Abstract
Introduction:
The DSM-5 explicitly states that the neural system model of specific phobia is centered on the amygdala. However, this hypothesis is predominantly supported by human studies on animal phobia, whereas visual cuing of other specific phobias, such as dental fear, do not consistently show amygdala activation. Considering that fear of anticipated pain is one of the best predictors of dental phobia, the current study investigated neural and autonomic activity of pain anticipation in individuals varying in the degree of fear of dental pain.
Method:
Functional brain activity (fMRI) was measured in women (n=31) selected to vary in the degree of self-reported fear of dental pain when under the threat of shock, in which one color signaled the possibility of receiving a painful electric shock and another color signaled safety.
Results:
Enhanced functional activity during threat, compared to safety, was found in regions including anterior insula and anterior/mid cingulate cortex. Importantly, threat reactivity in the anterior insula increased as reported fear of pain increased and further predicted skin conductance changes during pain anticipation.
Limitations:
The sample was comprised of women.
Conclusions:
Individual differences in fear of pain vary with activation in the anterior insula, rather than with the amygdala, indicating that fear is not uniquely associated with amygdala activation. Whereas coping techniques such as emotion regulation have been found to vary with activation in a frontal-amygdala circuit when confronted with visual cues, precision psychiatry may need to target specific brain circuits to diagnose and treat different types of specific phobia.
1. Introduction
Among specific phobias, dental fear is common, with prevalence rates varying between 13% and 19% (Oosterink et al., 2009). Specific phobias are characterized by excessive fear of phobic stimulation and are typically accompanied by persistent avoidance behavior, which, in the case of dental fear, is clearly a health risk. DSM-5 states that “Current neural system models for specific phobia emphasize the amygdala and related structures ...” (page 199; American Psychiatric Association, 2013). Neuroimaging evidence for a major role of the amygdala activation in specific phobia primarily comes from studies investigating animal fear, in which individuals reporting spider or snake fear show enhanced blood-oxygen-level-dependent (BOLD) activity in the amygdala when viewing phobogenic, compared to non-feared, scenes (Dilger et al., 2003; Sabatinelli et al., 2005; Straube et al., 2004, 2005, 2006; Veltman et al., 2004; Wendt, et al., 2008).
On the other hand, when visual cues have been used to investigate dental phobia, amygdala engagement for phobogenic visual images is not reliably found (Caseras et al., 2010; Hermann et al., 2007; Hilbert et al., 2014; Lueken et al., 2011, 2014; Schienle et al., 2013; for amygdala involvement, see Feldker et al., 2017). Considering that fear of anticipated pain is one of the best predictors of dental phobia, the current study investigated neural correlates and autonomic activity of pain anticipation in a sample of individuals varying in the degree of fear of dental pain. To the extent that fear of pain is mediated by differences in a functional network involving the amygdala, we expected heightened amygdala activation when high fear participants anticipated receiving painful stimulation.
In the laboratory, fear of pain has been investigated by threatening individuals with the possibility of receiving a painful shock, with one cue associated with potential shock exposure, while another cue signals safety. In healthy participants, threat of shock reliably elicits defensive reactivity, evidenced by potentiation of the startle reflex during threat, compared, to safety (e.g., Bradley, Moulder, Lang, 2005; Bublatzky et al., 2013; Costa, Bradley, Lang, 2015; Dunning, DelDonno, Hajcak. 2013; Grillon et al., 1991, 1993) as well as enhanced skin conductance responses (Bradley et al., 2017; Costa et al., 2015; Kopacz & Smith, 1971), and heightened event-related positive potentials over centroparietal EEG sensors (Böcker et al., 2004; Bublatzky and Schupp, 2013). Importantly, participants reporting high dental fear show enhanced psychophysiological defensive reactivity when under threat of shock - a potentially painful stimulus that is not specifically related to dental or facial pain (Bradley, Silakowski, & Lang, 2008).
The DSM-5 suggests that amygdala involvement is a central node in fear processing, based, in part, on data from threat of shock studies. Early neuroimaging studies reported enhanced functional activity in the amygdala when under threat of shock, compared to safety (Alvarez et al., 2011; Phelps et al., 2001). A number of recent studies investigating pain anticipation in healthy participants during threat of shock, however, reported increased activation in the anterior insula, as well as in mid and anterior cingulate cortex, supramarginal gyrus, and parietal associative cortex (Alvarez et al., 2015; Baldestron et al., 2017; Kirlic et al., 2019; Mechias, Etkin, Kalish, 2010; Reicherts, Wiemer, Gerdes, Schulz, Pauli, & Wiser, 2017) rather than in the amygdala. In the current study, the neural circuit underlying fear of pain anticipation was further studied as it varied in individuals reporting high or low fear of pain. To the extent that different neural regions underlie fear expression in specific aversive contexts, individual differences in fear of pain might covary with functional activity in the consistently replicated pain anticipation network, including the anterior insula. Because threat of shock prompts increased skin conductance changes that covary with activation of the anterior insula (Kirlik et al., 2019) or the amygdala (Phelps et al., 2001), skin conductance was continuously measured during threat and safe periods.
Understanding the neural networks underlying affective disorders (such as fear) represents a major advancement in precision psychiatry (Fernandes, Williams, Steiner, Leboyer, Carvalho, & Berk, 2017), because it allows targeting specific brain regions when assessing or treating the underlying dysfunctional emotional response. For instance, it has been proposed that frontal-limbic circuitry that includes the amygdala mediates dysfunctional emotion regulation (Morawetz, Bode, Baudewig, Heekeren, 2017; Ochsner et al., 2002, 2004; Porta-Casteras, et al., 2020; Urry et al., 2006), with treatment focusing on this circuit to regulate and reduce fear response (Hartley & Phelps, 2010). If fear of pain does not reliably engage the amygdala, on the other hand, different therapeutic techniques may be required.
2. Method
Thirty-four female students (M age = 18.8, SD = 0.8) from the University of Florida participated for course credit or $20. The University of Florida Institutional Review Board approved the study, and informed consent was obtained before entering the scanner. Data from two participants were discarded for excessive motion and from one participant for scanner malfunction, resulting in a final sample of 31 participants.
Considering gender differences in fear response strategies (Schienle et al., 2013; Shansky. 2019) and previous studies on similar populations that used a sample sizes approaching 30 (e.g., Schienle et al., 2013). a planned sample size of approximately 30–35 women was targeted. To include participants with varying degrees of fear of dental pain. three items from the Dental Fear Survey (DFS; Kleinknecht. Klepac. and Alexander. 1973; items 1. 14. and 20) were administered in a prescreening survey to students taking an introductory psychology course. To allow a complete coverage of the fear continuum. women that varied in reported fear. from low to high (score above the 95th percentile at the three items used during the screening). were contacted by phone and invited to participate.
On the day of the fMRI session. all participants completed the entire DFS (which consists of 20 items). In addition. the Fear of Dental Pain questionnaire. which probes specific pain scenarios involved in dentistry (FDP; van Wijk & Hoogstraten. 2003). and the Fear of Pain Questionnaire-III (FPQ-III; McNeil and Reinwater. 1998) which assesses fear of pain more broadly were completed. The State-Trait Anxiety Inventory (STAI; Spielberger et al., 1983). and the Beck Depression Inventory (BDI-II; Beck et al., 1996) were administered to assess individual differences in temperament and mood.
2.1. Materials and Design
Stimuli consisted of 16 red and blue squares equivalent in hue and color saturation. Half (8) of the cues signaled that an electric shock could be delivered through an electrode attached to the leg. whereas half (8) signaled safety. Cues were presented for 12 s followed by a 12 s inter-trial interval consisting of a black screen. Stimulus presentation was controlled using a PC-compatible computer running E-Prime (Psychology Software Tools. Inc., Sharpsburg. PA). In addition to the critical 16 anticipatory trials. an additional set of trials presented standard (i.e., not phobogenic) emotional and neutral scenes that were analyzed separately (Sambuco. Costa. Lang. & Bradley. 2020).
A single mild shock (2.6 mA, 250 ms duration), was delivered through a bar electrode to the right ankle, was generated using a Powerlab stimulator attached to a ML180 Stimulus Isolator (AD Instruments, Inc., Colorado Springs, CO), following completion of all 32 anticipatory trials.
2.2. Procedure
Prior to entering the scanner, participants were instructed that hen ne color (red or blue, counterbalanced across participants) was displayed, an electric shock could be delivered through a stimulating bar electrode attached to their right ankle, and that no shock was possible during presentations of the other color.
In the scanner, the shock electrode was attached to the participant’s right ankle and the shock contingencies were repeated. Participants were not informed about the strength of the electric shock, and only a single mild shock was administered after all the experimental trials, before leaving the bore of the scanner.
After leaving the scanner, each participant rated the unpleasantness of shock anticipation and exposure on a 7-point scale ranging from unpleasant (1) to pleasant (7). Confirming the administration of the mild shock, participants rated the single shock only mildly unpleasant (M=4; SD = 1.7).
2.3. Data Acquisition and Analysis
2.3.1. functional MRI
A Tl-weighted anatomical volume was acquired using a Siemens 3T Allegra MR scanner. A total of 288 functional volumes (50 coronal slices, 2.5 mm thick, .5 mm gap) were collected using a T2* weighted echo planar imaging sequence (3 s TR, 35 ms TE, 160 mm FOV, 64 × 64 acquisition matrix). Offline, the functional data were slice-time adjusted, motion corrected, spatially smoothed (5.0 mm FWHM Gaussian kernel), and converted to percent BOLD signal change (for each voxel based on the mean across the entire time series) using the Analysis of Functional Neuroimages software (AFNI, Cox, 1996).
Hemodynamic responses were deconvolved using a multiple linear regression model with a cubic spline response function (24-s) that coded threat and safe trials and motion parameters (6) as variables. A whole-brain ANOVA was conducted on the peak BOLD activity (6-s after cues onset) with threat or safe cue as the independent variable. The alpha-level for voxelwise analysis was determined by using simulations (3dClustSim in AFNI) that model spatial fMRI noise as a mixture of Gaussian plus mono-exponential distributions. Based on a voxel-level uncorrected p-value of 0.001, simulations indicated a minimum cluster extent of 19 voxels (2.5 mm3) for a cluster-level corrected alpha of 0.05.
Based on the results of group-level analysis and the relationship between BOLD activity and reported fear of dental pain, additional follow-up analyses were performed to further test the relationship between regional functional activity and reported fear of pain. BOLD activity (threat minus safe) in the functional clusters of anterior insula, mid cingulate cortex, and supramarginal gyrus were linearly combined with a principal component analysis (PCA), where the resulting single component (eigenvalue > 1 and 71% of the variance explained) eigenvalues used to predict individual differences in reported fear of pain.
Additionally, a region of interest (ROI) analysis on the bilateral amygdala was performed to test the difference in BOLD activity during threat compared to safety, with amygdala identified in one of the structural atlases provided in AFNI (TT_N27).
2.3.2. Skin conductance
Skin conductance was recorded during scanning using two large Ag/Ag-CL electrodes attached to the left hypothenar eminence of the left palm. A low, constant voltage AC excitation (22 mV at 75 Hz) was supplied to one of n electrodes using a Powerlab ML116 –GSR amplifier. Skin conductance levels were sampled continuously at 1000 Hz using a laptop computer running Chart software (v 5.5.1, ADInstruments, Inc., Colorado Springs, CO). Activity was filtered offline in MATLAB (Mathworks, Inc., Natick, MA) using a 3 Hz low pass filter, then averaged into half-second bins, baseline deviated from mean activity 1 s prior to cue onset. Based on the resulting waveforms, skin conductance change was averaged from 4 to 12 s following cue onset and log transformed. Trials in which the skin conductance change was greater than 2 standard deviation from the mean, were excluded to reduce the effect of outliers (.07 trials removed). Because skin conductance changes were not normally distributed (Shapiro-Wilk test: W = .75, p < 0.001), a non-parametric test (Spearman p) was used to investigate the relationship between skin conductance and BOLD activity.
3. Results
Questionnaires measuring fear of dental pain, fear of pain, and dental fear were each normally distributed (Shapiro-Wilk test: W = .97, .95, .97 p = 0.45, 0.57, and 0.21, respectively). Fear of dental pain was significantly related both to reported dental fear (r = .74, p < 0.001) and to fear of general pain (r = .66, p < 0.001).
3.1. BOLD Activity
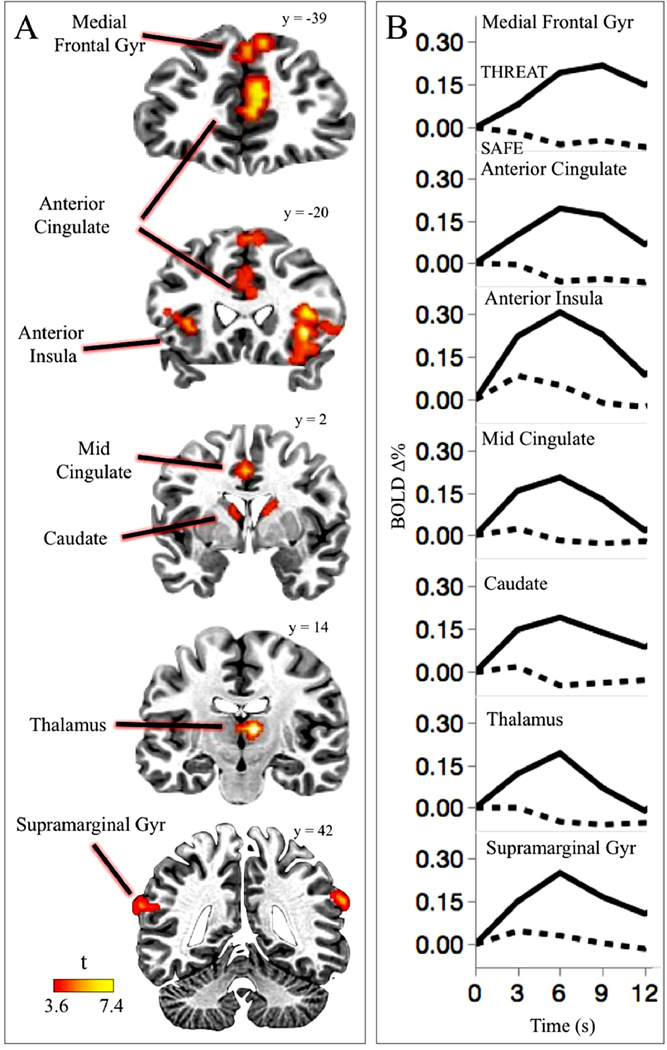
Figure 1 illustrates regions in which BOLD activity was significantly enhanced during threat compared to safety, and prominently included the bilateral anterior insula, as well as the supramarginal gyrus, anterior cingulate cortex, mid cingulate cortex, medial frontal gyrus, thalamus, dorsal caudate, orbitofrontal cortex, and precuneus (see also Table 1).
Figure 1.
Regions activated during threat of shock. Enhanced functional activity during threat compared to safety was found in the anterior insula, medial frontal gyrus, anterior and mid cingulate cortex, thalamus, caudate, and supramarginal gyrus (A). Across the regions involved in threat processing, the BQLD signal change time course shows a similar pattern of neural activity across all the regions, characterized by heightened changes in the context of a cue signaling possible electric shock, compared to safety, with a larger difference between threat and safety found at 6-s after the cue onset (B). Note: Brain slices are displayed in the neurological orientation (left hemisphere is on the left; right hemisphere is on the right).
Table 1.
Pain anticipation network. Regions showing significantly enhanced BOLD activity during threat compared to safety.
| Region | BOLD Δ% Mean (SD) | Cluster Size | Peak t | +LPI coordinates in Talairach space | ||||
|---|---|---|---|---|---|---|---|---|
| Threat | Safe | X | Y | Z | ||||
| Anterior Insula | R | .31 (.22) | .05 (.20) | 203 | 7.45 | −34 | −16 | 11 |
| L | .30 (.28) | .06 (.17) | 51 | 5.14 | 29 | −26 | −1 | |
| Dorsal Anterior Cingulate Cortex | M | .19 (.17) | −.06 (.14) | 150 | 5.33 | −9 | −41 | −21 |
| Mid Cingulate Cortex | M | .20 (.26) | −.02 (.19) | 20 | 4.62 | −1 | 1 | −39 |
| Medial Frontal Gyr | L | .19 (.16) | −.06 (.19) | 88 | 5.65 | −14 | −44 | −44 |
| Caudate | R | .20 (.22) | −.06 (.16) | 74 | 4.90 | −14 | −1 | −14 |
| L | .18 (.19) | −.03 (.14) | 23 | 5.56 | 6 | −6 | −9 | |
| Thalamus | R | .19 (.16) | −.05 (.14) | 69 | 7.63 | −9 | 14 | −6 |
| Supramarginal Gyr | R | .30 (.21) | .10 (.16) | 24 | 5.50 | −64 | 41 | −21 |
| L | .19 (.24) | −.04 (.21) | 26 | 4.94 | 51 | 54 | −9 | |
| Precuneus | M | .13 (.26) | −.10 (.21) | 29 | 4.86 | 1 | 56 | −41 |
| Orbitofrontal | M | .16 (.37) | −.25 (.31) | 19 | 4.22 | −6 | −54 | 9 |
Note. R = right, L = left, M = medial; Cluster size expressed as number of significant voxel for the comparison threat vs. safe; Location of the peak t-statistic is reported in +L +P +I coorainates in Taiairach space.
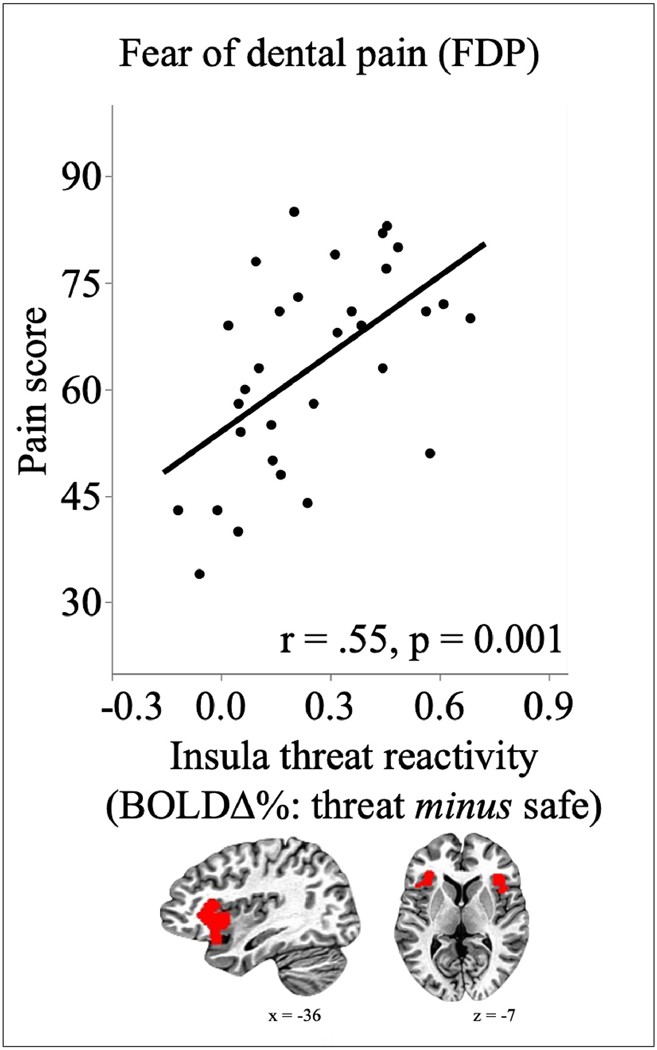
Enhanced functional activity in the anterior insula during threat, compared to safety, was consistent across the sample, with 90.3% of the participants showing a positive increase in functional activity in the anterior insula during cues signaling shock threat, compared to safety. More importantly, as illustrated in Figure 2, activation of the insula during threat, compared to safety, was significantly related to fear of dental pain (r = .55, p = 0.001), but not dental fear (r = .17, p = 0.35), with the difference in functional activation during threat and safe periods increasing as fear of pain increased. This effect was driven by an increase in insula activity during threat (r = .27), and a decrease during safety (r = −.40) as reported fear of dental pain increases.
Figure 2.
Threat reactivity in the anterior insula. Enhanced BQLD activity during threat compared to safety in the anterior insula (bilateral functional cluster resulting from whole brain analysis showed underneath the X axis) is significantly related with reported fear of dental pain.
In addition to anterior insula activation, functional activity during threat, compared to safety, increased significantly with heightened fear of dental pain in both the mid-cingulate cortex (r = .52, p = 0.003) and the supramarginal gyrus (r = .50, p = 0.004). When activation in these three regions was linearly combined in a principal component analysis, the first principal component from this analysis correlated with fear of dental pain, r = .62, p < 0.001, and reported fear of pain, r = .46, p = 0.009. Enhanced threat reactivity in this network was also related to higher rated aversiveness of anticipated shock exposure (r = .41, p = 0.02).
No voxels in the amygdala were significant in the whole-brain analysis of threat compared to safety. A follow-up ROI analysis was performed to re-assess whether there is any evidence of amygdala activation during threat of shock, resulting in a small but significant increase in functional activity in the amygdala during threat (M = .12, SD = .20), compared to safety (M = .002, SD = .11), F(1,30) = 9.84, p = 0.004, ηp2 = .25. However, there were no significant relationships between magnitude of amygdala activation and reported fear of dental pain (r = .08, p = 0.66) or fear of general pain (r = .19, p = 0.30).
3.2. Skin conductance.
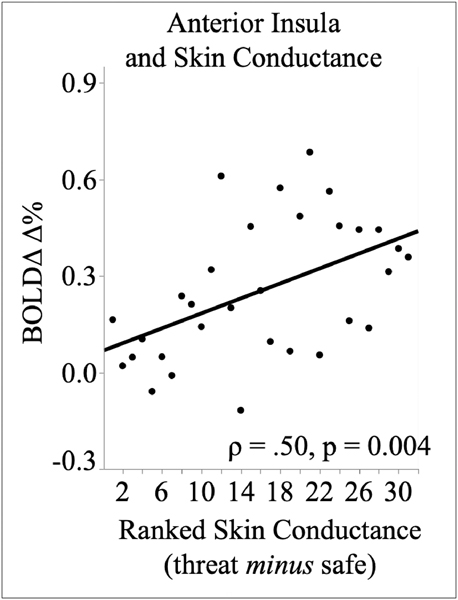
Skin conductance changes were greater during threat (M = .06 log μS), compared to safety (M = .02 log μS), F(1,30) = 8.37, p = 0.007, ηp2 = .22. As illustrated in Figure 3, skin conductance reactivity during threat, compared to safety, significantly increased with threat activation in the anterior insula (Spearman ρ = .50, p = 0.004), the mid cingulate cortex (Spearman ρ = .49, p = 0.005) and the supramarginal gyrus (Spearman p = .40, p = 0.027). The amygdala was not related to differences in skin conductance threat reactivity (Spearman ρ = .21, p = 0.27).
Figure 3.
Scatter plots show the correlation (Spearman’s rho) between skin conduct. (scores ranked in ascending order) with BQLD threat reactivity in the anterior ins
Skin conductance during threat, compared to safety, was also significantly related to fear of dental pain (Spearman ρ = .50, p = 0.004), with skin conductance changes to threat, compared to safety, increases as reported fear of dental p .increased.
3.3. Shock ratings
Consistent with the mild shock that was delivered following the anticipatory phase, neither fear of pain nor dental fear was significantly related to the rated unpleasantness of the shock (respectively, r = −.08, p = 0.66, and r = .17, p = 0.36). Neither anxiety nor depression was related to reported questionnaire scores or to aversiveness ratings during threat of shock (all Fs < 1).
4. Discussion
Anticipating a painful electric shock prompted enhanced functional activity in a network of regions including the anterior insula, mid cingulate cortex, and supramarginal gyrus, as well as in middle frontal gyrus, thalamus, precuneus, caudate, and anterior cingulate cortex. This network consists of regions reported in previous studies measuring neural activity during anticipation of painful stimulation (e.g., Alvarez et al., 2015; Kirlic et al., 2019 Mechias, Etkin, Kalish, 2010; Meyer, Padmala, Pessoa, 2019; Palermo et al., 2015). More importantly, activation in the anterior insula during threat of shock significantly covaried with individual differences in reported fear of dental pain, with anterior insula enhancement when anticipating painful stimulation increasing as reported fear of pain increased. The magnitude of threat reactivity in the anterior insula when anticipating exposure to a potentially painful event not only covaried with individual differences in the fear of experiencing dental pain, but uniquely, prompted heightened skin conductance activity when anticipating pain.
Whereas a whole brain analysis found no evidence of amygdala activation during threat of shock, compared to safety, a follow-up ROI analysis suggested a small, but significant increase threat effect in the amygdala. However, functional activity in this region did not covary with individual differences in fear of dental pain, fear of general pain, or with skin conductance response during threat compared to safety. A small, threat related difference in amygdala activation is consistent with the results of a meta-analysis which did not find reliable activation of the amygdala during threat, compared to safety, in studies investigating instructed fear (Mechias, Etkin, & Kalisch, 2009), as well as in a more recent meta-analysis of fear processing, which reported reliable activation of both the anterior insula and mid-cingulate cortex, but not the amygdala (Fullana et al., 2016). Amygdala activation may be less reliable during threat of shock because the threat cue signals only the possibility of shock exposure, compared to actual exposure to pain in acute (Kano et al., 2013) or chronic pain contexts (DaSilva & Seminowicz, 2019).
Because the homology of the human neocortex, including anterior insula and anterior/mid cingulate cortex, is absent or unclear in species such as rats (Carlén, 2017; Vogt et al., 2013, 2014), it appears that fear learning in humans may involve different specialized circuits, depending on the feared object. When the feared situation is anticipated pain, the anterior insula and mid cingulate cortex - but not the amygdala - are not only the most reliably activated regions, but also covary with individual differences in fear of pain as well as autonomic reactivity. In humans, the amygdala’s role in fear processing may be specific to situations in which the feared stimulus is cued by a sensory (e.g., visual) representation, such as in animal phobia, consistent with data finding reliable amygdala activation when processing visual cues (Brown et al., 2011; Lang & Bradley, 2010; Lang & Davis, 2006; Sambuco, Bradley, Herring, Lang, 2020; Satpute et al., 2015).
The anterior insula (together with the mid cingulate cortex) may represent a key functional region that integrates information from other anatomical regions involved in the threat of shock. For example, threat activation in the supramarginal gyrus may be reflect constant monitoring the location of lower-limbs, including the ankles to which the shock electrode was attached in the current study (e.g., Goosens et al., 2019; Iandolo et al., 2018), consistent with a hypothesis of heightened vigilance toward the sensory region in which painful stimulation was expected (Baas, Milstein, Donlevy, & Grillon. 2006; Grillon & Davis, 1997). Additionally, the increased activation in the thalamus (medio-dorsal nucleus) found in the context of cues signaling shock threat is also found when viewing aversive scenes (Frank & Sabatinelli, 2014; Sambuco et al., 2020), suggesting broad involvement in sensory processing of cues signaling aversiveness. Moreover, activation of the dorsal portion of the caudate has been reported in learning contexts involving uncertainty (Delgado, Li, Schiller & Phelps, 2008; Balleine, Delgado, & Hikosaka, 2007), which was present in multiple ways in the current study, as participants were given no information regarding the intensity, timing, or probability of possible exposure to shock.
All these sources of information might be integrated in the anterior insula, which is proposed to be a multisesory integrative hub (Chen, Michels, Supekar, Kochalka, Ryali, & Menon, 2015; Namkung, Kim, Sawa, 2017; Satpute et al., 2015) that is primarily involved in interoceptive representation and autonomic activation (Craig, 2009; Critchley et al., 2004). Together with additional evidence showing that functional activity in the anterior insula covaries with subjective expectations in painful contexts (Wiech et al., 2010), the current data suggest that fear of pain may lead to overestimation of pain intensity, providing a marker sensitive to these individual differences.
5. Limitations
Because women are generally more sensitive to pain, as well as disproportionately represented in clinical contexts involving acute or chronic pain, the current study focused on this sensitive population. Of interest is a recent study (Horn, Alappattu, Gay, Bishop, 2014), assessing pain sensitivity to thermal stimulation, in which gender differences were found that were hypothesized to be due to differences in fear of pain. Considering the accumulating evidences on gender differences in fear response strategies (Li & Graham, 2020; Schienle et al., 2013; Shansky, 2019), the current study provides the foundation to further asses potential gender differences in threat reactivity in this sensitive population.
6. Conclusions
When anticipating exposure to potentially painful stimulation, enhanced functional activity is found in a specific subset of regions in the pain matrix -- anterior insula, mid cingulate cortex, and supramarginal gyrus -- that covary significantly with individual differences in reported fear of pain. Functional activity in the amygdala, on the other hand, was not consistently involved in threat processing and did not predict individual differences in reported fear or pain or autonomic reactivity to threat cues, suggesting that treatments such as emotion regulation, which rely on amygdala activation (Morawetz, et al., 2017; Ochsner et al., 2002, 2004; Porta-Casteràs, et al., 2020; Urry et al., 2006) may be unsuccessful. More broadly, the current data challenge the assumption of a single, amygdala-centered, fear circuit that is activated in all aversive contexts. Instead, these data suggests that high fear of anticipated pain involves a brain circuit - also involved in pain perception - that includes anterior insula and cingulate cortex. Taken together, the data suggest the need to (1) identify neural markers underlying different types of phobia and (2) better characterize the role of amygdala in fear processing in humans. Ultimately, understanding the neural circuits engaged during fear processing will lead to appropriate therapeutic treatment for fear-related disorders that acknowledge potentially different neural substrates in specific fear contexts.
Highlights.
According to DSM-5, the neural system model of specific phobia is centered on the amygdala
Fear of dental pain was modeled here in a context of pain anticipation
In pain anticipation, fear of dental pain covaried with anterior insula activity, but not amygdala
Different neural substrates mediate fear response in different types of specific phobia
Acknowledgements
This research was supported in part by grants from the National Institute of Dental and Craniofacial Research (DE 13956) and the National Institute of Mental Health (P50 MH 72850).
Footnotes
Uncited References
Bublatzkv and Schupp, 2012, Cauda et al., 2011, Coghill et al., 1999, Deen et al., 2011, Liddell and Locker, 1997, Menon V. Salience Network. In 2015, Mesulam et al., 1982, Ploghaus et al., 1999, Ploner et al., 2010, Preuschoff et al., 2008, Seymour et al., 2004, Weinstein, 1990
Declaration of Competing Interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez et al., 2011.Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C. (2011). Phasic and sustained fear in humans elicits distinct patterns of brain activity. Neuroimage, 55, 389–400, 10.1016/j.neuroimage.2010.11.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez et al., 2015.Alvarez RP, Kirlic N, Misaki M, Bodurka J, Rhudy JL, Paulus MP, & Drevets WC (2015). Increased anterior insula activity in anxious individuals is linked to diminished perceived control. Translational Psychiatry, e59, 1–9. 10.1038/tp.2015.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association 2013.American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Balleine et al., 2007.Balleine BW, Delgado MR, & Hikosaka O. (2007). The role of the dorsal striatum in reward and decision-making. Journal of Neuroscience, 27,8161–8165. 10.1523/JNEUROSCI.1554-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas et al., 2006.Baas JM, Milstein J, Donlevy M, & Grillon C. (2006). Brainstem correlates of defensive states in humans. Biological Psychiatry, 59,588––593. 10.1016/j.biopsych.2005.09.009 [DOI] [PubMed] [Google Scholar]
- Balderston et al., 2017.Balderston NL, Liu L, Roberson-Nay R, Ernst M, Grillon C. (2017). The relationship between dlPFC activity during unpredictable threat and CO2-induced panic symptoms. Translational Psychiatry,7, 1266 10.1038/s41398-017-0006-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck et al., 1996.Beck AT, Steer RA, & Brown GK (1996) Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Böcker et al., 2004.Böcker KB, Baas JM, Kenemans JL, & Verbaten MN (2004). Differences in startle modulation during instructed threat and selective attention. Biological Psychology, 67, 343––58. [DOI] [PubMed] [Google Scholar]
- Bradley et al., 2005.Bradley MM, Moulder B, Lang PJ (2005). When good things go bad: The reflex physiology of defense. Psychological Science, 16,468––473. 10.1111/j.0956-7976.2005.01558.x [DOI] [PubMed] [Google Scholar]
- Bradley et al., 2008.Bradley MM, Silakowski T, Lang PJ (2008). Fear of pain and defensive activation. Pain,137,156––163. 10.1016/j.pain.2007.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown et al., 2011.Brown S, Gao X, Tisdelle L, Eickhoff SB, & Liotti M. (2011). Naturalizing aesthetics: Brain areas for aesthetic appraisal across sensory modalities. NeuroImage, 58, 250––258. 10.1016/j.neuroimage.2011.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bublatzky et al., 2013.Bublatzky F, Guerra PM, Pastor MC, Schupp HT, & Vila J. (2013). Additive effects of threat-of-shock and picture valence on startle reflex modulation. PLOS ONE, 8, 1––6. 10.1371/journal.pone.0054003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bublatzky and Schupp, 2012.Bublatzky F, & Schupp HT (2012). Pictures cueing threat: Brain dynamics in viewing explicitly instructed danger cues. Social Cognitive and Affective Neuroscience, 7, 611–22. 10.1093/scan/nsr032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caseras et al., 2010.Caseras X, Giampietro V, Lamas A, Brammer M, Villaroya O, Carmonsa S, … & Mataix-Cols D. (2010). The functional neuroanatomy of blood-injection-injury phobia: a comparison with spider phobics and healthy controls. Psychological Medicine, 40, 125––34. 10.1017/S0033291709005972 [DOI] [PubMed] [Google Scholar]
- Carlén, 2017.Carlén M. (2017). What constitutes the prefrontal cortex? Science, 358, 478–482. https://goi.org/10.1126/science.aan8868 [DOI] [PubMed] [Google Scholar]
- Cauda et al., 2011.Cauda F, D’Agata F, Sacco K, Duca S, Geminiani G, Vercelli A (2011). Functional connectivity of the insula in the resting brain. NeuroImage,55, 8–23. 10.1016/j.neuroimage.2010.11.049 [DOI] [PubMed] [Google Scholar]
- Coghill et al., 1999.Coghill RC, Sang CN, Maisog JM, Iadarola MJ (1999). Pain intensity processing within the human brain: A bilateral, distributed mechanism. Journal of Neurophysiology,82, 1934––1943. 10.1152/jn.1999.82.4.1934 [DOI] [PubMed] [Google Scholar]
- Costa et al., 2015.Costa VD, Bradley MM, Lang PJ (2015). From threat to safety: Instructed reversal of defensive reactions. Psychophysiology, 52, 325–332. 10.1111/psyp.12359 [DOI] [PubMed] [Google Scholar]
- Cox, 1996.Cox RW (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–173. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Craig, 2009.Craig AD (2009). How do you feel—now? The anterior insula and human awareness. Nature Review Neuroscience, 10, 59––70. 10.1038/nrn2555 [DOI] [PubMed] [Google Scholar]
- Critchley et al., 2004.Critchley HD, Wiens S, Rotshtein P, Ohman A, & Dolan RJ (2004). Neural systems supporting interoceptive awareness. Nature Neuroscience, 7, 189––195. 10.1038/nn1176 [DOI] [PubMed] [Google Scholar]
- DaSilva and Seminowicz, 2019.DaSilva J, & Seminowicz D. (2019). Neuroimaging of pain in animal models: a review of recent literature. PAIN Report, 4, e732. 10.1097/PR9.0000000000000732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen et al., 2011.Deen B, Pitskel NB, Pelphrey KA (2011). Three systems of insular functional connectivity identified with cluster analysis. Cerebral Cortex, 21, 1498––506. 10.1093/cercor/bhq186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado et al., 2008.Delgado MR, Li J, Schiller D, & Phelps EA (2008). The role of the striatum in aversive learning and aversive prediction errors. Philosophical Transactions of the Royal Society, 363, 3787––3800. 10.1098/rstb.2008.0161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger et al., 2003.Dilger S, Straube T, Mentzel HJ, Fitzek C, Reichenbach JR, Hecht H, Miltner WH (2003). Brain activation to phobia-related pictures in spider phobic humans: An event-related functional magnetic resonance imaging study. Neuroscience Letters, 348, 29––32. 10.1016/S0304-3940(03)00647-5 [DOI] [PubMed] [Google Scholar]
- Dunning et al., 2013.Dunning JP, DelDonno S, & Hajcak G. (2013). The effects of contextual threat and anxiety on affective startle modulation. Biological Psychology, 94, 130––135. 10.1016/j.biopsycho.2013.05.013 [DOI] [PubMed] [Google Scholar]
- Feldker et al., 2017.Feldker K, Heitmann C, Neumeister P, Tupak S, Schrammen E, Moeck R, . . . Straube T. (2017). Transdiagnostic brain responses to disorder-related threat across four psychiatric disorders. Psychological Medicine, 47(4), 730–743. 10.1016/10.1017/S0033291716002634 [DOI] [PubMed] [Google Scholar]
- Fernandes et al., 2017.Fernandes BS, Williams LM, Steiner J, Leboyer M, Carvalho AF, & Berk M. (2017). The new field of ‗precision psychiatry’. BMC Med, 15, 80 10.1186/s12916-017-0849-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank and Sabatinelli, 2014.Frank DW, & Sabatinelli D. (2014). Human thalamic and amygdala modulation in emotional scene perception. Brain Research, 1587, 69–76. 10.1016/j.brainres.2014.08.061 [DOI] [PubMed] [Google Scholar]
- Fullana et al., 2016.Fullana MA, Harrison BJ, Soriano-Mas C, Vervliet B, Cardoner N, Àvila-Parcet A, Radua J. (2016). Neural signatures of human fear conditioning: An updated and extended meta-analysis of fMRI studies. Molecular Psychiatry, 21, 500–508. 10.1038/mp.2015.88 [DOI] [PubMed] [Google Scholar]
- Goossens et al., 2019.Goossens N, Janssens L, Caeyenberghs K, Albouy G, Brumagne S. (2019). Differences in brain processing of proprioception related to postural control in patients with recurrent non-specific low back pain and healthy controls. Neuroimage: Clinical, 23, 101881. 10.1016/j.nicl.2019.101881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon et al., 1991.Grillon C, Ameli R, Woods SW, Merikangas K, Davis M. (1991). Fear-potentiated startle in humans: Effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology, 28, 588––595. 10.1111/j.1469-8986.1991.tb01999.x [DOI] [PubMed] [Google Scholar]
- Grillon et al., 1993.Grillon C, Ameli R, Woods SW, Merikangas K, Davis M. (1993). Measuring the time-course of anxiety using the fear-potentiated startle reflex. Psychophysiology, 30, 340–346. 10.1111/j.1469-8986.1993.tb02055.x [DOI] [PubMed] [Google Scholar]
- Grillon and Davis, 1997.Grillon C, & Davis M. (1997). Effects of stress and shock anticipation on prepulse inhibition of the startle reflex. Psychophysiology, 34, 511––517. 10.1111/j.1469-8986.1997.tb01737.x [DOI] [PubMed] [Google Scholar]
- Hermann et al., 2007.Hermann A, Schafer A, Walter B, Stark R, Vaitl D, Schienle A. (2007). Diminished medial prefrontal cortex activity in blood-injection-injury phobia. Biological Psychology, 75, 124––30. 10.1016/j.biopsycho.2007.01.002 [DOI] [PubMed] [Google Scholar]
- Hilbert et al., 2014.Hilbert K, Evens R, Maslowski NI, Wittchen H-U, & Lueken U. (2014). Fear processing in dental phobia during crossmodal symptom provocation: an fMRI study. Biomed Res. Int, 1––9. 10.1155/2014/196353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn et al., 2014.Horn ME, Alappattu MJ, Gay CW, Bishop M. (2014). Fear of severe pain mediates sex differences in pain sensitivity responses to thermal stimuli. Pain Research and Treatment, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iandolo et al., 2018.Iandolo R, Bellini A, Saiote C, Marre I, Bommarito G, Oesingmann N, Fleysher L, Mancardi GL, Casadio M, Inglese M. (2018). Neural correlates of lower limbs proprioception: an fMRI study of foot position matching. Human Brain Mapping, 39, 1929––1944. 10.1002/hbm.23972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano et al., 2013.Kano M, Farmer AD, Aziz Q, Giampietro V, Brammer MJ, Williams SC, Fakundo S, Coen SJ (2013). Sex differences in brain response to anticipated and experienced visceral pain in healthy subjects. American Journal of Physiology: Gastrointestinal and Liver Physiology, 304, G687––G699. 10.1152/ajpgi.00385.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirlic et al., 2019.Kirlic N, Aupperle RL, Rhudy JL, Misaki M, Kuplicki R, Sutton A, Alvarez RP (2019). Latent variable analysis of negative affect and its contributions to neural responses during shock anticipation. Neuropsychopharmacology, 44, 695––702. 10.1038/s41386-018-0187-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinknecht et al., 1973.Kleinknecht R, Klepac R, Alexander L. (1973). Origins and characteristics of fear of dentistry. Journal of the American Dental Association, 86, 842–848. 10.14219/jada.archive.1973.0165 [DOI] [PubMed] [Google Scholar]
- Kopacz and Smith, 1971.Kopacz KM, Smith BD (1971). Sex differences in skin conductance measures as a function of shock threat. Psychophysiology, 8, 293––303. 10.1111/j.1469-8986.1971.tb00459.x [DOI] [PubMed] [Google Scholar]
- Lang and Bradley, 2010.Lang PJ, & Bradley MM (2010). Emotion and the motivational brain. Biological Psychology, 84(3), 437––450. 10.1016/j.biopsycho.2009.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang and Davis, 2006.Lang PJ, & Davis M. (2006). Emotion, motivation, and the brain: Reflex foundations in animal and human research. Progress in Brain Research, 156, 3–29. 10.1016/S0079-6123(06)56001-7 [DOI] [PubMed] [Google Scholar]
- Li and Graham, 2020.Li SH, & Graham BM (2020). Progesterone levels predict reductions in behavioral avoidance following cognitive restructuring in women with spider phobia. Journal of Affective Disorders, 270, 1–8. 10.1016/j.jad.2020.03.039 [DOI] [PubMed] [Google Scholar]
- Liddell and Locker, 1997.Liddell A, & Locker D. (1997). Gender and age differences in attitudes to dental pain and dental control. Community Dentistry and Oral Epidemiology, 25, 314––318. 10.1111/j.1600-0528.1997.tb00945.x [DOI] [PubMed] [Google Scholar]
- Lueken et al., 2011.Lueken U, Kruschwitz JD, Muehlhan M, Siegert J, Hoyer J, Wittchen H-U (2011). How specific is specific phobia? Different neural response patterns in two subtypes of specific phobia. Neuroimage, 56, 363––372. 10.1016/j.neuroimage.2011.02.015 [DOI] [PubMed] [Google Scholar]
- Lueken et al., 2014.Lueken U, Hilbert K, Stolyar V, Maslowski NI, Beesdo-Baum K, Wittchen H-U (2014). Neural substrates of defensive reactivity in two subtypes of specific phobia. Soc. Cogn. Affect. Neurosci, 9, 1668––1675. 10.1093/scan/nst159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil and Rainwater, 1998.McNeil DW, Rainwater AJ (1998). Development of the fear of pain questionnaire-III. Journal of Behavioral Medicine, 21, 389–410. 10.1023/A:1018782831217 [DOI] [PubMed] [Google Scholar]
- Mechias et al., 2010.Mechias ML, Etkin A, Kalisch R. (2010). A meta-analysis of instructed fear studies: Implications for conscious appraisal of threat. NeuroImage, 49, 1760–1768. 10.1016/j.neuroimage.2009.09.040 [DOI] [PubMed] [Google Scholar]
- Menon V. Salience Network. In 2015.Menon V. Salience Network In: Toga AW, editor. Brain Mapping: An Encyclopedic Reference, Vol. 2, Academic Press: Elsevier; 2015. pp. 597–611. [Google Scholar]
- Mesulam et al., 1982.Mesulam M-M, Bishop GA, Carson KA &, King JS Tracing neural connections with horseradish peroxidase In Mesulam M-M (Ed.), Methods in the neurosciences: IBRO Handbook series. (pp. 127––130). Chichester, New York: IBRO Handbook Series; (1982). [Google Scholar]
- Meyer et al., 2019.Meyer C, Padmala S, Pessoa L. 2019. Dynamic threat processing. Journal of Cognitive Neuroscience, 31, 522––542. 10.1162/jocn_a_01363, PMID: 30513044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawetz et al., 2017.Morawetz C, Bode S, Baudewig J, & Heekeren HR (2017): Effective amygdala-prefrontal connectivity predicts individual differences in successful emotion regulation. Soc Cogn Affect Neurosci, 569––585. 10.1093/scan/nsw169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner et al., 2002.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE (2002). Rethinking feelings: An fmri study of the cognitive regulation of emotion. J. Cognitive Neuroscience, 14, 1215––29. 10.1162/089892902760807212 [DOI] [PubMed] [Google Scholar]
- Ochsner et al., 2004.Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, & Gross JJ (2004). For better or for worse: neural systems supporting the cognitive down- and upregulation of negative emotion. Neuroimage, 23, 483––99. 10.1016/j.neuroimage.2004.06.030 [DOI] [PubMed] [Google Scholar]
- Oosterink et al., 2009.Oosterink FMD, de Jongh A, & Hoogstraten J. (2009). Prevalence of dental fear and phobia relative to other fear and phobia subtypes. European Journal of Oralal Sciences 117, 135––143. 10.1111/j.1600-0722.2008.00602.x [DOI] [PubMed] [Google Scholar]
- Palermo et al., 2015.Palermo S, Benedetti F, Costa T, Amanzio M. (2015). Pain anticipation: An activation likelihood estimation meta-analysis of brain imaging studies. Human Brain Mapping, 36, 1648–1661. 10.1002/hbm.22727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps et al., 2001.Phelps EA, O’Connor KJ, Gatenby C, Gore JC, Grillon C, Davis M. (2001), Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience, 4, 437––441. 10.1038/86110 [DOI] [PubMed] [Google Scholar]
- Ploghaus et al., 1999.Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JN (1999). Dissociating pain from its anticipation in the human brain. Science, 284, 1979––1981. 10.1126/science.284.5422.1979 [DOI] [PubMed] [Google Scholar]
- Ploner et al., 2010.Ploner M, Lee MC, Wiech K, Bingel U, & Tracey I. (2010). Prestimulus functional connectivity determines pain perception in humans. PNAS, 107, 355––360. 10.1073/pnas.0906186106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta-Casteràs et al., 2020.Porta-Casteràs D, Fullana MA, Tinoco D, Martínez-Zalacaín I, Puyol J, Palao DJ, et al. (2020). Prefrontal-amygdala connectivity in trait anxiety and generalized anxiety disorder: Testing the boundaries between healthy and pathological worries. Journal of Affective Disorders, 267, 211–219. 10.1016/j.jad.2020.02.029 [DOI] [PubMed] [Google Scholar]
- Preuschoff et al., 2008.Preuschoff K, Quartz SR, Bossaerts P. (2008). Human insula activation reflects risk prediction errors as well as risk. Journal of Neuroscience, 28, 2745––2752. 10.1523/JNEUROSCI.4286-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reicherts et al., 2017.Reicherts J, Wiemer A, Gerdes ABS, Schulz SM, Pauli P, Wieser MJ (2017). Anxious anticipation and pain: The influence of instructed vs conditioned threat on pain. Social Cognitive & Affective Neuroscience, 12, 544–554. 10.1093/scan/nsw181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli et al., 2005.Sabatinelli D, Bradley MM, Fitzsimmons JR, & Lang PJ (2005). Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage, 24, 1265––1270. 10.1016/j.neuroimage.2004.12.015 [DOI] [PubMed] [Google Scholar]
- Sambuco et al., 2020.Sambuco N, Bradley MM, Herring DR, Lang PJ (2020). Common circuit or paradigm shift? The functional brain in emotional scene perception and emotional imagery. Psychophysiology, 57(4), 10.1111/psyp.13522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambuco et al., 2020.Sambuco N, Costa VD, Lang PJ, & Bradley MM (2020). Aversive perception in a threat context: Separate and independent neural activation. Biological Psychology. 10.1016/j.biopsycho.2020.107926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpute et al., 2015.Satpute A, Kang J, Bickart K, Yardley H, Wager T, & Barrett LF (2015). Involvement of sensory regions in affective experience: A meta-analysis. Frontiers in Psychology, 6, 1860 10.3389/fpsyg.2015.01860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienle et al., 2013.Schienle A, Scharmuller W, Leutgeb V, Schafer A, Stark R. (2013). Sex differences in the functional and structural neuroanatomy of dental phobia. Brain Structure and Function, 218, 779––87. 10.1007/s00429-012-0428-z [DOI] [PubMed] [Google Scholar]
- Seymour et al., 2004.Seymour B, O’Doherty JP, Dayan P, Koltzenburg M, Jones AK, Dolan RJ, Friston KJ, Frackowiak RS (2004). Temporal difference models describe higher-order learning in humans. Nature, 429, 664––667. 10.1038/nature02581 [DOI] [PubMed] [Google Scholar]
- Shansky, 2019.Shansky RM (2019). Are hormons a ―“female problem” for animal research? Science, 364, 825-- 10.1126/science.aaw7570 [DOI] [PubMed] [Google Scholar]
- Spielberger et al., 1983.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Straube et al., 2004.Straube T, Kolassa IT, Glauer M, Mentzel H, Miltner W. (2004). Effect of task conditions on brain responses to threatening faces in social phobics: An event-related functional magnetic resonance imaging study. Biological Psychiatry, 56, 921––30. 10.1016/j.biopsych.2004.09.024 [DOI] [PubMed] [Google Scholar]
- Straube et al., 2005.Straube T, Mentzel H-J, Miltner WHR (2005). Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology, 52, 163–168. 10.1159/000087987 [DOI] [PubMed] [Google Scholar]
- Straube et al., 2006.Straube T, Glauer M, Dilger S, Mentzel HJ, Miltner WH (2006). Effects of cognitive-behavioral therapy on brain activation in specific phobia. Neuroimage, 29, 125––135. [DOI] [PubMed] [Google Scholar]
- Urry et al., 2006.Urry HL, van Reekum CM, Johnston T, Kalin NH, Thurow ME, Shaefer HS, et al. (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience, 26, 4415––25. 10.1523/JNEUROSCI.3215-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk and Hoogstraten, 2003.van Wijk AJ, Hoogstraten J. (2003). The fear of dental pain questionnaire; construction and validity. European Journal of Oral Sciences, 111, 12–18. 10.1016/j.neuroimage.2005.07.007 [DOI] [PubMed] [Google Scholar]
- Veltman et al., 2004.Veltman DJ, Tuinebreijer WE, Winkelman D, Lamertsma AA, Witter MP, Dolan RJ, Emmelkamp PMG (2004). Neurophysiological correlates of habituation during exposure in spider phobia. Psychiatry Research, 132, 149––158. [DOI] [PubMed] [Google Scholar]
- Vogt et al., 2013.Vogt BA, Hof PR, Zilles K, Vogt LJ, Herold C, & Palomero-Gallagher N. (2013). Cingulate area 32 homologies in mouse, rat, macaque and human: Cytoarchitecture and receptor architecture. J Comp Neurol, 4189–4204. 10.1002/cne.23409 [DOI] [PubMed] [Google Scholar]
- Wendt et al., 2008.Wendt J, Lotze M, Weike AI, Hosten N, & Hamm Al.O. (2008). Brain activation and defensive response mobilization during sustained exposure to phobia-related and other affective pictures in spider phobia. Psychophysiology, 45, 205–215. 10.1111/j.1469-8986.2007.00620.x [DOI] [PubMed] [Google Scholar]
- Weinstein, 1990.Weinstein P. (1990). Breaking the worldwide cycle of pain, fear and avoidance: Uncovering risk factors and promoting prevention. Annals of Behavioral Medicine, 1990, 12, 141––47. 10.1093/abm/12.4.141 [DOI] [Google Scholar]
- Wiech et al., 2010.Wiech K, Lin CS, Brodersen KH, Bingel U, Pioner M, Tracey I. (2010). Anterior insula integrates information about salience into perceptual decisions about pain. Journal of Neuroscience, 2010;30:16324–31. 10.1523/JNEUROSCI.2087-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]