Abstract
The evolution of the immune system, diet, and the microbiome are interconnected. Dietary metabolites modulate the cells of the immune system, both directly and indirectly, via shifts in the composition of the intestinal microbiota and its products. As a result, overconsumption and malnutrition can have substantial effects on immune responses and inflammation. In resource-rich nations, diets high in processed foods, fat and sugar can contribute to chronic inflammatory conditions, which are on the rise worldwide. Conversely, in resource poor countries, malnutrition associated with food security can lead to immunodeficiencies and shifts in the microbiome that drive intestinal inflammation. Developing a deeper understanding of the relationship between diet, microbiota, and the immune system is of huge importance given its impact on inflammatory diseases and potential as an easily modifiable mediator of immunomodulation.
Introduction:
330 million years ago our ancestors evolved the ability to sustain themselves on a variety of different plants, in part, via the acquisition of an intestinal microbiome (1). The microorganisms of the microbiota (bacteria, archaea, fungi, protists and viruses) allowed herbivores to digest complex carbohydrates and they soon dominated the Earth. The acquisition of a complex microbiome also required evolution of host cellular and biochemical processes, including the adaptive immune system (2, 3). Adaptive immunity can mediate responses to repeat microbial encounters in an efficient manner, both protecting against invasive organisms and also fostering symbiosis with beneficial members of the microbiota (3, 4). Since the evolution of the host/microbiome relationship was driven by nutrition and this was intertwined with the evolution of the immune system, it is not surprising that diet and nutrition affect the immune response. Here we will review how diet affects immunity both directly, by modifying immune cells, and also indirectly, via inducing changes to either the microbiota or non-immune tissues. In particular, we will focus on how nutrition contributes to immune-mediated disease. We will address both overnutrition in High-income nations and undernutrition and malnutrition of Low-to-Middle-income settings. For clarity, we will not discuss at length the separate process where the host sequesters key nutrients and minerals from pathogens, which has been reviewed elsewhere (5, 6). Additionally, we have focused on how specific nutrients modulate immune responses, as these studies more often provide mechanistic insights into interactions with specific cells.
Overnutrition and immunity
Recent advances in food production and distribution have reduced food insecurity and famine for billions of people. However, increasing caloric intake has been associated with a global rise of non-communicable diseases, such as cardiovascular disease, diabetes and cancer, which are the leading causes of death in High-income countries (HICs) (7). The ‘Western’ diet is characterized by increased consumption of total calories and a diet that is overly reliant on animal fat, refined grains and sugar and too little fruits and vegetables (8). Overnutrition has led to the staggering rise in global obesity rates, which have nearly tripled over the last 40 years (9, 10). The association of obesity and chronic inflammatory disease is well-established and extensively covered in previous reviews (11–15). Here, we will focus on how specific components of the Western Diet alter immune function acutely, contributing to inflammatory disease.
Dietary fat activates immunity and inflammation
Long-term high-fat diets (HFD) have been used in mice to induce obesity and the associated immune-metabolic shifts, such as low-grade adipose inflammation and insulin resistance (12). However, short-term consumption of high-fat diet in mice also results in altered immune function and inflammation, prior to the onset of obesity (16–18). The first site where the diet interacts with the host is the gastrointestinal epithelium and HFD has important effects on the constitution of this layer. The intestinal epithelium maintains a physical barrier between the microbiome and the host via tight junctions, which prevent intracellular entry of exogenous products (19). Feeding mice HFD for one week reduces the expression and alters the distribution of tight junction proteins, which is associated with increased intestinal permeability and bacterial product translocation (20, 21). This has been seen in humans as well, where high energy intake, in the form of excessive fat consumption, is correlated with higher levels of serum LPS levels, or endotoxemia (22). However, fatty acids can also support a healthy epithelium by driving increased ‘stemness’ and proliferative capacity in Lgr5+ crypt base columnar stem cells (23). Therefore, the negative effects of HFD on barrier function are likely the result of immune activation in the intestine, rather than direct effects on the epithelium itself. Indeed, mice fed HFD for 4 weeks had increased expression of chemokines (CCL1, CCL2) and chemokine receptors (CCR2), leading to the infiltration of proinflammatory monocytes and lymphocytes to intestinal tissues (24). In specific contexts, innate immune responses can be directly induced by free fatty acids, which are elevated in the blood after high fat meals and directly activate TLR4 on adipocytes and macrophages and TLR2 on monocytes to induce cytokine production (25, 26). Further, saturated fatty acids, such as palmitate, can directly activate the NLRP3-ASC inflammasome in myeloid cells to support IL-1β processing (27). Cytokines and inflammatory lipid molecules produced by innate immune cells, such as TNFα, IL-1β IFNγ, iNOS, COX-2 and IL-6, increase intestinal permeability (28–30). As evidence for the immune-mediated effects of HFD, HFD-fed IFNγ-deficient mice show reduced intestinal permeability compared to controls, (31, 32). Therefore, HFD-induced increases in circulating bacterial products also likely leads to the potent induction of inflammatory cytokines (21, 33). In support of this notion, germ-free mice, which lack a microbiome, have reduced expression of TNFα when fed a HFD (28). Thus, the over consumption of fat affects the intestinal barrier by directly activating macrophages, monocytes and T cells, leading to increased intestinal permeability and endotoxemia and further contributing to a systemic proinflammatory response. Short-term HFD feeding in mice also leads to a loss of hematopoietic stem cells in bone marrow and reduced hematopoietic reconstitution potential via disruption of TGFβ receptors in lipid rafts (34). Finally, dietary fat can have direct effects on adaptive immune cells where long-chain fatty acids, such as lauric acid, support the differentiation of T helper 17 (Th17) T cells and contribute to pathology in animal models of neuroinflammation (35).
Dietary fat and the microbiome
In addition to direct effects on host immune cells, HFD can quickly and reversibly change the composition of the microbiome. In mice, diets high in fat and sugar lead to a transient dysbiosis of the microbiota, characterized by reduced diversity and an expansion of opportunistic pathogens (36). Human microbiome studies have also associated increased Proteobacteria and Firmicutes and decreased Bacteroidetes to dyslipidemia, insulin resistance, and inflammation (37, 38). The importance of these associations is indicated my experiments where transfer of the microbiota from obese mice and humans to germ-free mice results in obesity in the recipient mice, while antibiotic treatment of obese mice reduces adipose inflammation and adiposity (21, 39, 40). Accordingly germ-free mice fed a HFD are resistant to the development of obesity (28). Conversely, there are members of the microbiota that suppress HFD-induced inflammatory responses, such as Akkermansia muciniphilia, which is negatively correlated with metabolic syndrome and has been shown to reduce systemic LPS and inflammation (41, 42). It is important to note that HFD-induced changes to the microbiome may also be indirectly affected by immune-mediated inflammation which is associated with the ‘blooming’ of Enterobacteriaceae that thrive in this environment (43, 44). For example, rats that are genetically susceptible to diet-induced obesity have an increase in LPS-producing Enterobacteriaceae which may enhance TLR signaling and HFD-induced inflammation (45). HFD and associated inflammation may also foster pathogen colonization as seen in murine L. monocytogenes infections, where HFD-induced microbiome changes are exacerbated and mice are more susceptible to infection due to a dysregulated immune response (46).
Food additives (sugar, salt and emulsifiers) increase inflammation
The Western diet is characterized by increased additives, such as emulsifiers, salt and sugar to make them more durable and hyper-palatable (8, 47). Sugar consumption has become dominant in the Western diet and it is estimated that an average person in the United States will consume over 70kg of sugar per year (48). High sugar consumption can have direct effects on host organs such as in models of Non-Alcoholic Fatty Liver Disease where excess dietary fructose leads to exacerbated hepatosteatosis (49). Sugar is also an important direct modifier of the immune response. For example, glucose is the preferred fuel source for Type 1 immunity, because it is important in the differentiation, proliferation and function of Th1 CD4+ T cells, neutrophils, pro-inflammatory macrophages and activated dendritic cells (50–53). Glucose is also the preferred substrate for proliferating CD8+ T lymphocytes that need to use glycolysis so that components of the tricarboxylic acid cycle can be used for translation (54). High glucose intake also increases Th17 differentiation and exacerbates mouse models of colitis and autoimmune encephalomyelitis (55). Conversely, while glucose can support the proliferation of regulatory T cells (Tregs), Glut1 expression and glycolysis in these cells is associated with less potent suppression of inflammation (56). Accordingly, limiting sugar in the diet has shown substantial efficacy in the treatment of pediatric Inflammatory Bowel Disease (57). Glucose is also necessary for the proliferation and function of B cells as blocking glucose utilization decreases B cell number and antibody production in mice (58). Indeed, glycolysis-inhibitors, such as dimethyl fumarate and pyruvate kinase, have been found to improve autoimmune disease in mice and humans by downregulating aerobic glycolysis in activated lymphoid and myeloid cells (59, 60).
An additional issue, that compounds the effects of sugar consumption, is that processed foods often contain “acellular” sugar that, unlike sugar in fruits and vegetables, does not need to be digested and are immediately available to the host and microbiota. Gordon and colleagues, using gnotobiotic mice with a defined microbiome, reported that high sucrose diets led to an enrichment for enzymes for processing simple sugars (40). Such enzymes are often found in families of facultative anaerobic bacteria such as Enterobacteriaceae that bloom and contribute to intestinal inflammation (61). Processed foods are often deficient in fiber, so a high sugar diet may select against the symbiotic bacteria that help our digestion and select for bacteria that can best use simple sugars to proliferate quickly (61).
Other additives such as emulsifiers and salt have also sharply increased in the Western Diet, due to their preservative properties. Emulsifiers are detergents in food products, that, when fed to mice at low doses, leads to microbial encroachment and transferrable dysbiosis, which increases myeloperoxidase activity in the gut and results in endotoxemia (62). Excessive salt consumption is thought to contribute to the rise in hypertension and cardiovascular disease seen in high-income nations (63). Increasing salt concentration also has a direct effect on immune cells due to a salt sensing kinase (SGK1) on CD4+ T cells that stabilizes IL-23R expression and enhances Th17 differentiation (64). These changes led to greater induction of Th17 cells in vivo with upregulation of pro-inflammatory cytokines GM-CSF, TNFα and IL-2 and worse autoimmunity in mice fed a high salt diet (65). SGK1 can also be activated downstream of the MTORC2 complex to increase Th2 differentiation and inhibit Th1 differentiation (66). Accordingly, high salt diets also exacerbate colitis in mouse models (67). In the future it will be important to determine the role of these food additives on immune-mediated disease in humans.
Fiber and short chain fatty acids are microbiome dependent immune regulators
The typical diet of HICs both overfeeds and undernourishes, in that there are too many calories but not the correct nutrients, including too little fiber. The evolution of the microbiome was driven by the necessity to digest complex polysaccharides into usable metabolites so it is intuitive that a lack of fiber would have negative effects on the health of both the microbiome and host (68, 69). Similar to HFD and diets high in sugar, low fiber diets are associated with a low diversity and pro-inflammatory microbiota (70). The loss of diversity from a low fiber diet is pernicious since the loss of the bacterial strains may lead to a reduction in microbiome functionality that can only be restored from external sources (71). How reduced microbial diversity induces inflammation is not entirely clear, but we are beginning to understand some potential mechanisms. Reduced intestinal diversity is associated with domination of the microbiome by Gram negative bacteria (Bacteroidetes, Proteobacteria, Verrucomicrobia) which could lead to increased activation of the host immune response through increased levels of lipopolysaccharide (72). Indeed, in gnotobiotic mice with a defined consortium of bacteria, a fiber-free diet induced Akkermansia muciniphilia (a Verrucomicrobia) to consume and deplete the mucus barrier of the colon, leaving the host more susceptible to colonization and infection with the Proteobacteria pathogen, Citrobacter rodentium (73).
Perhaps the best studied mechanism via which low fiber diet and reduced microbiome diversity can contribute to inflammation is via a reduction in short chain fatty acids (SCFAs). SCFAs, namely acetate, propionate and butyrate, are metabolites produced by microbial digestion of complex carbohydrates. SCFAs are a major carbon source for epithelial cells of the intestine and are critical to the proper anaerobic function of the gut (74, 75). SCFAs also have important effects on immune cells, both by binding to G protein coupled receptors and Histone Deacetylases, which, in almost all cases, dampen inflammation (76). Thus, it has been posited that these metabolites act as a surrogate signal by which the immune system can measure microbiome health (4). Specifically, SCFAs dampen the inflammatory responses of myeloid cells both locally in the intestine and at peripheral sites such as the lung and bone marrow (77, 78). SCFAs have potent effects on adaptive immune cells as well. In particular, a lack of SCFA production, as is experienced by germ-free mice, leads to a substantial reduction in colonic Tregs (79–81). These SCFA-supported colonic Tregs are likely important for preventing inflammatory immune responses against innocuous antigens, derived either from diet, host or microbiota (79–84). However, SCFAs do not dampen immune responses indiscriminately. CD8+ cytotoxic T cell responses are supported by SCFA signaling and a diet high in fiber was shown to reduce pathology and increase viral clearance in a mouse model of influenza infection (78, 85). SCFAs can also affect B cell responses. In support of the hypothesis that SCFAs drive host microbiota homeostasis, acetate production in the small intestine increases retinoic acid production by dendritic cells and thus can support class switch recombination to IgA (86). The direct effects of butyrate and propionate are more controversial, with different groups showing either augmentation or suppression of systemic antibody production which may be explained by differences in microbiome composition and experimental approach (87, 88).
In concert with the anti-inflammatory effects of fiber and SCFAs, both animal models and clinical studies generally support the notion that a diet high in fiber is protective against chronic inflammatory disease (76). For instance, a recent clinical study showed that a defined high fiber diet could improve outcomes of type 2 diabetic patients via shifts in the microbiota, as measured by hemoglobin A1C levels (89). Similar, though perhaps less significant effects have been seen in other studies (90, 91). The mechanism by which dietary fiber is producing these positive effects is unknown and potentially quite complex given their pleiotropic effects on immune activation, metabolism and the microbiome.
Plant-based ligands for the Aryl Hydrocarbon Receptor (Ahr) activate IL-22 production in the intestine
Ahr is a transcription factor expressed in a variety of immune cells and in particular in the lymphocytes found at barrier surfaces. Ahr was initially studied for its response to toxins such as dioxin (92), but it is also activated by indole compounds, derived from the digestion of vegetables from the Brassicaceae family (cauliflower, broccoli, cabbage etc.) (93). One of the potent effects of Ahr activation is the production of IL-22 from innate lymphocytes at the intestinal surface (94). Ahr activation is necessary for IL-22 production from Innate Lymphoid cell type 3 (ILC3s) and Intraepithelial γδ T cells at the intestinal barrier (93–95). IL-22 signaling is important for keeping bacteria out of the base of the intestinal crypt via the induction of anti-microbial peptides, thereby protecting the Lgr5+ CBC stem cells and maintaining the regenerative capacity of the intestine in the face of genotoxic/inflammatory stress (96–98).
Thus, via multiple mechanisms the immune system is regulated by the proper digestion of plant products in the diet by the microbiota. Conversely, it is clear that the Western diet, which is low in plant products and high in fat and sugar, is contributing to dysbiosis of the gut microbiome and increased intestinal and systemic inflammation, contributing to substantial increases in chronic inflammatory diseases (Figure 1).
Figure 1. The Westernized diet drives mucosal inflammation.
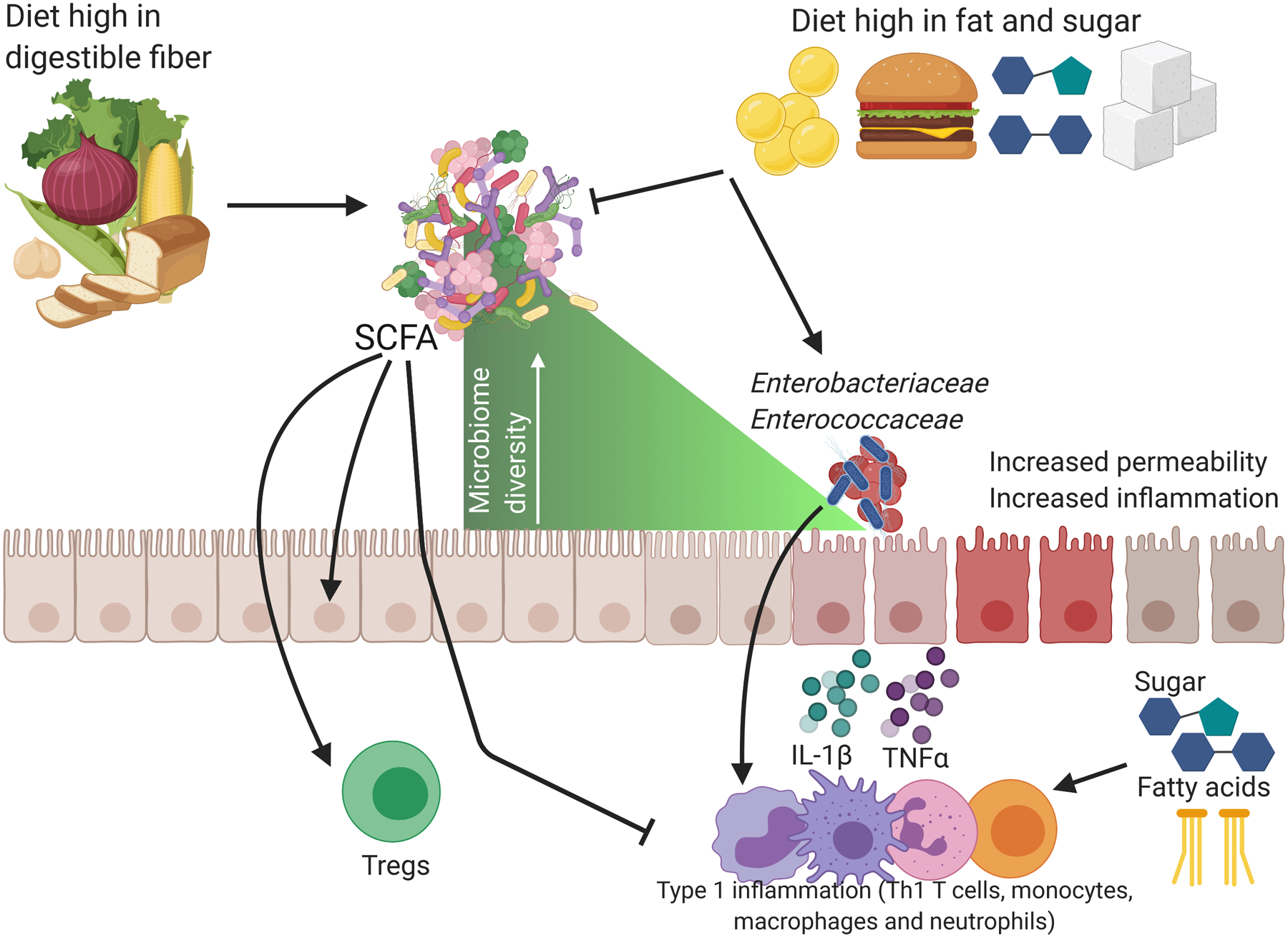
Diets high in fiber support microbiome diversity and the production of short-chain fatty acids (SCFAs) which act as an energy source for the intestinal epithelium, decrease oxygen in the intestinal lumen, increase colonic Tregs and suppress inflammatory immune cells. Conversely diets high in fat and sugar contribute to reduced microbiome diversity and activate inflammatory type 1 immune cells (Th1 T cells, monocytes, macrophages and neutrophils) to produce cytokines such as IL-1β and TNFβ. These immune cells increase inflammation in the intestine which allows for ‘blooms’ of Enterobacteriaceae and Enterococcaceae, a loss of microbiome diversity and increased permeability through the opening of tight junctions. Figure created using biorender.com.
Undernutrition, malnutrition and immunity
In contrast to the diseases of overnutrition of HICs, many people in low- and middle-income countries. (LMICs) are subject to malnutrition and undernutrition. Despite recent laudable reductions in incidence, undernutrition and malnutrition continue to be a significant global public health concern and contribute to ~45% of mortality in children under five years old living in LMICs (99). Though they often coincide and thus the terms are used interchangeably, for the purposes of this review we will define undernutrition as insufficient caloric intake and malnutrition as insufficient quantities of specific nutrients and vitamins. Here we will first address how specific deficiencies in micronutrients affect immunity followed by a discussion of how under and malnutrition shape the immune response more generally.
Micronutrient deficiencies subvert immunity
Micronutrient (vitamins and mineral) deficits, can lead to immune dysfunction. For instance, Vitamin A deficiency can lead to immune dysfunction and increased susceptibility to infection (100). Vitamin A is absorbed from fruits and vegetables in the diet by the intestine and then enzymatically converted to retinoic acid (RA), which is a critical signal for multiple immune processes. During embryogenesis, RA supports the development of lymphoid tissue (101). Critically RA supports the differentiation of ILC3s, of which a sub-type, lymphoid tissue inducer cells, are important for the production of lymph nodes (102, 103). RA production is modulated by the microbiome, specifically Clostridia spp., which downregulates the expression of enzymes necessary for the production of RA, thereby curbing ILC3s and downstream anti-microbial peptide production (104). CD103+ intestinal dendritic cells also require RA and the transcription factor it activates, RARα, for their development from precursors (105). Beyond development, RA is also critical for the activation of mucosal adaptive immune responses. Conversion of RA by CD103+ DCs trafficking from the intestinal mucosa to the gut-draining lymph nodes induces homing markers, such as α4β7 and CCR9, for gut homing on activated T cells and B cells (106, 107). RA also influences humoral immunity as RA produced by follicular dendritic cells in Peyer’s patches induce B cell proliferation and the generation of IgA+ B cells (108). The role of RA on CD4+ T cell differentiation is more complex. A complete lack of RA signals and RARα activation leads to a substantial failure of signal transduction downstream of the T cell receptor, leading to deficits in T cell responses to infection (109). However, as RA production from CD103+ DCs increases, it skews differentiation of CD4+ T cells towards Tregs, contributing to the immune tolerance to innocuous food and microbiota-derived antigens(110–113). Thus, vitamin A deficiency represents a critical issue for the mucosal immune response, as it cannot adequately respond to infection and also lacks the ability to regulate responses to the microbiota, perhaps explaining why vitamin A deficiency is associated with increased incidence and severity of infection.
Zinc is a trace element that is essential for immunity and lack of adequate dietary zinc is common in sub-Saharan Africa and South Asia where it is associated with increased mortality (114). Even mild zinc deficiency is sufficient to induce an imbalance between Th1 cell and Th2 cell functions, as well as impair NK cell function (115). In contrast, zinc supplementation promotes survival and regulates inflammation. The zinc transporter, SLC39A8 (ZIP8), inhibits pro-inflammatory responses via zinc-mediated down-modulation of IKK activity (116), and reduces inflammation during sepsis (117).
Iron deficiency is the most common micronutrient deficiency in the world, affecting more than 25% people globally. Since iron is required for monocyte to macrophage differentiation and for macrophages to successfully ward off intracellular bacteria by the NADPH mediated oxidative burst, it is critical for innate immune responses to bacteria (118). Iron deficiency is also associated with lower CD4/CD8 T cell counts and defects in IgG mediated humoral immunity (119, 120). However, for both zinc and iron, the advantages imparted by oral supplementation are complex, as the microbiota, in particular the more pathogenic members, compete for and sequester iron (121). Additionally, high amounts of iron in the blood make the host prone to lethal bacteremia, which is in part, why iron is tightly regulated by the body (122). Therefore, attempts to restore levels of these metals must be undertaken carefully, particularly in LMICs where enteric bacterial infection is often endemic.
Undernutrition, the microbiome and childhood development
The intestinal microbiome has not only evolved with its host but also adapts in concert with the development of the host. Infants are first colonized with facultative anaerobes (Enterobacteriaceae), then Bifidobacteria and finally as children transition to solid food, Clostridia and Bacteroides, that assist in fiber digestion (123–125). During the first 1000 days of life these sequential changes in the composition of the juvenile gut microbiota are essential for healthy development and disturbances in the establishment of the microbiota may have deleterious effects (126). Undernourished children from LMICs exhibit a delayed maturation of the microbiome and maintain Enterobacteriaceae for longer periods of time (127). The effects of an ‘immature’ microbiome extend well beyond childhood as transfer of the microbiota from undernourished infants into germ-free mice leads to stunted growth, clearly indicating that the microbiome can contribute to development during early years of life (128).
The consequences of undernourishment are not limited to hunger, growth defects and delayed development of the microbiome. As discussed above, immune cells have specific requirements for food-derived metabolites and the development of the immune system is affected by metabolite deficiencies. Protein-energy malnutrition (PEM) affects the development of the immune system by decreasing hematopoiesis (129) and inducing thymic atrophy and thymocyte apoptosis, leading to an impairment in peripheral T cells (130). PEM can also affect T cell activation, as DCs from PEM mice are less competent for antigen presentation and eliciting T cell activation (131). Accordingly, children with undernutrition/malnutrition are at an increased risk of death due to infectious diseases such as influenza, diarrhea, pneumonia and malaria (132, 133). The effects of undernourishment can extend long after caloric intake is restored as altered nutritional and environmental conditions during early life can program cells to behave differently to the same stimuli later (134). For example, undernutrition in during development predisposes individuals to immune-related chronic inflammatory disorders such as type 2 diabetes, obesity and cardiovascular disease (135). Even intermittent fasting can have substantial effects on the distribution and function of both memory T cells and circulating inflammatory monocytes (136, 137). However the effects of more prolonged caloric restriction on long-lived tissue resident immunity is less well understood and may be critical to explaining the long-term pro-inflammatory effects of undernutrition.
Undernutrition and Environmental Enteric Dysfunction
Undernourishment and malnutrition do not occur in a vacuum. In LMICs, scarcity of food is driven by poverty, which goes hand-in-hand with lack of access to clean water and adequate sanitation. Intestinal infections caused by environmental contamination with pathogens can exacerbate the effects of undernutrition/malnutrition as these two conditions can function in a positive feedback loop. As mentioned above, undernourished individuals have a greater susceptibility to enteric pathogens and, in turn chronic inflammation and subsequent metabolic energy loss (138). Perhaps the best example of how malnutrition is both a cause and consequence of enteric dysfunction is the gastrointestinal disease Environmental Enteric Dysfunction (EED) (139). EED is most impactful in children, where it contributes to permanent stunting of both stature and brain development (138, 140). EED is characterized by villous blunting, lymphocytic infiltration, reduced absorptive capacity, and reduced barrier function (141). EED-associated malabsorption significantly exacerbates malnutrition because when diet is restored, nutrient uptake is still impacted, complicating diet-based health initiatives (142). While the etiology of EED is not known, it is possible that malnutrition and chronic inflammation combine to impede effective mucosal immunity, leading to elongated infectious courses and loss of absorptive capacity in the small intestine (Figure 2). Tragically, reduced mucosal immunity in areas where EED is endemic may also contribute to a reduced efficacy of oral vaccination, further complicating public health efforts to reduce infection and restore impaired pediatric development (143–146).
Figure 2. Proposed mechanism for the development of Environmental Enteric Dysfunction.
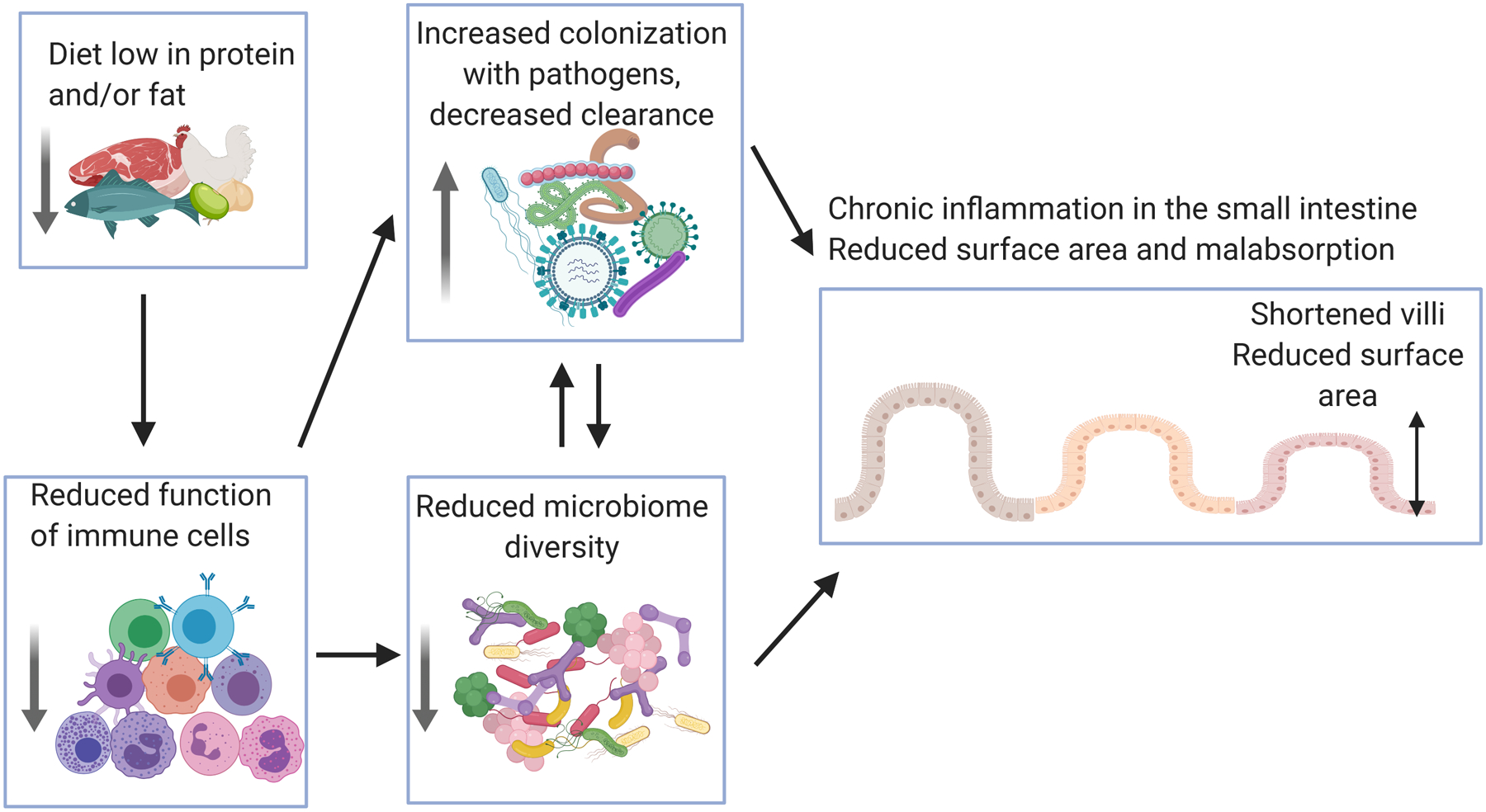
Insufficient protein and fat in the diet can reduce the effectiveness of the immune response, leading to improper control of intestinal infection and a loss of a healthy diverse microbiome. Together malnutrition, chronic infection and microbiome dysbiosis lead to intestinal inflammation, reduced epithelial surface area and malabsorption exacerbating the effects of malnutrition. Figure created using biorender.com.
Conclusion
The diet that humans evolved to eat is hard to define. For humans, culture, customs and technology make observing our ‘natural’ diet very difficult, but nonetheless it is likely that few if any people eat the diet of our evolutionary ancestors (147). Additionally much of what we eat, is modified by the microbiota and our response to any given diet is shaped by our individualized and malleable microbiomes (148). While the immune system and microbiome are adaptable to the diet, there are limits, and unfortunately, the diet of HICs is testing the boundaries of both systems and contributing to chronic disease on a massive scale. For example, it seems that the immune system has mechanisms to detect whether the microbiota is properly digesting dietary plant products via the measurement of microbiome-derived SCFAs. One potentially unfortunate outcome from this mechanism is that a Westernized diet low in plant products may ‘fool’ the immune system into thinking that the microbiome is not functioning, leading to unnecessary inflammation. Alternatively, in LMICs the combined effects of malnutrition and undernutrition lead to inhibited development of protective mucosal immunity. As a result, chronic inflammation and malabsorption in LMICs can lead to developmental delays that are difficult to restore and have health effects that extend well past the pediatric developmental period. As we seek to increase wealth and food security in LMICs worldwide it will be important to not repeat our errors in shifting to a hyper-processed, high sugar and low fiber diet which might exchange one set of problems for another.
Finally, though this review has focused on the negative effects of consuming diet that is ill-suited for health, we should not lose sight of the tremendous opportunity we have to augment immunity with deeper knowledge of diet and metabolites. Indeed, there is evidence that anti-microbial immunity can be both aided and inhibited by nutrition levels and macronutrients (149, 150). A deeper understanding of the interacting network that connects diet, the microbiome and the immune system will be important so that we can design diets to better resolve disease.
Acknowledgments:
We would like to thank S. Spencer for critical reading of the manuscript. We apologize that not all of the studies in this developing field could be discussed and referenced in this brief review.
Funding: National Institutes of Health (R21AI142051, TWH), the Kenneth Rainin Foundation (TWH) and UPMC Children’s Hospital of Pittsburgh (TWH and AB)
References
- 1.Romano M 2017. Gut Microbiota as a Trigger of Accelerated Directional Adaptive Evolution: Acquisition of Herbivory in the Context of Extracellular Vesicles, MicroRNAs and Inter-Kingdom Crosstalk. Front Microbiol 8: 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kostic AD, Howitt MR, and Garrett WS. 2013. Exploring host-microbiota interactions in animal models and humans. Genes Dev 27: 701–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McFall-Ngai M 2007. Adaptive immunity: care for the community. Nature 445: 153. [DOI] [PubMed] [Google Scholar]
- 4.Belkaid Y, and Hand TW. 2014. Role of the microbiota in immunity and inflammation. Cell 157: 121–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hood MI, and Skaar EP. 2012. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10: 525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nunez G, Sakamoto K, and Soares MP. 2018. Innate Nutritional Immunity. J Immunol 201: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gersh BJ, Sliwa K, Mayosi BM, and Yusuf S. 2010. Novel therapeutic concepts: the epidemic of cardiovascular disease in the developing world: global implications. Eur Heart J 31: 642–648. [DOI] [PubMed] [Google Scholar]
- 8.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O’Keefe JH, and Brand-Miller J. 2005. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr 81: 341–354. [DOI] [PubMed] [Google Scholar]
- 9.Flegal KM, Carroll MD, Kuczmarski RJ, and Johnson CL. 1998. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord 22: 39–47. [DOI] [PubMed] [Google Scholar]
- 10.Ogden CL, Carroll MD, Kit BK, and Flegal KM. 2014. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 311: 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandra RK 1981. Immune response in overnutrition. Cancer Res 41: 3795–3796. [PubMed] [Google Scholar]
- 12.Daryabor G, Kabelitz D, and Kalantar K. 2019. An update on immune dysregulation in obesity-related insulin resistance. Scand J Immunol 89: e12747. [DOI] [PubMed] [Google Scholar]
- 13.Fantuzzi G 2005. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol 115: 911–919; quiz 920. [DOI] [PubMed] [Google Scholar]
- 14.Kanneganti TD, and Dixit VD. 2012. Immunological complications of obesity. Nat Immunol 13: 707–712. [DOI] [PubMed] [Google Scholar]
- 15.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, and Dosch HM. 2009. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med 15: 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bleau C, Karelis AD, St-Pierre DH, and Lamontagne L. 2015. Crosstalk between intestinal microbiota, adipose tissue and skeletal muscle as an early event in systemic low-grade inflammation and the development of obesity and diabetes. Diabetes Metab Res Rev 31: 545–561. [DOI] [PubMed] [Google Scholar]
- 17.Ji Y, Sun S, Xia S, Yang L, Li X, and Qi L. 2012. Short term high fat diet challenge promotes alternative macrophage polarization in adipose tissue via natural killer T cells and interleukin-4. J Biol Chem 287: 24378–24386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woodland DC, Liu W, Leong J, Sears ML, Luo P, and Chen X. 2016. Short-term high-fat feeding induces islet macrophage infiltration and beta-cell replication independently of insulin resistance in mice. Am J Physiol Endocrinol Metab 311: E763–E771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okumura R, and Takeda K. 2017. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp Mol Med 49: e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amar J, Chabo C, Waget A, Klopp P, Vachoux C, Bermudez-Humaran LG, Smirnova N, Berge M, Sulpice T, Lahtinen S, Ouwehand A, Langella P, Rautonen N, Sansonetti PJ, and Burcelin R. 2011. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol Med 3: 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, and Burcelin R. 2008. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57: 1470–1481. [DOI] [PubMed] [Google Scholar]
- 22.Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC, Chamontin B, and Ferrieres J. 2008. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr 87: 1219–1223. [DOI] [PubMed] [Google Scholar]
- 23.Beyaz S, Mana MD, Roper J, Kedrin D, Saadatpour A, Hong SJ, Bauer-Rowe KE, Xifaras ME, Akkad A, Arias E, Pinello L, Katz Y, Shinagare S, Abu-Remaileh M, Mihaylova MM, Lamming DW, Dogum R, Guo G, Bell GW, Selig M, Nielsen GP, Gupta N, Ferrone CR, Deshpande V, Yuan GC, Orkin SH, Sabatini DM, and Yilmaz OH. 2016. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 531: 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawano Y, Nakae J, Watanabe N, Kikuchi T, Tateya S, Tamori Y, Kaneko M, Abe T, Onodera M, and Itoh H. 2016. Colonic Pro-inflammatory Macrophages Cause Insulin Resistance in an Intestinal Ccl2/Ccr2-Dependent Manner. Cell Metab 24: 295–310. [DOI] [PubMed] [Google Scholar]
- 25.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, and Flier JS. 2006. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116: 3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snodgrass RG, Huang S, Choi IW, Rutledge JC, and Hwang DH. 2013. Inflammasome-mediated secretion of IL-1beta in human monocytes through TLR2 activation; modulation by dietary fatty acids. J Immunol 191: 4337–4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, and Ting JP. 2011. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 12: 408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NM, Magness S, Jobin C, and Lund PK. 2010. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One 5: e12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim KA, Gu W, Lee IA, Joh EH, and Kim DH. 2012. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One 7: e47713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida H, Miura S, Kishikawa H, Hirokawa M, Nakamizo H, Nakatsumi RC, Suzuki H, Saito H, and Ishii H. 2001. Fatty acids enhance GRO/CINC-1 and interleukin-6 production in rat intestinal epithelial cells. J Nutr 131: 2943–2950. [DOI] [PubMed] [Google Scholar]
- 31.Al-Sadi RM, and Ma TY. 2007. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol 178: 4641–4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luck H, Tsai S, Chung J, Clemente-Casares X, Ghazarian M, Revelo XS, Lei H, Luk CT, Shi SY, Surendra A, Copeland JK, Ahn J, Prescott D, Rasmussen BA, Chng MH, Engleman EG, Girardin SE, Lam TK, Croitoru K, Dunn S, Philpott DJ, Guttman DS, Woo M, Winer S, and Winer DA. 2015. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab 21: 527–542. [DOI] [PubMed] [Google Scholar]
- 33.Burcelin R, Garidou L, and Pomie C. 2012. Immuno-microbiota cross and talk: the new paradigm of metabolic diseases. Semin Immunol 24: 67–74. [DOI] [PubMed] [Google Scholar]
- 34.Hermetet F, Buffiere A, Aznague A, Pais de Barros JP, Bastie JN, Delva L, and Quere R. 2019. High-fat diet disturbs lipid raft/TGF-beta signaling-mediated maintenance of hematopoietic stem cells in mouse bone marrow. Nat Commun 10: 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haghikia A, Jorg S, Duscha A, Berg J, Manzel A, Waschbisch A, Hammer A, Lee DH, May C, Wilck N, Balogh A, Ostermann AI, Schebb NH, Akkad DA, Grohme DA, Kleinewietfeld M, Kempa S, Thone J, Demir S, Muller DN, Gold R, and Linker RA. 2015. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity 43: 817–829. [DOI] [PubMed] [Google Scholar]
- 36.Zhang C, Zhang M, Pang X, Zhao Y, Wang L, and Zhao L. 2012. Structural resilience of the gut microbiota in adult mice under high-fat dietary perturbations. ISME J 6: 1848–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jorgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clement K, Dore J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker JD, Raes J, Hansen T, H. I. T. c. Meta, Bork P, Wang J, Ehrlich SD, and Pedersen. O 2013. Richness of human gut microbiome correlates with metabolic markers. Nature 500: 541–546. [DOI] [PubMed] [Google Scholar]
- 38.Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, Knight R, Ahima RS, Bushman F, and Wu GD. 2009. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 137: 1716–1724 e1711–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, and Gordon JI. 2006. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027–1031. [DOI] [PubMed] [Google Scholar]
- 40.Turnbaugh PJ, Backhed F, Fulton L, and Gordon JI. 2008. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, and Cani PD. 2013. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 110: 9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneeberger M, Everard A, Gomez-Valades AG, Matamoros S, Ramirez S, Delzenne NM, Gomis R, Claret M, and Cani PD. 2015. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep 5: 16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winter SE, and Baumler AJ. 2014. Dysbiosis in the inflamed intestine: chance favors the prepared microbe. Gut Microbes 5: 71–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carvalho FA, Koren O, Goodrich JK, Johansson ME, Nalbantoglu I, Aitken JD, Su Y, Chassaing B, Walters WA, Gonzalez A, Clemente JC, Cullender TC, Barnich N, Darfeuille-Michaud A, Vijay-Kumar M, Knight R, Ley RE, and Gewirtz AT. 2012. Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell Host Microbe 12: 139–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, and Raybould HE. 2010. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol 299: G440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Las Heras V, Clooney AG, Ryan FJ, Cabrera-Rubio R, Casey PG, Hueston CM, Pinheiro J, Rudkin JK, Melgar S, Cotter PD, Hill C, and Gahan CGM. 2019. Short-term consumption of a high-fat diet increases host susceptibility to Listeria monocytogenes infection. Microbiome 7: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monteiro CA, Moubarac JC, Cannon G, Ng SW, and Popkin B. 2013. Ultra-processed products are becoming dominant in the global food system. Obes Rev 14 Suppl 2: 21–28. [DOI] [PubMed] [Google Scholar]
- 48.Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, Gersch MS, Benner S, and Sanchez-Lozada LG. 2007. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr 86: 899–906. [DOI] [PubMed] [Google Scholar]
- 49.Bergheim I, Weber S, Vos M, Kramer S, Volynets V, Kaserouni S, McClain CJ, and Bischoff SC. 2008. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol 48: 983–992. [DOI] [PubMed] [Google Scholar]
- 50.Freemerman AJ, Johnson AR, Sacks GN, Milner JJ, Kirk EL, Troester MA, Macintyre AN, Goraksha-Hicks P, Rathmell JC, and Makowski L. 2014. Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J Biol Chem 289: 7884–7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerriets VA, Kishton RJ, Nichols AG, Macintyre AN, Inoue M, Ilkayeva O, Winter PS, Liu X, Priyadharshini B, Slawinska ME, Haeberli L, Huck C, Turka LA, Wood KC, Hale LP, Smith PA, Schneider MA, MacIver NJ, Locasale JW, Newgard CB, Shinohara ML, and Rathmell JC. 2015. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J Clin Invest 125: 194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palmer CS, Ostrowski M, Balderson B, Christian N, and Crowe SM. 2015. Glucose metabolism regulates T cell activation, differentiation, and functions. Front Immunol 6: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thwe PM, Pelgrom LR, Cooper R, Beauchamp S, Reisz JA, D’Alessandro A, Everts B, and Amiel E. 2017. Cell-Intrinsic Glycogen Metabolism Supports Early Glycolytic Reprogramming Required for Dendritic Cell Immune Responses. Cell Metab 26: 558–567 e555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Delgoffe GM, and Powell JD. 2015. Sugar, fat, and protein: new insights into what T cells crave. Curr Opin Immunol 33: 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang D, Jin W, Wu R, Li J, Park SA, Tu E, Zanvit P, Xu J, Liu O, Cain A, and Chen W. 2019. High Glucose Intake Exacerbates Autoimmunity through Reactive-Oxygen-Species-Mediated TGF-beta Cytokine Activation. Immunity 51: 671–681 e675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gerriets VA, Kishton RJ, Johnson MO, Cohen S, Siska PJ, Nichols AG, Warmoes MO, de Cubas AA, MacIver NJ, Locasale JW, Turka LA, Wells AD, and Rathmell JC. 2016. Foxp3 and Toll-like receptor signaling balance Treg cell anabolic metabolism for suppression. Nat Immunol 17: 1459–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suskind DL, Wahbeh G, Cohen SA, Damman CJ, Klein J, Braly K, Shaffer M, and Lee D. 2016. Patients Perceive Clinical Benefit with the Specific Carbohydrate Diet for Inflammatory Bowel Disease. Dig Dis Sci 61: 3255–3260. [DOI] [PubMed] [Google Scholar]
- 58.Caro-Maldonado A, Wang R, Nichols AG, Kuraoka M, Milasta S, Sun LD, Gavin AL, Abel ED, Kelsoe G, Green DR, and Rathmell JC. 2014. Metabolic reprogramming is required for antibody production that is suppressed in anergic but exaggerated in chronically BAFF-exposed B cells. J Immunol 192: 3626–3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Angiari S, Runtsch MC, Sutton CE, Palsson-McDermott EM, Kelly B, Rana N, Kane H, Papadopoulou G, Pearce EL, Mills KHG, and O’Neill LAJ. 2020. Pharmacological Activation of Pyruvate Kinase M2 Inhibits CD4(+) T Cell Pathogenicity and Suppresses Autoimmunity. Cell Metab 31: 391–405 e398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kornberg MD, Bhargava P, Kim PM, Putluri V, Snowman AM, Putluri N, Calabresi PA, and Snyder SH. 2018. Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science 360: 449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamada N, Kim YG, Sham HP, Vallance BA, Puente JL, Martens EC, and Nunez G. 2012. Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science 336: 1325–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, and Gewirtz AT. 2015. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 519: 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mohan S, and Campbell NR. 2009. Salt and high blood pressure. Clin Sci (Lond) 117: 1–11. [DOI] [PubMed] [Google Scholar]
- 64.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, and Kuchroo VK. 2013. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 496: 513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, and Hafler DA. 2013. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496: 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heikamp EB, Patel CH, Collins S, Waickman A, Oh MH, Sun IH, Illei P, Sharma A, Naray-Fejes-Toth A, Fejes-Toth G, Misra-Sen J, Horton MR, and Powell JD. 2014. The AGC kinase SGK1 regulates TH1 and TH2 differentiation downstream of the mTORC2 complex. Nat Immunol 15: 457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tubbs AL, Liu B, Rogers TD, Sartor RB, and Miao EA. 2017. Dietary Salt Exacerbates Experimental Colitis. J Immunol 199: 1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, and Sonnenburg JL. 2016. Diet-induced extinctions in the gut microbiota compound over generations. Nature 529: 212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sonnenburg ED, and Sonnenburg JL. 2014. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab 20: 779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Flint HJ, Scott KP, Louis P, and Duncan SH. 2012. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol 9: 577–589. [DOI] [PubMed] [Google Scholar]
- 71.Ng KM, Aranda-Diaz A, Tropini C, Frankel MR, Van Treuren W, O’Laughlin CT, Merrill BD, Yu FB, Pruss KM, Oliveira RA, Higginbottom SK, Neff NF, Fischbach MA, Xavier KB, Sonnenburg JL, and Huang KC. 2019. Recovery of the Gut Microbiota after Antibiotics Depends on Host Diet, Community Context, and Environmental Reservoirs. Cell Host Microbe 26: 650–665 e654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hand TW, Vujkovic-Cvijin I, Ridaura VK, and Belkaid Y. 2016. Linking the Microbiota, Chronic Disease, and the Immune System. Trends Endocrinol Metab 27: 831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, Pudlo NA, Kitamoto S, Terrapon N, Muller A, Young VB, Henrissat B, Wilmes P, Stappenbeck TS, Nunez G, and Martens EC. 2016. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 167: 1339–1353 e1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Byndloss MX, Olsan EE, Rivera-Chavez F, Tiffany CR, Cevallos SA, Lokken KL, Torres TP, Byndloss AJ, Faber F, Gao Y, Litvak Y, Lopez CA, Xu G, Napoli E, Giulivi C, Tsolis RM, Revzin A, Lebrilla CB, and Baumler AJ. 2017. Microbiota-activated PPAR-gamma signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 357: 570–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roediger WE 1992. Oxidative and synthetic functions of n-Butyrate in colonocytes. Dis Colon Rectum 35: 511–512. [DOI] [PubMed] [Google Scholar]
- 76.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, and Macia L. 2014. The role of short-chain fatty acids in health and disease. Adv Immunol 121: 91–119. [DOI] [PubMed] [Google Scholar]
- 77.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, and Mackay CR. 2009. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461: 1282–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trompette A, Gollwitzer ES, Pattaroni C, Lopez-Mejia IC, Riva E, Pernot J, Ubags N, Fajas L, Nicod LP, and Marsland BJ. 2018. Dietary Fiber Confers Protection against Flu by Shaping Ly6c(−) Patrolling Monocyte Hematopoiesis and CD8(+) T Cell Metabolism. Immunity 48: 992–1005 e1008. [DOI] [PubMed] [Google Scholar]
- 79.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, and Rudensky AY. 2013. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504: 451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, and Ohno H. 2013. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504: 446–450. [DOI] [PubMed] [Google Scholar]
- 81.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, and Garrett WS. 2013. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341: 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iweala OI, and Nagler CR. 2019. The Microbiome and Food Allergy. Annu Rev Immunol 37: 377–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marino E, Richards JL, McLeod KH, Stanley D, Yap YA, Knight J, McKenzie C, Kranich J, Oliveira AC, Rossello FJ, Krishnamurthy B, Nefzger CM, Macia L, Thorburn A, Baxter AG, Morahan G, Wong LH, Polo JM, Moore RJ, Lockett TJ, Clarke JM, Topping DL, Harrison LC, and Mackay CR. 2017. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat Immunol 18: 552–562. [DOI] [PubMed] [Google Scholar]
- 84.Tan J, McKenzie C, Vuillermin PJ, Goverse G, Vinuesa CG, Mebius RE, Macia L, and Mackay CR. 2016. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell Rep 15: 2809–2824. [DOI] [PubMed] [Google Scholar]
- 85.Bachem A, Makhlouf C, Binger KJ, de Souza DP, Tull D, Hochheiser K, Whitney PG, Fernandez-Ruiz D, Dahling S, Kastenmuller W, Jonsson J, Gressier E, Lew AM, Perdomo C, Kupz A, Figgett W, Mackay F, Oleshansky M, Russ BE, Parish IA, Kallies A, McConville MJ, Turner SJ, Gebhardt T, and Bedoui S. 2019. Microbiota-Derived Short-Chain Fatty Acids Promote the Memory Potential of Antigen-Activated CD8(+) T Cells. Immunity 51: 285–297 e285. [DOI] [PubMed] [Google Scholar]
- 86.Wu W, Sun M, Chen F, Cao AT, Liu H, Zhao Y, Huang X, Xiao Y, Yao S, Zhao Q, Liu Z, and Cong Y. 2017. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol 10: 946–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim M, Qie Y, Park J, and Kim CH. 2016. Gut Microbial Metabolites Fuel Host Antibody Responses. Cell Host Microbe 20: 202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sanchez HN, Moroney JB, Gan H, Shen T, Im JL, Li T, Taylor JR, Zan H, and Casali P. 2020. B cell-intrinsic epigenetic modulation of antibody responses by dietary fiber-derived short-chain fatty acids. Nat Commun 11: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, Fu H, Xue X, Lu C, Ma J, Yu L, Xu C, Ren Z, Xu Y, Xu S, Shen H, Zhu X, Shi Y, Shen Q, Dong W, Liu R, Ling Y, Zeng Y, Wang X, Zhang Q, Wang J, Wang L, Wu Y, Zeng B, Wei H, Zhang M, Peng Y, and Zhang C. 2018. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 359: 1151–1156. [DOI] [PubMed] [Google Scholar]
- 90.Silva FM, Kramer CK, Crispim D, and Azevedo MJ. 2015. A high-glycemic index, low-fiber breakfast affects the postprandial plasma glucose, insulin, and ghrelin responses of patients with type 2 diabetes in a randomized clinical trial. J Nutr 145: 736–741. [DOI] [PubMed] [Google Scholar]
- 91.Soare A, Khazrai YM, Del Toro R, Roncella E, Fontana L, Fallucca S, Angeletti S, Formisano V, Capata F, Ruiz V, Porrata C, Skrami E, Gesuita R, Manfrini S, Fallucca F, Pianesi M, and Pozzilli P. 2014. The effect of the macrobiotic Ma-Pi 2 diet vs. the recommended diet in the management of type 2 diabetes: the randomized controlled MADIAB trial. Nutr Metab (Lond) 11: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schmidt JV, and Bradfield CA. 1996. Ah receptor signaling pathways. Annu Rev Cell Dev Biol 12: 55–89. [DOI] [PubMed] [Google Scholar]
- 93.Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, Wilhelm C, and Veldhoen M. 2011. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 147: 629–640. [DOI] [PubMed] [Google Scholar]
- 94.Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, and Zhou L. 2012. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 36: 92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stockinger B, Hirota K, Duarte J, and Veldhoen M. 2011. External influences on the immune system via activation of the aryl hydrocarbon receptor. Semin Immunol 23: 99–105. [DOI] [PubMed] [Google Scholar]
- 96.Gronke K, Hernandez PP, Zimmermann J, Klose CSN, Kofoed-Branzk M, Guendel F, Witkowski M, Tizian C, Amann L, Schumacher F, Glatt H, Triantafyllopoulou A, and Diefenbach A. 2019. Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature 566: 249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hanash AM, Dudakov JA, Hua G, O’Connor MH, Young LF, Singer NV, West ML, Jenq RR, Holland AM, Kappel LW, Ghosh A, Tsai JJ, Rao UK, Yim NL, Smith OM, Velardi E, Hawryluk EB, Murphy GF, Liu C, Fouser LA, Kolesnick R, Blazar BR, and van den Brink MR. 2012. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity 37: 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lindemans CA, Calafiore M, Mertelsmann AM, O’Connor MH, Dudakov JA, Jenq RR, Velardi E, Young LF, Smith OM, Lawrence G, Ivanov JA, Fu YY, Takashima S, Hua G, Martin ML, O’Rourke KP, Lo YH, Mokry M, Romera-Hernandez M, Cupedo T, Dow L, Nieuwenhuis EE, Shroyer NF, Liu C, Kolesnick R, van den Brink MRM, and Hanash AM. 2015. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 528: 560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Caulfield LE, de Onis M, Blossner M, and Black RE. 2004. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr 80: 193–198. [DOI] [PubMed] [Google Scholar]
- 100.Hall JA, Grainger JR, Spencer SP, and Belkaid Y. 2011. The role of retinoic acid in tolerance and immunity. Immunity 35: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van de Pavert SA, Olivier BJ, Goverse G, Vondenhoff MF, Greuter M, Beke P, Kusser K, Hopken UE, Lipp M, Niederreither K, Blomhoff R, Sitnik K, Agace WW, Randall TD, de Jonge WJ, and Mebius RE. 2009. Chemokine CXCL13 is essential for lymph node initiation and is induced by retinoic acid and neuronal stimulation. Nat Immunol 10: 1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Spencer SP, Wilhelm C, Yang Q, Hall JA, Bouladoux N, Boyd A, Nutman TB, Urban JF Jr., Wang J, Ramalingam TR, Bhandoola A, Wynn TA, and Belkaid Y. 2014. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science 343: 432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Beijer MR, Kraal G, and den Haan JM. 2014. Vitamin A and dendritic cell differentiation. Immunology 142: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grizotte-Lake M, Zhong G, Duncan K, Kirkwood J, Iyer N, Smolenski I, Isoherranen N, and Vaishnava S. 2018. Commensals Suppress Intestinal Epithelial Cell Retinoic Acid Synthesis to Regulate Interleukin-22 Activity and Prevent Microbial Dysbiosis. Immunity 49: 1103–1115 e1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Klebanoff CA, Spencer SP, Torabi-Parizi P, Grainger JR, Roychoudhuri R, Ji Y, Sukumar M, Muranski P, Scott CD, Hall JA, Ferreyra GA, Leonardi AJ, Borman ZA, Wang J, Palmer DC, Wilhelm C, Cai R, Sun J, Napoli JL, Danner RL, Gattinoni L, Belkaid Y, and Restifo NP. 2013. Retinoic acid controls the homeostasis of pre-cDC-derived splenic and intestinal dendritic cells. J Exp Med 210: 1961–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, and Song SY. 2004. Retinoic acid imprints gut-homing specificity on T cells. Immunity 21: 527–538. [DOI] [PubMed] [Google Scholar]
- 107.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, and von Andrian UH. 2006. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 314: 1157–1160. [DOI] [PubMed] [Google Scholar]
- 108.Suzuki K, Maruya M, Kawamoto S, Sitnik K, Kitamura H, Agace WW, and Fagarasan S. 2010. The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin A generation in the gut. Immunity 33: 71–83. [DOI] [PubMed] [Google Scholar]
- 109.Hall JA, Cannons JL, Grainger JR, Dos Santos LM, Hand TW, Naik S, Wohlfert EA, Chou DB, Oldenhove G, Robinson M, Grigg ME, Kastenmayer R, Schwartzberg PL, and Belkaid Y. 2011. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity 34: 435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, and Belkaid Y. 2007. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med 204: 1775–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, and Powrie F. 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-{beta}- and retinoic acid-dependent mechanism. J Exp Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, and Cheroutre H. 2007. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317: 256–260. [DOI] [PubMed] [Google Scholar]
- 113.Mucida D, Pino-Lagos K, Kim G, Nowak E, Benson MJ, Kronenberg M, Noelle RJ, and Cheroutre H. 2009. Retinoic acid can directly promote TGF-beta-mediated Foxp3(+) Treg cell conversion of naive T cells. Immunity 30: 471–472; author reply 472–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wessells KR, and Brown KH. 2012. Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS One 7: e50568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dardenne M 2002. Zinc and immune function. Eur J Clin Nutr 56 Suppl 3: S20–23. [DOI] [PubMed] [Google Scholar]
- 116.Liu MJ, Bao S, Galvez-Peralta M, Pyle CJ, Rudawsky AC, Pavlovicz RE, Killilea DW, Li C, Nebert DW, Wewers MD, and Knoell DL. 2013. ZIP8 regulates host defense through zinc-mediated inhibition of NF-kappaB. Cell Rep 3: 386–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wong HR, Shanley TP, Sakthivel B, Cvijanovich N, Lin R, Allen GL, Thomas NJ, Doctor A, Kalyanaraman M, Tofil NM, Penfil S, Monaco M, Tagavilla MA, Odoms K, Dunsmore K, Barnes M, Aronow BJ, and Genomics of Pediatric SSSI. 2007. Genome-level expression profiles in pediatric septic shock indicate a role for altered zinc homeostasis in poor outcome. Physiol Genomics 30: 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cherayil BJ 2011. The role of iron in the immune response to bacterial infection. Immunol Res 50: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hassan TH, Badr MA, Karam NA, Zkaria M, El Saadany HF, Abdel Rahman DM, Shahbah DA, Al Morshedy SM, Fathy M, Esh AM, and Selim AM. 2016. Impact of iron deficiency anemia on the function of the immune system in children. Medicine (Baltimore) 95: e5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Das I, Saha K, Mukhopadhyay D, Roy S, Raychaudhuri G, Chatterjee M, and Mitra PK. 2014. Impact of iron deficiency anemia on cell-mediated and humoral immunity in children: A case control study. J Nat Sci Biol Med 5: 158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moretti D, Goede JS, Zeder C, Jiskra M, Chatzinakou V, Tjalsma H, Melse-Boonstra A, Brittenham G, Swinkels DW, and Zimmermann MB. 2015. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood 126: 1981–1989. [DOI] [PubMed] [Google Scholar]
- 122.Ganz T 2006. Hepcidin--a peptide hormone at the interface of innate immunity and iron metabolism. Curr Top Microbiol Immunol 306: 183–198. [DOI] [PubMed] [Google Scholar]
- 123.La Rosa PS, Warner BB, Zhou Y, Weinstock GM, Sodergren E, Hall-Moore CM, Stevens HJ, Bennett WE Jr., Shaikh N, Linneman LA, Hoffmann JA, Hamvas A, Deych E, Shands BA, Shannon WD, and Tarr PI. 2014. Patterned progression of bacterial populations in the premature infant gut. Proc Natl Acad Sci U S A 111: 12522–12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, and Ley RE. 2011. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A 108 Suppl 1: 4578–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Subramanian S, Blanton LV, Frese SA, Charbonneau M, Mills DA, and Gordon JI. 2015. Cultivating healthy growth and nutrition through the gut microbiota. Cell 161: 36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Robertson RC, Manges AR, Finlay BB, and Prendergast AJ. 2019. The Human Microbiome and Child Growth - First 1000 Days and Beyond. Trends Microbiol 27: 131–147. [DOI] [PubMed] [Google Scholar]
- 127.Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, Benezra A, DeStefano J, Meier MF, Muegge BD, Barratt MJ, VanArendonk LG, Zhang Q, Province MA, Petri WA Jr., Ahmed T, and Gordon JI. 2014. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 510: 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Blanton LV, Charbonneau MR, Salih T, Barratt MJ, Venkatesh S, Ilkaveya O, Subramanian S, Manary MJ, Trehan I, Jorgensen JM, Fan YM, Henrissat B, Leyn SA, Rodionov DA, Osterman AL, Maleta KM, Newgard CB, Ashorn P, Dewey KG, and Gordon JI. 2016. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Borelli P, Barros FE, Nakajima K, Blatt SL, Beutler B, Pereira J, Tsujita M, Favero GM, and Fock RA. 2009. Protein-energy malnutrition halts hemopoietic progenitor cells in the G0/G1 cell cycle stage, thereby altering cell production rates. Braz J Med Biol Res 42: 523–530. [DOI] [PubMed] [Google Scholar]
- 130.Savino W, and Dardenne M. 2010. Nutritional imbalances and infections affect the thymus: consequences on T-cell-mediated immune responses. Proc Nutr Soc 69: 636–643. [DOI] [PubMed] [Google Scholar]
- 131.Conzen SD, and Janeway CA. 1988. Defective antigen presentation in chronically protein-deprived mice. Immunology 63: 683–689. [PMC free article] [PubMed] [Google Scholar]
- 132.Taylor AK, Cao W, Vora KP, De La Cruz J, Shieh WJ, Zaki SR, Katz JM, Sambhara S, and Gangappa S. 2013. Protein energy malnutrition decreases immunity and increases susceptibility to influenza infection in mice. J Infect Dis 207: 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schaible UE, and Kaufmann SH. 2007. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med 4: e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Barker DJ 2007. The origins of the developmental origins theory. J Intern Med 261: 412–417. [DOI] [PubMed] [Google Scholar]
- 135.Kolcic I 2012. Double burden of malnutrition: A silent driver of double burden of disease in low- and middle-income countries. J Glob Health 2: 020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Collins N, Han SJ, Enamorado M, Link VM, Huang B, Moseman EA, Kishton RJ, Shannon JP, Dixit D, Schwab SR, Hickman HD, Restifo NP, McGavern DB, Schwartzberg PL, and Belkaid Y. 2019. The Bone Marrow Protects and Optimizes Immunological Memory during Dietary Restriction. Cell 178: 1088–1101 e1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jordan S, Tung N, Casanova-Acebes M, Chang C, Cantoni C, Zhang D, Wirtz TH, Naik S, Rose SA, Brocker CN, Gainullina A, Hornburg D, Horng S, Maier BB, Cravedi P, LeRoith D, Gonzalez FJ, Meissner F, Ochando J, Rahman A, Chipuk JE, Artyomov MN, Frenette PS, Piccio L, Berres ML, Gallagher EJ, and Merad M. 2019. Dietary Intake Regulates the Circulating Inflammatory Monocyte Pool. Cell 178: 1102–1114 e1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Guerrant RL, DeBoer MD, Moore SR, Scharf RJ, and Lima AA. 2013. The impoverished gut--a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol 10: 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Korpe PS, and Petri WA Jr. 2012. Environmental enteropathy: critical implications of a poorly understood condition. Trends Mol Med 18: 328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Keusch GT, Denno DM, Black RE, Duggan C, Guerrant RL, Lavery JV, Nataro JP, Rosenberg IH, Ryan ET, Tarr PI, Ward H, Bhutta ZA, Coovadia H, Lima A, Ramakrishna B, Zaidi AK, Hay Burgess DC, and Brewer T. 2014. Environmental enteric dysfunction: pathogenesis, diagnosis, and clinical consequences. Clin Infect Dis 59 Suppl 4: S207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Crane RJ, Jones KD, and Berkley JA. 2015. Environmental enteric dysfunction: an overview. Food Nutr Bull 36: S76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Petri WA Jr., Naylor C, and Haque R. 2014. Environmental enteropathy and malnutrition: do we know enough to intervene? BMC Med 12: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Naylor C, Lu M, Haque R, Mondal D, Buonomo E, Nayak U, Mychaleckyj JC, Kirkpatrick B, Colgate R, Carmolli M, Dickson D, van der Klis F, Weldon W, Steven Oberste M, teams P. s., Ma JZ, and Petri WA Jr. 2015. Environmental Enteropathy, Oral Vaccine Failure and Growth Faltering in Infants in Bangladesh. EBioMedicine 2: 1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Johnson RA, Uddin T, Aktar A, Mohasin M, Alam MM, Chowdhury F, Harris JB, LaRocque RC, Bufano MK, Yu Y, Wu-Freeman Y, Leung DT, Sarracino D, Krastins B, Charles RC, Xu P, Kovac P, Calderwood SB, Qadri F, and Ryan ET. 2012. Comparison of immune responses to the O-specific polysaccharide and lipopolysaccharide of Vibrio cholerae O1 in Bangladeshi adult patients with cholera. Clin Vaccine Immunol 19: 1712–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Grassly NC, Jafari H, Bahl S, Durrani S, Wenger J, Sutter RW, and Aylward RB. 2009. Mucosal immunity after vaccination with monovalent and trivalent oral poliovirus vaccine in India. J Infect Dis 200: 794–801. [DOI] [PubMed] [Google Scholar]
- 146.Santos N, and Hoshino Y. 2005. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev Med Virol 15: 29–56. [DOI] [PubMed] [Google Scholar]
- 147.Katz DL, and Meller S. 2014. Can we say what diet is best for health? Annu Rev Public Health 35: 83–103. [DOI] [PubMed] [Google Scholar]
- 148.Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M, Suez J, Mahdi JA, Matot E, Malka G, Kosower N, Rein M, Zilberman-Schapira G, Dohnalova L, Pevsner-Fischer M, Bikovsky R, Halpern Z, Elinav E, and Segal E. 2015. Personalized Nutrition by Prediction of Glycemic Responses. Cell 163: 1079–1094. [DOI] [PubMed] [Google Scholar]
- 149.Wang A, Huen SC, Luan HH, Baker K, Rinder H, Booth CJ, and Medzhitov R. 2018. Glucose metabolism mediates disease tolerance in cerebral malaria. Proc Natl Acad Sci U S A 115: 11042–11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang A, Huen SC, Luan HH, Yu S, Zhang C, Gallezot JD, Booth CJ, and Medzhitov R. 2016. Opposing Effects of Fasting Metabolism on Tissue Tolerance in Bacterial and Viral Inflammation. Cell 166: 1512–1525 e1512. [DOI] [PMC free article] [PubMed] [Google Scholar]


