potential drug targets, and also antibody raised against SARS-CoV could at least partly protect against SARS-CoV-2 infection and both the serine protease TMPRSS2 and the ACE 2 could be a possible target for therapeutic intervention (Hoffmann et al., 2020).
Broad-spectrum antiviral drugs such as IFN-alpha, protease inhibitors like the lopinavir, nucleoside analogs, including ribavirin, and Neuraminidase inhibitors such as remdesivir can be used as a potential antiviral treatment for the coronavirus infected patients. These antiviral agents can interfere at different stages of the viral replication cycle.
The antiviral property of pentoxifylline against tick-borne encephalitis virus, herpes simplex virus, vaccinia virus, and rotavirus has been shown in vitro, indicating the wide-spectrum of antiviral activity of pentoxifylline (Amvros' eva et al., 1993). This also accords with the previous report, which demonstrated the suppression of the human immunodeficiency virus (HIV) type 1 replication in human peripheral blood mononuclear cells (Fazely et al., 1991). Furthermore, in a randomized, controlled trial, adjunctive therapy with pentoxifylline in HIV patients with tuberculosis resulted in a statistically significant overall reduction in plasma HIV RNA compared to the control group (Wallis et al., 1996). The evaluation of pentoxifylline in HIV patients demonstrated a transient positive trend of change in CD4+ and CD8+ cells (Clerici et al., 1997)
There are limited, precise details on the antiviral mechanism of pentoxifylline. Concerning this question, research has been done to identify the stage at which pentoxifylline inhibits the replication of the Japanese encephalitis virus. The finding of this study provides further support for the hypothesis that the drug most likely exerted its inhibitory action on virus assembly and/or release (Sebastian et al., 2009). A significant increase in the percentage of patients with the sustained virological response, a gold standard for assessment of treatment response in chronic hepatitis C, has shown the antiviral efficacy of pentoxifylline following adding it to the conventional hepatitis C treatment (Jiménez-Luévano et al., 2015) and, therefore, may have the potential to use a similar inhibitory effect ( Fig. 1 ) on SARS-CoV-2 virus as well.
3. Caffeine
3.1. Caffeine as the other potential adjuvant therapy for COVID-19 related respiratory symptoms
Among the range of methylxanthines, caffeine is most commonly used for preterm infants with apnea receiving non-invasive respiratory support (Clark et al., 2006). Ventilating preterm infants may result in severe pulmonary adverse like bronchopulmonary dysplasia (BPD) (Moschino et al., 2020). Therefore, as non-invasive respiratory support, caffeine has already shown to reduce apnea of prematurity along with its associated improved lung function at 11 years of age (Jobe, 2017). Within an hour of caffeine dose, infant apnea in pertussis was resolved effectively by preventing respiratory failure and improving respiratory drive (Evered et al., 2018). Caffeine treatment on preterm infants at birth also showed significant improvement in minute ventilation and tidal volume (Dekker et al., 2017) as well as extubation success (Henderson-Smart and Davis, 2003). In addition to that, caffeine is also of the few known drugs shown to reduce the risk of BPD at 36 weeks post-menstrual age (PMA) (Dobson et al., 2014). Caffeine acts as a non-selective phosphodiesterase inhibitor, increasing cAMP levels, thus directly relaxing infant pulmonary vascular muscle and improving oxygenation and therefore early caffeine therapy strongly decrease the risk of pulmonary hypertension linked to chronic lung disease of prematurity (Vyas-Read et al., 2017). These evidences suggest a potential role of caffeine to treat the respiratory symptoms in infants with COVID-19 (Dulson and Bishop, 2016). Furthermore, caffeine showed asthma improvement in adults as well. It was reported that people with mild to moderate asthma improved lung function even at a low dose of 5 mg/kg body weight (Welsh et al., 2010). Caffeine also showed a significant bronchodilator effect in young patients with asthma (Becker et al., 1984). Among the various proposed mechanisms for the bronchodilator effect, the most well-established mechanism phosphodiesterase inhibition and adenosine receptor antagonism (Tilley, 2011). Along with the well-established role in improving pulmonary functions and respiratory symptoms as well as its bronchodilatory role on the upper respiratory tract of patients, caffeine makes itself a possible candidate as adjuvant therapy for COVID-19 patients showing respiratory symptoms.
3.2. Further potential roles of caffeine as an adjuvant to treat COVID-19 symptoms
Caffeine has been shown to affect cytokine production, lymphocyte proliferation, antibody production, natural killer cell (NK) function, histamine release, and immune cell apoptosis (Horrigan et al., 2006). In line with caffeine's role in immunomodulation, a bolus dose of 6 mg/kg caffeine caused an increase in total lymphocyte and CD8+ T cell count and an increase of CD4+CD69+ T cells before and after exercise with pre-exercise caffeine ingestion only (Bishop et al., 2005). More recent studies on human cells showed that high doses and prolonged exposure to caffeine most likely to promote NK cell activity in humans (Chen et al., 2012; Dulson and Bishop, 2016). In addition, caffeine effectively suppresses the proinflammatory cytokines (Horrigan et al., 2004). A more recent study on human samples showed that caffeine can also suppress phagocytosis by inhibiting cAMP phosphodiesterase (Steck et al., 2015). Multiple clinical trials reported improvement of serum pro-inflammatory markers like IL-18 in response to pure coffee administration and reported that caffeine of coffee contributed to the effect (Kempf et al., 2010, 2015; Wedick et al., 2011). However, caffeine also resulted in increase in pro-inflammatory IL-6 levels compared to control group (Wedick et al., 2011) and the inconsistency of IL-6 levels in across studies suggest that effect of caffeine on IL-6 levels (Tauler et al., 2013) requires further research. Taken together, the role of caffeine in promoting NK cell activity, increase in CD8+ T cells as well as suppression of proinflammatory cytokines allowed us to speculate its potential role in the suppression of hyper immune response in COVID-19 patients. However, further studies involving COVID-19 patients and control group will be required to confirm this speculation.
During lung injury, the NOD-like receptor 3 (NLRP3) inflammasome plays a key role in the innate immune response (Wu et al., 2013). When macrophages sense external pathogens, it results in the formation of NLRP3 inflammasome and secretion of the proinflammatory cytokines IL-1β and IL-18 in macrophages to amplify inflammation (Lamkanfi and Dixit, 2012). Furthermore, caffeine was proven to significantly reduce NLRP3 expression and associated caspase cleavage as well as inhibit NLRP3 inflammasome activation by suppressing the mitogen-activated protein kinase (MAPK)/NF-κB signaling and therefore suppressed the secretion of IL-1β and IL-18 in human derived THP-1 macrophages (human monocytic cell line) (Guo et al., 2015; Zhao et al., 2019). NLRP1 inflammasome is correlated to lung diseases caused by influenza A virus, bacteria, and syncytial virus (Shen et al., 2020; Tate et al., 2016; Yang et al., 2006). Along with the aforementioned compelling evidence, we propose a potential immunomodulatory role of caffeine in COVID-19 related respiratory inflammation.
Early studies showed that caffeine inhibited viral RNA synthesis and viral protein synthesis in infected cells and thereby inhibiting growth and propagation of Newcastle disease virus, influenza virus, poliovirus, herpes simplex virus type 1 (HSV-1), HIV, vaccinia virus and polyomavirus (Dahl et al., 2005; Daniel et al., 2005; Olson and Consigli, 1979; Shiraki and Rapp, 1988; Yamazaki and Tagaya, 1980). Later studies further confirmed that caffeine preferentially causes cytopathic effect (CPE) and the death of HSV-1 infected cells (Murayama et al., 2008). In a more recent meta-analysis on the effect of caffeine in patients with chronic hepatitis C, it was shown that patients with higher caffeine intake showed 61% reduced risk of developing advanced hepatic fibrosis compared to lower caffeine intake patient group (Jaruvongvanich et al., 2017). Specifically, for hepatitis C, an in-vitro study also demonstrated that caffeine efficiently inhibited the replication of hepatitis C (Batista et al., 2015). A similar antiviral behavior of caffeic acid through suppression of multiplication as well as CPE changes of the infected cells has been shown against Influenza A Virus (Utsunomiya et al., 2014). Overall, there is substantial evidence for the broad-spectrum antiviral role of caffeine, and preferential CPE of caffeine on virally infected cells could potentially induce selective death of virus-infected cells of COVID-19 patients.
4. Side effects and toxicity
Among methylxanthines pentoxifylline is well tolerated. With a usual therapeutic dose of 1.2 g per day, a few rare events have been reported worldwide. The main reported adverse effects are gastrointestinal and more serious side effects such as cardiac arrhythmia and seizure have been observed rarely through post-marketing investigation (McCarty et al., 2016). Pentoxifylline should be used cautiously in hepatic and renal impairment as well as in special population such as elderly and pregnant.
Some studies did not find association between maternal caffeine intake and birth defects (Bech et al., 2007; Browne et al., 2011), other more recent studies reported detrimental effects, malformation incidence and risk of miscarriage (Chen et al., 2012; Group, 2008; Hoyt et al., 2014; Weng et al., 2008). Moderate intake of caffeine is considered safe and acute toxicity is rare but gastrointestinal disturbances, insomnia, nervousness, headache, tachycardia, arrhythmia, nausea, seizures may result from caffeine intoxication (Nawrot et al., 2003). Chronic effects of caffeine may include dysfunction of the liver, musculature and the gastrointestinal and renal systems (Nawrot et al., 2003).
Despite the global usage of theophylline as bronchodilator, it has a very narrow therapeutic window and its interaction with various drugs has led to the limitation of its use (Journey and Bentley, 2018). Toxic effects of theophylline observed when plasma concentrations exceed 20 mcg/mL (Journey and Bentley, 2018) but sometimes toxic effects can be observed within therapeutic levels as well. Theophylline normally have more toxic effects than caffeine including nausea, vomiting, increased acid secretion and gastroesophageal reflux as well as cardiac arrhythmias at higher concentrations (Barnes, 2013). CNS symptoms like dizziness, irritability, and lightheadedness can also occur (Jilani et al., 2019). At higher doses, theophylline causes increase in the levels of catecholamine which eventually led to adverse effects like metabolic acidosis, hyperglycemia and hypokalemia. Cardiac arrhythmias, seizures and even death may result at high serum levels of 80–100 mcg/mL (Journey and Bentley, 2018).
5. Conclusion
The world has faced a new pandemic that has no proven pharmacotherapy despite all the attempts to develop a new effective medicine or vaccine with an acceptable safety profile. The current review has provided a collective data on the potential beneficial properties of methylxanthines like pentoxifylline and caffeine as an adjuvant therapy to treat COVID-19 patients. Addressing the broad spectrum of COVID-19 symptoms including respiratory failure due to hypersensitivity and exaggerated immune response, is challenging. Pentoxifylline and in some aspects, caffeine with extensively proven therapeutic properties like anti-inflammatory, antioxidant, immunomodulatory, antiviral, as well as their beneficial effects in the alleviation of respiratory symptoms can be considered as an adjuvant treatment in COVID-19 patients. Moreover, pentoxifylline can also address the treatment of thrombotic complications, a recently identified manifestation of COVID-19. Although direct evidence of pentoxifylline and caffeine in COVID-19 patients are yet to be studied and also possible side effects should be considered, the insights gained from this review highlighted their efficacy and safety in COVID-19 disease and can be used to develop additional strategies to tackle this global challenge.
Funding
This research received no external funding.
Credit author statement
Faezeh Monji: literature review, Writing - original draft, preparation, All authors have read and agreed to the published version of the manuscript. Abrar Al-Mahmood Siddiquee: literature review, Writing - original draft, preparation, Writing - review & editing, All authors have read and agreed to the published version of the manuscript. Farshad Hashemian: Conceptualization, Writing - review & editing, All authors have read and agreed to the published version of the manuscript.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
The authors wish to thank Dr. Shahrooz Faghihroohi for his support to design the figure.
References
- Al-Tawfiq J.A., Al-Homoud A.H., Memish Z.A. Remdesivir as a possible therapeutic option for the COVID-19. Travel Med. Inf. Disp. 2020;34:101615. doi: 10.1016/j.tmaid.2020.101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S., Mustafa S.J., Metzger W.J. Adenosine-induced bronchoconstriction in an allergic rabbit model: antagonism by theophylline aerosol. Agents Actions. 1992;37:165–167. doi: 10.1007/BF02028098. [DOI] [PubMed] [Google Scholar]
- Almario B., Wu S., Peng J., Alapati D., Chen S., Sosenko I.R. Pentoxifylline and prevention of hyperoxia-induced lung -injury in neonatal rats. Pediatr. Res. 2012;71:583–589. doi: 10.1038/pr.2012.14. [DOI] [PubMed] [Google Scholar]
- Amvros' eva T., Votiakov V., Andreeva O., Vladyko G., Nikolaeva S., Orlova S., Azarova I., Zgirovskaia A. New properties of trental as an inhibitor of viral activity with a wide range of activity. Vopr. Virusol. 1993;38:230–233. [PubMed] [Google Scholar]
- Barnes P.J. Theophylline. Am. J. Respir. Crit. Care Med. 2013;188:901–906. doi: 10.1164/rccm.201302-0388PP. [DOI] [PubMed] [Google Scholar]
- Batista M.N., Carneiro B.M., Braga A.C., Rahal P. Caffeine inhibits hepatitis C virus replication in vitro. Arch. Virol. 2015;160:399–407. doi: 10.1007/s00705-014-2302-1. [DOI] [PubMed] [Google Scholar]
- Bauer T.T., Ewig S., Rodloff A.C., Muller E.E. Acute respiratory distress syndrome and pneumonia: a comprehensive review of clinical data. Clin. Infect. Dis. 2006;43:748–756. doi: 10.1086/506430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bech B.H., Obel C., Henriksen T.B., Olsen J. Effect of reducing caffeine intake on birth weight and length of gestation: randomised controlled trial. BMJ. 2007;24(334):409. doi: 10.1136/bmj.39062.520648.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker A.B., Simons K.J., Gillespie C.A., Simons F.E. The bronchodilator effects and pharmacokinetics of caffeine in asthma. N. Engl. J. Med. 1984;310:743–746. doi: 10.1056/NEJM198403223101202. [DOI] [PubMed] [Google Scholar]
- Bishop N.C., Fitzgerald C., Porter P.J., Scanlon G.A., Smith A.C. Effect of caffeine ingestion on lymphocyte counts and subset activation in vivo following strenuous cycling. Eur. J. Appl. Physiol. 2005;93:606–613. doi: 10.1007/s00421-004-1271-6. [DOI] [PubMed] [Google Scholar]
- Bohn S.K., Ward N.C., Hodgson J.M., Croft K.D. Effects of tea and coffee on cardiovascular disease risk. Food Funct. 2012;3:575–591. doi: 10.1039/c2fo10288a. [DOI] [PubMed] [Google Scholar]
- Brie D., Sahebkar A., Penson P.E., Dinca M., Ursoniu S., Serban M.C., Zanchetti A., Howard G., Ahmed A., Aronow W.S., Muntner P., Lip G.Y., Wong N.D., Rysz J., Banach M., Lipid B.P. Effects of pentoxifylline on inflammatory markers and blood pressure: a systematic review and meta-analysis of randomized controlled trials. J. Hypertens. 2016;34:2318–2329. doi: 10.1097/HJH.0000000000001086. [DOI] [PubMed] [Google Scholar]
- Browne M.L., Hoyt A.T., Feldkamp M.L., Rasmussen S.A., Marshall E.G., Druschel C.M., Romitti P.A. Maternal caffeine intake and risk of selected birth defects in the National Birth Defects Prevention Study. Birth Defects Res. Clin. Mol. Teratol. 2011;91:93–101. doi: 10.1002/bdra.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce M., Scott N., Shine P., Lader M. Anxiogenic effects of caffeine in patients with anxiety disorders. Arch. Gen. Psychiatr. 1992;49:867–869. doi: 10.1001/archpsyc.1992.01820110031004. [DOI] [PubMed] [Google Scholar]
- Cao Y.C., Deng Q.X., Dai S.X. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: an evaluation of the evidence. Trav. Med. Infect. Dis. 2020;35:101647. doi: 10.1016/j.tmaid.2020.101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J., Xing F., Liu J., Yip C.C., Poon R.W., Tsoi H.W., Lo S.K., Chan K.H., Poon V.K., Chan W.M., Ip J.D., Cai J.P., Cheng V.C., Chen H., Hui C.K., Yuen K.Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., Perlman S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Bell E.M., Browne M.L., Druschel C.M., Romitti P.A., Schmidt R.J., Burns T.L., Moslehi R., Olney R.S., National Birth Defects Prevention S. Maternal caffeine consumption and risk of congenital limb deficiencies. Birth Defects Res. Clin. Mol. Teratol. 2012;94:1033–1043. doi: 10.1002/bdra.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng V.C.C., Lau S.K.P., Woo P.C.Y., Yuen K.Y. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin. Microbiol. Rev. 2007;20:660–694. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E., de Wit H. Enhanced mood and psychomotor performance by a caffeine-containing energy capsule in fatigued individuals. Exp. Clin. Psychopharmacol. 2008;16:13–21. doi: 10.1037/1064-1297.16.1.13. [DOI] [PubMed] [Google Scholar]
- Clark R.H., Bloom B.T., Spitzer A.R., Gerstmann D.R. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics. 2006;117:1979–1987. doi: 10.1542/peds.2005-1707. [DOI] [PubMed] [Google Scholar]
- Clerici M., Piconi S., Balotta C., Trabattoni D., Capetti A., Fusi M.L., Ruzzante S., Longhi R., Colombo M.C., Moroni M., Milazzo F. Pentoxifylline improves cell-mediated immunity and reduces human immunodeficiency virus (HIV) plasma viremia in asymptomatic HIV-seropositive persons. J. Infect. Dis. 1997;175:1210–1215. doi: 10.1086/593570. [DOI] [PubMed] [Google Scholar]
- Coccheri S., Bignamini A.A. Pharmacological adjuncts for chronic venous ulcer healing. Phlebology. 2016;31:366–367. doi: 10.1177/0268355515619562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Normile D. New SARS-like virus in China triggers alarm. Science. 2020;367:234–235. doi: 10.1126/science.367.6475.234. [DOI] [PubMed] [Google Scholar]
- Conti P., Ronconi G., Caraffa A., Gallenga C.E., Ross R., Frydas I., Kritas S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34:1. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- Corbel M., Boichot E., Lagente V. Role of gelatinases MMP-2 and MMP-9 in tissue remodeling following acute lung injury. Braz. J. Med. Biol. Res. 2000;33:749–754. doi: 10.1590/s0100-879x2000000700004. [DOI] [PubMed] [Google Scholar]
- Dahl J., You J., Benjamin T.L. Induction and utilization of an ATM signaling pathway by polyomavirus. J. Virol. 2005;79:13007–13017. doi: 10.1128/JVI.79.20.13007-13017.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel R., Marusich E., Argyris E., Zhao R.Y., Skalka A.M., Pomerantz R.J. Caffeine inhibits human immunodeficiency virus type 1 transduction of nondividing cells. J. Virol. 2005;79:2058–2065. doi: 10.1128/JVI.79.4.2058-2065.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J., Hooper S.B., van Vonderen J.J., Witlox R.S.G.M., Lopriore E., Te Pas A.B. Caffeine to improve breathing effort of preterm infants at birth: a randomized controlled trial. Pediatr. Res. 2017;82:290–296. doi: 10.1038/pr.2017.45. [DOI] [PubMed] [Google Scholar]
- Deree J., Martins J., De Campos T., Putnam J.G., Loomis W.H., Wolf P., Coimbra R. Pentoxifylline attenuates lung injury and modulates transcription factor activity in hemorrhagic shock. J. Surg. Res. 2007;143:99–108. doi: 10.1016/j.jss.2007.03.083. [DOI] [PubMed] [Google Scholar]
- Dettelbach H.R., Aviado D.M. Clinical pharmacology of pentoxifylline with special reference to its hemorrheologic effect for the treatment of intermittent claudication. J. Clin. Pharmacol. 1985;25:8–26. doi: 10.1002/j.1552-4604.1985.tb02796.x. [DOI] [PubMed] [Google Scholar]
- Dobson N.R., Patel R.M., Smith P.B., Kuehn D.R., Clark J., Vyas-Read S., Herring A., Laughon M.M., Carlton D., Hunt C.E. Trends in caffeine use and association between clinical outcomes and timing of therapy in very low birth weight infants. J. Pediatr. 2014;164:992–998. doi: 10.1016/j.jpeds.2013.12.025. e993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyen D., Moceri P., Ducreux D., Dellamonica J. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet. 2020;395:1516. doi: 10.1016/S0140-6736(20)30912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., Zhou M., Chen L., Meng S., Hu Y., Peng C., Yuan M., Huang J., Wang Z., Yu J., Gao X., Wang D., Yu X., Li L., Zhang J., Wu X., Li B., Xu Y., Chen W., Peng Y., Hu Y., Lin L., Liu X., Huang S., Zhou Z., Zhang L., Wang Y., Zhang Z., Deng K., Xia Z., Gong Q., Zhang W., Zheng X., Liu Y., Yang H., Zhou D., Yu D., Hou J., Shi Z., Chen S., Chen Z., Zhang X., Yang X. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. U. S. A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulson D.K., Bishop N.C. Effect of a high and low dose of caffeine on human lymphocyte activation in response to antigen stimulation. Appl. Physiol. Nutr. Metabol. 2016;41:224–227. doi: 10.1139/apnm-2015-0456. [DOI] [PubMed] [Google Scholar]
- Evered J., Pfeifer E., Gracianette M. Caffeine to prevent respiratory failure and improve outcome in infant pertussis. BMJ. Case Rep. 2018:1–14. doi: 10.1136/bcr-2017-223102. bcr-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazely F., Dezube B.J., Allen-Ryan J., Pardee A.B., Ruprecht R.M. Pentoxifylline (Trental) decreases the replication of the human immunodeficiency virus type 1 in human peripheral blood mononuclear cells and in cultured T cells. Blood. 1991;77:1653–1656. [PubMed] [Google Scholar]
- Fernandes J.L., de Oliveira R.T.D., Mamoni R.L., Coelho O.R., Nicolau J.C., Blotta M., Serrano C.V., Jr. Pentoxifylline reduces pro-inflammatory and increases anti-inflammatory activity in patients with coronary artery disease--a randomized placebo-controlled study. Atherosclerosis. 2008;196:434–442. doi: 10.1016/j.atherosclerosis.2006.11.032. [DOI] [PubMed] [Google Scholar]
- Fleming C.M., He H., Ciota A., Perkins D., Finn P.W. Administration of pentoxifylline during allergen sensitization dissociates pulmonary allergic inflammation from airway hyperresponsiveness. J. Immunol. 2001;167:1703–1711. doi: 10.4049/jimmunol.167.3.1703. [DOI] [PubMed] [Google Scholar]
- Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;16:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- Gautret P., Lagier J.-C., Parola P., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gonzalez-Amaro R., Portales-Perez D., Baranda L., Redondo J.M., Martinez-Martinez S., Yanez-Mo M., Garcia-Vicuna R., Cabanas C., Sanchez-Madrid F. Pentoxifylline inhibits adhesion and activation of human T lymphocytes. J. Immunol. 1998;161:65–72. [PubMed] [Google Scholar]
- Gonzalez-Espinoza L., Rojas-Campos E., Medina-Perez M., Pena-Quintero P., Gomez-Navarro B., Cueto-Manzano A.M. Pentoxifylline decreases serum levels of tumor necrosis factor alpha, interleukin 6 and C-reactive protein in hemodialysis patients: results of a randomized double-blind, controlled clinical trial. Nephrol. Dial. Transplant. 2012;27:2023–2028. doi: 10.1093/ndt/gfr579. [DOI] [PubMed] [Google Scholar]
- Graham R.L., Baric R.S. Recombination, reservoirs, and the modular spike: mechanisms of coronavirus cross-species transmission. J. Virol. 2010;84:3134–3146. doi: 10.1128/JVI.01394-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.-X. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group C.S. Maternal caffeine intake during pregnancy and risk of fetal growth restriction: a large prospective observational study. BMJ. 2008;337 doi: 10.1136/bmj.a2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Callaway J.B., Ting J.P. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat. Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M., George A., Sen R., Rath S., Durdik J.M., Bal V. Presence of pentoxifylline during T cell priming increases clonal frequencies in secondary proliferative responses and inhibits apoptosis. J. Immunol. 1999;162:689–695. [PubMed] [Google Scholar]
- Haas F., Bevelaqua F., Levin N., Salazar-Schicchi J., Reggiani J.L., Axen K., Pineda H. Pentoxifylline improves pulmonary gas exchange. Chest. 1990;97:621–627. doi: 10.1378/chest.97.3.621. [DOI] [PubMed] [Google Scholar]
- Hanania A.N., Mainwaring W., Ghebre Y.T., Hanania N.A., Ludwig M. Radiation-induced lung injury: assessment and management. Chest. 2019;156:150–162. doi: 10.1016/j.chest.2019.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S., Rasyid A., Nurhayati E., Prihartono J. Atlantis Press; 2017. Selected Benefits of Pentoxifylline in Acute Ischemic Stroke Management: Consideration of Risk Factors, Health Science International Conference (HSIC 2017) [Google Scholar]
- Hayashi M., Tsuchiya H., Yamamoto N., Karita M., Shirai T., Nishida H., Takeuchi A., Tomita K. Caffeine-potentiated chemotherapy for metastatic carcinoma and lymphoma of bone and soft tissue. Anticancer Res. 2005;25:2399–2405. [PubMed] [Google Scholar]
- Henderson-Smart D.J., Davis P.G. Cochrane Database Syst. Rev.; 2003. Prophylactic methylxanthines for extubation in preterm infants; p. CD000139. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan L.A., Kelly J.P., Connor T.J. Caffeine suppresses TNF-alpha production via activation of the cyclic AMP/protein kinase A pathway. Int. Immunopharm. 2004;4:1409–1417. doi: 10.1016/j.intimp.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Horrigan L.A., Kelly J.P., Connor T.J. Immunomodulatory effects of caffeine: friend or foe? Pharmacol. Ther. 2006;111:877–892. doi: 10.1016/j.pharmthera.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Hoyt A.T., Browne M., Richardson S., Romitti P., Druschel C., National Birth Defects Prevention S. Maternal caffeine consumption and small for gestational age births: results from a population-based case-control study. Matern. Child Health J. 2014;18:1540–1551. doi: 10.1007/s10995-013-1397-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C.Y., Norris J.W., Hogan E.L., Bladin P., Dinsdale H.B., Yatsu F.M., Earnest M.P., Scheinberg P., Caplan L.R., Karp H.R. Pentoxifylline in acute nonhemorrhagic stroke. A randomized, placebo-controlled double-blind trial. Stroke. 1988;19:716–722. doi: 10.1161/01.str.19.6.716. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Neely G.G., Yaghubian-Malhami R., Perkmann T., van Loo G., Ermolaeva M., Veldhuizen R., Leung Y.H.C., Wang H. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaruvongvanich V., Sanguankeo A., Klomjit N., Upala S. Effects of caffeine consumption in patients with chronic hepatitis C: a systematic review and meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2017;41:46–55. doi: 10.1016/j.clinre.2016.05.012. [DOI] [PubMed] [Google Scholar]
- Jilani T.N., Preuss C.V., Sharma S. StatPearls Publishing; 2019. Theophylline, StatPearls [Internet] [PubMed] [Google Scholar]
- Jiménez-Luévano M.Á., Lerma-Díaz J.M., Hernández-Flores G., Jiménez-Partida M.Á., Bravo-Cuellar A. Addition of pentoxifylline to pegylated interferon-alpha-2a and ribavirin improves sustained virological response to chronic hepatitis C virus: a randomized clinical trial. Ann. Hepatol. 2015;12:248–255. [PubMed] [Google Scholar]
- Jobe A.H. Caffeine: a lung drug for all very low birth weight preterm infants? Am. J. Respir. Crit. Care Med. 2017;196:1241–1243. doi: 10.1164/rccm.201707-1402ED. [DOI] [PubMed] [Google Scholar]
- Joob B., Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue. J. Am. Acad. Dermatol. 2020;82:e177. doi: 10.1016/j.jaad.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journey J.D., Bentley T.P. StatPearls 122Publishing; 2018. Theophylline Toxicity. StatPearls [Internet] [Google Scholar]
- Josset L., Menachery V.D., Gralinski L.E., Agnihothram S., Sova P., Carter V.S., Yount B.L., Graham R.L., Baric R.S., Katze M.G. Cell host response to infection with novel human coronavirus EMC predicts potential antivirals and important differences with SARS coronavirus. mBio. 2013;4:e00165–113. doi: 10.1128/mBio.00165-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley J. Cytokines of the lung. Am. Rev. Respir. Dis. 1990;141:765–788. doi: 10.1164/ajrccm/141.3.765. [DOI] [PubMed] [Google Scholar]
- Kempf K., Herder C., Erlund I., Kolb H., Martin S., Carstensen M., Koenig W., Sundvall J., Bidel S., Kuha S., Tuomilehto J. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: a clinical trial. Am. J. Clin. Nutr. 2010;91:950–957. doi: 10.3945/ajcn.2009.28548. [DOI] [PubMed] [Google Scholar]
- Kempf K., Kolb H., Gartner B., Bytof G., Stiebitz H., Lantz I., Lang R., Hofmann T., Martin S. Cardiometabolic effects of two coffee blends differing in content for major constituents in overweight adults: a randomized controlled trial. Eur. J. Nutr. 2015;54:845–854. doi: 10.1007/s00394-014-0763-3. [DOI] [PubMed] [Google Scholar]
- Kimura H., Tsuchiya H., Shirai T., Nishida H., Hayashi K., Takeuchi A., Ohnari I., Tomita K. Caffeine-potentiated chemotherapy for metastatic osteosarcoma. J. Orthop. Sci. 2009;14:556–565. doi: 10.1007/s00776-009-1372-5. [DOI] [PubMed] [Google Scholar]
- Klok F., Kruip M., van der Meer N., Arbous M., Gommers D., Kant K., Kaptein F., van Paassen J., Stals M., Huisman M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam A., Newhouse M.T. Management of asthma and chronic airflow limitation. Are methylxanthines obsolete? Chest. 1990;98:44–52. doi: 10.1378/chest.98.1.44. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M., Dixit V.M. Inflammasomes and their roles in health and disease. Annu. Rev. Cell Dev. Biol. 2012;28:137–161. doi: 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]
- Lara D.R. Caffeine, mental health, and psychiatric disorders. J. Alzheimers Dis. 2010;20(Suppl. 1):S239–S248. doi: 10.3233/JAD-2010-1378. [DOI] [PubMed] [Google Scholar]
- Li H., Tan G., Tong L., Han P., Zhang F., Liu B., Sun X. Pentoxifylline inhibits pulmonary inflammation induced by infrarenal aorticcross-clamping dependent of adenosine receptor A2A. Am. J. Transl. Res. 2016;8:2210–2221. [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly C.M., Sandhu J.S., Ishizaka A., Harada H., Yonemaru M., Larrick J.W., Shi T.-X., O'Hanley P.T., Raffin T.A. Pentoxifylline prevents tumor necrosis factor-induced lung injury1-3. Am. Rev. Respir. Dis. 1989;139:1361–1368. doi: 10.1164/ajrccm/139.6.1361. [DOI] [PubMed] [Google Scholar]
- Lipton R.B., Diener H.C., Robbins M.S., Garas S.Y., Patel K. Caffeine in the management of patients with headache. J. Headache Pain. 2017;18:107. doi: 10.1186/s10194-017-0806-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty M.F., O'Keefe J.H., DiNicolantonio J.J. Pentoxifylline for vascular health:a brief review of the literature. Open Heart. 2016;3 doi: 10.1136/openhrt-2015-000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson M., Höglund P., Johansson K., Jönsson C., Killander F., Malmström P., Weddig A., Kjellén E. Pentoxifylline and vitamin E treatment for prevention of radiation-induced side-effects in women with breast cancer: a phase two, double-blind, placebo-controlled randomised clinical trial (Ptx-5) Eur. J. Canc. 2009;45:2488–2495. doi: 10.1016/j.ejca.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Manzini S., Perretti F., Abelli L., Evangelista S., Seeds E.A., Page C.P. Isbufylline, a new xanthine derivative, inhibits airway hyperresponsiveness and airway inflammation in Guinea pigs. Eur. J. Pharmacol. 1993;249:251–257. doi: 10.1016/0014-2999(93)90519-n. [DOI] [PubMed] [Google Scholar]
- Martín J.F.B., Jiménez J.L., MuEóz-Fernández A. Pentoxifylline and severe acute respiratory syndrome (SARS): a drug to be considered. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2003;9:SR29–SR34. [PubMed] [Google Scholar]
- Matthay M.A., Aldrich J.M., Gotts J.E. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir. Med. 2020;8:433–434. doi: 10.1016/S2213-2600(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, P., McAuley, D.F., Brown, M., Sanchez, E., Tattersall, R.S., Manson, J.J., Hlh across Speciality Collaboration, U.K., 2020. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 395, 1033-1034. [DOI] [PMC free article] [PubMed]
- Mensah-Brown E.P., Stosic Grujicic S., Maksimovic D., Jasima A., Shahin A., Lukic M.L. Downregulation of apoptosis in the target tissue prevents low-dose streptozotocin-induced autoimmune diabetes. Mol. Immunol. 2002;38:941–946. doi: 10.1016/s0161-5890(02)00021-4. [DOI] [PubMed] [Google Scholar]
- Misirlioglu C.H., Demirkasimoglu T., Kucukplakci B., Sanri E., Altundag K. Pentoxifylline and alpha-tocopherol in prevention of radiation-induced lung toxicity in patients with lung cancer. Med. Oncol. 2007;24:308–311. doi: 10.1007/s12032-007-0006-z. [DOI] [PubMed] [Google Scholar]
- Montravers P., Fagon J.Y., Gilbert C., Blanchet F., Novara A., Chastre J. Pilot study of cardiopulmonary risk from pentoxifylline in adult respiratory distress syndrome. Chest. 1993;103:1017–1022. doi: 10.1378/chest.103.4.1017. [DOI] [PubMed] [Google Scholar]
- Moschino L., Zivanovic S., Hartley C., Trevisanuto D., Baraldi E., Roehr C.C. Caffeine in preterm infants: where are we in 2020? ERJ Open Res. 2020;6 doi: 10.1183/23120541.00330-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama M., Tsujimoto K., Uozaki M., Katsuyama Y., Yamasaki H., Utsunomiya H., Koyama A.H. Effect of caffeine on the multiplication of DNA and RNA viruses. Mol. Med. Rep. 2008;1:251–255. [PubMed] [Google Scholar]
- Murthy S., Gomersall C.D., Fowler R.A. Care for critically ill patients with COVID-19. J. Am. Med. Assoc. 2020;323:1499–1500. doi: 10.1001/jama.2020.3633. [DOI] [PubMed] [Google Scholar]
- Nawrot P., Jordan S., Eastwood J., Rotstein J., Hugenholtz A., Feeley M. Effects of caffeine on human health. Food Addit. Contam. 2003;20:1–30. doi: 10.1080/0265203021000007840. [DOI] [PubMed] [Google Scholar]
- Okuno T., Sugiyama T., Tominaga M., Kojima S., Ikeda T. Effects of caffeine on microcirculation of the human ocular fundus. Jpn. J. Ophthalmol. 2002;46:170–176. doi: 10.1016/s0021-5155(01)00498-1. [DOI] [PubMed] [Google Scholar]
- Olson N.J., Consigli R.A. Production of labile Newcastle disease virus progeny after infection of chicken embryo cells in the presence of caffeine. Am. J. Vet. Res. 1979;40:387–392. [PubMed] [Google Scholar]
- Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Chaudhary M.J., Tiwari P.C., Nath R., Babu S., Pant K.K. Pharmacological studies on the anti-inflammatory and immunomodulatory role of pentoxifylline and its interaction with nitric oxide (NO) in experimental arthritis in rats. Inflammopharmacology. 2016;24:221–231. doi: 10.1007/s10787-016-0281-4. [DOI] [PubMed] [Google Scholar]
- Park M.K., Fontana, Babaali H., Gilbert-McClain L.I., Stylianou M., Joo J., Moss J., Manganiello V.C. Steroid-sparing effects of pentoxifylline in pulmonary sarcoidosis. Sarcoidosis Vasc. Diffuse Lung Dis. 2009;26:121–131. [PMC free article] [PubMed] [Google Scholar]
- Pauwels R. The effects of theophylline on airway inflammation. Chest. 1987;92:32S–37S. doi: 10.1378/chest.92.1_supplement.32s. [DOI] [PubMed] [Google Scholar]
- Poulakis N., Androutsos G., Kazi D., Bastas A., Provata A., Bitsakou C., Kontozoglou T., Polyzogopoulou C., Tassiopoulou A. The differential effect of pentoxifylline on cytokine production by alveolar macrophages and its clinical implications. Respir. Med. 1999;93:52–57. doi: 10.1016/s0954-6111(99)90077-x. [DOI] [PubMed] [Google Scholar]
- Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020 doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020;ciaa248 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J. Eur. Acad. Dermatol. Venereol. 2020;34:e212–e213. doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- Rogers M.C., Williams J.V. Quis custodiet ipsos custodes? Regulation of cell-mediated immune responses following viral lung infections. Annu. Rev. Virol. 2018;5:363–383. doi: 10.1146/annurev-virology-092917-043515. [DOI] [PubMed] [Google Scholar]
- Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma J.V., Ward P.A. Oxidants and redox signaling in acute lung injury. Comp. Physiol. 2011;1:1365–1381. doi: 10.1002/cphy.c100068. [DOI] [PubMed] [Google Scholar]
- Sayed R., El Wakeel L., Saad A.S., Kelany M., El-Hamamsy M. Pentoxifylline and vitamin E reduce the severity of radiotherapy-induced oral mucositis and dysphagia in head and neck cancer patients: a randomized, controlled study. Med. Oncol. 2020;37:8. doi: 10.1007/s12032-019-1334-5. [DOI] [PubMed] [Google Scholar]
- Sebastian L., Desai A., Madhusudana S.N., Ravi V. Pentoxifylline inhibits replication of Japanese encephalitis virus: a comparative study with ribavirin. Int. J. Antimicrob. Agents. 2009;33:168–173. doi: 10.1016/j.ijantimicag.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp C., Millar A.B., Medford A.R. Advances in understanding of the pathogenesis of acute respiratory distress syndrome. Respiration. 2015;89:420–434. doi: 10.1159/000381102. [DOI] [PubMed] [Google Scholar]
- Shaw S.M., Shah M.K., Williams S.G., Fildes J.E. Immunological mechanisms of pentoxifylline in chronic heart failure. Eur. J. Heart Fail. 2009;11:113–118. doi: 10.1093/eurjhf/hfn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C., Zhang Z., Xie T., Ji J., Xu J., Lin L., Yan J., Kang A., Dai Q., Dong Y. Rhein suppresses lung inflammatory injury induced by human respiratory syncytial virus through inhibiting NLRP3 inflammasome activation via NF-κB pathway in mice. Front. Pharmacol. 2020;10:1600. doi: 10.3389/fphar.2019.01600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraki K., Rapp F. Effects of caffeine on herpes simplex virus. Intervirology. 1988;29:235–240. doi: 10.1159/000150050. [DOI] [PubMed] [Google Scholar]
- Shulla A., Heald-Sargent T., Subramanya G., Zhao J., Perlman S., Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J. Virol. 2011;85:873–882. doi: 10.1128/JVI.02062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille Y., Reynolds H.Y. Macrophages and Polymorphonuclear neutrophils in lung defense and Injury1-2. Am. Rev. Respir. Dis. 1990;141:471–501. doi: 10.1164/ajrccm/141.2.471. [DOI] [PubMed] [Google Scholar]
- Smits S.L., de Lang A., van den Brand J.M., Leijten L.M., van I.W.F., Eijkemans M.J., van Amerongen G., Kuiken T., Andeweg A.C., Osterhaus A.D., Haagmans B.L. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck R.P., Hill S.L., Weagel E.G., Weber K.S., Robison R.A., O'Neill K.L. Pharmacologic immunosuppression of mononuclear phagocyte phagocytosis by caffeine. Pharmacol. Res. Perspect. 2015;3 doi: 10.1002/prp2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan P., Bekir S., Jaffar Z., Page C., Jeffery P., Costello J. Anti-inflammatory effects of low-dose oral theophylline in atopic asthma. Lancet. 1994;343:1006–1008. doi: 10.1016/s0140-6736(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Sunil V.R., Vayas K.N., Cervelli J.A., Malaviya R., Hall L., Massa C.B., Gow A.J., Laskin J.D., Laskin D.L. Pentoxifylline attenuates nitrogen mustard-induced acute lung injury, oxidative stress and inflammation. Exp. Mol. Pathol. 2014;97:89–98. doi: 10.1016/j.yexmp.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh R., Vig M., Bhatia S., Goodspeed E.P., John B., Kandpal U., Srivastava S., George A., Sen R., Bal V. Pentoxifylline functions as an adjuvant in vivo to enhance T cell immune responses by inhibiting activation-induced death. J. Immunol. 2002;169:4262–4272. doi: 10.4049/jimmunol.169.8.4262. [DOI] [PubMed] [Google Scholar]
- Talik P., Krzek J., Ekiert R.J. Analytical techniques used for determination of methylxanthines and their analogues—recent advances. Separ. Purif. Rev. 2012;41:1–61. [Google Scholar]
- Tate M.D., Ong J.D.H., Dowling J.K., McAuley J.L., Robertson A.B., Latz E., Drummond G.R., Cooper M.A., Hertzog P.J., Mansell A. Reassessing the role of the NLRP3 inflammasome during pathogenic influenza A virus infection via temporal inhibition. Sci. Rep. 2016;6:27912. doi: 10.1038/srep27912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauler P., Martinez S., Moreno C., Monjo M., Martinez P., Aguilo A. Effects of caffeine on the inflammatory response induced by a 15-km run competition. Med. Sci. Sports Exerc. 2013;45:1269–1276. doi: 10.1249/MSS.0b013e3182857c8a. [DOI] [PubMed] [Google Scholar]
- Thébaud B., Abman S.H. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am. J. Respir. Crit. Care Med. 2007;175:978–985. doi: 10.1164/rccm.200611-1660PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley S.L. Springer Berlin Heidelberg; Berlin, Heidelberg: 2011. Methylxanthines in Asthma, Methylxanthines; pp. 439–456. [Google Scholar]
- Tong Z., Dai H., Chen B., Abdoh Z., Guzman J., Costabel U. Inhibition of cytokine release from alveolar macrophages in pulmonary sarcoidosis by pentoxifylline: comparison with dexamethasone. Chest. 2003;124:1526–1532. doi: 10.1378/chest.124.4.1526. [DOI] [PubMed] [Google Scholar]
- Torii K., Iida K., Miyazaki Y., Saga S., Kondoh Y., Taniguchi H., Taki F., Takagi K., Matsuyama M., Suzuki R. Higher concentrations of matrix metalloproteinases in bronchoalveolar lavage fluid of patients with adult respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1997;155:43–46. doi: 10.1164/ajrccm.155.1.9001287. [DOI] [PubMed] [Google Scholar]
- Tynell J., Westenius V., Rönkkö E., Munster V.J., Melén K., Österlund P., Julkunen I. Middle East respiratory syndrome coronavirus shows poor replication but significant induction of antiviral responses in human monocyte-derived macrophages and dendritic cells. J. Gen. Virol. 2016;97:344. doi: 10.1099/jgv.0.000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsunomiya H., Ichinose M., Ikeda K., Uozaki M., Morishita J., Kuwahara T., Koyama A.H., Yamasaki H. Inhibition by caffeic acid of the influenza A virus multiplication in vitro. Int. J. Mol. Med. 2014;34:1020–1024. doi: 10.3892/ijmm.2014.1859. [DOI] [PubMed] [Google Scholar]
- van Koert R.R., Bauer P.R., Schuitema I., Sander J.W., Visser G.H. Caffeine and seizures: a systematic review and quantitative analysis. Epilepsy Behav. 2018;80:37–47. doi: 10.1016/j.yebeh.2017.11.003. [DOI] [PubMed] [Google Scholar]
- Vyas-Read S., Kanaan U., Shankar P., Stremming J., Travers C., Carlton D.P., Fitzpatrick A. Early characteristics of infants with pulmonary hypertension in a referral neonatal intensive care unit. BMC Pediatr. 2017;17:163. doi: 10.1186/s12887-017-0910-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis R.S., Nsubuga P., Whalen C., Mugerwa R.D., Okwera A., Oette D., Jackson J.B., Johnson J.L., Ellner J.J. Pentoxifylline therapy in human immunodeficiency virus-seropositive persons with tuberculosis: a randomized, controlled trial. J. Infect. Dis. 1996;174:727–733. doi: 10.1093/infdis/174.4.727. [DOI] [PubMed] [Google Scholar]
- Wang L., He W., Yu X., Hu D., Bao M., Liu H., Zhou J., Jiang H. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J. Infect. 2020;80:639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedick N.M., Brennan A.M., Sun Q., Hu F.B., Mantzoros C.S., van Dam R.M. Effects of caffeinated and decaffeinated coffee on biological risk factors for type 2 diabetes: a randomized controlled trial. Nutr. J. 2011;10:93. doi: 10.1186/1475-2891-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh E.J., Bara A., Barley E., Cates C.J. Cochrane Database Syst. Rev.; 2010. Caffeine for asthma; p. CD001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W.X., Lee S.Y., Siang R., Koh R.Y. Repurposing pentoxifylline for the treatment of fibrosis: an overview. Adv. Ther. 2017;34:1245–1269. doi: 10.1007/s12325-017-0547-2. [DOI] [PubMed] [Google Scholar]
- Weng X., Odouli R., Li D.K. Maternal caffeine consumption during pregnancy and the risk of miscarriage: a prospective cohort study. Am. J. Obstet. Gynecol. 2008;198:279 e271–278. doi: 10.1016/j.ajog.2007.10.803. [DOI] [PubMed] [Google Scholar]
- Whitfield K., Rambaldi A., Wetterslev J., Gluud C. Cochrane Database Syst. Rev.; 2009. Pentoxifylline for alcoholic hepatitis; p. CD007339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020;53:368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Yan Z., Schwartz D.E., Yu J., Malik A.B., Hu G. Activation of NLRP3 inflammasome in alveolar macrophages contributes to mechanical stretch-induced lung inflammation and injury. J. Immunol. 2013;190:3590–3599. doi: 10.4049/jimmunol.1200860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Liu C., Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., Zhang X., Pan A., Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. U. S. A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Z., Tagaya I. Antiviral effects of atropine and caffeine. J. Gen. Virol. 1980;50:429–431. doi: 10.1099/0022-1317-50-2-429. [DOI] [PubMed] [Google Scholar]
- Yang C.-Y., Chen C.-S., Yiang G.-T., Cheng Y.-L., Yong S.-B., Wu M.-Y., Li C.-J. New insights into the immune molecular regulation of the pathogenesis of acute Respiratory Distress Syndrome. Int. J. Mol. Sci. 2018;19:588. doi: 10.3390/ijms19020588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R.-Z., Lee M.-J., Hu H., Pollin T.I., Ryan A.S., Nicklas B.J., Snitker S., Horenstein R.B., Hull K., Goldberg N.H. Acute-phase serum amyloid A: an inflammatory adipokine and potential link between obesity and its metabolic complications. PLoS Med. 2006;3:e287. doi: 10.1371/journal.pmed.0030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Ren Y., Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav. Immun. 2020;S0889–1591:30465–30467. doi: 10.1016/j.bbi.2020.04.017. 0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Ma L., Cai C., Gong X. Caffeine inhibits NLRP3 inflammasome activation by suppressing MAPK/NF-kappaB and A2aR signaling in LPS-induced THP-1 macrophages. Int. J. Biol. Sci. 2019;15:1571–1581. doi: 10.7150/ijbs.34211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W., China Novel Coronavirus I., Research T. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zito P.M., Murgia R.D., III Pentoxifylline (trental) in venous insufficiency and venous leg ulcers. J. Dermatol. Nurses Assoc. 2018;10:294–296. [Google Scholar]
- Zumla A., Chan J.F., Azhar E.I., Hui D.S., Yuen K.Y. Coronaviruses - drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]