This cohort study evaluates the association between congenital cytomegalovirus and the prevalence at birth of microcephaly in the United States.
Key Points
Question
What is the association of congenital cytomegalovirus with the prevalence at birth of microcephaly in the United States?
Findings
In this population-based cohort study of 2.3 million pregnancies identified in health care data from 2000 to 2015 in the United States, the prevalence of microcephaly was 2.1 to 7.7 per 10 000 live births, depending on case definition. Congenital cytomegalovirus diagnosis was the strongest measured risk factor for microcephaly, increasing the risk by at least 7-fold.
Meaning
Congenital cytomegalovirus is an important cause of microcephaly and other newborn neurologic outcomes in the United States and warrants greater attention from public health and medical fields.
Abstract
Importance
Congenital cytomegalovirus (cCMV) has received far less clinical and public health attention as a teratogenic infection than the Zika virus epidemic. However, cCMV may be responsible for a large fraction of microcephaly cases in the United States.
Objective
To evaluate the association between cCMV and the prevalence at birth of microcephaly in the United States.
Design, Setting, and Participants
This population-based cohort study included pregnant women and their newborns identified in 2 insurance claims databases from the United States: Medicaid Analytic eXtract (January 1, 2000, to December 31, 2013) and IBM Research MarketScan, a database for employer-sponsored private health insurance (January 1, 2011, to September 30, 2015). All pregnancies that resulted in live births in women with full health benefits were included. Analysis began June 2016 and ended May 2020.
Exposures
Congenital cytomegalovirus infection documented in inpatient or outpatient newborn claims records.
Main Outcomes and Measures
The primary outcome was microcephaly at birth documented in inpatient or outpatient newborn and/or maternal claims records. Cases with chromosomal abnormalities or neural tube defects were excluded. The association between cCMV and microcephaly was estimated in the pooled cohort using prevalence ratios (PRs) and 95% CIs.
Results
In the pooled cohort of 2 338 580 pregnancies (2 075 410 pregnancies [88.7%] were among women younger than 35 years), 336 infants (0.014%) had a cCMV diagnosis. The prevalence of microcephaly among newborns with and without a cCMV diagnosis was 655 and 2.8 per 10 000 live births, respectively (PR, 232; 95% CI, 154-350). After restricting to CMV-tested newborns (572 [0.024%]) to correct for preferential testing of infants with microcephaly, the PR was 15 (95% CI, 5.2-41). However, this PR is biased if other cCMV-related outcomes (eg, hearing loss) trigger testing because cCMV prevalence in tested infants, with ([46%]) or without microcephaly (22 of 559 [3.9%]), would overestimate that in the source population. Therefore, the prevalence of cCMV in overall infants with microcephaly (22 of 669 [3.2%]) was compared with that from an external unbiased sample of US infants screened at birth (449 of 100 332 [0.45%]) to estimate a PR of 7.4 (95% CI, 4.8-11.5) as a conservative lower bound.
Conclusions and Relevance
Congenital cytomegalovirus infection increases the prevalence of microcephaly at birth by at least 7-fold. Prevention of CMV infection during pregnancy might substantially reduce the number of newborns with microcephaly and other cCMV-related outcomes in the United States.
Introduction
Microcephaly is a congenital malformation that gained public attention after high prevalence was observed in parts of South America following an outbreak of Zika virus in 2015. Maternal Zika virus infection affects fetal brain development in utero and leads to undersized heads. The Zika virus epidemic is accepted as the cause of the observed elevated prevalence at birth of microcephaly in South America.1,2,3,4,5,6
Other infections have been associated with microcephaly, including cytomegalovirus (CMV), herpes simplex virus, varicella-zoster virus, toxoplasmosis, and rubella. All of these viruses are capable of transplacental fetal infection and exhibit tropism for the brain. In the United States, congenital rubella has been eradicated, intrauterine herpes simplex virus is rare,7 and the incidences of acute primary infection in pregnancy for toxoplasmosis and varicella-zoster virus are estimated to be under 0.2 per 1000 pregnant women.8,9,10 Meanwhile, more than half of adults in the United States are infected with CMV by age 40 years, and 1% to 4% of seronegative women have a primary infection in pregnancy.11,12 Vertical transmission occurs with both primary (30%-40%) and nonprimary (1%-2%) infections.13 The mean prevalence of congenital CMV (cCMV) infection among infants in the United States is estimated to be 0.4% to 0.5%.14,15 Thus, CMV may be responsible for a large proportion of potentially preventable microcephaly cases in the United States. In addition to microcephaly, cCMV infection is associated with other newborn neurologic outcomes including neonatal seizures, chorioretinitis, and sensorineural hearing loss.16,17 Yet, cCMV has received much less attention as a teratogenic infection than Zika virus.18
Although the association between cCMV and microcephaly is well described,19,20 the absolute effect of cCMV remains difficult to assess owing to its underdiagnosis. In clinical practice, newborns are more frequently tested for CMV if they exhibit clinical findings suspicious for intrauterine infection.21 However, about 80% to 90% of newborns with cCMV are asymptomatic and may only develop symptoms such as hearing loss later in life.22 Therefore, the majority of neonatal cCMV infections go undiagnosed.23
To evaluate the association between cCMV and the prevalence at birth of microcephaly in the United States, we conducted a population-based cohort study using 2 large health care databases. We used sensitivity analyses to address bias introduced by underdiagnosis of and differential testing for cCMV.
Methods
Data Source and Study Cohort
We identified 2 cohorts of pregnant women and their newborns: 1 of publicly insured women nested in the United States, Medicaid Analytic eXtract (MAX) from January 1, 2000, to December 31, 2013, and another of commercially insured women nested in the IBM Research MarketScan (MarketScan) database from January 1, 2011, to September 30, 2015. The use of these databases for research was approved by the institutional review board at Brigham and Women’s Hospital, which waived the need for informed consent.
Data for the first cohort were abstracted from MAX for 46 states and the District of Columbia.24 The cohort included all pregnancies in women aged 12 to 55 years that resulted in live births for which Medicaid covered the health care expenses. The MAX data set contains individual-level demographic and Medicaid enrollment information, as well as all physician services and hospitalizations, their accompanying diagnoses and procedures, and all filled outpatient medication prescriptions. We excluded women who had supplemental private insurance or restricted medical benefits and those who did not have an appropriate enrollment type.24,25 Infants were required to meet the same eligibility criteria for Medicaid as their mothers until at least 90 days after birth, unless they died sooner, in which case we allowed an eligibility period of shorter duration. The last menstrual period was defined based on validated algorithms.26 We excluded pregnancies with a documented chromosomal abnormality and pregnancies with exposure to known teratogenic medications during the first trimester (ie, lithium, antineoplastic agents, retinoids, and thalidomide) under the assumption that the pathophysiologic mechanisms of malformations in these pregnancies are likely related to these known causes.
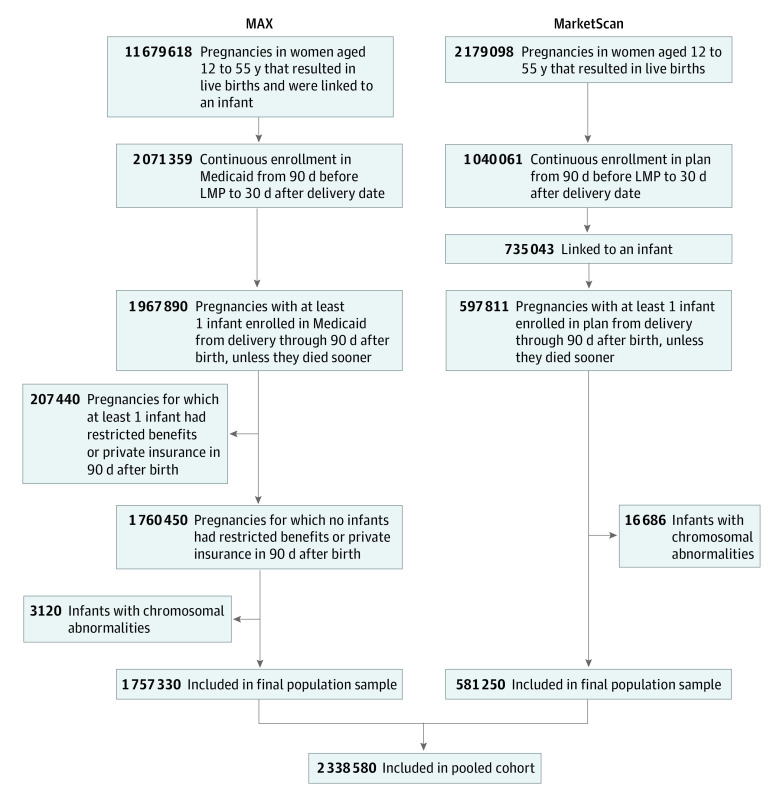
The same approach was applied to MarketScan, a large nationwide data set that contains the claims of employees, their spouses, and dependents who are covered by employer-sponsored private health insurance in the United States. The structure and composition of these data are similar to those of MAX, and the methods we used to create the linked cohort and analyze the data followed comparable protocols. The algorithms used for the development of the MAX24,26 and MarketScan25 cohorts have been described previously and are summarized in Figure 1.
Figure 1. Overview of the Pooled Cohort Creation Using Medicaid Analytic eXtract (MAX) (2000-2013) and MarketScan (2011-2015) Databases.
LMP indicates last menstrual period.
Exposure and Covariate Definitions
The primary exposure was cCMV, defined as 2 or more codes in the infant records for cCMV infection or disease (International Classification of Diseases, Ninth Revision [ICD-9] codes 771.1, 078.5) between delivery and 90 days after delivery. Covariates of interest included potential risk factors for cCMV infection, CMV testing, and/or microcephaly (Table 1). We also assessed other infectious risk factors for microcephaly recorded in maternal claims between last menstrual period and delivery such as Herpesviridae (including varicella-zoster virus, herpes simplex virus, and Epstein-Barr virus), rubella, and toxoplasmosis. Information about race/ethnicity was available in MAX only and was classified as recorded in the Medicaid enrollment file.
Table 1. Prevalence at Birth of cCMV and of Microcephaly Without Comorbid Neural Tube Defects Stratified by Patient Characteristics in Pooled Medicaid Analytic eXtract (2000-2013) and MarketScan (2011-2015) Databases.
| Characteristic | No. (%) | Prevalence per 10 000 (95% CI) | Infants with microcephaly, No. (%) | Prevalence per 10 000 (95% CI) | |
|---|---|---|---|---|---|
| Pregnancies | Infants with cCMV diagnosis | ||||
| Total No. | 2 338 580 | 336 | 1.4 (1.3-1.6) | 679 | 2.9 (2.7-3.1) |
| Maternal age, y | |||||
| ≤19 | 284 564 (12.2) | 79 (23.5) | 2.8 (2.2-3.4) | 87 (12.8) | 3.1 (2.4-3.7) |
| 20-24 | 746 301 (31.9) | 105 (31.3) | 1.4 (1.1-1.7) | 204 (30.0) | 2.7 (2.4-3.1) |
| 25-29 | 588 227 (25.1) | 63 (18.8) | 1.1 (0.8-1.3) | 189 (27.8) | 3.2 (2.8-3.7) |
| 30-34 | 456 318 (19.5) | 52 (15.5) | 1.1 (0.8-1.4) | 115 (16.9) | 2.5 (2.1-3.0) |
| 35-39 | 212 381 (9.1) | 29 (8.6) | 1.4 (0.9-1.9) | 70 (10.3) | 3.3 (2.5-4.1) |
| ≥40 | 50 789 (2.2) | <11a | NA | 14 (2.1) | 2.8 (1.3-4.2) |
| Infant sexb | |||||
| Male | 1 192 507 (51.0) | 186 (55.4) | 1.6 (1.3-1.8) | 263 (38.7) | 2.2 (1.9-2.5) |
| Female | 1 136 353 (48.6) | 147 (43.8) | 1.3 (1.1-1.5) | 414 (61.0) | 3.6 (3.3-4.0) |
| Gestation | |||||
| Multiple | 80 186 (3.4) | 38 (11.3) | 4.7 (3.2-6.2) | 55 (8.1) | 6.9 (5.0-8.7) |
| Single | 2 258 394 (96.6) | 298 (88.7) | 1.3 (1.2-1.5) | 624 (91.9) | 2.8 (2.5-3.0) |
| Race/ethnicity | |||||
| White | 707 087 (30.2) | 97 (28.9) | 1.4 (1.1-1.6) | 204 (30.0) | 2.9 (2.5-3.3) |
| American Indian or Alaskan Native | 30 497 (1.3) | <11a | NA | 12 (1.8) | 3.9 (1.7-6.2) |
| Asian | 68 785 (2.9) | 12 (3.6) | 1.7 (0.8-2.7) | 18 (2.7) | 2.6 (1.4-3.8) |
| Black or African American | 571 303 (24.4) | 109 (32.4) | 1.9 (1.5-2.3) | 230 (33.9) | 4.0 (3.5-4.5) |
| Hispanic or Latino | 251 696 (10.8) | 18 (5.4) | 0.7 (0.4-1.0) | 36 (5.3) | 1.4 (1.0-1.9) |
| Otherc | 127 962 (5.5) | 18 (5.4) | 1.4 (0.8-2.1) | 42 (6.2) | 3.3 (2.3-4.3) |
| Parityd | |||||
| Multipara | 1 343 179 (57.4) | 156 (46.4) | 1.2 (1.0-1.3) | 398 (58.6) | 3.0 (2.7-3.3) |
| Primipara | 414 151 (17.7) | 103 (30.7) | 2.5 (2.0-3.0) | 144 (21.2) | 3.5 (2.9-4.0) |
| Maternal conditions | |||||
| Hypertension (preexisting) | 55 941 (2.4) | <11a | NA | 30 (4.4) | 5.4 (3.4-7.3) |
| Preeclampsia | 100 319 (4.3) | 29 (8.6) | 2.9 (1.8-3.9) | 44 (6.5) | 4.4 (3.1-5.7) |
| Placental abruption | 24 521 (1.0) | 12 (3.6) | 4.9 (2.1-7.7) | 26 (3.8) | 10.6 (6.5-14.7) |
| Diabetes (preexisting) | 41 639 (1.8) | <11a | NA | 42 (6.2) | 10.1 (7.0-13.1) |
| Fever in pregnancy | 42 407 (1.8) | 16 (4.8) | 3.8 (1.9-5.6) | <11a | NA |
| Asthma (preexisting and gestational onset) | 147 819 (6.3) | 25 (7.4) | 1.7 (1.0-2.4) | 55 (8.1) | 3.7 (2.7-4.7) |
| Maternal substance use in pregnancy | |||||
| Any anticonvulsants | 25 979 (1.1) | <11a | NA | 15 (2.2) | 5.8 (2.9-8.7) |
| Tobacco | 197 789 (8.4) | 25 (7.4) | 1.3 (0.8-1.8) | 83 (12.2) | 4.2 (3.3-5.1) |
| Other drug abusee | 35 005 (1.5) | <11a | NA | 23 (3.4) | 6.6 (3.9-9.3) |
| Fetal conditions | |||||
| Preterm birth | 247 312 (10.6) | 174 (51.8) | 7.0 (6.0-8.1) | 277 (40.8) | 11.2 (9.9-12.5) |
| Small for gestational age | 326 261 (14.0) | 138 (41.1) | 4.2 (3.5-4.9) | 255 (37.6) | 7.8 (6.9-8.8) |
Abbreviations: cCMV, congenital cytomegalovirus; NA, not applicable.
Cell contains fewer than 11 individuals. Therefore, to provide summary prevalence ratios while maintaining anonymity of participants, no additional frequency numbers are provided for this row.
A total of 9722 infants were classified as sex unknown and were excluded from analysis.
The other race/ethnicity category included Native Hawaiian or other Pacific Islander, Hispanic or Latino and 1 or more races, more than 1 race (Hispanic or Latino not indicated), and unknown.
Sociodemographic and parity data available only for the Medicaid Analytic eXtract data set.
Includes dependent or nondependent abuse of cannabis, hallucinogens, sedatives, opioids, cocaine, amphetamines, antidepressants, mixed or unspecified drugs, and drug-induced mental disorders (International Classification of Diseases, Ninth Revision codes 292.xx, 304.xx, 305.2x-305.9x, 648.4 from last menstrual period plus 1 day to delivery).
Outcome Definition
The primary outcome was the presence of microcephaly in the infant documented in inpatient or outpatient infant records between delivery and 90 days after delivery or in maternal records between delivery and 30 days after delivery. We required 1 code for microcephaly (ICD-9 code 742.1) and at least 1 of the following: (1) 1 or more additional microcephaly code(s) on a different date; (2) 1 or more procedure code(s) relevant for microcephaly workup, including magnetic resonance imaging of brain or brain stem, computed tomography scan of head, or other diagnostic imaging of head (ICD-9 codes 88.91, 87.03, 87.04; Common Procedural Technology codes 70551-3, 70450, 70460, 70470) or abnormal brain scan findings (ICD-9 code 794.09); or (3) documented infant death within 30 days after delivery.
Maternal records were considered because claims for infants are sometimes recorded in maternal files during the first weeks after birth. We required 2 or more diagnostic and/or procedural codes for the primary definition of microcephaly to increase specificity. We excluded cases with coexisting neural tube defects (ICD-9 codes 740.xx-742.xx other than 742.1x) to exclude infants with microcephaly resulting from failed neural tube closure.
We also differentiated disproportionate microcephaly (ie, undersized head relative to body) from proportionate microcephaly (ie, newborns at similarly low percentiles for weight, length, and head circumference). Disproportionate microcephaly is associated with worse neurocognitive outcomes and prognosis than proportionate microcephaly, yet the 2 phenotypes are not routinely distinguished in studies.27 To attempt to isolate the disproportionate phenotype, we conducted a sensitivity analysis in a subcohort excluding newborns who were small for gestational age (SGA) or preterm (preterm infants may be mistakenly coded with microcephaly).
Secondary Outcome Definitions
Secondary outcomes were those associated with the brain tropism observed for CMV, including eye anomalies (ICD-9 code 743.xx, excluding isolated 743.6x and 743.8x), chorioretinitis (ICD-9 code 363.20), and neonatal seizures (ICD-9 codes 345.xx and 780.3x, excluding isolated 780.31 or 780.32) recorded in infant records between delivery and 30 days after delivery, and hearing loss (ICD-9 code 389.xx) recorded in infant records between delivery and 90 days after delivery.
Analysis
We first identified live births with diagnosed cCMV and described associated risk factors. We then estimated the prevalence at birth of microcephaly and described associated risk factors. Because the main results from each cohort were not statistically or clinically heterogenous and the case numbers were small within each, prevalence estimates were presented as pooled estimates using summary prevalence ratios (PR) with Mantel-Haenszel weights. Analysis began June 2016 and ended May 2020.
We estimated unadjusted summary PRs from the 2 cohorts using Mantel-Haenszel weights with 95% CIs for microcephaly in live births comparing infants with and without a recorded cCMV diagnosis.
We performed several sensitivity analyses to test the robustness of the primary findings. To characterize the range in prevalence estimates for microcephaly depending on the phenotype specification and ascertainment from claims data, we estimated the pooled prevalence of microcephaly using differing case definition algorithms (eTable 1 in the Supplement). To account for the lack of universal screening for cCMV in newborns and for the differential CMV testing and diagnosis in infants with or without microcephaly, we restricted the analyses for the cCMV-microcephaly association to pregnancies with a documented CMV test in the first month of life (antibody test, direct fluorescent antibody test, enzyme immunoassay, or DNA/RNA nucleic acid test; Common Procedural Technology codes 86444-5, 87271, 87332, and 87495-87497 in infant claims from birth to 30 days). We also performed sensitivity analyses for CMV testing and diagnosis time windows (eTable 2 in the Supplement). The 30-day testing and 90-day diagnosis windows were chosen based on laboratory guidelines for cCMV diagnosis, which recommend testing in the first 3 weeks of life (90 days for diagnosis allows for buffer time after testing).
Because only a subset of infants were tested for CMV, we reviewed claims profiles for all tested infants with microcephaly and a random sample of tested infants without microcephaly. Both groups often had other cCMV-related symptoms that may have triggered testing, suggesting that tested infants likely had a higher prevalence of cCMV than the source population. Therefore, we used a case-control approach to estimate the odds ratio of cCMV exposure in infants with microcephaly compared with infants without microcephaly, which approximates the PR for microcephaly under the rare disease assumption. First, we calculated the prevalence of cCMV among infants with microcephaly in our data, including tested infants (among whom the cCMV proportion was likely an overestimate) and infants overall (among whom the cCMV proportion was likely an underestimate since a small proportion was tested, and lack of test was classified as lack of diagnosis). Second, we obtained external estimates of the prevalence of cCMV-positive infants among tested infants without microcephaly from a multicenter study that screened all infants from March 2007 to March 2012.15 Because microcephaly is a rare condition, cCMV prevalence in the source population will approximate prevalence among infants without microcephaly. We converted prevalence estimates into odds and calculated the odds ratio for cCMV comparing infants with vs without microcephaly in the source population.
Finally, to isolate the potential association between cCMV and disproportionate microcephaly, we restricted the population to newborns with no indication of SGA or prematurity. The associations between cCMV and microcephaly with secondary cCMV-related neurologic neonatal outcomes were estimated using summary PRs and 95% CIs.
Results
Study Cohort
The study cohort included 1 757 330 pregnancies in publicly insured women (MAX) and 581 250 pregnancies in commercially insured women (MarketScan). Pregnancies among women aged 20 to 24 years comprised the largest proportion of the cohort (746 301 [31.9%]), while pregnancies among women aged 35 to 39 years comprised the smallest proportion (212 381 [9.1%]). Overall, 88.7% (n = 2 075 410) of pregnancies occurred among women younger than 35 years.
Congenital CMV
The prevalence of diagnosed cCMV in the pooled cohort was 1.4 per 10 000 live births. Factors associated with increased prevalence of diagnosed cCMV included maternal age younger than 20 years, fever during pregnancy, placental abruption, preeclampsia, multiple gestation, SGA, and preterm birth. In MAX, Black or African American race/ethnicity was associated with higher risk of diagnosed cCMV, while Hispanic or Latino race/ethnicity was associated with lower risk (Table 1).
Microcephaly
The pooled prevalence of microcephaly was 2.9 per 10 000 live births. In case definition sensitivity analyses, the pooled prevalence estimate of microcephaly ranged from 2.1 to 7.7 per 10 000 live births (eTable 1 in the Supplement). The prevalence at birth of microcephaly was higher among female infants compared with male infants; multiple gestation compared with singletons; infants born to women who used tobacco or who misused drugs compared with nonusers; and infants born to women with preexisting diabetes, preexisting hypertension, preeclampsia, or placental abruption compared with women without these conditions (Table 1). In MAX, the prevalence was higher among infants born to Black or African American women and lower among infants born to Hispanic or Latino women (4.0 and 1.4 per 10 000, respectively).
cCMV-Microcephaly Association
The pooled prevalence of microcephaly was 655 per 10 000 in newborns with a cCMV diagnosis and 2.8 per 10 000 in those without one (summary PR, 232; 95% CI, 154-350) (Table 2). Thirteen of 679 infants (1.9%) with a microcephaly diagnosis had a recorded CMV test compared with 559 of 2 337 901 infants without a microcephaly diagnosis (0.02%). Among tested infants, the pooled prevalence of microcephaly comparing those with and without a cCMV diagnosis was 2143 and 129 per 10 000, respectively (summary PR, 15; 95% CI, 5.6-41).
Table 2. Prevalence at Birth of Microcephaly and Other Neurologic Outcomes Recorded Within First 90 Days of Life Among Infants With and Without a cCMV Diagnosis in a Cohort of Pregnancies Nested Within Pooled Medicaid Analytic eXtract (2000-2013) and MarketScan (2011-2015) Databases.
| Outcome | Total, No. | No. (%) | Summary prevalence ratio (95% CI)a | |
|---|---|---|---|---|
| cCMV diagnosis (n = 336) | No cCMV diagnosis (n = 2 338 244) | |||
| Microcephaly | 679 | 22 (6.5) | 657 (0.03) | 232 (154-350) |
| Neonatal seizures | 5922 | <11b | NA | 8 (4-17) |
| Hearing loss | 4605 | 28 (8.3) | 4577 (0.2) | 46 (31-68) |
| Chorioretinitis or eye anomaliesc | 1025 | <11b | NA | 69 (37-128) |
Abbreviations: cCMV, congenital cytomegalovirus; NA, not applicable.
Prevalence ratio of outcome among infants with a cCMV diagnosis divided by that in those without a cCMV diagnosis.
Cell contains fewer than 11 individuals. Therefore, to provide summary prevalence ratios while maintaining anonymity of participants, no additional frequency numbers are provided for this row.
Chorioretinitis and eye anomalies were combined owing to small case counts.
Because the prevalence of cCMV in tested infants with (46.15%) and without (22 of 559 [3.94%]) microcephaly was likely an overestimation of that in all infants with and without microcephaly in the source population, we compared the prevalence of cCMV diagnosis in all infants with microcephaly (22 of 679 [3.24%]) with the expected prevalence of cCMV infections in the source population (449 of 100 332 [0.45%]), obtained from an external reference study in which all infants were tested (odds ratio, 7.4; 95% CI, 4.8-11.5).15 Given the low prevalence of microcephaly, this is an approximation of the PR of microcephaly comparing infants with vs without cCMV.
Small for gestational age and prematurity were more prevalent among infants diagnosed with cCMV and among infants diagnosed with microcephaly (Table 1). After excluding infants diagnosed as SGA and/or preterm from the cohort (n = 525 684) to isolate the disproportionate microcephaly phenotype, the pooled prevalence of microcephaly was 1.5 per 10 000 live births overall and 476 and 1.5 per 10 000 live births among infants with and without a cCMV diagnosis, respectively (summary PR, 327; 95% CI, 138-775). Owing to the small number of infants with microcephaly and recorded toxoplasmosis, rubella, and non-CMV herpesvirus infections (including herpes zoster, herpes simplex, and Epstein-Barr virus), it was not possible to calculate stable PRs for these infections.
Secondary cCMV-related Neurologic Outcomes
The prevalence of hearing loss, neonatal seizures, chorioretinitis, or eye anomalies was higher among infants with a cCMV diagnosis compared with infants without a cCMV diagnosis (Table 2), as well as among infants with a microcephaly diagnosis compared with those without one (68 of 679 infants with microcephaly [10%] vs 11 369 of 2 337 901 infants without microcephaly [0.49%], respectively; Table 3).
Table 3. Prevalence at Birth of Select Central Nervous System Outcomes Recorded Within First 90 Days of Life Among Infants With and Without a Diagnosis of Microcephaly in a Cohort of Pregnancies Nested Within Pooled Medicaid Analytic eXtract (2000-2013) and MarketScan (2011-2015) Databases.
| Outcome | Total, No. | No. (%) | Summary prevalence ratio (95% CI)a | |
|---|---|---|---|---|
| Microcephaly diagnosis (n = 679) | No microcephaly diagnosis (n = 2 337 901) | |||
| Neonatal seizures | 5922 | 44 (6.5) | 5878 (0.2) | 25 (19-34) |
| Hearing loss | 4605 | 14 (2.1) | 4591 (0.2) | 11 (6.3-18) |
| Chorioretinitis or eye anomaliesb | 1025 | 13 (1.9) | 1012 (0.04) | 45 (26-77) |
Prevalence ratio of outcome among infants with microcephaly compared with infants without microcephaly.
Chorioretinitis and eye anomalies were combined owing to small case counts.
Discussion
In a population-based cohort of more than 2 million pregnancies in the United States, cCMV infection was associated with at least a 7-fold increased prevalence of microcephaly and was also associated with hearing loss, neonatal seizures, chorioretinitis, and eye anomalies in newborns.
We estimated a prevalence of microcephaly of approximately 3 per 10 000 live births between 2000 and 2015, which is within the range of estimates generated by established surveillance systems. In the United States, the National Birth Defects Prevention Network28 reported microcephaly prevalence ranging from 0.5 to 19.0 per 10 000 live births between 2006 and 2010.29 This variation is partially due to differences in the clinical definition of microcephaly, case ascertainment methods, and the timing and setting of diagnosis.28,30 Our results further illustrate the role of case definition. Microcephaly is a clinical diagnosis defined as occipitofrontal head circumference at birth 2 or more SDs below the median for gestational age and sex,31,32,33 although this definition is not necessarily followed in clinical practice. Outside of cases resulting from congenital syndromes or failures in neural tube closure, undersized heads can result from reduced brain growth or from SGA with proportionally small heads. Although cCMV infection can result in small infants through effects on prematurity and growth restriction, we were interested in the disproportionate microcephaly phenotype originating from neurotropic effects in the brain, which is more associated with long-term neurodevelopmental sequelae. Our results suggest that the relative risk of microcephaly associated with cCMV diagnosis is stronger for the disproportionate phenotype.
The association between cCMV and microcephaly has been previously described in small samples of infants with cCMV.16,34,35 We estimated this association at the population level with large case numbers (despite underdiagnosis) by leveraging the power of health care databases with analytic techniques to correct for testing bias. In our data, the prevalence of diagnosed cCMV was 0.014% among all newborns compared with 0.4% to 0.5% in US surveillance studies,14,15 suggesting substantial underestimation likely due to lack of universal testing, and 4.9% among those tested for CMV, suggesting selective testing of newborns with suspicious symptoms. Indeed, 1.9% of infants with microcephaly and 0.024% of infants without microcephaly had a recorded CMV test, and 0.59% and 0.023% of newborns with and without diagnosed hearing loss, respectively, had a recorded test. These low testing prevalence measures are consistent with a medical record review study conducted in the United States, which found that just 0.7% of all newborns were screened for CMV.36
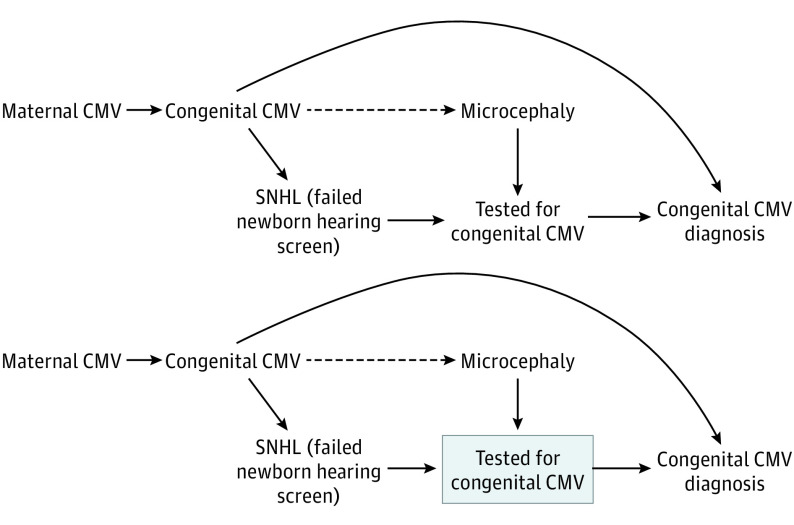
The directed acyclic graph in Figure 2 represents how differential exposure misclassification biases the effect estimates for risk factors such as cCMV that receive targeted screening among those with the outcome of interest. As shown on the directed acyclic graph, there is an open noncausal path from microcephaly to cCMV diagnosis through CMV testing, which is also triggered by other cCMV signs such as hearing loss. This surveillance bias may result in overestimation of the association between cCMV and microcephaly in the overall population (PR, 232). Restricting the cohort to infants tested for CMV introduces selection bias because tested infants likely had other cCMV-related symptoms that triggered testing, resulting in overestimation of the cCMV prevalence in both infants with and without microcephaly and biased estimation of the association (PR, 15). This is represented on the directed acyclic graph by conditioning on a CMV test, a common effect of hearing loss and microcephaly, which opens a new noncausal path between microcephaly and cCMV diagnosis. Using unbiased estimates of the prevalence of cCMV infection in the source population, we estimated that the PR for the association between cCMV and microcephaly is at least 7.4. This is likely a conservative lower bound because only a small proportion of infants with microcephaly (but all infants without microcephaly) are tested for cCMV to construct this estimate. This PR estimate would be 190 if we had used the prevalence of cCMV in tested infants with microcephaly rather than all infants with microcephaly; however, this PR may represent the association with disproportionate microcephaly (the phenotype triggering testing) more than on small head size overall.
Figure 2. Directed Acyclic Graphs for the Association Between Congenital Cytomegalovirus (CMV) and Microcephaly.
Newborns with microcephaly are more likely to be tested for congenital CMV than newborns without microcephaly, as are newborns with sensorineural hearing loss (SNHL) who fail the newborn hearing screen (or have other congenital CMV–related symptoms). Thus, there are more newborns tested, and more with a positive CMV test result, among those with microcephaly, resulting in upwardly biased estimates of the association of congenital CMV with microcephaly. To avoid this surveillance bias, we restricted to newborns with CMV testing, as represented by the box around CMV testing in the second directed acyclic graph. However, among tested newborns, both individuals with and without microcephaly may be more likely to have congenital CMV–related symptoms such as SNHL that triggered the test. Thus, among tested newborns, there would be an overrepresentation of CMV-positive infants in both the case and referent groups, resulting in biased estimates of the association of congenital CMV with microcephaly.
Limitations
There are 2 main limitations to our study. First, microcephaly ascertainment based on claims data can be inaccurate, and we did not validate our algorithm with medical records. However, we were able to replicate known risk factors and neurologic comorbidities for microcephaly, indirectly supporting the validity of our outcome ascertainment algorithm. This limitation is on top of a challenging microcephaly phenotype specification that exists even for US Centers for Disease Control and Prevention surveillance studies.37 Second, the study had substantial underascertainment of cCMV infection that was differential by outcome owing to selective testing. However, we used the described triangulation approach to place boundaries on the true effect estimate. Under any scenario, cCMV was the strongest risk factor for microcephaly identified in this study.
Conclusions
Although more than 20 000 infants with cCMV are born each year in the United States,13 fewer than half of obstetricians in the United States council their patients on CMV prevention, and testing is usually only performed if infection is suspected.38 Although our results do not speak to the risks and benefits of prevention strategies or screening programs, combined with surveillance data on the incidence of CMV during pregnancy in different populations, they provide important evidence on the large proportion of potentially preventable microcephaly cases. Cytomegalovirus remains one of the strongest known teratogenic infections and increases the risk of microcephaly among newborns with congenital infection by at least 7-fold. Unlike Zika virus, it has been responsible for a large number of cases in the United States in recent decades.
eTable 1. Sensitivity analyses for the case definition of microcephaly for Medicaid Analytic eXtract (MAX) (2000-2013) and MarketScan (2011-2015) databases
eTable 2. Sensitivity analyses for congenital cytomegalovirus (cCMV) diagnosis and testing windows in infant records in pooled Medicaid Analytic eXtract (MAX) (2000-2013) and MarketScan (2011-2015) databases
References
- 1.Brasil P, Pereira JP Jr, Moreira ME, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med. 2016;375(24):2321-2334. doi: 10.1056/NEJMoa1602412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Driggers RW, Ho CY, Korhonen EM, et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med. 2016;374(22):2142-2151. doi: 10.1056/NEJMoa1601824 [DOI] [PubMed] [Google Scholar]
- 3.Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016;374(16):1552-1563. doi: 10.1056/NEJMra1602113 [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects: reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981-1987. doi: 10.1056/NEJMsr1604338 [DOI] [PubMed] [Google Scholar]
- 5.Calvet G, Aguiar RS, Melo ASO, et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. 2016;16(6):653-660. doi: 10.1016/S1473-3099(16)00095-5 [DOI] [PubMed] [Google Scholar]
- 6.Mlakar J, Korva M, Tul N, et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374(10):951-958. doi: 10.1056/NEJMoa1600651 [DOI] [PubMed] [Google Scholar]
- 7.Marquez L, Levy ML, Munoz FM, Palazzi DL. A report of three cases and review of intrauterine herpes simplex virus infection. Pediatr Infect Dis J. 2011;30(2):153-157. doi: 10.1097/INF.0b013e3181f55a5c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maldonado YA, Read JS; Committee on Infectious Diseases . Diagnosis, treatment, and prevention of congenital toxoplasmosis in the United States. Pediatrics. 2017;139(2):e20163860. doi: 10.1542/peds.2016-3860 [DOI] [PubMed] [Google Scholar]
- 9.Zhang HJ, Patenaude V, Abenhaim HA. Maternal outcomes in pregnancies affected by varicella zoster virus infections: population-based study on 7.7 million pregnancy admissions. J Obstet Gynaecol Res. 2015;41(1):62-68. doi: 10.1111/jog.12479 [DOI] [PubMed] [Google Scholar]
- 10.Stagno S, Whitley RJ. Herpesvirus infections of pregnancy: part II: herpes simplex virus and varicella-zoster virus infections. N Engl J Med. 1985;313(21):1327-1330. doi: 10.1056/NEJM198511213132105 [DOI] [PubMed] [Google Scholar]
- 11.Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the National Health And Nutrition Examination Surveys, 1988-2004. Clin Infect Dis. 2010;50(11):1439-1447. doi: 10.1086/652438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stagno S, Pass RF, Cloud G, et al. Primary cytomegalovirus infection in pregnancy: incidence, transmission to fetus, and clinical outcome. JAMA. 1986;256(14):1904-1908. doi: 10.1001/jama.1986.03380140074025 [DOI] [PubMed] [Google Scholar]
- 13.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17(4):253-276. doi: 10.1002/rmv.535 [DOI] [PubMed] [Google Scholar]
- 14.Murph JR, Souza IE, Dawson JD, et al. Epidemiology of congenital cytomegalovirus infection: maternal risk factors and molecular analysis of cytomegalovirus strains. Am J Epidemiol. 1998;147(10):940-947. doi: 10.1093/oxfordjournals.aje.a009384 [DOI] [PubMed] [Google Scholar]
- 15.Fowler KB, McCollister FP, Sabo DL, et al. ; CHIMES Study . A targeted approach for congenital cytomegalovirus screening within newborn hearing screening. Pediatrics. 2017;139(2):e20162128. doi: 10.1542/peds.2016-2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boppana SB, Pass RF, Britt WJ, Stagno S, Alford CA. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J. 1992;11(2):93-99. doi: 10.1097/00006454-199202000-00007 [DOI] [PubMed] [Google Scholar]
- 17.Fowler KB, Boppana SB. Congenital cytomegalovirus infection. Semin Perinatol. 2018;42(3):149-154. doi: 10.1053/j.semperi.2018.02.002 [DOI] [PubMed] [Google Scholar]
- 18.Butler D. Zika raises profile of more common birth-defect virus. Nature. 2016;535(7610):17. doi: 10.1038/535017a [DOI] [PubMed] [Google Scholar]
- 19.Weller TH, Hanshaw JB. Virologic and clinical observations on cytomegalic inclusion disease. N Engl J Med. 1962;266:1233-1244. doi: 10.1056/NEJM196206142662401 [DOI] [PubMed] [Google Scholar]
- 20.Hanshaw JB. Cytomegalovirus complement-fixing antibody in microcephaly. N Engl J Med. 1966;275(9):476-479. doi: 10.1056/NEJM196609012750905 [DOI] [PubMed] [Google Scholar]
- 21.Leung J, Cannon MJ, Grosse SD, Bialek SR. Laboratory testing and diagnostic coding for cytomegalovirus among privately insured infants in the United States: a retrospective study using administrative claims data. BMC Pediatr. 2013;13(90):90. doi: 10.1186/1471-2431-13-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ornoy A, Diav-Citrin O. Fetal effects of primary and secondary cytomegalovirus infection in pregnancy. Reprod Toxicol. 2006;21(4):399-409. doi: 10.1016/j.reprotox.2005.02.002 [DOI] [PubMed] [Google Scholar]
- 23.Cannon MJ, Griffiths PD, Aston V, Rawlinson WD. Universal newborn screening for congenital CMV infection: what is the evidence of potential benefit? Rev Med Virol. 2014;24(5):291-307. doi: 10.1002/rmv.1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmsten K, Huybrechts KF, Mogun H, et al. Harnessing the Medicaid Analytic eXtract (MAX) to evaluate medications in pregnancy: design considerations. PLoS One. 2013;8(6):e67405. doi: 10.1371/journal.pone.0067405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacDonald SC, Cohen JM, Panchaud A, McElrath TF, Huybrechts KF, Hernández-Díaz S. Identifying pregnancies in insurance claims data: methods and application to retinoid teratogenic surveillance. Pharmacoepidemiol Drug Saf. 2019;28(9):1211-1221. doi: 10.1002/pds.4794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Margulis AV, Setoguchi S, Mittleman MA, Glynn RJ, Dormuth CR, Hernández-Díaz S. Algorithms to estimate the beginning of pregnancy in administrative databases. Pharmacoepidemiol Drug Saf. 2013;22(1):16-24. doi: 10.1002/pds.3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanzlik E, Gigante J. Microcephaly. Children (Basel). 2017;4(6):E47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cragan JD, Isenburg JL, Parker SE, et al. ; National Birth Defects Prevention Network . Population-based microcephaly surveillance in the United States, 2009 to 2013: an analysis of potential sources of variation. Birth Defects Res A Clin Mol Teratol. 2016;106(11):972-982. doi: 10.1002/bdra.23587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mai CT, Kucik JE, Isenburg J, et al. ; National Birth Defects Prevention Network . Selected birth defects data from population-based birth defects surveillance programs in the United States, 2006 to 2010: featuring trisomy conditions. Birth Defects Res A Clin Mol Teratol. 2013;97(11):709-725. doi: 10.1002/bdra.23198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mai CT, Cassell CH, Meyer RE, et al. ; National Birth Defects Prevention Network . Birth defects data from population-based birth defects surveillance programs in the United States, 2007 to 2011: highlighting orofacial clefts. Birth Defects Res A Clin Mol Teratol. 2014;100(11):895-904. doi: 10.1002/bdra.23329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashwal S, Michelson D, Plawner L, Dobyns WB; Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society . Practice parameter: evaluation of the child with microcephaly (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2009;73(11):887-897. doi: 10.1212/WNL.0b013e3181b783f7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization . Screening, assessment and management of neonates and infants with complications associated with Zika virus exposure in utero. Updated August 30, 2016. Accessed August 7, 2020. https://www.who.int/csr/resources/publications/zika/assessment-infants/en/ [Google Scholar]
- 33.Leviton A, Holmes LB, Allred EN, Vargas J. Methodologic issues in epidemiologic studies of congenital microcephaly. Early Hum Dev. 2002;69(1-2):91-105. doi: 10.1016/S0378-3782(02)00065-8 [DOI] [PubMed] [Google Scholar]
- 34.Kylat RI, Kelly EN, Ford-Jones EL. Clinical findings and adverse outcome in neonates with symptomatic congenital cytomegalovirus (SCCMV) infection. Eur J Pediatr. 2006;165(11):773-778. doi: 10.1007/s00431-006-0172-6 [DOI] [PubMed] [Google Scholar]
- 35.Noyola DE, Demmler GJ, Nelson CT, et al. ; Houston Congenital CMV Longitudinal Study Group . Early predictors of neurodevelopmental outcome in symptomatic congenital cytomegalovirus infection. J Pediatr. 2001;138(3):325-331. doi: 10.1067/mpd.2001.112061 [DOI] [PubMed] [Google Scholar]
- 36.Mahajan V, Patel J. Presentations of and testing practices for congenital CMV. Pediatrics. 2019;144(2 Meeting Abstract 674). doi: 10.1542/peds.144.2_MeetingAbstract.674 [DOI] [Google Scholar]
- 37.Graham KA, Fox DJ, Talati A, et al. Prevalence and clinical attributes of congenital microcephaly: New York, 2013-2015. MMWR Morb Mortal Wkly Rep. 2017;66(5):125-129. doi: 10.15585/mmwr.mm6605a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention (CDC) . Knowledge and practices of obstetricians and gynecologists regarding cytomegalovirus infection during pregnancy: United States, 2007. MMWR Morb Mortal Wkly Rep. 2008;57(3):65-68. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Sensitivity analyses for the case definition of microcephaly for Medicaid Analytic eXtract (MAX) (2000-2013) and MarketScan (2011-2015) databases
eTable 2. Sensitivity analyses for congenital cytomegalovirus (cCMV) diagnosis and testing windows in infant records in pooled Medicaid Analytic eXtract (MAX) (2000-2013) and MarketScan (2011-2015) databases