Abstract
Background
Artemisia annua is the most common outdoor aeroallergen throughout Northern China; however, no multicenter study has investigated sublingual immunotherapy (SLIT) as a treatment option for Artemisia annua-induced allergic rhinitis (AR). The aim of this study was to evaluate the efficacy and safety of an innovative SLIT for Artemisia annua-related AR.
Methods
This was a randomized, double-blind, placebo-controlled, multicenter, phase 3 clinical trial conducted in China (NCT XXX). A total of 702 Artemisia annua-sensitized eligible patients were randomized in a ratio of 2:1 to receive Artemisia annua-SLIT or placebo. The treatment lasted 32 weeks; including 5-weeks up-dosing phase and 27-weeks maintenance phase. The primary endpoint was the daily combined score of medication and rhinoconjunctivitis symptom (CSMRS), and secondary endpoints were daily total nasal symptom score (dTNSS) and daily rescue medication score (dRMS) during peak pollen period. Safety of treatment was evaluated according to adverse events (AEs) experienced.
Results
Mean daily CSMRS was significantly improved during the peak pollen period in the SLIT group compared with the placebo group (1.46 ± 0.47 vs 1.88 ± 0.42, P < 0.0001 in full analysis set [FAS]; 1.49 ± 0.52 vs 1.95 ± 0.46, P < 0.0001 in per protocol set [PPS]); representing a 22.3% and 23.6% reduction, respectively, relative to placebo. In specifically Artemisia annua monosensitized patients, mean daily CSMRS reductions were demonstrated as 24.1% and 27.0% in the FAS and PPS populations, respectively, when comparing the active treatment to placebo treatment. Similarly, SLIT decreased dTNSS in peak pollen period by 19.0% in FAS and 22.3% in PPS, respectively, relative to placebo. In coincidence, dRMS in peak pollen period was reduced by 22.0% in FAS and 26.0% in PPS. 65.8% patients in SLIT group experienced treatment-related AEs, none of which was serious.
Conclusion
This study indicates that SLIT with Artemisia annua drops is an effective and safe treatment option in Chinese patients with Artemisia Annua-induced AR.
Keywords: Sublingual immunotherapy, Allergic rhinitis, Artemisia annua, Efficacy, Safety
Introduction
Allergic rhinitis (AR) is a global allergic disease, which has a pronounced negative impact on quality of life and leads to an increased economic and health-related burden on both the affected individual and society.1 In the recent decade, the prevalence of self-reported AR in China has increased markedly from 11.1% to 17.6%.2 A recent study has reported that the incidence of physician-diagnosed AR is as high as 32.4% in Northern China,3 with the majority of patients having seasonal allergic rhinitis (SAR) due to outdoor aeroallergens.
Artemisia species, or mugwort, is the most important outdoor aeroallergen throughout China.4,5 It has been reported that about 11.3% of the Chinese patients with respiratory allergies are sensitized to Artemisia pollen, with the number of patients sensitized to this allergen being over 50% in Northern China.5 The commonly occurring species of Artemisia in China is Artemisia annua, or sweet sage wort, which is mainly distributed in the area around the northern part of Yangtze River.4 This plant produces a large quantity of small pollen grains and causes widespread SAR over a long period from the beginning of July until the end of September each year.
Medications generally used to relieve AR symptoms have been shown to be partially effective and without long-term benefits in 40% of patients with hay fever in a general practice setting.6 In contrast, allergen specific immunotherapy (AIT) is the only treatment modality able to induce prolonged remission and alter disease progression.7 Although subcutaneous immunotherapy (SCIT) has been introduced into clinical practice for over 100 years and confirmed to be efficacious,8,9 considering the inconvenience of injection and potential risks of adverse events (AEs) in SCIT, sublingual immunotherapy (SLIT) has been developed as an alternative to facilitate access to AIT and to provide a very favorable safety profile. By enhancing the benefit-risk ratio in favor of superior convenience of self-administration without supervision of medical personnel, SLIT has been shown to be preferred as an effective and safer alternative to SCIT in recent years.10 Moreover, most clinical trials and meta-analysis confirm a significantly more reliable efficacy and better safety profile of SLIT compared with placebo for management of pollen-induced AR or allergic rhinoconjunctivitis.11, 12, 13, 14, 15, 16, 17
Compared to pollen specific SLIT widely performed in AR patients in Europe18 and North America,19, 20, 21 little research has focused on the efficacy and safety profile of AIT with Artemisia annua. Recently, Artemisia annua drops have been developed as the first registered, standardized pollen allergen extracts for SLIT in patients with Artemisia pollen-induced SAR; with phase 1 and phase 2 clinical trials in a small group of Chinese patients with Artemisia annua-sensitized SAR. These trials indicated that this preparation did not result in any serious adverse events and also determined the effective safe dose for Artemisia annua SLIT (unpublished data). The aim of the present study was therefore to evaluate the efficacy and safety of SLIT with this new preparation in a large cohort of Chinese SAR patients sensitized to Artemisia annua, using a placebo-controlled, double-blind, randomized, multicentre clinical trial design.
Methods
Study design
This phase 3, randomized, double-blind, placebo-controlled study (registered at clinicaltrial.gov as NCT03990272) was conducted from March of 2017 (approximate 4 months before the local natural Artemisia pollen season) to October of 2017, and involved patients from 13 centers across Northern China.
A total of 702 eligible patients were recruited and randomized into SLIT group and placebo group at the ratio of 2:1 according to a computer-generated block-randomization scheme. All patients and investigators were blinded to the study treatments by use of a placebo, which was identical to Artemisia drops adjunct in taste and appearance, and blinding was maintained through the trial until the database was locked.
All patients received treatment for 32 weeks involving a “5-week” up-dosing phase (1.25 biological unit to 2400 biological unit) and a “27-week” maintenance phase (2400 biological unit) with daily sublingual drops (Fig. 1), which were provided by Zhejiang Wolwo Bio-Pharmaceutical Co., Ltd., China. The allergen extract used for immunotherapy was glycerinated allergen extract of Artemisia annua, with the main allergenic components Art an1 and Art an3, while the placebo drops were physically identical to active medication, but without allergenic components. The drops were administered sublingually for 1 min before swallowing and nothing was allowed by mouth for 5 min after the administration of the drops. The participants were provided with rescue medications to use in a stepwise manner during the treatment, to alleviate AR symptoms as needed. Treatment compliance was evaluated with daily diary card, in which antiallergy medications, symptoms and adverse events were required to be recorded. The investigator inspected the vials and residue medications to confirm adherence to the regimen.
Fig. 1.
Study design. The treatment began approximately 4 months before start of the Artemisia pollen season and continued throughout a single pollen season
Treatment began approximate 4 months before the expected start of the pollen season according to the peak period of outpatients with SAR in the previous two autumns.3,4 The start of the natural peak pollen period22 in 2017 was defined as the first of 3 consecutive days with a pollen count of 50 pollen/m3 or greater each day. The end of peak pollen period was defined as the last occurrence of 3 consecutive days with ≥50 pollen/m3 each day.
The study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the corresponding review board in each center, and written informed consent was obtained from each subject prior to entry into the study.
Subjects
Patients with allergic rhinitis due to Artemisia pollens were recruited into the study. All patients had experienced Artemisia-related SAR for over 2 years; with symptom scores for 2 or more nasal symptoms ≥2 points during July to October in 2016. Artemisia annua allergy was further verified by the presence of specific immunoglobulin E (sIgE) ≥ 3.5 kU/L, using UniCAP system (Phadia, Uppsala, Sweden). Polysensitization was defined as Humulus- or Artemisiifolia-sIgE levels (ImmunoCAP) ≥0.7 kU/L (ImmunolCAP), but < Artemisia-sIgE level.
Patients were excluded from the study if they had previously received AIT, suffered from persistent or unstable asthma, perennial allergic rhinitis (eg, house dust mite sensitized patients with perennial allergic symptoms), cancer, or other serious diseases that could render them unfit to receive allergen immunotherapy, or any nasal condition that could confound the results of the study (chronic rhinitis, chronic rhinosinusitis with/without polyps). Similarly, patients who were non-compliant with the study protocol and treatment, pregnant and sexually active women of childbearing potential, patients taking β-antagonists or those who had taken systemic corticosteroids in last 4 weeks, and patients whose Humulus- or Artemisiifolia-sIgE levels (ImmunoCAP) were ≥ Artemisia-sIgE level, were also excluded.
Demographic and clinical data including age, sex, atopic status, comorbid allergic diseases, sIgE, and nasal/ocular symptom scores at the previous pollen season were collected before initiation of treatment.
Efficacy and safety assessments
The primary efficacy endpoint was set as the daily combined scores of medication and rhinoconjunctivitis symptoms (CSMRS) (ranging from 0 to 6); which was calculated as the combined score of daily average scores of 6 rhinoconjunctivitis symptoms (rhinorrhea, nasal congestion, nasal itching, sneezing, ocular pruritus, and watery eyes) and the daily rescue medication score (RMS).23
Nasal and ocular symptoms were rated on a 4-point scale of 0–3; with 0 = no symptoms, 1 = mild symptoms (sign/symptom clearly present, but minimal awareness; easily tolerated), 2 = moderate symptoms (definite awareness of sign/symptom that is bothersome but tolerable), and 3 = severe symptoms (sign/symptom that is hard to tolerate; causes interference with activities of daily living and/or sleeping). Relief medication was scored for daily use as follows: 0 = no use of rescue medication, 1 = use of oral and/or topical non-sedative H1 antihistamines (Clarityne or Patanol), 2 = use of intranasal corticosteroids (Rhinocort) with/without H1 antihistamines, and 3 = use of oral corticosteroids with/without intranasal corticosteroids, with/without H1 antihistamines.
Two secondary endpoints were investigated in the current study. The daily total nasal symptom score (dTNSS) was the sum of four nasal symptom scores for nasal congestion, discharge, itching and sneezing (ranging from 0 to 12). The daily RMS (dRMS) was assessed as the rescue medication score (ranging from 0 to 3).
Safety was evaluated by the occurrence and severity of adverse events (AEs) and the casual relationship between AEs and the experimental drug. All AEs were recorded in dairy card and categorized as mild (no impact on the activities of daily living), moderate (decreased or affected performance of the activities of daily living) or severe (an inability to perform the activities of daily living or death).
Statistical analyses
The full analysis set (FAS) population, same as the intent-to-treat population, included all randomized subjects who had received at least 1 dose of study medication during the peak pollen season and who had at least 1 post-dose efficacy assessment and rescue medication score. The per protocol set (PPS) population included all subjects in FAS population who completed the study with no serious violation of the protocol. The safety analysis was performed with the safety set (SS) population, which included all randomized subjects who had received at least 1 dose of the study medication in the study.
The sample size was estimated by power statistics using the CSMRS as the primary outcome measure. We estimated that using a 2:1 group design, at least 582 subjects would be required between the two treatment groups to detect a mean difference of 0.32 (combined SD, 1.12) (according to previous data in phase 2 clinical trials in a small group of Chinese patients with Artemisia annua-sensitized SAR), with 90% power and a 2-tailed α value of 0.05. Considering a drop-out rate of 20% and randomization block size as 6, we recruited 702 participants, with 468 subjects in the SLIT group and 234 patients in the placebo group. The last observation carry forward was used for missing values of the primary outcome.
SPSS statistical software, Version 22.0 (IBM Corp., Armonk, NY, USA) was used for data analysis, and two-side tests were performed with a significance level of 0.05. Descriptive statistics were used for baseline and post-treatment variables. Efficacy endpoints were assessed in the FAS and PPS population, respectively. Analysis of covariance (ANCOVA) was performed to compare differences in efficacy endpoints. Mean and median differences between groups were estimated. ANCOVAs were performed on scores adjusted for baseline, treatment center, age and gender. An interaction term (treatment group ∗ sensitization status) was included in the regression model. The minimum clinically significant difference (MCSD), the important threshold of change in the outcomes between SLIT and Placebo groups in both the FAS and PPS populations, was estimated as one half of the SD (baseline or control) according to Norman and colleagues.24 If the left limit of the covariance-based 95% confidence interval (CI) surrounding the between-group difference was greater than MCSD, the clinical significance was certain.
Results
Demographic characteristics
A total of 966 subjects with allergic rhinitis symptoms were screened for eligibility and 702 subjects were randomized at a ratio of 2:1 into the SLIT group (n = 468) and placebo group (n = 234) as shown in Fig. 2. Data for 30 participants (20 participants in SLIT group and 10 participants in placebo group) from one center (Center 13) were excluded from the SS population, as the sealed envelope containing the blinding information on treatment was lost. Furthermore, 35 patients withdrew their consent before the pollen season and did not accept the treatment, whereas 5 patients were lost to follow-up. Thus, the FAS population included 632 subjects. A total of 42 patients were excluded from PPS, primarily as a result of change of residence over 7 days during the pollen season.
Fig. 2.
Subjects disposition. A total of 702 subjects were recruited and randomized into the two treatment groups to receive either Artemisia annua sublingual immunotherapy (SLIT) or placebo. Overall, 672, 632 and 590 subjects were included in the safety set (SS), full analysis set (FAS) and per protocol set (PPS) population, respectively
Both groups were comparable in age, gender, atopic status and co-morbidity of allergic asthma, allergic conjunctivitis and atopic dermatitis (Table 1). Similarly, nasal and ocular symptoms scores at pollen season of 2016 were generally comparable between both groups (Table 1).
Table 1.
Demographic characteristics of the subjects in full analysis set population.
| Variables | SLIT (n = 421) | Placebo (n = 211) |
|---|---|---|
| Age (year) | 37.5 ± 8.73 | 38.7 ± 9.43 |
| Sex, n (%) | ||
| Male | 192 (45.6%) | 103 (48.8%) |
| Female | 229 (54.4%) | 108 (51.2%) |
| Height (m) | 1.68 ± 0.079 | 1.678 ± 0.077 |
| Weight (kg) | 66.58 ± 12.30 | 66.91 ± 11.36 |
| BMI (kg/m2) | 23.58 ± 3.19 | 23.64 ± 2.791 |
| Nasal symptom scores at the previous pollen season | 9.4 ± 1.83 | 9.3 ± 1.90 |
| Nasal inching | 2.2 ± 0.79 | 2.1 ± 0.85 |
| Sneezing | 2.5 ± 0.58 | 2.5 ± 0.59 |
| Rhinorrhea | 2.5 ± 0.65 | 2.4 ± 0.71 |
| Nasal obstruction | 2.2 ± 0.85 | 2.2 ± 0.87 |
| Ocular symptom scores at the previous pollen season | 3.7 ± 1.30 | 3.8 ± 1.42 |
| Ocular pruritus | 2.4 ± 0.68 | 2.5 ± 0.72 |
| Watery eyes | 1.3 ± 0.95 | 1.3 ± 1.03 |
| Specific IgE (kU/L) | 4.1 ± 0.79 | 4.2 ± 0.78 |
| Allergic Asthma, n (%) | 9 (2.1%) | 4 (1.9%) |
| Allergic conjunctivitis, n (%) | 388 (92.2%) | 198 (93.8%) |
| Atopic dermatitis, n (%) | 28 (6.7%) | 10 (4.7%) |
| Food Allergy, n (%) | 6 2 (14.7%) | 29 (13.7%) |
| Drug Allergy, n (%) | 44 (10.5%) | 32 (15.2%) |
| Other allergic disease, n (%) | 10 (2.4%) | 4 (1.9%) |
| Polysensitization, n (%) | 75 (17.8%) | 38 (18.0%) |
BMI, body mass index; SLIT, sublingual immunotherapy
Primary endpoint of clinical efficacy
CSMRS in peak pollen period was significantly improved in Artemisia annua-SLIT group compared to placebo group. There was a 22.3% reduction in the mean CSMRS (1.46 ± 0.47 vs. 1.88 ± 0.42, P < 0.0001) in the active treatment group relative to the placebo treatment group in the FAS population (Table 2). The median values were found to be 1.44 vs. 1.87 (a 23.0% reduction) in Artemisia annua SLIT group and placebo group, respectively (Supplementary Table E1). Similarly, there was a 23.6% reduction in the mean CSMRS in the active treatment group relative to placebo treatment group in the PPS population (1.49 ± 0.52 vs. 1.95 ± 0.46, P < 0.0001) (Table 2). The median values in PPS were found to be 1.49 vs. 1.93 (a 22.8% reduction) in Artemisia annua SLIT and placebo groups, respectively (Supplementary Table E1). The MCSDs in CSMRS between SLIT and placebo groups in FAS and PPS populations were estimated to be 0.21 and 0.23, respectively. As the left limit of 95% CI of adjusted mean difference in improvement of CSMRS was 0.289 and 0.321, respectively, which was greater than the MCSD, this indicated that the improvement of CSMRS in SLIT group was clinically significant.
Table 2.
Daily combined scores of medication and rhinoconjunctivitis symptoms during the peak pollen period.
| FAS Population |
PPS Population |
|||
|---|---|---|---|---|
| SLIT | Placebo | SLIT | Placebo | |
| N | 421 | 211 | 395 | 195 |
| Mean ± SD | 1.46 ± 0.47 | 1.88 ± 0.42 | 1.49 ± 0.52 | 1.95 ± 0.46 |
| P value | <0.0001 | <0.0001 | ||
| Improvement | 22.3% | 23.6% | ||
| Adjusted mean difference (95% CI) | 0.371 (0.289–0.453) | 0.420 (0.321–0.518) | ||
FAS, full analysis set; PPS, per protocol set; SLIT, sublingual immunotherapy; SAR, seasonal allergic rhinitis; CI, confidence interval
Analysis of the improvements in CSMRS using a regression model, including the interaction term between treatment variable (SLIT or placebo) and sensitization status (monosensitization or polysensitization), indicated evidence of difference based on sensitization status (P < 0.001 in both FAS and PPS populations). In specifically Artemisia annua monosensitized patients, Artemisia annua SLIT significantly reduced mean daily CSMRS by 24.1% (1.48 ± 0.54 vs. 1.95 ± 0.44) and 27.0% (1.43 ± 0.55 vs 1.96 ± 0.44) in the FAS and PPS populations, respectively. However, the reduction in mean daily CSMRS in polysensitized patients was not significantly different between SLIT and placebo groups in the FAS (1.39 ± 1.01 vs. 1.38 ± 0.94) and PPS (1.39 ± 1.00 vs. 1.45 ± 0.94) populations.
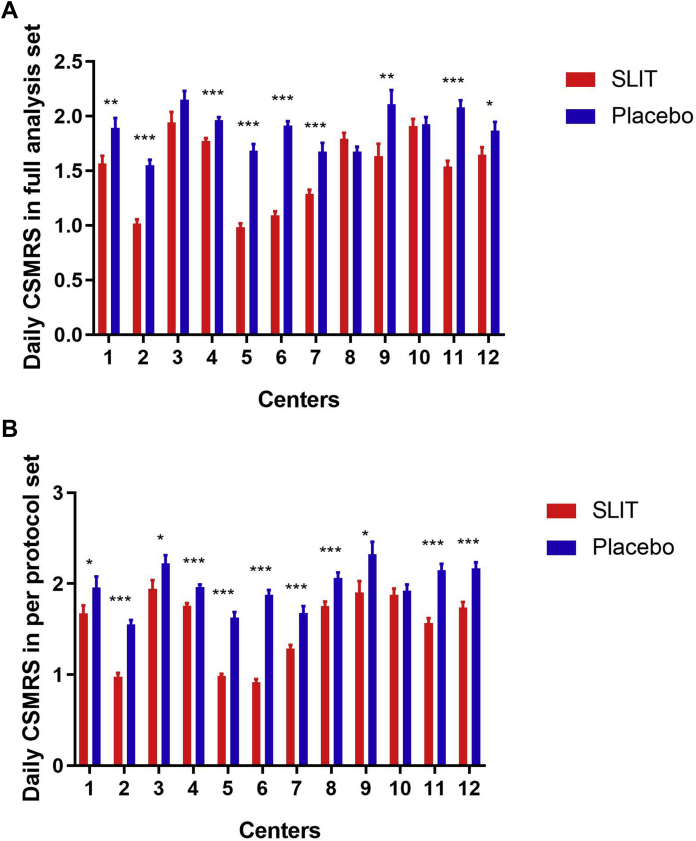
As far as each center was concerned, the mean daily CSMRS in peak pollen period for active treatment in nine centers indicated a significant advantage over the placebo in FAS population (Fig. 3A); whereas in PPS population, only in one center (Center 10), the mean daily CSMRS for active treatment demonstrated no significant difference from that for placebo (Fig. 3B).
Fig. 3.
Effect of Artemisia annua sublingual immunotherapy on daily combined scores of medication and rhinoconjunctivitis symptoms (CSMRS) in separate centers. (A) daily CSMRS during peak pollen period in full analysis set population; (B) daily CSMRS during peak pollen period in per protocol set population. FAS, full analysis set; PPS, per protocol set. ∗, P < 0.05; ∗∗, P < 0.01, ∗∗∗, P < 0.001
Secondary endpoints of clinical efficacy
The secondary efficacy endpoints of mean daily TNSS and mean daily RMS were also significantly improved with Artemisia annua SLIT compared to placebo (Table 3). Patients receiving active treatment reported a 19.0% reduction in mean dTNSS during the peak pollen period relative to placebo recipients (3.84 ± 1.06 vs. 4.74 ± 0.98, P < 0.0001) in FAS population. In PPS population, the mean values were found to be 3.79 ± 1.08 vs. 4.88 ± 1.01 in Artemisia annua SLIT and placebo groups (P < 0.0001), respectively, which reported a 22.3% reduction. The MCSDs in dTNSS PPS population was estimated to be 0.505, less than the left limit of 95% CI of adjusted mean difference (0.672); indicating that the difference in improvement of dTNSS between the Artemisia annua SLIT group and placebo group was also clinically meaningful.
Table 3.
Summary of average daily total nasal symptom score and daily rescue medication scores during peak pollen period.
| FAS population |
PPS population |
|||
|---|---|---|---|---|
| SLIT | Placebo | SLIT | Placebo | |
| N | 421 | 211 | 395 | 195 |
| Daily total nasal symptom score | ||||
| Mean ± SD | 3.84 ± 1.06 | 4.74 ± 0.98 | 3.79 ± 1.08 | 4.88 ± 1.01 |
| P value | <0.0001 | <0.001 | ||
| Improvement | 19.0% | 22.3% | ||
| Adjusted mean difference (95% CI) | 0.663 (0.447–0.879) | 0.883 (0.672–1.094) | ||
| Daily rescue medication scores | ||||
| Mean ± SD | 0.56 ± 0.27 | 0.71 ± 0.26 | 0.54 ± 0.29 | 0.73 ± 0.27 |
| P value | <0.0001 | <0.0001 | ||
| Improvement | 22.0% | 26.0% | ||
| Adjusted mean difference (95% CI) | 0.154 (0.101–0.206) | 0.131 (0.088–0.175) | ||
FAS, full analysis set; PPS, per protocol set; SLIT, sublingual immunotherapy; CI, confidence intervals
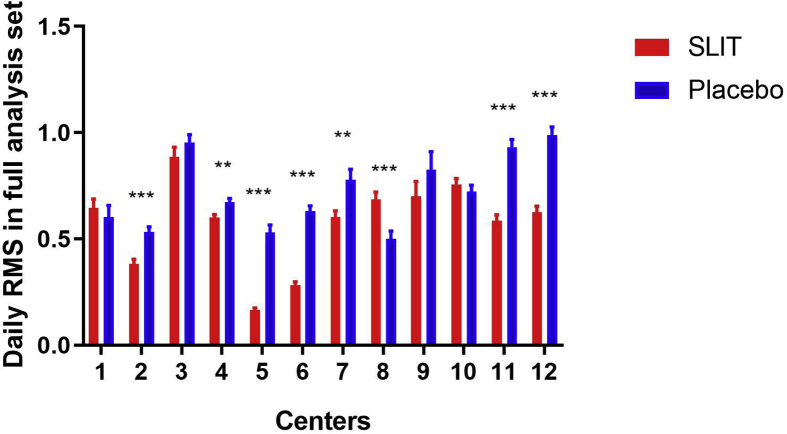
Subjects who received active Artemisia annua SLIT treatment reported a 22.0% reduction in mean dRMS throughout the peak pollen period relative to placebo recipients (0.56 ± 0.27 vs. 0.71 ± 0.26, P < 0.0001) in FAS and a 26.0% reduction (0.54 ± 0.29 vs. 0.73 ± 0.27, P < 0.0001) in PPS. The estimated MCSDs of dRMS in FAS population and PPS population was 0.13 and 0.135, respectively. The 95% CIs surrounding the adjusted between-group difference (0.101–0.206 in FAS and 0.088–0.175 in PPS) included values less than the estimated MCSDs, therefore, the clinical significance of rescue medication use reduction in the Artemisia annua SLIT group is uncertain. When each center was considered separately, the mean daily RMS in the Artemisia annua SLIT group during peak pollen period was found to be significantly reduced in seven centers (Center 2, 4, 5, 6, 7, 11 and 12) compared to the placebo SLIT group in statistics (Supplementary Figure E1 A and B).
Safety
Artemisia annua SLIT was well tolerated, with no deaths, drug-related serious allergic reactions, anaphylaxis, or life-threatening events during the whole treatment. Among the 6245 AEs reported in the current study, 2344 events were considered as drug-related and occurred in 425/672 patients (63.2%) included in the SS population. The percentage of participants reporting AEs was 91.5% (410/448 in Artemisia annua SLIT group and 205/224 in the control group). Similarly, the incidence of AEs per subject was also comparable in both groups during the entire 32 weeks of treatment (10.11 vs 10.24, respectively). Although the percentage of subjects who reported drug-related AEs was higher in Artemisia annua SLIT group (295/448, 65.8%) compared with control group (130/224, 58.0%), the incidence of drug-related AEs per subject were similar in both groups (5.51 vs. 5.53). Most AEs were early onset, transient, self-limiting reactions; with the most common drug-related AEs in the Artemisia annua SLIT group reported to oral paresthesia (36.8%). Table 4 lists the AEs with an incidence of 5% or greater. Overall, four patients in the active group and five patients in the placebo group each reported a severe AE (Table 5), none of which was considered to be associated with the study drug.
Table 4.
Adverse events experienced by 5% or more of subjects in safety set population.
| Adverse events No. (%) | Treatment emergent |
Treatment related |
||
|---|---|---|---|---|
| SLITa | Placebob | SLITa | Placebob | |
| Oral paresthesia | 169 (37.7) | 56 (25.0) | 165 (36.8) | 55 (24.6) |
| Nasopharyngitis | 126 (28.1) | 62 (27.7) | 2 (0.4) | 2 (0.9) |
| Sneezing | 116 (25.9) | 66 (29.5) | 46 (10.3) | 26 (11.6) |
| Nasal pruritus | 100 (22.3) | 47 (21.0) | 37 (8.3) | 18 (8.0) |
| Rhinorrhea | 91 (20.3) | 56 (25.0) | 32 (7.1) | 19 (8.5) |
| Eye pruritus | 88 (19.6) | 59 (26.3) | 41 (9.2) | 23 (10.3) |
| Nasal congestion | 80 (17.9) | 49 (21.9) | 25 (5.6) | 15 (6.7) |
| Throat irritation | 59 (13.2) | 22 (9.8) | 40 (8.9) | 15 (6.7) |
| Oropharyngeal pain | 51 (11.4) | 28 (12.5) | 14 (3.1) | 9 (4.0) |
| Cough | 41 (9.2) | 26 (11.6) | 9 (2.0) | 8 (3.6) |
| URTI | 41 (9.2) | 19 (8.5) | 1 (0.2) | 0 |
| Ear pruritus | 40 (8.9) | 11 (4.9) | 27 (6.0) | 7 (3.1) |
| Headache | 32 (7.1) | 14 (6.3) | 6 (1.3) | 2 (0.9) |
| Throat-clearing | 30 (6.7) | 17 (7.6) | 5 (1.1) | 4 (1.8) |
| Diarrhea | 29 (6.5) | 13 (5.8) | 5 (1.1) | 1 (0.4) |
| Tongue itching | 29 (6.5) | 2 (0.9) | 29 (6.5) | 2 (0.9) |
| Swollen tongue | 24 (5.4) | 0 | 24 (5.4) | 0 |
SLIT, sublingual immunotherapy. URTI, upper respiratory tract infection.
N = 448 in SLIT group.
N = 224 in Placebo group
Table 5.
Severe adverse events and its relation to the treatment.
| Case No. | Group | Adverse events | Relation to the treatment |
|---|---|---|---|
| 1 | Placebo | Tibial fracture because of accident | Not treatment-related |
| 2 | SLIT | Eczema requiring hospitalization | Probably not treatment-relateda |
| 3 | SLIT | Bronchopneumonia | Probably not treatment-relatedb |
| 4 | SLIT | Forearm fracture because of accident | Not treatment-related |
| 5 | Placebo | Incomplete intestinal obstruction | Not treatment-related |
| 6 | Placebo | Ureteral calculus | Not treatment-related |
| 7 | Placebo | Benign paroxysmal positional vertigo | Not treatment-related |
| 8 | Placebo | Lumbar disc herniation | Not treatment-related |
| 9 | SLIT | Anterior cruciate ligament rupture | Not treatment-related |
SLIT, sublingual immunotherapy.
The patient had a medical history of continuous eczema since October 2016, which is six months before initiation of the treatment.
Bronchopneumonia was diagnosed on the last day previous to the last visit
Discussion
Artemisia annua is generally considered as the main aeroallergen responsible for SAR during late summer and autumn in Northern China. Rigorous clinical trials have confirmed that AIT can significantly relieve AR symptoms and reduce the use of medications, not only during but also after discontinuation of AIT.25, 26, 27 Despite the widespread and adverse socioeconomic impact of Artemisia allergy in China, not much attention has been paid to date to either research focused on AIT with Artemisia annua allergen or the development of commercially available allergen extracts for Artemisia annua-AIT. To our knowledge, this is the first confirmatory randomized, double-blinded, placebo-controlled, multicenter, phase 3 clinical trial to investigate the clinical efficacy of SLIT with a liquid preparation of Artemisia annua extract in a Chinese adult population with Artemisia annua-associated SAR. Our study demonstrated that Artemisia annua SLIT was a safe and significantly efficacious therapy; as indicated by elicitation of a significantly greater reduction in combined scores of symptoms and the rescue medication compared to placebo SLIT, in Artemisia annua-sensitized individuals during the pollen season. Indeed, our findings has further indicated that Artemisia annua SLIT is likely to have more pronounced effects in Artemisia annua-monosensitized individuals than in polysensitized individuals. When applied in future clinical practice of SLIT, Artemisia annua drops is promising to greatly benefit the most prevalent SAR patients sensitized to Artemisia annua in China.
In this regard the present study demonstrated that the mean CSMRS was reduced by 22.3% in the FAS population and by 23.6% in PPS population, after Artemisia annua SLIT, relative to placebo SLIT in peak pollen period. These results meet the clinically relevant efficacy criteria established by World Allergy Organization criteria,28 a difference of at least 20% between active and placebo groups. In this regard, our data for dTNSS in the PPS population have also indicated that patients receiving Artemisia annua SLIT had greater and highly statistically significant reductions of greater than 20% compared with placebo during peak pollen period. Furthermore, these results also compared favorably with a recent Cochrane meta-analysis, which compared efficacy of different pharmacologic agents for allergic rhinitis29 and demonstrated that compared with placebo, the effect sizes for leukotriene pathway modifiers, antihistamines, and intranasal corticosteroids were, respectively, 5%, 7%, and 18%.
Several clinical trials of SLIT have reported significant improvements in the clinical endpoints for active intervention compared to placebo. Our results are similar to the reduction scores generally observed in SLIT clinical trials of other allergens for treatment of AR.20,21,30,31 The improvements of CSMRS and TNSS were statistically and clinically significant. The mean values for differences in dRMS in peak pollen period observed in PPS population in the current study were similar to those observed in the North American timothy grass AIT study (26%).20 However, the improvements of RMS were statistically significant but may not be clinically significant, because 95% CIs of adjusted mean difference included values of uncertain clinical significance. Both rescue medication use and symptoms in the Artemisia annua SLIT group were significantly reduced in seven centers in statistics as indicated by CSMRS and RMS decrease. In other centers, although there was similar use of rescue medication between the Artemisia annua SLIT group and placebo SLIT group, significant improvement of CSMRS was probably achieved because of remarkable alleviation of symptoms in the Artemisia annua SLIT group. Similarly, Blaiss and colleagues21 have reported a 22% improvement in the median daily symptom score in grass pollen-induced ARC patients following timothy grass AIT versus placebo AIT, which is comparable to a 22.3% improvement in the mean dTNSS observed in the present study. Moreover, our finding that Artemisia annua SLIT was more effective in Artemisia annua-monosensitized patients than in polysensitized patients is also in accordance with the recommendations of the World Allergy Organization (WAO) taskforce, which has been suggested that exposure to confounding allergen/s during the relevant allergen season is likely to be a critical factor that reduces the statistical power to detect meaningful differences in clinical AIT trials.32
The optimal stage to begin AIT treatment is of paramount importance for clinical outcome. In accordance with other RCT clinical trials,18,20,21,31,33,34 AIT treatment in the present study was also started approximate 4 months in advance of the estimated pollen season. Although both Durham and colleagues30 and Dahl and colleagues34 have demonstrated efficacy of SLIT with a particular preparation of grass allergen tablets in two separate randomized multicentre trials in SAR patients, these studies demonstrated that starting SLIT only 8 weeks before the PS, resulted in a much smaller treatment effect over placebo (16% reduction in symptoms),30 compared to starting SLIT 16 weeks before the pollen season (30% reduction in symptoms).34
Apart from satisfactory clinical efficacy, our results also revealed a favorable safety profile for Artemisia annua SLIT. Although 65.8% patients in the SLIT group experienced drug-related AEs, these AEs were early onset, transient, and self-limiting; with no serious AEs. The rate and severity of AEs observed in this study in this study are in accordance with those reported in other SLIT studies. One recent study investigating the long-term efficacy of Japanese cedar pollen SLIT in AR patients sensitized to Japanese cedar pollen has demonstrated a cumulative AEs incidence rate of 93.0% within 8 weeks of treatment,35 which was comparable to the 91.5% AEs rate observed in the present clinical trial. The percentage of subjects who reported drug-related AEs in the present was 65.8% in Artemisia annua SLIT group, which was lower than the 84.9% of subjects experiencing AEs in the 5-grass-pollen SLIT study in Europe,18 but higher than the 12% of subjects experiencing AEs to ragweed sublingual-liquid immunotherapy trial in North America.19 It is possible that the differences in drug-related AEs in these studies may be a consequence of differences in both the types and potencies of the allergens employed for SLIT.
The current study, however, is limited in some ways. Firstly, the duration of treatment and the follow-up period were relatively short compared to many other studies investigating the long-term effects of AIT in patients with allergic disease. Although Lou and colleagues36 have reported that Artemisia annua-SLIT significantly reduced nasal symptoms in Artemisia annua-sensitized patients and that the improvement in symptoms was sustained throughout to the next year, this study was conducted in a relatively small number of patients from a single center. Thus, further long-term immunotherapy studies are needed to determine the optimal duration of treatment regimens and evaluate the carry-over effect. Secondly, immunological biomarkers of immunotherapy, such as specific allergen antibody levels (IgE and immunoglobulin G4), were not supervised throughout the treatment. Thus, further investigations to determine the effect of Artemisia-specific SLIT on the immune system are also warranted.
Conclusion
In conclusion, this multicentre, randomized, double-blind, placebo-controlled phase 3 clinical trial demonstrated that the standardized liquid drop preparation containing Artemisia annua allergen extracts employed for SLIT was well tolerated and effective in reducing the symptoms of allergic rhinitis caused by Artemisia annua pollen in a Chinese adult population. When applied in future SLIT, the registered Artemisia annua drop will provide great potential to benefit the huge amount of SAR patients sensitized to Artemisia annua in China. Although this preparation indicated first-season efficacy when administered as pre-seasonal and co-seasonal specific immunotherapy, these findings need to be confirmed over the long-term. Further studies are also needed to identify the immunologic mechanisms involved, which might point the way forward in better management of AR, as well prevention of its progression to asthma in susceptible individuals.
Abbreviations
- AIT
allergen specific immunotherapy
- ANCOVA
Analysis of covariance
- AR
allergic rhinitis
- AE
adverse event
- CSMRS
combined scores of medication and rhinoconjunctivitis symptoms
- CI
confidence interval
- FAS
full analysis set
- sIgE
specific immunoglobulin E
- PPS
per protocol Set
- (d)RMS
(daily) rescue medication score
- SS
safety set
- SAR
seasonal allergic rhinitis
- SCIT
subcutaneous immunotherapy
- SLIT
sublingual immunotherapy
- (d)TNSS
(daily) total nasal symptom score
Consent for publication
All authors agreed to the publication of this work.
Availability of data and materials
The datasets generated during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the corresponding review board in each center, and written informed consent was obtained from each subject prior to entry into the study.
Funding
This work was supported by grants from the national natural science foundation of China (81630023, 81420108009, 81970850, 81870698, 81400444 and 81470678), China, National Key R & D Program of China (2018YFC0116800), China, Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2019-I2M-5-022), China, the Program for Changjiang Scholars and Innovative Research Team (IRT13082), China, Beijing Municipal Administration of Hospitals' Mission Plan (SML20150203), China, Beijing Municipal Administration of Hospitals’ Youth Program (QML20150202), China, Beijing Natural Science Foundation (Z141107002514122), China, Capital Health Development Foundation (2016-1-2052), China, and Beijing Advanced Innovation Center for Food Nutrition and Human Health, Beijing Technology and Business University (BTBU) (20181045), China, the Public Welfare Development and Reform Pilot Project (2019-10), China.
Authors’ contributions
All the authors contributed significantly to the study: HL, XW, QW, CZ, ZX, QZ, JM, SZ, HZ, RM, HZ, HL, WX collected the data. HL and XW performed statistical analyses. HL, CW and LZ wrote the manuscript. CW and LZ designed and supervised the study.
Declaration of competing interest
All authors declare no financial or commercial conflicts of interest.
Acknowledgments
We thank Dr. Hui Chen for her assistance in statistical analysis.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2020.100458.
Contributor Information
Chengshuo Wang, Email: wangcs830@126.com.
Luo Zhang, Email: dr.luozhang@139.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Brozek J.L., Bousquet J., Agache I. Allergic rhinitis and its impact on asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017;140:950–958. doi: 10.1016/j.jaci.2017.03.050. [DOI] [PubMed] [Google Scholar]
- 2.Wang X.D., Zheng M., Lou H.F. An increased prevalence of self-reported allergic rhinitis in major Chinese cities from 2005 to 2011. Allergy. 2016;71:1170–1180. doi: 10.1111/all.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X.Y., Ma T.T., Wang X.Y. Prevalence of pollen-induced allergic rhinitis with high pollen exposure in grasslands of northern China. Allergy. 2018;73:1232–1243. doi: 10.1111/all.13388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang R., Sun J.L., Yin J., Li Z. Artemisia allergy research in China. BioMed Res Int. 2015;2015:179426. doi: 10.1155/2015/179426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao Z., Fu W.Y., Sun Y. Artemisia pollen allergy in China: component-resolved diagnosis reveals allergic asthma patients have significant multiple allergen sensitization. Allergy. 2019;74:284–293. doi: 10.1111/all.13597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bousquet J., Van Cauwenberge P., Khaltaev N., Aria Workshop G., World Health O. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108:S147–S334. doi: 10.1067/mai.2001.118891. [DOI] [PubMed] [Google Scholar]
- 7.Bousquet J., Lockey R., Malling H.J. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102:558–562. doi: 10.1016/s0091-6749(98)70271-4. [DOI] [PubMed] [Google Scholar]
- 8.Noon L., Cantar B.O. Prophylactic inoculations against hay fever. Lancet. 1911;1:1572–1573. [Google Scholar]
- 9.Freeman J. Further observations on the treatment of hay fever by hypodermic inoculations of pollen vaccine. Historical document. Ann Allergy. 1960;18:427–434. [PubMed] [Google Scholar]
- 10.Canonica G.W., Bachert C., Hellings P. Allergen immunotherapy (AIT): a prototype of precision medicine. World Allergy Organ J. 2015;8:31. doi: 10.1186/s40413-015-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson H., Cartier S., Allen-Ramey F., Lawton S., Calderon M.A. Network meta-analysis shows commercialized subcutaneous and sublingual grass products have comparable efficacy. J Allergy Clin Immunol Pract. 2015;3:256–266 e253. doi: 10.1016/j.jaip.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Durham S.R., Penagos M. Sublingual or subcutaneous immunotherapy for allergic rhinitis? J Allergy Clin Immunol. 2016;137:339–349 e310. doi: 10.1016/j.jaci.2015.12.1298. [DOI] [PubMed] [Google Scholar]
- 13.Moingeon P., Cox L. Relevance of a 5-grass sublingual tablet for immunotherapy of patients with grass pollen allergy in North America. Expet Rev Clin Immunol. 2016;12:617–623. doi: 10.1586/1744666X.2016.1147349. [DOI] [PubMed] [Google Scholar]
- 14.Maloney J., Durham S., Skoner D. Safety of sublingual immunotherapy Timothy grass tablet in subjects with allergic rhinitis with or without conjunctivitis and history of asthma. Allergy. 2015;70:302–309. doi: 10.1111/all.12560. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y., Zhou W., Chen A. Efficacy of sublingual immunotherapy for cedar pollinosis: a systematic review and meta-analysis. Ann Allergy Asthma Immunol. 2016;117:348–353. doi: 10.1016/j.anai.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 16.Feng B., Wu J., Chen B. Efficacy and safety of sublingual immunotherapy for allergic rhinitis in pediatric patients: a meta-analysis of randomized controlled trials. Am J Rhinol Allergy. 2017;31:27–35. doi: 10.2500/ajra.2017.31.4382. [DOI] [PubMed] [Google Scholar]
- 17.Nurmatov U., Dhami S., Arasi S. Allergen immunotherapy for allergic rhinoconjunctivitis: a systematic overview of systematic reviews. Clin Transl Allergy. 2017;7:24. doi: 10.1186/s13601-017-0159-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wahn U., Tabar A., Kuna P. Efficacy and safety of 5-grass-pollen sublingual immunotherapy tablets in pediatric allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2009;123:160–166 e163. doi: 10.1016/j.jaci.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Creticos P.S., Esch R.E., Couroux P. Randomized, double-blind, placebo-controlled trial of standardized ragweed sublingual-liquid immunotherapy for allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2014;133:751–758. doi: 10.1016/j.jaci.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 20.Nelson H.S., Nolte H., Creticos P., Maloney J., Wu J., Bernstein D.I. Efficacy and safety of timothy grass allergy immunotherapy tablet treatment in North American adults. J Allergy Clin Immunol. 2011;127:72–80. doi: 10.1016/j.jaci.2010.11.035. 80 e71-72. [DOI] [PubMed] [Google Scholar]
- 21.Blaiss M., Maloney J., Nolte H., Gawchik S., Yao R., Skoner D.P. Efficacy and safety of timothy grass allergy immunotherapy tablets in North American children and adolescents. J Allergy Clin Immunol. 2011;127:64–71. doi: 10.1016/j.jaci.2010.11.034. 71 e61-64. [DOI] [PubMed] [Google Scholar]
- 22.Pfaar O., Bastl K., Berger U. Defining pollen exposure times for clinical trials of allergen immunotherapy for pollen-induced rhinoconjunctivitis – an EAACI position paper. Allergy. 2017;72:713–722. doi: 10.1111/all.13092. [DOI] [PubMed] [Google Scholar]
- 23.Pfaar O., Demoly P., Gerth van Wijk R. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI Position Paper. Allergy. 2014;69:854–867. doi: 10.1111/all.12383. [DOI] [PubMed] [Google Scholar]
- 24.Norman G.R., Sloan J.A., Wyrwich K.W. Interpretation of changes in health - related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 25.Bergmann K.C., Demoly P., Worm M. Efficacy and safety of sublingual tablets of house dust mite allergen extracts in adults with allergic rhinitis. J Allergy Clin Immunol. 2014;133:1608–1614 e1606. doi: 10.1016/j.jaci.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 26.Eng P.A., Borer-Reinhold M., Heijnen I.A., Gnehm H.P. Twelve-year follow-up after discontinuation of preseasonal grass pollen immunotherapy in childhood. Allergy. 2006;61:198–201. doi: 10.1111/j.1398-9995.2006.01011.x. [DOI] [PubMed] [Google Scholar]
- 27.Jacobsen L., Niggemann B., Dreborg S. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–948. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 28.Canonica G.W., Bousquet J., Casale T. Sub-lingual immunotherapy: world allergy organization position paper 2009. World Allergy Organ J. 2009;2:233–281. doi: 10.1097/WOX.0b013e3181c6c379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson A.M., O'Byrne P.M., Parameswaran K. Leukotriene receptor antagonists for allergic rhinitis: a systematic review and meta-analysis. Am J Med. 2004;116:338–344. doi: 10.1016/j.amjmed.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 30.Durham S.R., Yang W.H., Pedersen M.R., Johansen N., Rak S. Sublingual immunotherapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117:802–809. doi: 10.1016/j.jaci.2005.12.1358. [DOI] [PubMed] [Google Scholar]
- 31.Okamoto Y., Okubo K., Yonekura S. Efficacy and safety of sublingual immunotherapy for two seasons in patients with Japanese cedar pollinosis. Int Arch Allergy Immunol. 2015;166:177–188. doi: 10.1159/000381059. [DOI] [PubMed] [Google Scholar]
- 32.Canonica G.W., Baena-Cagnani C.E., Bousquet J. Recommendations for standardization of clinical trials with Allergen Specific Immunotherapy for respiratory allergy. A statement of a World Allergy Organization (WAO) taskforce. Allergy. 2007;62:317–324. doi: 10.1111/j.1398-9995.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- 33.Didier A., Malling H.J., Worm M. Optimal dose, efficacy, and safety of once-daily sublingual immunotherapy with a 5-grass pollen tablet for seasonal allergic rhinitis. J Allergy Clin Immunol. 2007;120:1338–1345. doi: 10.1016/j.jaci.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 34.Dahl R., Kapp A., Colombo G. Efficacy and safety of sublingual immunotherapy with grass allergen tablets for seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;118:434–440. doi: 10.1016/j.jaci.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Gotoh M., Yonekura S., Imai T. Long-term efficacy and dose-finding trial of Japanese cedar pollen sublingual immunotherapy tablet. J Allergy Clin Immunol Pract. 2019;7:1287–1297 e1288. doi: 10.1016/j.jaip.2018.11.044. [DOI] [PubMed] [Google Scholar]
- 36.Lou H., Huang Y., Ouyang Y. Artemisia annua-sublingual immunotherapy for seasonal allergic rhinitis: a randomized controlled trial. Allergy. 2020;75:2026–2036. doi: 10.1111/all.14218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.