Abstract
This study describes changes in blood donor demographics and seroreactivity after testing of blood donations for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies began and was publicized in the US in mid-June 2020.
The coronavirus disease 2019 (COVID-19) pandemic has challenged the adequacy of the blood supply. To attract new donors and support the collection of convalescent plasma,1 many blood collection organizations have implemented and publicized routine testing of donations for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies. We examined whether testing of donations for SARS-CoV-2 antibodies was associated with changes in donor characteristics and reactivity of donated blood.
Methods
The American Red Cross collects about 40% of blood in the US from 44 states, and initiated testing of all donations on June 15, 2020, using the Ortho VITROS anti–SARS-CoV-2 S1 Total Ig assay detecting total immunoglobulin. Sensitivity is 90% and specificity is 100%, as reported using a limited data set.2 Each donation sample is tested once; results are reported to donors electronically. Samples with signal levels above the manufacturer-defined cutoff are defined as reactive. Routine donor information is collected and identified only by code. The change in first-time vs repeat donors was compared in the first 2 weeks before testing was initiated (June 1-14, 2020) vs after testing (June 15–August 23, 2020). Temporal changes in seroreactivity rates overall and by US Census regions were evaluated over the study period (June 15–August 23) by linear regression. Bivariable analyses were conducted to compare the proportions of donor characteristics with χ2 tests. Multivariable logistic regression compared reactive rates among subgroups, adjusting for sex, age group, race/ethnicity, and region, including interactions.
We conducted all analyses using SAS Software (version 9.4; SAS Institute Inc). Two-sided P values less than .05 defined statistical significance; data are presented with 95% CIs. The institutional review board of the American Red Cross considered the study exempt as human subjects research; each donor is provided a research study information sheet as part of the donation consent process.
Results
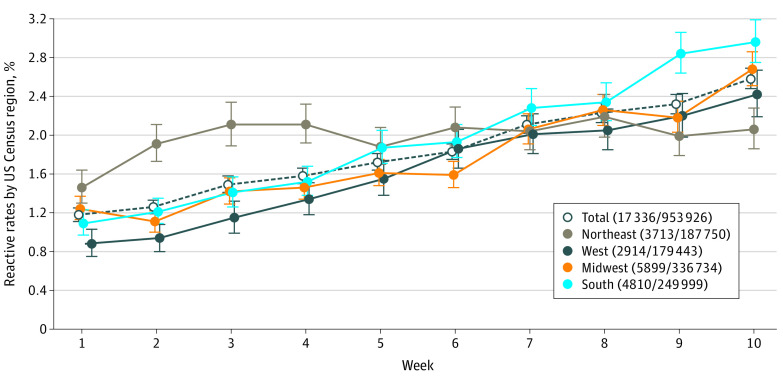
Of 953 926 donations tested, 17 336 (1.82% [95% CI, 1.79%-1.84%]) were reactive; 4786 (28%) were from first-time donors and 12 550 (72%) from repeat donors for anti–SARS-CoV-2 rates of 2.99% (95% CI, 2.90%-3.07%) among first-time donors and 1.58% (95% CI, 1.55%-1.61%) among repeat donors (P < .001) (Table). In the 2 weeks prior to initiation of testing, 11% of donors were first-time donors compared with 17% (P < .001) after that time. By multivariable analysis, the odds of reactivity were higher in donors aged 18 to 24 years compared with donors who were aged 55 years and older (odds ratio [OR], 2.43 [95% CI, 1.94-3.04]; P < .001), African American (OR, 2.58 [95% CI, 1.71-3.88]; P < .001), and Hispanic (OR, 2.31 [95% CI, 1.77-3.00]; P < .001) compared with White donors, and donors from the Northeast compared with the West (OR, 1.83 [95% CI, 1.57-2.12]; P < .001). Reactive rates increased over the study period, from 1.18% (95% CI, 1.11%-1.25%) to 2.58% (95% CI, 2.48%-2.69%; P < .001). Rates increased significantly in all Census regions except the Northeast (1.46% [95% CI, 1.3%-1.64%] to 2.06% [95% CI, 1.86%-2.28%]; P = .09), with the greatest increases in the South (1.09% [95% CI, 0.97%-1.23%] to 2.96% [95% CI, 2.75%-3.19%]; P < .001) and West (0.88% [95% CI, 0.75%-1.03%] to 2.42% [95% CI, 2.19%-2.67%]; P < .001) (Figure).
Table. Analysis of Donor Population Characteristics Associated With American Red Cross Blood Donations and SARS-CoV-2 Antibody–Reactive Donors.
| Variable | Total, No. (%) | Reactive donations, No. (% of total) | Bivariable analysis, OR (95% CI)a | Multivariable analysisa | |
|---|---|---|---|---|---|
| OR (95% CI) | P value | ||||
| All | 953 926 (100) | 17 336 (1.82) | |||
| Donation status | |||||
| First-time | 160 328 (16.81) | 4786 (2.99) | 1.92 (1.85-1.98) | ||
| Repeat | 793 598 (83.19) | 12 550 (1.58) | 1 [Reference] | ||
| Sex | |||||
| Female | 524 607 (54.99) | 9392 (1.79) | 1 [Reference] | 1 [Reference] | |
| Male | 429 319 (45.01) | 7944 (1.85) | 1.03 (1.00-1.07) | 0.97 (0.87-1.08) | .54 |
| Age, y | |||||
| 16-17 | 8375 (0.88) | 188 (2.44) | 1.75 (1.51-2.02) | 1.79 (1.03-3.12) | .04 |
| 18-24 | 51 763 (5.43) | 2003 (3.87) | 3.06 (2.91-3.22) | 2.43 (1.94-3.04) | <.001 |
| 25-39 | 204 407 (21.43) | 4684 (2.29) | 1.78 (1.71-1.85) | 1.98 (1.69-2.31) | <.001 |
| 40-54 | 262 912 (27.56) | 4919 (1.87) | 1.45 (1.39-1.51) | 1.35 (1.15-1.58) | <.001 |
| ≥55 | 426 469 (44.71) | 5542 (1.30) | 1 [Reference] | 1 [Reference] | |
| Race/ethnicityb | |||||
| African American | 19 185 (2.01) | 788 (4.11) | 2.56 (2.38-2.75) | 2.58 (1.71-3.88) | <.001 |
| Asian | 20 639 (2.16) | 471 (2.28) | 1.39 (1.27-1.53) | 1.91 (1.33-2.75) | <.001 |
| White | 861 863 (90.35) | 14 221 (1.65) | 1 [Reference] | 1 [Reference] | |
| Hispanic | 31 769 (3.33) | 1381 (4.35) | 2.71 (2.56-2.86) | 2.31 (1.77-3.00) | <.001 |
| Multiracial/ethnicc | 9996 (1.06) | 196 (1.96) | 1.19 (1.03-1.37) | 2.00 (1.2-3.34) | .01 |
| Native American | 2574 (0.27) | 55 (2.14) | 1.30 (1.00-1.70) | 1.84 (0.72-4.71) | .21 |
| Otherd | 4601 (0.48) | 130 (2.83) | 1.73 (1.46-2.07) | 0.79 (0.35-1.79) | .58 |
| Prefer not to answer | 3299 (0.35) | 94 (2.85) | 1.75 (1.42-2.15) | 1.14 (0.51-2.54) | .76 |
| US Census region | |||||
| Midwest | 336 734 (35.30) | 5899 (1.75) | 1.08 (1.03-1.13) | 1.30 (1.13-1.51) | <.001 |
| Northeast | 187 750 (19.68) | 3713 (1.98) | 1.22 (1.16-1.28) | 1.83 (1.57-2.12) | <.001 |
| South | 249 999 (26.21) | 4810 (1.92) | 1.1 9 (1.14-1.25) | 1.21 (1.03-1.41) | .02 |
| West | 179 443 (18.81) | 2914 (1.62) | 1 [Reference] | 1 [Reference] | |
Abbreviations: OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
By bivariable analyses, rates for all variables were significantly different at P < .001 except sex at P = .03. By multivariable analysis, not all ORs were statistically significant. Four statistically significant interactions occurred between age and region (P < .001), sex and region (P = .01), race/ethnicity and region (P = .01), and age and race/ethnicity (P = .04).
Race and ethnicity are self-determined and are routinely collected at donation.
Donor provides more than 1 of the named categories.
Donor provides a category that is not among those named.
Figure. Frequency of Reactivity in Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibody Testing by Week, June 15 to August 23, 2020, in the 4 US Census Regions.
Each region is represented by a different line; the dashed line represents the total percentage seroreactivity. Each point is the mean of the overall data for that week (for each of the 10 weeks), with error bars representing the associated 95% CIs. The text provides the total number of anti–SARS-CoV-2 reactives and the key also provides the total number reactive/total number of donations by US Census region, including the number of donations tested.
Discussion
This study found that, after the introduction of antibody testing, the proportion of first-time donors increased, and donations from younger and racial and ethnic minority donors were more likely to be reactive. In addition, reactivity rates increased with time. This increase may be due to donors with higher rates of prior exposure donating to obtain antibody test results, particularly first-time donors, but may also reflect increased exposure in the general population or increased recognition of the need for convalescent plasma.3
The distribution of anti–SARS-CoV-2 reactive test results was similar to results reported for patients with clinically diagnosed COVID-19, with higher rates among African American and Hispanic donors and those from the Northeast.4,5 However, blood donors are not representative of the overall population. Additionally, first-time donors differ from repeat donors largely because repeat donors have already been screened for transfusion-transmissible infections and other health conditions. Blood donors reactive for anti–SARS-CoV-2 are not deferred from future donation because SARS-CoV-2, to date, is not transmissible by transfusion.6
The main limitations include that testing results represent cross-sectional findings over a relatively short period, and American Red Cross collection areas in the US underrepresent areas such as New York City, south Florida, and some Western states. Also, reactive results were not confirmed, and thus the data may overrepresent blood donor seropositivity.
Section Editor: Jody W. Zylke, MD, Deputy Editor.
References
- 1.US Food and Drug Administration Investigational COVID-19 convalescent plasma: guidance for industry. Published April 2020. Updated May 2020. Accessed August 10, 2020. https://www.fda.gov/media/136798/download
- 2.US Food and Drug Administration Instructions for use: Vitros Immunodiagnostics Anti-SARS-Cov-2 IgG. Accessed August 10, 2020. https://www.fda.gov/media/137363/download
- 3.Roback JD, Guarner J. Convalescent plasma to treat COVID-19: possibilities and challenges. JAMA. 2020;323:1561-1562. doi: 10.1001/jama.2020.4940 [DOI] [PubMed] [Google Scholar]
- 4.CDC COVID-19 Response Team Geographic differences in COVID-19 cases, deaths, and incidence: United States, February 12-April 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):465-471. doi: 10.15585/mmwr.mm6915e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tenforde MW, Billig Rose E, Lindsell CJ, et al. ; CDC COVID-19 Response Team . Characteristics of adult outpatients and inpatients with COVID-19: 11 academic medical centers, United States, March-May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(26):841-846. doi: 10.15585/mmwr.mm6926e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz LM. Is SARS-CoV-2 transfusion transmitted? Transfusion. 2020;60(6):1111-1114. doi: 10.1111/trf.15831 [DOI] [PMC free article] [PubMed] [Google Scholar]