Abstract
This study examines cost sharing for novel second-line diabetes treatment agents under Medicare Part D.
Diabetes affects 1 in 3 Medicare beneficiaries.1 Treatment guidelines recommend that most patients start treatment with metformin followed by second-line drugs until glycemic goals are reached. For years, these second-line drugs were predominantly inexpensive generic drugs, such as sulfonylureas and thiazolidinediones (TZDs).2 Recent guidelines, however, endorse costly, predominantly brand-name drugs, such as sodium-glucose cotransporter-2 inhibitors (SGLT2is) and glucagon-like peptide-1 receptor agonists (GLP-1RAs), as preferred second-line medications for patients with established or increased risk for atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease.3 Some SGLT2is and GLP-1RAs offer cardiovascular benefits, and certain SGLT2is are protective against renal disease.3 A third novel class, dipeptidyl peptidase-4 inhibitors (DPP-4is), is also increasingly used after metformin.2 Because the cost of medications affects adherence,4 we examined cost sharing for these novel diabetes agents under Medicare Part D, which covers 45 million people.5
Methods
We reviewed 6 drug classes using first quarter 2019 Medicare formulary and pricing files, which contain drug prices and cost sharing for all Part D plans, including stand-alone Part D plans and Medicare Advantage plans. We calculated the average monthly price and out-of-pocket cost for each drug across all plans nationwide. For each class, we also calculated the average cost for a patient to be treated with the least-expensive drug covered by their plan along with metformin, atorvastatin, lisinopril, and no other drugs. We projected annual out-of-pocket costs for each regimen according to the 4 phases of a 2019 standard Part D plan, including a $415 deductible; an initial coverage phase until total drug costs reach $3820; a coverage gap in which beneficiaries pay 25% of brand-name drug costs until out-of-pocket spending reaches $5100; and a catastrophic coverage phase, with 5% cost-sharing for the remainder of the year (with a minimum copay of $3.40 for generics and $8.50 for brands).5 We did not include premiums in the projections. Analyses used SAS, version 9.4 (SAS Institute). An institutional review board waiver was provided by the University of Hawaii Office of Research Compliance. Patient consent was not needed because deidentified national datasets purchased from the Centers for Medicare & Medicaid Services were used.
Results
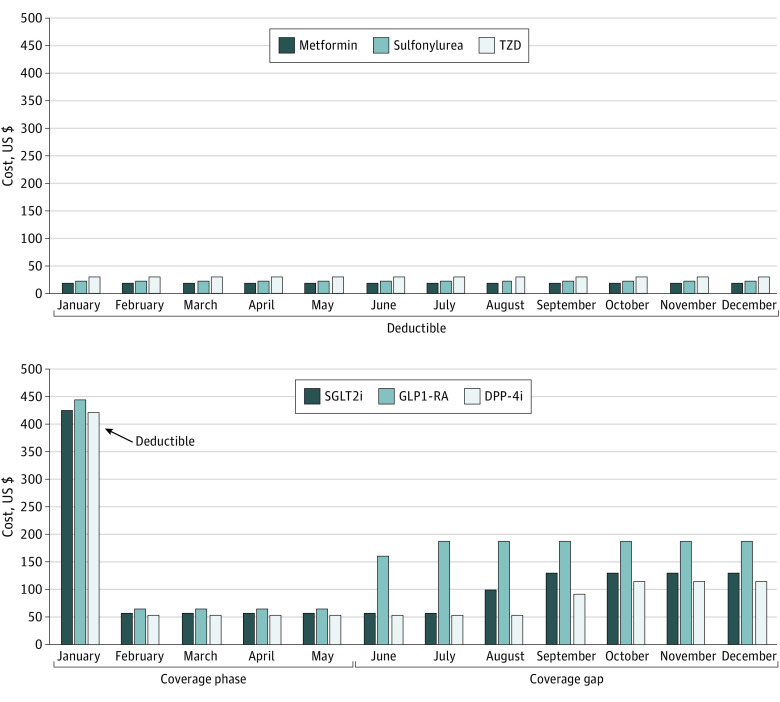
Across 3323 Part D plans nationwide, commonly covered GLP-1RAs, SGLT2is, and DPP-4is had monthly list prices between $434 and $935 (Table), compared with $3 to $11 for metformin, sulfonylureas, and TZDs. Projected copayments for novel drug regimens varied substantially by month, from $53 to $65 during the initial coverage phase to $116 to $186 during the coverage gap (Figure).
Table. Coverage and Out-of-Pocket Costs for Diabetes Drugs Under Medicare Part D in 2019.
| Drug classa | Plan coverage, % | Total cost, $ (SD) | Out-of-pocket cost, $ | ||
|---|---|---|---|---|---|
| 30 db | Annual | 30 d (SD) | Projected annual | ||
| Metformin | 100 | 5 (3) | 63 (35) | 2 (2) | 63c |
| Traditional second-line drugs | |||||
| Sulfonylureas | |||||
| Glipizide | 100 | 3 (1) | 31 (17) | 2 (2) | 31 |
| Glimepiride | 100 | 7 (3) | 80 (38) | 2 (2) | 80 |
| Glyburide | 29 | 8 (4) | 101 (52) | 6 (7) | 101 |
| TZDsa | |||||
| Pioglitazone | 100 | 11 (9) | 136 (113) | 4 (4) | 136 |
| Novel second-line drugs | |||||
| SGLT2is | |||||
| Empagliflozin | 99 | 497 (41) | 5967 (486) | 51 (24) | 1298 |
| Dapagliflozin | 69 | 498 (47) | 5971 (565) | 87 (71) | 1615 |
| Canagliflozin | 51 | 510 (18) | 6118 (214) | 86 (75) | 1565 |
| GLP-1RAs | |||||
| Exenatide weekly | 95 | 723 (54) | 8681 (646) | 103 (108) | 2102 |
| Exenatide | 77 | 735 (47) | 8822 (562) | 214 (127) | 2520 |
| Dulaglutide | 90 | 742 (49) | 8903 (582) | 96 (97) | 2108 |
| Semaglutide | 73 | 808 (39) | 9690 (467) | 68 (53) | 2071 |
| Liraglutide | 89 | 935 (52) | 11 225 (627) | 68 (60) | 2230 |
| DPP-4is | |||||
| Saxagliptin | 39 | 434 (14) | 5202 (165) | 112 (61) | 1639 |
| Linagliptin | 90 | 441 (38) | 5288 (452) | 58 (40) | 1250 |
| Sitagliptin | 94 | 455 (38) | 5461 (454) | 50 (21) | 1236 |
| Drug regimens of metformin, ACEi, and statin, plus | |||||
| Traditional second-line drugs | |||||
| Sulfonylureas | 100 | 21 | 250 | 7 | 250c |
| TZDs | 100 | 30 | 355 | 9 | 355c |
| Novel second-line drugs | |||||
| SGLT2is | 100 | 514 | 6164 | 56 | 1381 |
| GLP-1RAs | 100 | 735 | 8820 | 65 | 1981 |
| DPP-4is | 100 | 455 | 5454 | 53 | 1231 |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; DPP-4is, dipeptidyl peptidase-4 inhibitors; GLP-1RAs, glucagon-like peptide-1 receptor agonists; SGLT2is, sodium-glucose cotransporter-2 inhibitors; TZDs, thiazolidinediones.
Extended release formulations and drugs covered by less than 20% of plans are not presented: rosiglitazone (19% of plans), alogliptin (14%), ertugliflozin (7%), and lixisenatide (1%).
30-Day costs are reported during the initial coverage phase.
For patients taking metformin, sulfonylureas, or TZDs, alone or in combination with lisinopril and atorvastatin, projected annual costs were low enough to fall entirely within the $415 deductible phase, in which patients pay 100% of drug costs.
Figure. Monthly Out-of-Pocket Costs for Traditional and Novel Drug Classes for Diabetes.
TZDs indicates thiazolidinediones; SGLT2is, sodium-glucose cotransporter 2 inhibitors; DPP-4is, dipeptidyl peptidase-4 inhibitors; and GLP-1RAs, glucagon-like peptide-1 receptor agonists. For each drug class, monthly copayments were projected using the least expensive option covered by a patient’s plan, as part of a guideline-directed diabetes regimen with metformin, lisinopril, and atorvastatin. A patient receiving a novel agent (SGLT2i, GLP1-RA, or DPP-4i) would meet their $415 deductible in January, then pay $53 to $65 in the initial coverage period and $116 to $186 during the coverage gap. In contrast, a patient on a traditional regimen with sulfonylurea or TZD would not meet their $415 deductible before the end of the year.
Total annual costs for common novel agents were $5202 to $11 225, compared with $31 to $136 for traditional drugs. Projected annual out-of-pocket costs for a novel drug regimen were $1231 to $1981, compared with $250 to $355 for traditional regimens.
Discussion
Novel diabetes drugs are expensive, increasing costs for both Medicare Part D and its beneficiaries.2 Under Medicare Part D in 2019, we found that list prices for novel diabetes drugs (SGLT2is, GLP-1RAs, and DPP-4is) approached 40 to 360 times the cost of commonly covered sulfonylureas or TZDs, and exceeded $5000 annually. Switching patients to these classes of drugs could increase their yearly out-of-pocket costs by 3-fold to 8-fold, from less than $360 to $1200 or up to $2000. For SGLT2is and GLP-1RAs, this increase of out-of-pocket costs could effectively divide Medicare beneficiaries with diabetes into 2 groups: those who can afford guideline-directed therapies with cardiovascular or kidney benefit and those obligated to use lower-priced alternatives for cost reasons. Because higher copayments lead to poorer adherence and worse health outcomes,4,6 clinicians should discuss affordability with patients when changing diabetes regimens.
Limitations of this study include projecting annual out-of-pocket costs based on drug benefit design, not actual patient claims. We projected costs for patients using specific diabetes regimens and assuming use of no other medications. Analyses did not include premiums, which also contribute to beneficiaries’ overall out-of-pocket costs.
We found patients’ out-of-pocket costs to be unevenly distributed throughout the year, exceeding $400 in January and further increasing in the coverage gap. Although recent proposals to cap out-of-pocket spending at $2000 would address Part D enrollees’ overall financial burden, benefit redesign for the program should also target consistent, predictable monthly copays, as the month with the highest copay of the year may be a barrier to continuing therapy. As out-of-pocket costs for a single diabetes drug can approach the proposed $2000 cap, broader cost containment efforts should be considered, such as allowing Medicare to negotiate prices with pharmaceutical manufacturers. Part D plans should be able to cover at least 1 effective diabetes drug per class at a reduced, fixed monthly copayment.
References
- 1.Cubanski J, Neuman T, True S, Damico A. How much does Medicare spend on insulin? Henry J Kaiser Family Foundation. April 1, 2019. Accessed March 8, 2020. https://www.kff.org/medicare/issue-brief/how-much-does-medicare-spend-on-insulin/
- 2.Sumarsono A, Everett BM, McGuire DK, et al. Trends in aggregate use and associated expenditures of antihyperglycemic therapies among US Medicare beneficiaries between 2012 and 2017. JAMA Intern Med. 2019;180(1):141-144. doi: 10.1001/jamainternmed.2019.3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110. doi: 10.2337/dc20-S009 [DOI] [PubMed] [Google Scholar]
- 4.Tseng CW, Tierney EF, Gerzoff RB, et al. Race/ethnicity and economic differences in cost-related medication underuse among insured adults with diabetes: the Translating Research Into Action for Diabetes Study. Diabetes Care. 2008;31(2):261-266. doi: 10.2337/dc07-1341 [DOI] [PubMed] [Google Scholar]
- 5.An overview of the Medicare Part D prescription drug benefit. Henry J Kaiser Family Foundation. November 13, 2019. Accessed March 1, 2020. https://www.kff.org/medicare/fact-sheet/an-overview-of-the-medicare-part-d-prescription-drug-benefit/
- 6.Mojtabai R, Olfson M. Medication costs, adherence, and health outcomes among Medicare beneficiaries. Health Aff (Millwood). 2003;22(4):220-229. doi: 10.1377/hlthaff.22.4.220 [DOI] [PubMed] [Google Scholar]