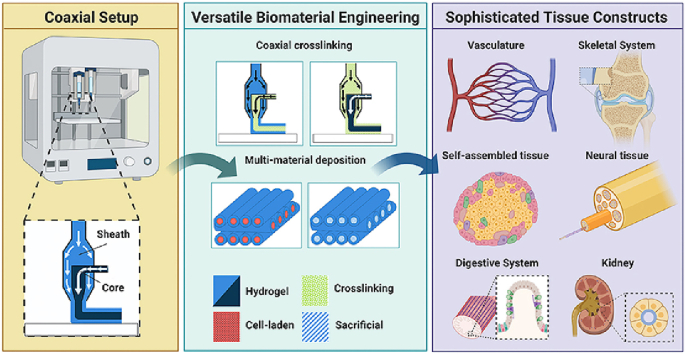
Abstract
Bioprinting is a rapidly developing technology for the precise design and manufacture of tissues in various biological systems or organs. Coaxial extrusion bioprinting, an emergent branch, has demonstrated a strong potential to enhance bioprinting's engineering versatility. Coaxial bioprinting assists in the fabrication of complex tissue constructs, by enabling concentric deposition of biomaterials. The fabricated tissue constructs started with simple, tubular vasculature but have been substantially developed to integrate complex cell composition and self-assembly, ECM patterning, controlled release, and multi-material gradient profiles. This review article begins with a brief overview of coaxial printing history, followed by an introduction of crucial engineering components. Afterward, we review the recent progress and untapped potential in each specific organ or biological system, and demonstrate how coaxial bioprinting facilitates the creation of tissue constructs. Ultimately, we conclude that this growing technology will contribute significantly to capabilities in the fields of in vitro modeling, pharmaceutical development, and clinical regenerative medicine.
Keywords: Coaxial nozzle, 3D bioprinting, Tissue engineering, Vascular constructs, Biofabrication strategies, Regenerative medicine
Graphical abstract
Highlights
-
•
Coaxial extrusion enables concentric multi-material deposition, a broader range of materials, and inline crosslinking.
-
•
Sophisticated tissue constructs including vasculature, skeletal, organoid, and neural systems have been demonstrated.
-
•
Novel applications of coaxial bioprinting are proposed; these benefit in vitro modeling and clinical regenerative medicine.
1. Introduction
Bioprinting is a rapidly developing technique for the precise manipulation and manufacture of complex tissues. There are a rapidly growing number of bioprinting techniques, including inkjet-based, extrusion-based, laser-assisted, and stereolithographic methods [1]. Of these, extrusion-based printing has received attention because it is compatible with a large number of materials, can position cells at high densities, and can utilize a variety of crosslinking methods [2]. Rapid commercialization has also aided in the widespread use of extrusion-based printing [3]. However, the technique is still in its nascency, and novel methods are being developed.
In this review article, we highlight the realized and untapped potential for a specific type of bioprinting technique: coaxial extrusion. Coaxial extrusion can be broadly defined as any extrusion technique which simultaneously deposits two or more flow streams in concentric rings, achieved by using a coaxial nozzle [4]. In the following sections, we briefly overview the history of coaxial printing and then present the mechanical hardware features necessary to implement the system for biomaterials. We illustrate various operating approaches and present tissue engineering applications to which coaxial bioprinting has demonstrated or potential applications. Finally, we briefly discuss which engineering design benchmarks will be crucial for future applications of the technology.
1.1. History
The roots of coaxial bioprinting technology go back at least 120 years. Electrospinning, a process that draws nanoscale fibers from an electrified solution, has a coaxial nozzle design in its first patent [5]. The coaxial nozzle was designed to keep fresh solvent around the nozzle to prevent clogging, a common problem even in modern systems. Electrospinning techniques are extensively reviewed elsewhere [6].
The first extrusion-based coaxial systems were developed in the 1930s, incorporating a coaxial hardening system for artificial silkworm silk [7]. Advancements in coaxial spinnerets were developed in the 1960s and 1970s [8,9], with the focus of the technology being hollow fibers, especially for use in dialysis. To our knowledge, the first biologically relevant application of a coaxial material (not just a hollow fiber or a sheath flow) was in cell encapsulation. In the 1980s, there were several studies on cell encapsulation that used a triaxial configuration (cell core, polymer sheath, and outer airflow sheath) that reduced shear stress in cells compared to other methods [10,11]. In 2003, a similar coaxial nozzle was used to electrospin two polymers into one core-sheath nanofiber. This allowed for the electrospinning of materials otherwise unsuited to the process by sheathing them in a material that is easy to electrospin or simplifies fabrication of tubes by using a sacrificial core [12].
While coaxial nozzles were being developed, the principles and motivations for coaxial geometries were being explored in fluidic systems. In 1953, Crossland Taylor made improvements on the Coulter cell counter by incorporating hydrodynamic focusing. Hydrodynamic focusing and cell counting have been researched together since 1972 [13] and have led to more recent applications in microfluidics [14]. Due to the laminar flow that characterizes microfluidic systems, they are naturally able to produce coaxial geometries, and this has been exploited in various material synthesis applications, both as threads [15] and as more complex tubes [16]. Hydrogel incorporation in these systems enabled cell-laden fibers [17,18].
While the first extrusion-based bioprinting was accomplished in 2002, the first coaxial bioprinting was done only four years later [19]. However, it was not achieved through traditional coaxial means. This study used the differences in viscosities of two materials extruded through the same syringe tip to produce a coaxial material. This “viscous encapsulation during extrusion” method, to our knowledge, has not been repeated. It was five more years before a true coaxial bioprinting setup, with control over the position of the extruded material and a coaxial extrusion syringe, was used [20]. The coaxial materials, in this case, included an alginate core and a collagen sheath. The combination of the materials allows the mechanical benefits of alginate and the biocompatibility benefits of collagen.
The intrinsic benefits of coaxial processing are consistent in both historical and current applications. These benefits can be broadly defined as (1) precise control of concentric multi-material deposition, (2) enabling a broader range of printable materials, (3) single-step deposition of sacrificial materials, (4) tunable release profiles, and (5) improved resolution through inline crosslinking. In the following sections, we demonstrate how these benefits can be exploited for various tissue engineering applications.
2. Bioprinter design
2.1. Instrumentation
2.1.1. Nozzle and extrusion
The key feature of coaxial extrusion is the use of concentric, multi-layered nozzles (Fig. 1A). Coaxial nozzles have an outer layer, which produces the sheath, and an inner layer, which produces the core. Nozzle sizes vary greatly depending on the application, and sheath nozzles with inner diameter up to 7 mm have been shown successful if proper curing and crosslinking procedures are implemented [21]. Core nozzle inner diameter can be as small as 210 μm [22]. Finer resolution has been demonstrated for single-material hybrid extrusion techniques [23]; however, inclusion of cells proves challenging as increased resolution and small nozzle sizes result in higher shear stress and damage to cells [24]. Very large nozzle sizes present opposite challenges, as the bioink yield stress may be overcome by gravity, making printing actuation impossible [25]. Other fabrication strategies may be more applicable in this case.
Fig. 1.
Key engineering components and features in coaxial bioprinting. (A) A typical extrusion coaxial bioprinter is uniquely equipped with a concentrically layered nozzle. This nozzle extrudes different materials in each layer, carried in sheath and core fluids, respectively. (B–M) By modulating hydrogels, sacrificial, cell-laden, and crosslinking materials within each fluid layer of the nozzle, as detailed in section 2.2, a variety of structures can be created to achieve co-cultures, perfusable tubular constructs, and high-resolution scaffolds. Scale bars: (B) 500 μm; (C) 250 μm; (D) 400 μm; (E) 20 μm; (F) 450 μm; (G) 300 μm; (H) 500 μm; (I) 1 mm; (J) 500 μm; (K) 200 μm; (L) 400 μm; (M) 50 μm. Adapted with permission from (B) [37], (C) [40], (D) [42], (E) [43], (F) Reprinted (adapted) with permission from Y. Wang, R. K. Kankala, K. Zhu, S.-B. Wang, Y. S. Zhang, and A.-Z. Chen, “Coaxial Extrusion of Tubular Tissue Constructs Using a Gelatin/GelMA Blend Bioink,” ACS Biomater. Sci. Eng., vol. 5, no. 10, pp. 5514–5524, Oct. 2019, https://doi.org/10.1021/acsbiomaterials.9b00926. Copyright (2019) American Chemical Society [62], (G) [44], (H) [29], (I) [30], (J) [45], (K) [79], (L) [48] and (M) [49].
For coaxial printing, the same nozzle dimensions can produce varying core to sheath ratios depending on the rate of extrusion, rheology of inks, and crosslinking parameters. Because the sheath and core fluids typically have distinct viscosities, differing extrusion rates are used for each flow stream [18]. Pneumatic and syringe pumps are used to independently control both sheath and core fluid flow. These flow rates are experimentally determined and affect the resulting dimensions. For example, increasing the flow rate of a layer will tend to thicken that layer of the resulting structure [26]. Often, increasing the flow rate of any hydrogel component will increase the diameter of the entire extruded fiber [[26], [27], [28]]. Flow rates also determine the resulting fiber morphology; by changing the ratio of core to sheath flow, straight, wavy, and helical fibers can be extruded. Triaxial and four-layered systems can also be created if printing parameters are optimized [29,30]. Arrangements of cell-laden material, crosslinking solution, hydrogel, or sacrificial material can produce different outcomes from the same nozzle, as discussed later, in Section 2.2.
The two main methods of extrusion can be classified broadly as piston-based or pneumatic. Piston extrusion, typical in custom setups, is achieved with syringe pumps whereas pneumatic extrusion, common in commercial bioprinters, is achieved by a pneumatic pump. Piston extrusion is volume defined, which allows for consistent flow of most inks, including non-homogenous mixtures [31]. This does, however, cause variations in pressure which can negatively affect cell viability if too high [32]. Pneumatic control circumvents this problem by carefully controlling pressure, but does require extensive ink tuning for precise volume deposition, and may fail with highly non-homogenous inks. While other extrusion mechanisms do exist, like cavity extrusion, they have not been utilized for coaxial bioprinting [33].
2.1.2. Crosslinking hardware
Crosslinking solidifies the printed construct and is a critical component in ensuring resolution and mechanical robustness. Crosslinking mechanisms fall into two categories; physical and chemical. Physical mechanisms rely on hydrophobic, electrostatic, or other non-covalent interactions. These interactions are inherently reversible, and are generally slow, although there are exceptions. A notable and widely used physical crosslinking method is the reaction of alginate with divalent anions such as Ca2+. This reaction is quicker than most physical methods and requires a bath [21], aerosol spray [29], or inline flow of calcium ions [30]. A vacuum under the stage may be used to remove the excess ion solution as deposition occurs [30]. Other physical crosslinking methods include temperature-based gelation, which requires a temperature-controlled printhead, print bed, or ideally both.
Chemical crosslinking involves the covalent reaction of bioprinted materials. The most ubiquitous chemical crosslinking method for bioprinting is UV-based reactions [34], although enzymatic methods are also utilized on rare occasions [32,35]. UV-based crosslinking requires a light source with wavelength and intensity compatible with the selected photoinitiator. Light-emitting diode light sources can be purchased at wavelengths of 365, 385, 395, 405, 455, or 477 nm and can vary greatly in intensity. Most researchers utilize light sources on the longest possible wavelength while still adequately crosslinking their constructs, as this minimizes cell damage [34,36]. While many studies have shown acceptable cell viability using UV-based crosslinking methods, researchers must make conscious choices to reduce the risk of photo-irradiating cells.
2.1.3. Stage
The stage of a bioprinter is where deposition of the bioink occurs, and bioprinter designs vary in the way deposition is guided to produce desired structures. Many bioprinters use a nozzle within a computer-guided biaxial printhead that moves both laterally and front to back, and a uniaxial stage that moves vertically. Other bioprinters use a stationary or uniaxial printhead and a moving stage. Ouyang et al. extruded onto a rotating rod to create a spiraled cylindrical structure resembling a spring [37]. More complex systems, such as six-axis printhead movement, are also commercially available [38]. In all cases, stepper motors guided by software input from the researcher's coded instructions actuate the movement. For optimal resolution, the rate of extrusion must match the speed of the printhead or stage [37].
Alternatively, a bioprinter without a stage or computer-controlled motion might be favorable. Bella et al. created a handheld bioprinter, or “biopen”, that can be used during surgery as a means of directly printing tissue scaffold material and cultured stem cells onto an area of damaged tissue [39]. To produce their biopen, Bella et al. created a handheld device containing a custom coaxial nozzle with feed tubes for each layer, and a crosslinking UV light source. The device could then be used in conventional surgical operation, providing another approach to coaxial bioprinting hardware.
2.2. Printing techniques
Coaxial extrusion provides for a basic core/sheath printing configuration, which can be adapted to fit the design considerations of the desired 3D structure or microenvironment. As previously stated, coaxial bioprinting can be used to control concentric multi-material deposition or improve resolution through inline crosslinking. Materials can include any combination of hydrogels, cell-laden materials, crosslinkers, or sacrificial materials. Depending on the selection of technique, the technology allows for the creation of fibers, tubular structures, composite 3D structures, and complex layered structures. Examples of each are shown in Fig. 1B – M.
2.2.1. Multi-material deposition
Multi-material deposition is used to achieve a complex structure composed of two different hydrogels capable of modulating mechanical or other properties to the structure. The technique may also allow integrating a sacrificial sheath or core, as well as co-extruding cell-laden layers for cell culture or co-culture models. Kim et al. demonstrated a mechanical property lending technique by creating multi-layer fibers with an alginate core and collagen sheath. This was done to improve mechanical properties that were not achieved with collagen fibers alone [20]. This method is generalizable to a number of materials, as demonstrated by Ouyang et al. (Fig. 1B) [37]. Structural support may also be provided by printing a more robust sheath around a soft core, such as cell-laden material. Dai et al. demonstrated this with a cell-laden fibrinogen core material, which was coaxially printed with an alginate/gelatin sheath (Fig. 1C). Encasing the dense cell laden core with a more robust sheath provided structure for fiber formation, while creating the desired microenvironment for cell growth [40].
Multi-material deposition techniques may also use sacrificial materials in either the core or sheath position. Sacrificial materials provide temporary support to the structure. Onoe et al. created meter long fibers using cell-laden extracellular matrix (ECM) protein core with an alginate sheath as a template until cell growth reached a point that the sheath could be removed [41]. Wang et al. followed a similar approach [42] (Fig. 1D). Another study demonstrated this method through the use of a sacrificial Pluronic F-127 core and an alginate/cell sheath to create bio-blood vessels for use in neovascularization [43] (Fig. 1E). Cannular structures can be obtained using a similar printing configuration (Fig. 1F) [30].
Cellular co-culture is common in coaxial bioprinting, as the coaxial configuration allows two different cell-laden materials to be co-deposited in defined layers. Kim et al. demonstrated this in an attempt to model the microvilli structures of the small intestine. Collagen/small intestinal submucosa 3D structures with human umbilical vein endothelial cells (HUVECs) were printed in the core, along with an epithelial colorectal adenocarcinoma (Caco-2) cell-laden sheath [44] (Fig. 1G). Another study used a four-layer coaxial configuration to create a co-culture model with HUVECs and human umbilical vein smooth muscle cells (HUVSMCs) separated by sodium alginate layers [29] (Fig. 1H). To create the co-culture, coaxially extruded hollow fibers were injected with both cell types after printing. Gao et al. used a combination of multi-material and sacrificial techniques to construct vascular grafts [43]. Endothelial cell-laden and smooth muscle cell-laden bioinks were printed against a sacrificial Pluronic F-127 core to create composite tubular structures suitable for vascular tissue reconstruction [43] (Fig. 1I).
2.2.2. Coaxial crosslinking
Coaxial crosslinking allows for the crosslinking reaction to begin during the extrusion process. Rather than extruding fibers into a crosslinking bath, a crosslinking solution can be printed coaxially along with the hydrogel, which can greatly improve printing resolution. One way to create tubular structures is by printing a single or multi-layer hydrogel sheath with a calcium chloride crosslinking solution core to create single layer and multi-layer tubular structures (Fig. 1J) [45]. Crosslinker in the core also promoted faster gelation, increased the print resolution, and allowed for further organization of printed tubes into scaffold structures (Fig. 1K) [46]. Costantini et al. showed that by using a crosslinking sheath around a hydrogel core, thick, multi-layered structures could be printed with fine resolution [47]. Cell-laden constructs can also be printed with a cross-linking sheath, as demonstrated by Salaris et al. with cortical neurons [48] (Figure 1L).
2.2.3. Hybrid techniques
The techniques discussed above can be used together to create unique structures requiring crosslinking and multi-material deposition methods. Colosi et al. used a combination of sheath crosslinking and microfluidic systems to create a composite fiber of two different hydrogels side by side [49] (Figure 1M). Other studies have shown microfluidic incorporation to achieve multi-material deposition or gradient mixing of components [28,[50], [51], [52]]. Heterogenous patterned filaments can be created by intermittently alternating core and sheath fluid [37,50]. Coaxial bioprinting techniques have also been used in conjunction with other modalities. For example, Cui et al. constructed a scaffold by alternating stereolithographic and coaxial extrusion techniques [53]. Hybridization of established and novel printing techniques will continue to expand this technology, allowing tissue-specific constructs (Fig. 2).
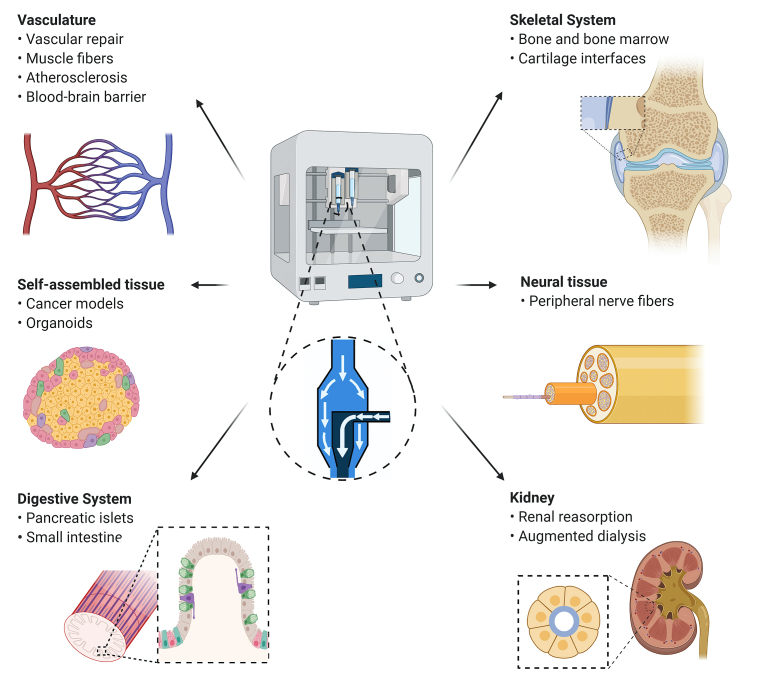
Fig. 2.
Schematic of tissue applications and key structural features that benefit from coaxial bioprinting. Various tissues can be created using coaxial printing strategies, including vasculature, skeletal tissue, self-assembled tissue, neural tissue, the digestive system, and the kidney. Specific features of interest are listed beneath each tissue type.
2.3. Bioink formulation
Selection and tuning of bioinks is central to designing bioprinting experiments. Bioink selection affects every aspect of the experiment, from required crosslinking components to ultimate cell viability. The ideal bioink would have a viscosity in the range of 30 mPa/s to 600 kPa/s [31], contain cell signaling and cell attachment molecules to induce a favorable cell response, possess mechanical properties appropriate for the application, and exhibit gelation processes that are both quick (to maintain resolution) and cytocompatible. This topic has been extensively reviewed elsewhere [31,32,35], and special attention has been given to the formulation of bioinks with photosensitive properties [34]. This topic, however, has not been discussed from the perspective of coaxial bioprinting.
In coaxial bioprinting, alginate and collagen were the first bioinks to be utilized [20], producing constructs that had the benefits of both collagen and alginate. As typically formulated, alginate has strong mechanical properties, allowing for superior resolution, whereas collagen is soft and allows for favorable cell interaction and aggregation. There has been success in both producing an alginate core with collagen sheath and an alginate sheath with collagen core [20,29]. As we discuss later, the latter approach – a low viscosity, high cell density core with a mechanically stable sheath – is a key benefit of coaxial bioprinting as it extends the range of printable bioinks.
While between 25 and 30 different materials have been used in bioprinting, coaxial bioprinting has only been used with a dozen bioinks [35]. In fact, more than half of all coaxial bioprinting publications utilize alginate or gelatin-based materials. A broader range of inks for coaxial bioprinting will help advance future experiments, as bioink requirements for cells can vary widely. This is especially the case for stem cells [54]. The desired properties of a final product can extend beyond the cells as well, and include requirements relating to functionalized peptides [55], electrical conductivity [56,57], or structural support. Coaxial bioprinting allows the possibility of even more diversity in material selection, since the core material does not necessarily need to meet traditional printability standards, as well as allowing for hollow constructs to be later perfused with other materials. For example, perfusion of decellularized ECM-based binding proteins, followed by a low viscosity cell-laden hydrogel could facilitate enhanced cell attachment and migration. In addition, to allow for a higher rate of diffusion in bioprinted constructs, sacrificial particles could be added to the sheath fluid that would later be removed by either leaching (in the case of salts) or thermal liquification (in the case of Pluronics).
3. Applications of coaxial printing
3.1. Vasculature
One major application for coaxial bioprinting has been in the creation of vasculature (Fig. 3), which is vital in most tissue engineering strategies [4]. A key clinical outcome of work in this field would be the development of fully functional, physiologically relevant tissue for vascular repair. In vitro and in vivo, lack of vascularization is often cited as one of the central challenges limiting the growth and function of tissue-engineered constructs, and significant attention is currently directed to solving this problem [58].
Fig. 3.
Vasculature applications of coaxial printing. (A) Tubular vessels were created by extruding sodium alginate onto a rotating rod (upper), and then assembled into multiscale vasculature (lower). (B) Alternatively, by crosslinking within a glass tube and varying the viscosity of the core and sheath fluids (left), coiled-rope structures (upper right) were created to provide space for the lumen formation of endothelial cells (lower right). (C) Artificial bio-blood vessels (BBV) were created using a sacrificial core fluid (left), showing enhanced limb salvage in a mouse model when laden with cells (EBBV) and statin drug (EABBV) (center right). (D) Coaxially printed vessels (left) showed better cell differentiation in vitro (center). They also significantly improved muscle-endothelial integration and blood vessel regeneration in a rat model, as compared to single fluid printing (right). Scale bars: (A) 5 mm; (B) 200 μm; (D) 200 μm. Reprinted (adapted) with permission from (A) Q. Gao et al., “3D Bioprinting of Vessel-like Structures with Multilevel Fluidic Channels,” ACS Biomater. Sci. Eng., vol. 3, no. 3, pp. 399–408, Mar. 2017, https://doi.org/10.1021/acsbiomaterials.6b00643. Copyright 2017 American Chemical Society. [61]; (B) [50]; (C) [43] and (D) [128].
Coaxially-bioprinted constructs can be used to model the fundamental multiscale characteristics of the circulatory system. Vascular structures include arteries and veins, which are 10–300 mm in diameter, arterioles and venules, which are 0.02–0.08 mm in diameter, and capillaries, which are typically 0.005–0.01 mm in diameter [59]. As presented previously, a typical coaxial nozzle resolution is 0.21 mm (inner diameter) - 7 mm (outer diameter), enabling the creation of tubes in the size range of arterioles and small arteries. Recent research has attempted to recapitulate these dimensions and multiscale arrangement. For example, a number of studies have coaxially printed macro-scale scaffolds composed of hollow fibers with the aim of increasing endothelial cell invasion and vascularization [22,46,60]. Multilevel fluidic channels can also be created by extruding onto a rotating platform: these channels could then be arranged into large, branched vasculature [61] (Fig. 3A). Thus, careful choice of nozzle dimensions and flow may enable a physiologically relevant scaffold organization.
Coaxial printing can also aid angiogenesis. Printing of cell-laden sheath and sacrificial or crosslinking core has also been shown to promote vessel-like arrangement and expression of endothelial-specific biomarkers [45,46,62,63]. Self-arranged lumen structures also emerge from cell migration through homogenous cell-laden core material [22,28] (Fig. 3B). Growth factor incorporation in coaxially extruded constructs can also achieve controlled release, whether the growth factor is incorporated in the core or the sheath fluid [21,60,64]. This can be extended for a variety of small molecules, such as angiogenic factors, sustained release of which can aid in endothelialization. As a significant step forward, Gao et al. showed that coaxially-bioprinted veins could aid in ischemic limb repair in a rodent model [43] (Fig. 3C). In their study, only cell-laden constructs were capable of limb salvage and were further improved by the inclusion of a statin drug. Further studies could follow a similar approach, taking these technologies closer to the clinic.
Models for disease and drug research are another compelling application for coaxially bioprinting vasculature. Current atherosclerosis models have been reviewed elsewhere [65,66] and can be built from 2D culture, micro-scale “on-a-chip” systems, or more traditional tissue-engineered scaffolds. Depending on the platform, challenges might include lack of three-dimensional arrangement, concerns about microfluidic sizes not modeling macro-scale processes well, complicated workflows, or incompatibility with bulk fluid flow [65]. Coaxially-bioprinted models can help advance the field as (1) coaxial printing is well suited for creating tubular structures, (2) the size scale is correct, (3) multi-layered constructs can be extruded in a single fabrication step, and (4) final constructs are perfusable. These ideas were recently demonstrated in a drug diffusion model of blood and lymphatic vessels, where coaxial printing simplified the fabrication process [67].
Coaxial prints may also aid disease models of the blood-brain barrier. The blood-brain barrier is critical for regulating transport to the brain and may be directly affected by inflammation and ischemia [68]. In vivo understanding of these processes is difficult; various 2D and 3D cell culture models have been developed to bridge this gap [69,70]. Recently, Liu et al. presented a coaxially printed model where they co-cultured brain microvascular endothelial cells, endothelial progenitor cells, and astrocytes [21]. After printing a hydrogel construct, the group sequentially deposited cell and material layers, resulting in a complex final structure. However, multi-layered coaxial printing has yet to be fully exploited and may provide simplified fabrication for novel insights into blood-brain barrier function and pathology. Additionally, key features of the blood-brain barrier, including transendothelial electrical resistance, permeability, and efflux transport, should be assayed in future work [69].
Coaxial vasculature strategies could be extended to a variety of tissues. For example, coaxial printing of endothelial cells has been successfully demonstrated in muscle fiber engineering. Coaxially-printed vasculature-laden constructs, after implantation, achieved superior re-vascularization, anastomosis, and innervation with the host tissue, likely due to the cells being geometrically compartmentalized (Fig. 3D) [43]. Another study accomplished drug testing in an endothelialized muscle construct printed with a coaxial nozzle [22]. Lastly, He et al. introduced a four-layer coaxial system for use with endothelial and smooth muscle cells [29]. These vascularization strategies are generally applicable and will provide strong motivation for the use of coaxial bioprinting amongst many different tissues.
3.2. Skeletal system
Articular cartilage, an avascular connective tissue that places a major role in the skeletal system, has received significant attention among tissue engineers [71]. Its well-characterized mechanical properties are unique in their ability to withstand loads and transfer forces through the musculoskeletal system [72,73]. The tissue is composed of anisotropic gradient layers of tissue, which are typically characterized as the superficial, middle, deep, and calcified layers [72]. Thus, in vitro assembly of functional, full-depth cartilage tissue must incorporate multiple material characteristics and gradient interfaces.
Several groups have utilized coaxial bioprinting in the creation of articular cartilage [39,47,51,74,75]. Kosik-Koziol et al. focused exclusively on the calcified cartilage zone, using core crosslinking extrusion to improve the resolution of the resulting scaffold [74]. Constantini et al. achieved a similar resolution with a microfluidic coaxial nozzle [47]. Multi-material gradient formation was recently demonstrated by Idaszek et al. [51]. The group integrated a passive microfluidic mixer within their coaxial extrusion nozzle, thereby allowing increased real-time control of the resulting print material. By modulating the flow with two gradients, a smooth transition between hyaline cartilage ink and calcified cartilage ink was achieved. Subsequently, collagen deposition and other favorable in vivo responses were observed. This approach demonstrated the potential for microfluidic-enhanced coaxial printing in biphasic, gradient systems.
Another noteworthy approach was presented by Bella et al. where a coaxial extrusion handheld bioprinter was designed [39,75] (Fig. 4A). In this case, the sheath hydrogel protected cells during extrusion and improved the mechanical properties of the final print. The use of a handheld printer facilitated onsite bespoke geometry and smooth incorporation within surgery. This initial study was a monophasic system; future work might incorporate biphasic printing in situ to extend the capabilities of the technology.
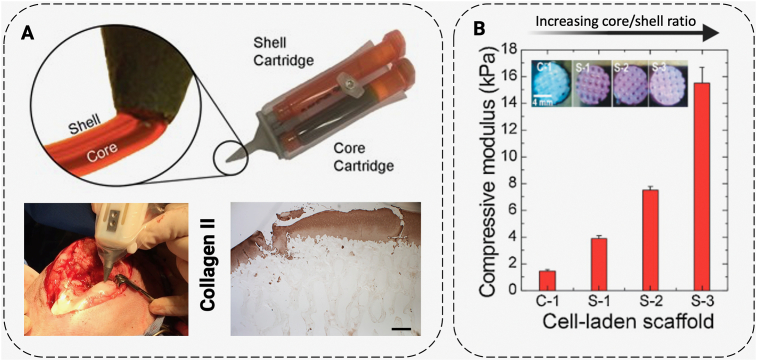
Fig. 4.
Cartilage applications of coaxial bioprinting. (A) For cartilage generation, Bella et al. developed a handheld coaxial extrusion printer, by miniaturizing the printer into a “biopen” pipette configuration (upper) that can be used for generation of cartilage on-site in a surgery (lower left). Upon implantation in a sheep model, favorable collagen formation was achieved (lower right). (B) Ahn et al. used an osteoblast precursor cell line as a middle layer in an alginate-based bioprinting system and identified an increase in mechanical properties as the core to sheath ratio increased in their constructs. Scale bars: (A) 50 μm. Adapted with permission from (A) [75] (upper) [39]; (lower) and (B) [80].
The skeletal system is also composed of bone marrow and ossified bone tissue. Bone marrow primarily consists of two types of stem cells: hemopoietic and stromal, with the former producing blood cells and the latter producing fat, cartilage, and bone. Hemopoietic cells are of particular interest as a means of producing blood ex vivo [76]. It has been recently shown that both the cell yield and enucleation increased through co-culture with stromal and macrophage cells. In addition, several studies have shown that hydrogel scaffolds are a promising method for culturing hemopoietic cells [77]. These results provide an opportunity to coaxially co-culture stromal and hemopoietic cells through coaxial bioprinting. While bioprinting of human mesenchymal stem cell-laden materials has been studied with promising results [78], the addition of a multi-layered co-culture technique would be of interest.
Relative to bone marrow, engineering of the bone itself is more straightforward. The key difference is the lack of blood production, which means that the primary concerns are cell viability and structural integrity instead of selective differentiation of hemopoietic cells into blood cells. While bioprinting for bone engineering is a well-studied field, cell infiltration and viability remain important issues [79]. One study used an osteoblast precursor cell line as a middle layer in an alginate-based bioprinting system and identified an increase in mechanical properties as a benefit of the coaxial design (Fig. 4B) [80]. Because this system incorporated cells directly, it did not have any issues with cell infiltration or non-uniform cell distribution. As was covered in 3.1.1, coaxial printing techniques could also be applied for enhanced vascularization, which is an outstanding problem in bone tissue engineering constructs [81].
3.3. Self-organized tissue (cancer and organoid models)
Proper cell motility and cell-cell contact are crucial for self-assembly of tissues, as evidenced by spheroid and organoid cultures [82,83]. Heterogenous, complex tissues have been robustly shown to self-organize from small populations of closely associated cells, such as embryoid bodies [84]. However, many cell-laden hydrogels hinder cell-cell contact instead of specifically promoting it [85]. Low density, dissociated cells seeded within hydrogels have limited intracellular junctions and migration, limiting self-assembly processes and significantly affecting cell differentiation and gene expression [86].
In cancer research, coaxial bioprinting offers unique opportunities to improve cell-cell contact and cell migration, by encapsulating larger aggregates. For example, Dai et al. demonstrated that an alginate-based sheath fluid could allow extrusion of a core fluid with soft mechanical properties and high cell density, which promoted cell migration and cell contact, respectively [40] (Fig. 5A). The high cell density allowed for better modeling of the cancer microenvironment, as evidenced by the high expression of cell specific biomarkers, as compared to alginate-based cell encapsulation. The same group extended their cancer platform for co-culture experiments and drug resistance modeling [42]. An advantage of this technique is its ability to exploit natural self-assembly processes. While not specifically used for cancer studies, similar self-assembly processes were achieved by Onoe et al. [41]. This bioprinting approach does, however, rely on a mechanically stable sheath, which can limit diffusion and waste removal; further development of ideal sheath material should be a focus of future research.
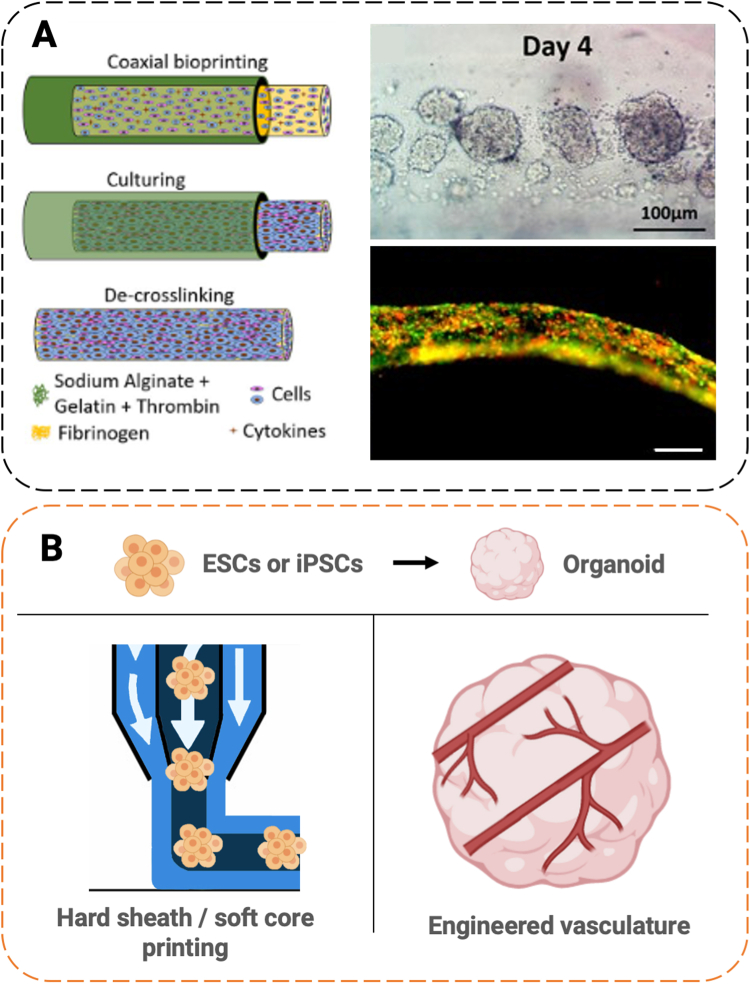
Fig. 5.
Applications of coaxial bioprinting in self-organized tissue and organoids. (A) In an example biomanufacturing process, cancer cells were extruded in a core of soft ECM material (fibrinogen) with a porous shell of crosslinking alginate (left). This allowed cells to aggregate by day 4 (upper right), and form fibers by day 7 (lower right). Adapted with permission from Dai et al. [40]. (B) Organoids, which model a number of tissues, are derived from embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs) (upper). In our future perspective, organoid fabrication can benefit from the coaxial printing by extruding stem cells in a hard sheath/soft core configuration, prioritizing cell-cell contact and protection (lower left). Alternatively, coaxial printing of endothelial/muscle combinations could be used at the time of cell aggregation to engineer vasculature (lower right). Scale bars (A) 100 μm (upper right); 200 μm (lower right).
Similar coaxial bioprinting techniques could be extended to the growing field of organoid engineering. Organoids, self-assembled tissues derived from stem cells, are valuable for recapitulating heterogenous early developmental processes and are increasingly being commercialized [87]. Organoids have been used in a staggering number of applications, including intestinal, cerebral, pancreatic, kidney, hepatic, retinal, lung, colonic, gastric, thyroid, prostate, salivary, mammary, lingual, placental, and spinal tissues, which are reviewed elsewhere [88]. Bioprinting has only recently seen application in organoid literature [[89], [90], [91], [92], [93], [94], [95], [96]]. In coaxial systems, developing organoid tissues might be printed within a sacrificial sheath hydrogel, allowing for mechanical strength and hierarchal arrangement, without compromising cell viability (Fig. 5B). Importantly, bioprinting within soft material preserves cell-cell contacts. A few studies have demonstrated spheroid or organoid bioprinting into pharmaceutical well-plate assays [89,90,97]. Coaxial bioprinting with a sacrificial sheath and soft core could augment these approaches, bringing the same benefits of enhanced cell-cell contact and self-assembly. Alternatively, coaxial bioprinting during organoid aggregation might aid in the creation of vascular structures within self-organized tissues, an outstanding unsolved problem [88].
3.4. Neural tissue
Although neural cell culture has been an extensive focus of microfabrication, bioprinting of neural cells has emerged more slowly [98,99]. Bioprinting with neurons is challenging, as neural cells are susceptible to their microenvironment, especially shear stress during the extrusion process [100]. Materials for neural cell culture should provide adhesion, cytocompatibility, and a soft elastic modulus, which is challenging to balance with the demands of printability. A few studies have demonstrated viable printing of neurons within a coaxial system. Onoe et al. encapsulated cortical cells within an ECM protein hydrogel, which formed nerve fibers [41]. Lozano et al. coaxially printed multi-layer structures of gellan gum-RGD hydrogel, achieving axon penetration through the construct [101]. A co-culture system of neuron and glia was developed by Salaris et al. to allow for electrophysiological measurements [48]. In the first example, a sacrificial sheath allowed for extrusion of soft ECM material. In the latter two cases, crosslinking/cell-laden approaches were used to improve the resolution of the resulting structure. Further innovation in bioprinting of neurons may provide novel insights into 3D culturing, precise network formation, and imbedded sensor strategies.
A major unexplored application of coaxial neural printing is the regeneration of peripheral nerve fibers. Peripheral nervous repair remains expensive, with success rates around 50% [102]. Thus, a bioengineered strategy is of great interest. Various biofabrication strategies for peripheral nervous system repair are reviewed elsewhere [103,104]. Notably, these include the creation of neural guidance conduits (Fig. 6). Neural guidance conduits are thin, tubular structures that typically have an inside diameter of 1–2 mm and lengths between 5 and 80 mm, depending on the in vivo model [103,105]. Because of the method's intrinsic benefits, coaxial bioprinting may be promising for peripheral nerve fiber repair [103]. In many ways, the technology uniquely satisfies the demands of neural guidance conduits as it enables precise, multi-layered, tubular structures at the correct size scale. For example, a sacrificial core might produce hollow neural guidance conduits, while growth factors might be incorporated into the sheath flow for enhanced neural growth. However, to our knowledge, this field remains largely unexplored.
Fig. 6.
Neural tissue applications of coaxial printing and future directions. (A) Neural guidance conduits have shown favorable results in rat models for peripheral nervous repair. As shown in their SEM images of sectional view (upper), neural guidance conduits are typically made of hollow tubes with a micro-porous wall. The dashed circle (center) shows a regenerated sciatic nerve, 16 weeks after implantation of a neural guidance conduit. (B) Coaxial bioprinting is well suited to create this structure, as shown in the schematic: this remains an untapped potential for neural guidance conduits. Scale bars (A) 200 μm (left); 1 mm (right); 10 mm (lower). Adapted with permission from Hu et al. [129].
3.5. Digestive system
Significant attention has been given to tissue engineering of the digestive system; other reviews have focused on bioprinting strategies for this organ system, so we present only enough background here to demonstrate the use for coaxial bioprinting in these tissues [[106], [107], [108], [109]]. Applications include pancreatic islet culture for diabetes treatment [110] and intestinal engineering [44,111].
For the treatment of diabetes, the end functional goal is to restore euglycemia through endogenous insulin production. Macroencapsulation is a potential solution [112]. In this technique, large numbers of transplanted cells are encapsulated and implanted, ideally producing insulin while avoiding immune cell interactions and response. Oxygen diffusion within macroencapsulated islets is often the limiting factor [112]. Liu et al. demonstrated a coaxial approach for multiscale printing pancreatic islets aimed to meet these requirements (Fig. 7A). The resulting bioprints showed multiscale organization, but failed to achieve active insulin production [113]. In an earlier study, cell-laden fibers were coaxially extruded and used as functionally active, removable implants in a mouse model [41]. The fibers were injected through a micro-catheter, allowing minimally invasive surgery. In the future, careful selection of hydrogel properties and extrusion parameters might allow for future macroscale control without compromising cell viability.
Fig. 7.
Digestive system applications of coaxial printing. (A) Pancreatic islet fibers inside an alginate/gelatin hydrogel sheath were coaxially printed (upper) and showed islet encapsulation and viability (lower) (B) Finger-like villus structures (upper) were mimicked through microscale positioning of intestinal cells using coaxial bioprinting (Caco-2 core and HUVEC endothelial sheath). End-closure was achieved by lifting the nozzle (red arrow). Immunocytochemistry revealed the development of the Caco-2/HUVEC encapsulation into matured endothelium monolayer and capillaries (lower). Scale bars (A) 200 μm (lower); (B) 100 μm (bottom left); 50 μm (lower right). Adapted with permission from (A) [113] and (B) [44]. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Coaxial printing has also been applied to intestinal tissue engineering. The intestines display multiscale organization, and are composed of canonical crypt-villus structures at a microscale level (features smaller than 1 mm) and a series of concentric tissue types at a macroscale level (features larger than 1 mm) [114]. One major focus of intestinal tissue engineering is in vitro models of drug absorption for pharmaceutical applications [115]. The most common approach, microfluidics, can achieve complex, multicellular, mechanically-active, flow-driven systems [116]. While these systems have shown their utility, they are complex to implement. Coaxial bioprinting might represent an opportunity for simplified workflows, as perfusable, multi-layered constructs can be extruded in a single step. As was discussed in section 3.3, cells could also be extruded at high density, promoting cell-cell contact and self-organization. Alternatively, crypt-villus-like structures could also be directly patterned. For example, Kim et al. achieved a crypt-villus-like structure with accurate positioning at the microscale, by simultaneously printing an endothelial cell-laden core with a Caco-2 cell-laden sheath (Fig. 7B). Further studies could apply similar methods and demonstrate drug adsorption [44] and toxicity screening. Other groups engaged in intestinal engineering aim to regenerate lost or damaged tissue, especially in the case of short bowel syndrome; coaxial bioprinting strategies may prove useful in this field as well [111,114,117].
3.6. Kidney
Kidney engineering falls into two main groups; regenerative therapy of the kidney or the modulation of dialysis systems. In the simplest sense, a kidney can be considered as a series of tubes that diffuse waste across a barrier [118]. Both dialysis and kidney modeling systems work on this basic principle. Despite the clear structural parallels between kidneys and coaxial bioprinting, to our best knowledge, no coaxial bioprinting has been used to study kidney tissue engineering.
Coaxial bioprinting can simplify kidney approaches. The use of bioprinting in kidney engineering was proposed in 2017 and realized the following year by a group from MIT [118,119]. A four-step process consisting of printing a path, casting, removing the printed material, and seeding the resulting tube with cells made significant progress in kidney-on-a-chip modeling [113]. By using coaxial bioprinting, the workflow could be simplified. A three-layered coaxial bioprinting system consisting of a crosslinking core, cell-laden middle layer, and supporting outer layer would increase speed and decrease processing steps.
In addition to improving kidney models, dialysis techniques are also a suitable application of coaxial bioprinting. Emergent techniques in dialysis rely on cell-laden tubes. Cell-laden dialysis systems incorporate the metabolic and endocrine function of a working kidney [120], and thus improve traditional dialysis systems. Upscaling has been shown in multi-step processes [121]. Future applications of coaxial bioprinting could produce cell-laden membranes in a single step. For example, cellulose based sheath flow with a low-viscosity core laden with kidney cells would be a viable option to produce living dialysis membranes.
4. Future perspective
4.1. Commercialization
Because the technology is fairly young, the majority of coaxial bioprinting studies have utilized custom-made bioprinting setups. This approach limits the repeatability of results between labs. Commerical coaxial bioprinting setups provide an opportunity for more widespread adoption of the techniques. Currently, available products range from basic setups, which can handle one core and one sheath fluid [122], to advanced microfluidic bioprinting strategies, which can be programmed to produce a variety of results [123]. Coaxial extrusion bioprinters include the Allevi 2/3 (Allevi, USA), RX1 Bioprintter (Aspect Biosystems, Canada), BioAssemblyBot (Advanced Solutions, USA), Bioscaffolder 3.1 (GeSiM, Germany), and 3DDiscovery Evolution (RegenHU, Switzerland) [38,[122], [123], [124], [125]].
4.2. Limitations
In the previous section, we have attempted to demonstrate the utility of coaxial bioprinting. However, challenges remain in terms of robust formulation, characterization, and standardization. For example, ink compatibility and formulation, especially in multi-material systems, remains a challenge [35]. While coaxial bioprinting can help improve printing resolution, extrusion-based bioprinting is still limited compared to other techniques, such as stereolithography [1]. Once a construct has been printed, mechanical characterization is important, especially in the case of load-bearing tissues. However, mechanical characterization is parameter-dependent, and characterization varies widely between studies [73]. In all cases, coaxial bioprinting studies are limited; future work will help standardize and validate the initial findings we have reviewed here.
Future work will also need to fulfill key biological requirements. For example, as coaxial printing allows for simplified co-culture experiments, experimenters need to address media optimization [126]. Current approaches include the use of minimal essential media [22,29,44] or ratios of more complex media formulations [30]. Additionally, nutrient exchange within constructs is a formidable barrier. While coaxial bioprinting of vasculature has demonstrated its ability to enable thicker tissue fabrication, these tissues still have relatively small volumes (usually all dimensions are under a centimeter), and future studies will need to continue to push this limit [63]. Time of fabrication for thick tissues is another key barrier that must be critically addressed, especially for sensitive cells [127]. This can in some ways be improved through the use of novel crosslinking approaches such as hybrid inline-UV crosslinking [24,34], but a lack of standardization both in intensity and duration of crosslinking still hinders development in this area. The optimal concentrations of photoinitiator and corresponding low intensity light sources will need to be characterized, especially as the field begins to engineer constructs that also change with respect to time (4D bioprinting).
4.3. Prospects
Coaxial bioprinting has demonstrated clear advantages for tissue fabrication: the technique enables a wider range of printable materials, a single-step deposition of sacrificial materials, and improved resolution through inline crosslinking. Importantly, coaxially-printed tissue constructs have also been shown to promote favorable biological outcomes, such as improved vascularization and host integration. A number of tissues, including cartilage and pancreatic islets, have been fabricated; coaxial bioprinting has clear, unexplored potential in neural guidance conduits, among the other areas we outline. The integration of self-assembled tissue bioprinting into pharmaceutical well-plate format assays also has a bright future [89,97]. The technology also broadens automation strategies for macro-scale tissue fabrication, which could benefit drug screening workflows. As the field matures, we envision that coaxial bioprinting will provide significant insights into the fields of in vitro modeling, pharmaceutical development, and clinical regenerative medicine.
CRediT authorship contribution statement
Andrew Kjar: Conceptualization, Writing - original draft, Writing - review & editing. Bailey McFarland: Writing - original draft, Writing - review & editing. Keetch Mecham: Writing - original draft, Writing - review & editing. Nathan Harward: Writing - original draft, Writing - review & editing. Yu Huang: Conceptualization, Writing - review & editing.
Declaration of competing interest
None.
Acknowledgment
We thank Utah State University's College of Engineering Undergraduate Research Program (EURP) for supporting Andrew Kjar and Bailey McFarland.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Li J., Chen M., Fan X., Zhou H. Recent advances in bioprinting techniques: approaches, applications and future prospects. J. Transl. Med. 2016;14(1):271. doi: 10.1186/s12967-016-1028-0. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao G., Kim B.S., Jang J., Cho D.-W. Recent strategies in extrusion-based three-dimensional cell printing toward organ biofabrication. ACS Biomater. Sci. Eng. 2019;5(3):1150–1169. doi: 10.1021/acsbiomaterials.8b00691. Mar. [DOI] [PubMed] [Google Scholar]
- 3.Choudhury D., Anand S., Naing M.W. The arrival of commercial bioprinters - towards 3D bioprinting revolution! Int. J. Bioprinting. 2018;4(2) doi: 10.18063/ijb.v4i2.139. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gu Z., Fu J., Lin H., He Y. Development of 3D bioprinting: from printing methods to biomedical applications. Asian J. Pharm. Sci., Dec. 2019 doi: 10.1016/j.ajps.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.J. F. Cooley, “Apparatus for electrically dispersing fluids,” US692631A, Feb. 04, 1902.
- 6.Xue J., Wu T., Dai Y., Xia Y. Electrospinning and electrospun nanofibers: methods, materials, and applications. Chem. Rev. Apr. 2019;119(8):5298–5415. doi: 10.1021/acs.chemrev.8b00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alwin S.H. ” US2046930A; 1936. Apparatus for Spinning Artificial Silk. Jul. 07. [Google Scholar]
- 8.G. Emil, “Spinnerette for the production of hollow filaments,” US3075242A, Jan. 29, 1963.
- 9.E. A. Mclain and H. I. Mahon, “Permselective hollow fibers and method of making,” US3423491A, Jan. 21, 1969.
- 10.Sefton M.V., Dawson R.M., Broughton R.L., Blysniuk J., Sugamori M.E. Microencapsulation of mammalian cells in a water-insoluble polyacrylate by coextrustion and interfacial precipitation. Biotechnol. Bioeng. 1987;29(9):1135–1143. doi: 10.1002/bit.260290914. [DOI] [PubMed] [Google Scholar]
- 11.Sucamori M., Sefton M. Microencapsulation of pancreatic islets in a water insoluble polyacrylate. ASAIO (Am. Soc. Artif. Intern. Organs) Trans. Dec. 1989;35(4):791–799. [PubMed] [Google Scholar]
- 12.Sun Z., Zussman E., Yarin A.L., Wendorff J.H., Greiner A. “Compound core–shell polymer nanofibers by Co-electrospinning. Adv. Mater. 2003;15(22):1929–1932. doi: 10.1002/adma.200305136. [DOI] [Google Scholar]
- 13.Shuler M.L., Aris R., Tsuchiya H.M. Hydrodynamic focusing and electronic cell-sizing techniques. Appl. Microbiol. Sep. 1972;24(3):384–388. doi: 10.1128/am.24.3.384-388.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyake R., Ohki H., Yamazaki I., Yabe R. A Development of Micro Sheath Flow Chamber,” in [1991] Proceedings. IEEE Micro Electro Mechanical Systems; Jan. 1991. pp. 265–270. [DOI] [Google Scholar]
- 15.Kenis P.J.A., Ismagilov R.F., Whitesides G.M. Microfabrication inside capillaries using multiphase laminar flow patterning. Science. 1999;285(5424):83–85. doi: 10.1126/science.285.5424.83. Jul. [DOI] [PubMed] [Google Scholar]
- 16.Jeong W., Kim J., Kim S., Lee S., Mensing G., Beebe D.J. “Hydrodynamic microfabrication via ‘on the fly’ photopolymerization of microscale fibers and tubes. Lab Chip. 2004;4(6):576–580. doi: 10.1039/B411249K. Nov. [DOI] [PubMed] [Google Scholar]
- 17.Shin S.-J. “‘On the fly’ continuous generation of alginate fibers using a microfluidic device. Langmuir. Aug. 2007;23(17):9104–9108. doi: 10.1021/la700818q. [DOI] [PubMed] [Google Scholar]
- 18.Lee K.H., Shin S.J., Park Y., Lee S.H. Synthesis of cell-laden alginate hollow fibers using microfluidic chips and microvascularized tissue-engineering applications. Small. Jun. 2009;5(11):1264–1268. doi: 10.1002/smll.200801667. [DOI] [PubMed] [Google Scholar]
- 19.Zein I., Hutmacher D.W., Tan K.C., Teoh S.H. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials. 2002;23(4):1169–1185. doi: 10.1016/S0142-9612(01)00232-0. Feb. [DOI] [PubMed] [Google Scholar]
- 20.Kim G., Ahn S., Kim Y., Cho Y., Chun W. “Coaxial structured collagen–alginate scaffolds: fabrication, physical properties, and biomedical application for skin tissue regeneration. J. Mater. Chem. Apr. 2011;21(17):6165–6172. doi: 10.1039/C0JM03452E. [DOI] [Google Scholar]
- 21.Liu L., Li X., Zhang X., Xu T. Biomanufacturing of a novel in vitro biomimetic blood-brain barrier model. Biofabrication. Apr. 2020;12(3) doi: 10.1088/1758-5090/ab4647. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y.S. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials. Dec. 2016;110:45–59. doi: 10.1016/j.biomaterials.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kjar A., Huang Y. Application of micro-scale 3D printing in pharmaceutics. Pharmaceutics. 2019;11(8):390. doi: 10.3390/pharmaceutics11080390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blaeser A., Campos D.F.D., Puster U., Richtering W., Stevens M.M., Fischer H. Controlling shear stress in 3D bioprinting is a key factor to balance printing resolution and stem cell integrity. Adv. Healthc. Mater. 2016;5(3):326–333. doi: 10.1002/adhm.201500677. [DOI] [PubMed] [Google Scholar]
- 25.Chimene D., Kaunas R., Gaharwar A.K. Hydrogel bioink reinforcement for additive manufacturing: a focused review of emerging strategies. Adv. Mater. 2020;32(1):1902026. doi: 10.1002/adma.201902026. [DOI] [PubMed] [Google Scholar]
- 26.Gao Q., He Y., Fu J., Liu A., Ma L. Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials. Aug. 2015;61:203–215. doi: 10.1016/j.biomaterials.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 27.Liu W. Coaxial extrusion bioprinting of 3D microfibrous constructs with cell-favorable gelatin methacryloyl microenvironments. Biofabrication. Jan. 2018;10(2) doi: 10.1088/1758-5090/aa9d44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shao L. Fiber-based mini tissue with morphology-controllable GelMA microfibers. Small. 2018;14(44):1802187. doi: 10.1002/smll.201802187. [DOI] [PubMed] [Google Scholar]
- 29.He J., Shao J., Li X., Huang Q., Xu T. Bioprinting of coaxial multicellular structures for a 3D co-culture model. Bioprinting. Sep. 2018;11 doi: 10.1016/j.bprint.2018.e00036. [DOI] [Google Scholar]
- 30.Gao G. Tissue-engineering of vascular grafts containing endothelium and smooth-muscle using triple-coaxial cell printing. Appl. Phys. Rev. Oct. 2019;6(4) doi: 10.1063/1.5099306. [DOI] [Google Scholar]
- 31.Hölzl K., Lin S., Tytgat L., Vlierberghe S.V., Gu L., Ovsianikov A. Bioink properties before, during and after 3D bioprinting. Biofabrication. Sep. 2016;8(3) doi: 10.1088/1758-5090/8/3/032002. [DOI] [PubMed] [Google Scholar]
- 32.Gungor-Ozkerim P.S., Inci I., Zhang Y.S., Khademhosseini A., Dokmeci M.R. Bioinks for 3D bioprinting: an overview. Biomater. Sci. May 2018;6(5):915–946. doi: 10.1039/C7BM00765E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisch P., Holub M., Zenobi-Wong M. ” Bioengineering; preprint: Jan. 2020. Improved Accuracy and Precision of Bioprinting through Progressive Cavity Pump-Controlled Extrusion. [DOI] [PubMed] [Google Scholar]
- 34.Lim K.S., Galarraga J.H., Cui X., Lindberg G.C.J., Burdick J.A., Woodfield T.B.F. Fundamentals and applications of photo-cross-linking in bioprinting. Chem. Rev. 2020 doi: 10.1021/acs.chemrev.9b00812. Apr. [DOI] [PubMed] [Google Scholar]
- 35.Hospodiuk M., Dey M., Sosnoski D., Ozbolat I.T. The bioink: a comprehensive review on bioprintable materials. Biotechnol. Adv. Mar. 2017;35(2):217–239. doi: 10.1016/j.biotechadv.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Oregon Health & Science University Department of Orthopaedics & Rehabilitation, OP31, 3181 Sam Jackson Park Road, Portland OR 97239, USA et al., “Visible light photoinitiation of mesenchymal stem cell-laden bioresponsive hydrogels. Eur. Cell. Mater. 2011;22:43–55. doi: 10.22203/eCM.v022a04. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ouyang L., Highley C.B., Sun W., Burdick J.A. A generalizable strategy for the 3D bioprinting of hydrogels from nonviscous photo-crosslinkable inks. Adv. Mater. 2017;29(8):1604983. doi: 10.1002/adma.201604983. [DOI] [PubMed] [Google Scholar]
- 38.“3D Bioprint & Vascularize Tissues,” Advanced Solutions. https://www.advancedsolutions.com accessed Jun. 16, 2020.
- 39.Bella C.D. In situ handheld three-dimensional bioprinting for cartilage regeneration. J. Tissue Eng. Regen. Med. 2018;12(3):611–621. doi: 10.1002/term.2476. [DOI] [PubMed] [Google Scholar]
- 40.Dai X. Coaxial 3D bioprinting of self-assembled multicellular heterogeneous tumor fibers. Sci. Rep. May 2017;7(1) doi: 10.1038/s41598-017-01581-y. Art. no. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Onoe H. Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat. Mater. Jun. 2013;12(6) doi: 10.1038/nmat3606. Art. no. 6. [DOI] [PubMed] [Google Scholar]
- 42.Wang X. Coaxial extrusion bioprinted shell-core hydrogel microfibers mimic glioma microenvironment and enhance the drug resistance of cancer cells. Colloids Surf. B Biointerfaces. Nov. 2018;171:291–299. doi: 10.1016/j.colsurfb.2018.07.042. [DOI] [PubMed] [Google Scholar]
- 43.Gao G. Tissue engineered bio-blood-vessels constructed using a tissue-specific bioink and 3D coaxial cell printing technique: a novel therapy for ischemic disease. Adv. Funct. Mater. 2017;27(33):1700798. doi: 10.1002/adfm.201700798. [DOI] [Google Scholar]
- 44.Kim W., Kim G.H. An intestinal model with a finger-like villus structure fabricated using a bioprinting process and collagen/SIS-based cell-laden bioink. Theranostics. Jan. 2020;10(6):2495–2508. doi: 10.7150/thno.41225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pi Q. Digitally tunable microfluidic bioprinting of multilayered cannular tissues. Adv. Mater. 2018;30(43):1706913. doi: 10.1002/adma.201706913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jia W. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials. Nov. 2016;106:58–68. doi: 10.1016/j.biomaterials.2016.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Costantini M. 3D bioprinting of BM-MSCs-loaded ECM biomimetic hydrogels for in vitro neocartilage formation. Biofabrication. Jul. 2016;8(3) doi: 10.1088/1758-5090/8/3/035002. [DOI] [PubMed] [Google Scholar]
- 48.Salaris F. 3D bioprinted human cortical neural constructs derived from induced pluripotent stem cells. J. Clin. Med. Oct. 2019;8(10) doi: 10.3390/jcm8101595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colosi C. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low viscosity bioink. Adv. Mater. Deerfield Beach Fla. Jan. 2016;28(4):677–684. doi: 10.1002/adma.201503310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shao L., Gao Q., Xie C., Fu J., Xiang M., He Y. Bioprinting of cell-laden microfiber: can it become a standard product? Adv. Healthc. Mater. 2019;8(9):1900014. doi: 10.1002/adhm.201900014. [DOI] [PubMed] [Google Scholar]
- 51.Idaszek J. 3D bioprinting of hydrogel constructs with cell and material gradients for the regeneration of full-thickness chondral defect using a microfluidic printing head. Biofabrication. Jul. 2019;11(4) doi: 10.1088/1758-5090/ab2622. [DOI] [PubMed] [Google Scholar]
- 52.Feng F., He J., Li J., Mao M., Li D. Multicomponent bioprinting of heterogeneous hydrogel constructs based on microfluidic printheads. Int. J. Bioprinting. Jul. 2019;5(2) doi: 10.18063/ijb.v5i2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cui H. In vitro and in vivo evaluation of 3D bioprinted small-diameter vasculature with smooth muscle and endothelium. Biofabrication. Oct. 2019;12(1) doi: 10.1088/1758-5090/ab402c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duarte Campos D.F. The stiffness and structure of three-dimensional printed hydrogels direct the differentiation of mesenchymal stromal cells toward adipogenic and osteogenic lineages. Tissue Eng. Feb. 2015;21(3–4):740–756. doi: 10.1089/ten.TEA.2014.0231. [DOI] [PubMed] [Google Scholar]
- 55.Loo Y., Lakshmanan A., Ni M., Toh L.L., Wang S., Hauser C.A.E. Peptide bioink: self-assembling nanofibrous scaffolds for three-dimensional organotypic cultures. Nano Lett. Oct. 2015;15(10):6919–6925. doi: 10.1021/acs.nanolett.5b02859. [DOI] [PubMed] [Google Scholar]
- 56.Mannoor M.S. 3D printed bionic ears. Nano Lett. Jun. 2013;13(6):2634–2639. doi: 10.1021/nl4007744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu K. Gold nanocomposite bioink for printing 3D cardiac constructs. Adv. Funct. Mater. Mar. 2017;27(12) doi: 10.1002/adfm.201605352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang G., Mahadik B., Choi J.Y., Fisher J.P. Vascularization in tissue engineering: fundamentals and state-of-art. Prog. Biomed. Eng. Jan. 2020;2(1) doi: 10.1088/2516-1091/ab5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones C.M. Measurement science in the circulatory system. Cell. Mol. Bioeng. Mar. 2014;7(1):1–14. doi: 10.1007/s12195-013-0317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Akkineni A.R., Ahlfeld T., Lode A., Gelinsky M. A versatile method for combining different biopolymers in a core/shell fashion by 3D plotting to achieve mechanically robust constructs. Biofabrication. Oct. 2016;8(4) doi: 10.1088/1758-5090/8/4/045001. [DOI] [PubMed] [Google Scholar]
- 61.Gao Q. 3D bioprinting of vessel-like structures with Multilevel fluidic channels. ACS Biomater. Sci. Eng. Mar. 2017;3(3):399–408. doi: 10.1021/acsbiomaterials.6b00643. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y., Kankala R.K., Zhu K., Wang S.-B., Zhang Y.S., Chen A.-Z. Coaxial extrusion of tubular tissue constructs using a gelatin/GelMA blend bioink. ACS Biomater. Sci. Eng. Oct. 2019;5(10):5514–5524. doi: 10.1021/acsbiomaterials.9b00926. [DOI] [PubMed] [Google Scholar]
- 63.Shao L., Gao Q., Xie C., Fu J., Xiang M., He Y. Directly coaxial 3D bioprinting of large-scale vascularized tissue constructs. Biofabrication. May 2020;12(3) doi: 10.1088/1758-5090/ab7e76. [DOI] [PubMed] [Google Scholar]
- 64.Moroni L., Schotel R., Sohier J., de Wijn J.R., van Blitterswijk C.A. Polymer hollow fiber three-dimensional matrices with controllable cavity and shell thickness. Biomaterials. Dec. 2006;27(35):5918–5926. doi: 10.1016/j.biomaterials.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 65.Simon L.R., Masters K.S. Disease-inspired tissue engineering: investigation of cardiovascular pathologies. ACS Biomater. Sci. Eng. May 2020;6(5):2518–2532. doi: 10.1021/acsbiomaterials.9b01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Savoji H. Cardiovascular disease models: a game changing paradigm in drug discovery and screening. Biomaterials. 2019;198:3–26. doi: 10.1016/j.biomaterials.2018.09.036. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cao X. A tumor-on-a-chip system with bioprinted blood and lymphatic vessel pair. Adv. Funct. Mater. 2019;29(31):1807173. doi: 10.1002/adfm.201807173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cho H. Three-dimensional blood-brain barrier model for in vitro studies of neurovascular pathology. Sci. Rep. Oct. 2015;5(1) doi: 10.1038/srep15222. Art. no. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DeStefano J.G., Jamieson J.J., Linville R.M., Searson P.C. Benchmarking in vitro tissue-engineered blood-brain barrier models. Fluids Barriers CNS. Dec. 2018;15(1):32. doi: 10.1186/s12987-018-0117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van der Helm M.W., van der Meer A.D., Eijkel J.C.T., van den Berg A., Segerink L.I. Microfluidic organ-on-chip technology for blood-brain barrier research. Tissue Barriers. Jan. 2016;4(1) doi: 10.1080/21688370.2016.1142493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huey D.J., Hu J.C., Athanasiou K.A. Unlike bone, cartilage regeneration remains elusive. Science. Nov. 2012;338(6109):917–921. doi: 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Little C.J., Bawolin N.K., Chen X. Mechanical properties of natural cartilage and tissue-engineered constructs. Tissue Eng. B Rev. Aug. 2011;17(4):213–227. doi: 10.1089/ten.TEB.2010.0572. [DOI] [PubMed] [Google Scholar]
- 73.Patel J.M., Wise B.C., Bonnevie E.D., Mauck R.L. A systematic review and guide to mechanical testing for articular cartilage tissue engineering. Tissue Eng. C Methods. Jul. 2019;25(10):593–608. doi: 10.1089/ten.tec.2019.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kosik-Kozioł A. 3D bioprinted hydrogel model incorporating β-tricalcium phosphate for calcified cartilage tissue engineering. Biofabrication. 03 2019;11(3) doi: 10.1088/1758-5090/ab15cb. [DOI] [PubMed] [Google Scholar]
- 75.Duchi S. Handheld Co-Axial Bioprinting: application to in situ surgical cartilage repair. Sci. Rep. Jul. 2017;7(1) doi: 10.1038/s41598-017-05699-x. Art. no. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seo Y., Shin K.-H., Kim H.H., Kim H.-S. Current advances in red blood cell generation using stem cells from diverse sources. Stem Cell. Int. 2019;29 doi: 10.1155/2019/9281329. https://www.hindawi.com/journals/sci/2019/9281329/ Jul. accessed Jun. 16, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fujimi A. Ex vivo large-scale generation of human red blood cells from cord blood CD34+ cells by co-culturing with macrophages. Int. J. Hematol. May 2008;87(4):339–350. doi: 10.1007/s12185-008-0062-y. [DOI] [PubMed] [Google Scholar]
- 78.Gao G., Schilling A.F., Yonezawa T., Wang J., Dai G., Cui X. Bioactive nanoparticles stimulate bone tissue formation in bioprinted three-dimensional scaffold and human mesenchymal stem cells. Biotechnol. J. 2014;9(10):1304–1311. doi: 10.1002/biot.201400305. [DOI] [PubMed] [Google Scholar]
- 79.Zhang L., Yang G., Johnson B.N., Jia X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019;84(15):16–33. doi: 10.1016/j.actbio.2018.11.039. [DOI] [PubMed] [Google Scholar]
- 80.Ahn S., Lee H., Kim G. Functional cell-laden alginate scaffolds consisting of core/shell struts for tissue regeneration. Carbohydr. Polym. Oct. 2013;98(1):936–942. doi: 10.1016/j.carbpol.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 81.Marrella A. Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration. Mater. Today. May 2018;21(4):362–376. doi: 10.1016/j.mattod.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yin X., Mead B.E., Safaee H., Langer R., Karp J.M., Levy O. Stem cell organoid engineering. Cell Stem Cell. Jan. 2016;18(1):25–38. doi: 10.1016/j.stem.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fiorini E., Veghini L., Corbo V. Modeling cell communication in cancer with organoids: making the complex simple. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lancaster M.A., Knoblich J.A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. Oct. 2014;9(10):2329–2340. doi: 10.1038/nprot.2014.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sthijns M.M.J.P.E., LaPointe V.L.S., van Blitterswijk C.A. Building complex life through self-organization. Tissue Eng. Oct. 2019;25(19–20):1341–1346. doi: 10.1089/ten.tea.2019.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen A.X., Hoffman M.D., Chen C.S., Shubin A.D., Reynolds D.S., Benoit D.S.W. Disruption of cell-cell contact-mediated notch signaling via hydrogel encapsulation reduces mesenchymal stem cell chondrogenic potential. J. Biomed. Mater. Res. 2015;103(4):1291–1302. doi: 10.1002/jbm.a.35383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Choudhury D., Ashok A., Naing M.W. Commercialization of organoids. Trends Mol. Med. Mar. 2020;26(3):245–249. doi: 10.1016/j.molmed.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 88.Ashok A., Choudhury D., Fang Y., Hunziker W. Towards manufacturing of human organoids. Biotechnol. Adv. Mar. 2020;39:107460. doi: 10.1016/j.biotechadv.2019.107460. [DOI] [PubMed] [Google Scholar]
- 89.Maloney E. Immersion bioprinting of tumor organoids in multi-well plates for increasing chemotherapy screening throughput. Micromachines. Feb. 2020;11(2) doi: 10.3390/mi11020208. Art. no. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Töpfer E. “Bovine colon organoids: from 3D bioprinting to cryopreserved multi-well screening platforms. Toxicol. Vitro. Dec. 2019;61:104606. doi: 10.1016/j.tiv.2019.104606. [DOI] [PubMed] [Google Scholar]
- 91.Reid J.A., Mollica P.M., Bruno R.D., Sachs P.C. Consistent and reproducible cultures of large-scale 3D mammary epithelial structures using an accessible bioprinting platform. Breast Cancer Res. 2018;20 doi: 10.1186/s13058-018-1045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reid J.A., Palmer X.-L., Mollica P.A., Northam N., Sachs P.C., Bruno R.D. A 3D bioprinter platform for mechanistic analysis of tumoroids and chimeric mammary organoids. Sci. Rep. May 2019;9(1) doi: 10.1038/s41598-019-43922-z. Art. no. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Skylar-Scott M.A. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci. Adv. Sep. 2019;5(9) doi: 10.1126/sciadv.aaw2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mollica P.A. 3D bioprinted mammary organoids and tumoroids in human mammary derived ECM hydrogels. Acta Biomater. Sep. 2019;95:201–213. doi: 10.1016/j.actbio.2019.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van Pel D.M., Harada K., Song D., Naus C.C., Sin W.C. Modelling glioma invasion using 3D bioprinting and scaffold-free 3D culture. J. Cell Commun. Signal. Dec. 2018;12(4):723–730. doi: 10.1007/s12079-018-0469-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gu Q., Tomaskovic‐Crook E., Wallace G.G., Crook J.M. 3D bioprinting human induced pluripotent stem cell constructs for in situ cell proliferation and successive multilineage differentiation. Adv. Healthc. Mater. 2017;6(17):1700175. doi: 10.1002/adhm.201700175. [DOI] [PubMed] [Google Scholar]
- 97.Higgins J.W. Dec. 2018. “Bioprinted Pluripotent Stem Cell-Derived Kidney Organoids Provide Opportunities for High Content Screening,” bioRxiv; p. 505396. [DOI] [Google Scholar]
- 98.Zhu W., O'Brien C., O'Brien J.R., Zhang L.G. 3D nano/microfabrication techniques and nanobiomaterials for neural tissue regeneration. Nanomed. May 2014;9(6):859–875. doi: 10.2217/nnm.14.36. [DOI] [PubMed] [Google Scholar]
- 99.Knowlton S., Anand S., Shah T., Tasoglu S. Bioprinting for neural tissue engineering. Trends Neurosci. Jan. 2018;41(1):31–46. doi: 10.1016/j.tins.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 100.B. Qiu et al., “Bioprinting neural systems to model central nervous system diseases,” Adv. Funct. Mater., vol. n/a, no. n/a, p. 1910250, doi: 10.1002/adfm.201910250. [DOI] [PMC free article] [PubMed]
- 101.Lozano R. 3D printing of layered brain-like structures using peptide modified gellan gum substrates. Biomaterials. Oct. 2015;67:264–273. doi: 10.1016/j.biomaterials.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 102.Grinsell D., Keating C.P. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. BioMed Res. Int. 2014;2014:1–13. doi: 10.1155/2014/698256. 698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sarker M., Naghieh S., McInnes A.D., Schreyer D.J., Chen X. Strategic design and fabrication of nerve guidance conduits for peripheral nerve regeneration. Biotechnol. J. 2018;13(7):1700635. doi: 10.1002/biot.201700635. [DOI] [PubMed] [Google Scholar]
- 104.Dixon A.R., Jariwala S.H., Bilis Z., Loverde J.R., Pasquina P.F., Alvarez L.M. Bridging the gap in peripheral nerve repair with 3D printed and bioprinted conduits. Biomaterials. Dec. 2018;186:44–63. doi: 10.1016/j.biomaterials.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 105.Farrukh A., Zhao S., del Campo A. Microenvironments designed to support growth and function of neuronal cells. Front. Mater. 2018;5 doi: 10.3389/fmats.2018.00062. [DOI] [Google Scholar]
- 106.Galliger Z., Vogt C.D., Panoskaltsis-Mortari A. 3D bioprinting for lungs and hollow organs. Transl. Res. Sep. 2019;211:19–34. doi: 10.1016/j.trsl.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heydari Z. Tissue engineering in liver regenerative medicine: insights into novel translational technologies. Cells. Feb. 2020;9:2. doi: 10.3390/cells9020304. Art. no. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Denost Q. Colorectal tissue engineering: prerequisites, current status and perspectives. Expet Rev. Med. Dev. Jul. 2013;10(4):501–507. doi: 10.1586/17434440.2013.811834. [DOI] [PubMed] [Google Scholar]
- 109.Arakelian L., Kanai N., Dua K., Durand M., Cattan P., Ohki T. Esophageal tissue engineering: from bench to bedside. Ann. N. Y. Acad. Sci. 2018;1434(1):156–163. doi: 10.1111/nyas.13951. [DOI] [PubMed] [Google Scholar]
- 110.Kim J., Kang K., Drogemuller C.J., Wallace G.G., Coates P.T. Bioprinting an artificial pancreas for type 1 diabetes. Curr. Diabetes Rep. Jul. 2019;19(8):53. doi: 10.1007/s11892-019-1166-x. [DOI] [PubMed] [Google Scholar]
- 111.Martin L.Y., Ladd M.R., Werts A., Sodhi C.P., March J.C., Hackam D.J. Tissue engineering for the treatment of short bowel syndrome in children. Pediatr. Res. Jan. 2018;83(1) doi: 10.1038/pr.2017.234. Art. no. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hwa A.J., Weir G.C. Transplantation of macroencapsulated insulin-producing cells. Curr. Diabetes Rep. Jun. 2018;18(8):50. doi: 10.1007/s11892-018-1028-y. [DOI] [PubMed] [Google Scholar]
- 113.Liu X. Development of a coaxial 3D printing platform for biofabrication of implantable islet-containing constructs. Adv. Healthc. Mater. 2019;8(7):1801181. doi: 10.1002/adhm.201801181. [DOI] [PubMed] [Google Scholar]
- 114.Clevers H. Tissue-engineering the intestine: the trials before the trials. Cell Stem Cell. Jun. 2019;24(6):855–859. doi: 10.1016/j.stem.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 115.Fetah K. The emergence of 3D bioprinting in organ-on-chip systems. Prog. Biomed. Eng. Jul. 2019;1(1) doi: 10.1088/2516-1091/ab23df. [DOI] [Google Scholar]
- 116.Bein A. Microfluidic organ-on-a-chip models of human intestine. Cell. Mol. Gastroenterol. Hepatol. Jan. 2018;5(4):659–668. doi: 10.1016/j.jcmgh.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Qi D. Repair and regeneration of small intestine: a review of current engineering approaches. Biomaterials. May 2020;240 doi: 10.1016/j.biomaterials.2020.119832. 119832. [DOI] [PubMed] [Google Scholar]
- 118.Morizane R., Bonventre J.V. Kidney organoids: a translational journey. Trends Mol. Med. 2017;23(3):246–263. doi: 10.1016/j.molmed.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lin N.Y.C. Renal reabsorption in 3D vascularized proximal tubule models. Proc. Natl. Acad. Sci. Unit. States Am. Mar. 2019;116(12):5399–5404. doi: 10.1073/pnas.1815208116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.van Gelder M.K. From portable dialysis to a bioengineered kidney. Expet Rev. Med. Dev. May 2018;15(5):323–336. doi: 10.1080/17434440.2018.1462697. [DOI] [PubMed] [Google Scholar]
- 121.Chevtchik N.V. Upscaling of a living membrane for bioartificial kidney device. Eur. J. Pharmacol. Nov. 2016;790:28–35. doi: 10.1016/j.ejphar.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 122.Coaxial Kit | Needles & Nozzles - Allevi. https://www.allevi3d.com/product/coaxial-kit/ accessed Jun. 16, 2020.
- 123.Microfluidic 3D Bioprinting Technology | Aspect Biosystems. https://www.aspectbiosystems.com/technology accessed Jun. 16, 2020.
- 124.3D bioprinters. https://www.regenhu.com/3d-bioprinters accessed Jun. 16, 2020.
- 125.Bioprinter - the modular solution for tissue engineering from GeSiM. https://gesim-bioinstruments-microfluidics.com/bioprinter/ accessed Jun. 16, 2020.
- 126.Battiston K.G., Cheung J.W.C., Jain D., Santerre J.P. Biomaterials in co-culture systems: towards optimizing tissue integration and cell signaling within scaffolds. Biomaterials. May 2014;35(15):4465–4476. doi: 10.1016/j.biomaterials.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 127.Corbett D.C., Olszewski E., Stevens K. A FRESH take on resolution in 3D bioprinting. Trends Biotechnol. Nov. 2019;37(11):1153–1155. doi: 10.1016/j.tibtech.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 128.Choi Y.-J. A 3D cell printed muscle construct with tissue-derived bioink for the treatment of volumetric muscle loss. Biomaterials. Jun. 2019;206:160–169. doi: 10.1016/j.biomaterials.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 129.Hu Y. 3D-engineering of cellularized conduits for peripheral nerve regeneration. Sci. Rep. Aug. 2016;6(1) doi: 10.1038/srep32184. Art. no. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]