Abstract
Background
Bone flare has been observed on 99mTc-MDP bone scans of patients with metastatic castration-resistant prostate cancer (mCRPC). This exploratory study investigates bone flare in mCRPC patients receiving androgen receptor (AR) inhibitors using 18F-NaF PET/CT.
Methods
Twenty-nine mCRPC patients undergoing AR-inhibiting therapy (abiraterone, orteronel, enzalutamide) received NaF PET/CT scans at baseline, week 6, and week 12 of treatment. SUV metrics were extracted globally for each patient (SUV) and for each individual lesion (iSUV). Bone flare was defined as increasing SUV metrics or lesion number at week 6 followed by subsequent week 12 decrease. Differences in metrics across timepoints were compared using Wilcoxon tests. Cox proportional hazard regression was conducted between global metrics and progression-free survival (PFS).
Results
Total SUV was most sensitive for flare detection and was identified in 14/23 (61%) patients receiving CYP17A1-inhibitors (abiraterone, orteronel), and not identified in any of six patients receiving enzalutamide. The appearance of new lesions did not account for initial increases in SUV metrics. iSUV metrics followed patient-level trends: bone flare positive patients showed a median of 72% (range: 0–100%) of lesions with total iSUV flare. Increasing mean SUV at week 6 correlated with extended PFS (HR = 0.58, p = 0.02).
Conclusion
NaF PET bone flare was present on 61% of mCRPC patients in the first 6 weeks of treatment with CYP17A1-inhibitors. Characterization provided in this study suggests favorable PFS in patients showing bone flare. This characterization of NaF flare is important for guiding treatment assessment schedules to better distinguish between patients showing bone flare and those truly progressing, and should be performed for all emerging mCRPC treatments and imaging agents.
Introduction
Mid-treatment response assessment in patients with bone metastatic castration-resistant prostate cancer (mCRPC) has found patients may show radiologic progression conflicting with serologic changes measured via prostate specific antigen (PSA) [1]. This apparent progression, identified on 99mTc methyl diphosphonate (MDP) bone scans, often subsided in later scans, suggesting initial changes in appearance can be classified as a bone flare reaction [1]. Rather than an unfavorable reaction, bone flare could be indicative of osteoblastic stimulation during the bone repair process [2–4], and had been associated with successful therapy [5, 6].
Bone flare in mCRPC patients has been associated with androgen receptor (AR) inhibitor therapies such as abiraterone [1, 7], which inhibits the CYP17A1 enzyme, indirectly preventing production of androgens and estrogens in adrenal glands and tumor tissue [8]. Newer AR-inhibiting therapies include orteronel, which inhibits the CYP17A1 pathway similar to abiraterone, and enzalutamide, which works via AR binding, blocking AR translocation and transcription and inhibiting downstream pathways [7]. Differentiation between early progression and bone flare in mCRPC patients receiving these therapies cannot be confirmed until follow-up scans are taken, thus understanding the dynamics of bone flare in emerging mCRPC treatments and imaging agents is essential to guide treatment response assessment schedules.
While planar MDP bone scanning remains the clinical standard of care, image interpretation can be variable, and detection of small changes in uptake is not feasible. Similar to MDP bone scans, 18F-sodium fluoride (NaF) positron emission tomography (PET) localizes in bone lesions as a surrogate of osteoblastic activity. NaF PET scans have a significantly higher detection sensitivity and specificity when paired with computed tomography (CT) scans compared to MDP bone scans [9] and thus can detect disease changes earlier. PET/CT also presents a higher spatial resolution [9], and allows for semi-quantitative response assessment using standardized uptake value (SUV) metrics. Recent studies have shown various NaF PET SUV metrics to be prognostic of progression-free survival (PFS) [10] and overall survival (OS) [11].
This exploratory study aims to identify and characterize bone flare in mCRPC patients receiving AR-directed treatments using 18F-NaF PET/CT. We aim to detect and quantify bone flare at both the global patient level and individual lesion level. Additionally, we examine the relationship between NaF PET flare and clinical PFS.
Methods
Patients and scan acquisition
This multi-institutional study included 33 mCRPC patients over the age of 18 enrolled as part of a larger prospective study evaluating the repeatability of NaF PET/CT [12] and its ability to measure response to therapy [10] (NCT01516866). Participating sites included University of Wisconsin Carbone Cancer Center, Memorial Sloan-Kettering Cancer Center, and the National Cancer Institute. Study protocols were approved by the Institutional Review Board at each institution and all patients signed a written informed consent. Patients underwent whole body NaF PET/CT imaging at baseline (pretreatment), week 6, and week 12 of treatment. Patients were injected intravenously with 163 ± 26MBq of 18F-NaF and imaged 63 ± 16 min post injection. Study design included a week 6 imaging timepoint to evaluate the presence of flare. Patients received treatment with either abiraterone, orteronel, or enzalutamide based on physician discretion.
Quantitative analysis
Using an image analysis software, Quantitative Total Bone Imaging [10, 12], imaging metrics (maximum, mean, and total uptake) were extracted from NaF PET/CT scans for each patient (SUV) and for each individual lesion (iSUV) at each timepoint. Lesion segmentation was completed on each NaF PET scan by applying a SUV > 15 g/mL threshold to lesions contained within skeletal regions [12, 13]. Any benign lesions identified by an experienced nuclear medicine physician still present after segmentation were manually removed. Articulated skeletal registration between scans was completed [14, 15], allowing individual lesions to be tracked across imaging timepoints.
Following previous studies [1], NaF flare was characterized by any increase in uptake on the first follow-up scan followed by any decrease on the subsequent scan, and was quantitatively assessed using each global SUV metric as well as lesion number. To study bone flare at a lesion level, only lesions in patients experiencing bone flare that were present with a functional volume greater than 1.5 cc at all three imaging timepoints [12] (baseline, week 6, and week 12) were analyzed.
PSA levels were measured 1 week before treatment start, and at approximately 4 week intervals for 4 treatment cycles. PSA levels were utilized with NaF SUV metrics to further analyze NaF flare. PFS was defined as the number of days from treatment initiation to a disease progression event (radiographic or clinical) or death, whichever comes first, with clinical progression including events related to changes in PSA and/or physician discretion. Physicians were not blinded to treatments. Patients not showing progression-related events by the end of follow-up period were censored to the last applicable examination date. For a full description of outcome evaluation, see [10].
Statistical analysis
Flare was assessed at a patient level and individual lesion level by characterizing the proportion of patients (lesions) showing flare in each SUV (iSUV) metric. To assess treatment effects of the patient population as a whole, differences in global SUV were summarized in medians and ranges and compared between timepoints using Wilcoxon signed-rank tests on absolute SUV values. iSUV metrics were compared using Wilcoxon signed-rank tests for clustered data to account for potential correlation of lesions within the same patient [16]. In order to assess the impact of flare on patient outcome, univariable Cox proportional hazard regression was conducted between baseline to week 6 changes in SUV metrics (ΔSUV) and PFS. Multivariable Cox proportional hazard regression analyses were conducted using univariable predictors (p < 0.2) to evaluate whether changes in global SUV metrics predict PFS independently from changes in PSA metrics. All reported p-values are two-sided and p < 0.05 was used to define statistical significance. Statistical analysis was conducted using R software (version 3.2.5; https://www.r-project.org/).
Results
Of the 33 patients enrolled in this study, 19 received abiraterone, seven received orteronel, and seven received enzalutamide. Relevant patient demographic information is in Supplementary Material: Table 1. Twenty-nine patients successfully completed baseline, week 6, and week 12 NaF PET/CT scans (N = 19 for abiraterone, N = 4 for orteronel, N = 6 for enzalutamide).
Patient level analysis
Evidence of global NaF flare was assessed in the 29 patients completing baseline and both follow-up NaF PET/CT scans (Table 1). One patient receiving enzalutamide showed minimal evidence of flare in any NaF PET metrics (SUVmean); however, SUVmean changes were small (+4% increase followed by −1% decrease) and discordant with response in other SUV metrics. No other patients receiving enzalutamide showed evidence of global NaF flare in any metrics including lesion number, thus these patients were separated from those receiving CYP17A1-inhibitors for further NaF flare analysis.
Table 1.
Statistics of patients receiving baseline, week 6, and week 12 scans
| NaF PET metric | All patients (N = 29) |
Patients receiving CYP17A1-inhibiting drugs (N = 23) |
||||
|---|---|---|---|---|---|---|
| Patients showing week 6 increase (%) | Patients showing flare (%) | Time-point | Median change (%) | Range | p-value | |
| Total patient uptake (SUVtotal) | 69 | 48 | Baseline – 6wk | 19.3 | (−42.2, 205.1) | 0.075 |
| 6wk – 12wk | −14.3 | (−76.3, 97.8) | 0.260 | |||
| Maximum patient uptake (SUVmax) | 45 | 34 | Baseline – 6wk | 5.2 | (−31.4, 61.9) | 0.423 |
| 6wk – 12wk | −15.4 | (−56.4, 39.8) | 0.015 | |||
| Average of all iSUVmean (SUVmean) | 55 | 38 | Baseline – 6wk | −0.5 | (−12.7, 37.0) | 0.689 |
| 6wk – 12wk | −6.1 | (−15.2, 20.5) | 0.004 | |||
| Number of lesions (Nlesion) | 34 | 17 | Baseline – 6wk | 0.0 | (−60, 100.0) | 0.355 |
| 6wk – 12wk | 15.9 | (−63.0, 115.0) | 0.879 | |||
Due to the similarities between the drug pathways and similar global response patterns, patients receiving abiraterone and orteronel were combined for the remaining analysis. Of the 23 patients receiving CYP17A1-inhibitors, 20 (87%) showed an initial increase at week 6 in one or more SUV metrics, with 17 (74%) showing evidence of patient-level NaF flare in one or more metrics. While nine patients receiving CYP17A1-inhibitors showed increasing lesion number at week 6, only five patients (22%) showed a subsequent decrease (flare) in lesion number.
All patients showing flare in SUVtotal also showed flare in SUVmean, SUVmax, or both, suggesting SUVtotal could be used individually to define flare response. Fourteen of the 23 patients receiving CYP17A1-inhibitors (61%) were thus classified as experiencing a NaF PET flare response to treatment (Fig. 1). Patients exhibited a wide range of treatment reactions, representative examples of extreme, median, and no evidence of bone flare are illustrated in Fig. 2.
Fig. 1.
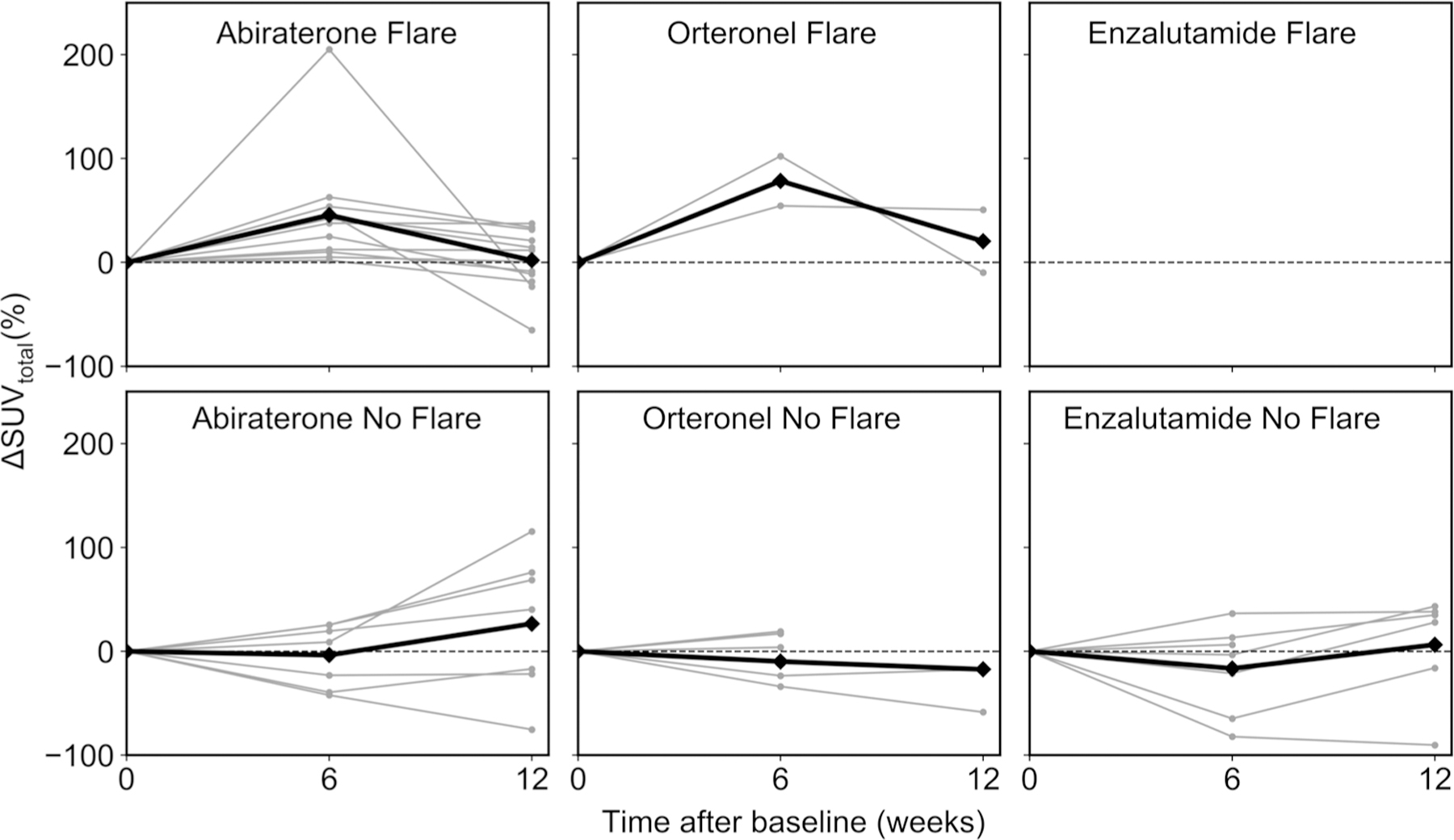
Change (%) in SUVtotal between follow-up and baseline scans for all 33 patients, separated by treatment and flare. Individual patients are indicated by the symbol (•) and patient averages in each category are marked by the symbol (♦). Only percent change between baseline and week 6 scans are shown for patients not receiving week 12 scans. Note no patients receiving enzalutamide showed a flare response
Fig. 2.
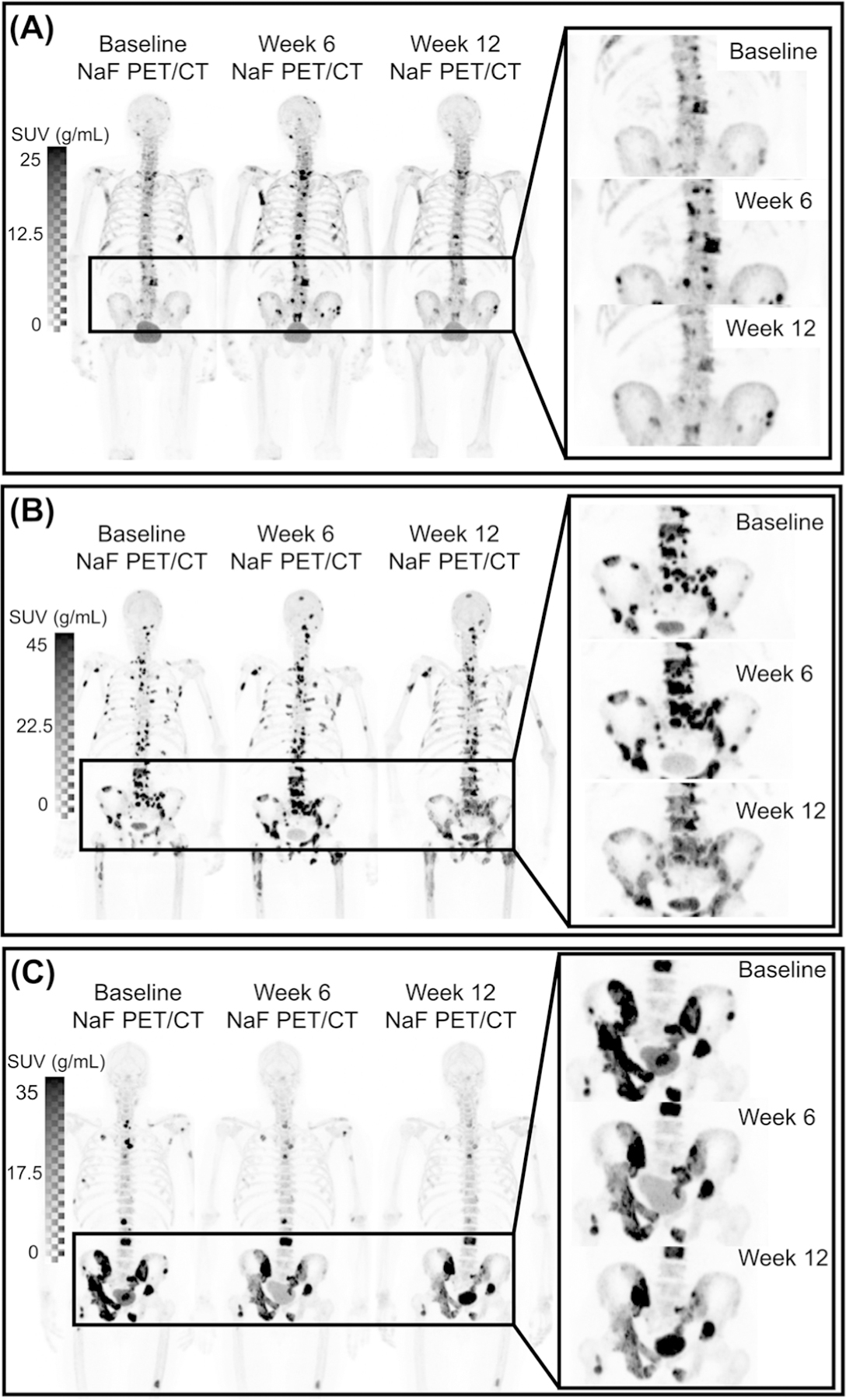
a A patient showing extreme NaF PET flare had an increase in SUVtotal of 205% (baseline-week 6) and −57% (week 6-week 12) with 38, 73, and 27 lesions on baseline, week 6, and week 12 scans, respectively. This patient showed signs of flare in all SUV metrics. b Patient showing average flare had change in SUVtotal of 53% (baseline-week 6) and −14% (week 6-week 12), with 113, 108, and 105 lesions on baseline, week 6, and week 12 scans, respectively. c Patient not showing bone flare showed change in SUVtotal of −34% (baseline-week 6) and −37% (week 6-week 12), with 32, 27, and 32 lesions on baseline, week 6, and week 12 scans, respectively
Table 1 shows median and range of changes in patient-level metrics between scan timepoints for patients receiving CYP17A1-inhibitors. Median changes in all SUV metrics show increasing values at week 6 relative to baseline scans, and decreasing values between week 6 and week 12 scans. Patient lesion number shows a stable median at week 6 followed by an increase at week 12.
Lesion level SUV changes
Sixty-two lesions with a segmented volume greater than 1.5 cc remained present on all three NaF PET scans of the 14 patients showing NaF PET flare, with a median of four longitudinally trackable lesions (range 1–21) per patient. Table 2 reports the median and range percentage of these individual lesions per patient that showed an increase in iSUV metrics at week 6 as well as lesions showing flare in each NaF PET metric. Similar to global SUV metrics, median changes between scans show an increase in all individual lesion uptake metrics between baseline and week 6 scans and subsequent decrease at week 12 (Table 2). Excluding change in iSUVmax at week 6, all iSUV metrics show significant changes between scans.
Table 2.
Statistics of individual lesions present on baseline, week 6, and week 12 scans (N = 62 lesions) in patients showing signs of NaF SUVtotal flare
| NaF PET Metric | Lesions increasing at week 6 Median % (range) | Flaring lesions Median % (range) | Time-point | Median change (%) | Range | p-value |
|---|---|---|---|---|---|---|
| Total lesion uptake (iSUVtotal) | 100 (50, 100) | 73 (0, 100) | Baseline – 6wk | 43.5 | (−39.6, 276.5) | 0.006 |
| 6wk – 12wk | −17.3 | (−76.6, 37.3) | 0.014 | |||
| Maximum lesion uptake (iSUVmax) | 66 (0, 100) | 54 (0, 100) | Baseline – 6wk | 7.1 | (−34.4, 85.1) | 0.061 |
| 6wk – 12wk | −17.0 | (−56.4, 21.0) | 0.004 | |||
| Average lesion uptake (iSUVmean) | 73 (0, 100) | 62 (0, 100) | Baseline – 6wk | 3.4 | (−18.5, 55.2) | 0.036 |
| 6wk – 12wk | −13.0 | (−41.1, 13.6) | 0.002 |
Relation to clinical outcome
Twenty-five patients receiving CYP17A1 inhibitors had paired baseline and week 6 scans for inclusion in outcome analysis. Median interval from start of treatment to date of progression was 7.1 months (range 2.4–29.5). A forest plot representing results from cox proportional hazard regression analysis (hazard ratio +/−95% confidence interval) is shown in Fig. 3 for changes in SUV NaF PET metrics between baseline and week 6 scans. An increase in SUVmean at week 6 was significantly associated with prolonged progression-free survival (HR = 0.57, p = 0.02). This relationship remained significant in a multivariable model with changes in PSA levels at week 8 (HR = 0.56, p = 0.02 for ΔSUVmean, HR = 1.97, p = 0.03 for ΔPSA). The change in total number of lesions, assessed at week 6, showed no relation to PFS. No other multivariable models including ΔSUV metrics showed significant relations to PFS after adjusting for changes in PSA metrics.
Fig. 3.

Forest plot representing hazard ratio +/−95% confidence interval for changes in between baseline and week 6 in patients receiving CYP17A1-inhibiting therapies
Correlation to PSA
PSA levels were used to further analyze bone flare in patients receiving baseline, week 6, and week 12 scans (N = 23). In comparing the change in SUVtotal between baseline and week 6 scans with change in PSA between baseline and week 8, all patients showed reactions in three categories: (1) increasing SUVtotal and increasing PSA, N = 5; (2) increasing SUVtotal and decreasing PSA, N = 13; or (3) decreasing SUVtotal and decreasing PSA, N = 5. Only one of the 14 patients showing NaF flare did not show a decrease in PSA, with an increase of 10% at week 8; however, showed signs of flare in both SUVmean and SUVtotal with a PFS longer than the median of 7.1 months (23.6 months).
Discussion
This exploratory study is the first to document and measure bone flare response to AR-directed therapies on NaF PET/CT scans of patients with mCRPC using both patient and individual lesion level metrics. Flare was detected in 61% (14/23) of patients receiving CYP17A1-inhibitors, with a majority of evaluable lesions in these patients showing an individual flare response occurring independent from uptake in normal bone. Patients receiving enzalutamide did not show characteristics of NaF flare. Patients could be grouped into three categories of treatment reaction: classical responders (decrease in NaF uptake and decrease in PSA, N = 5), classical progression (increase in NaF uptake and increase in PSA, N = 5), or bone flare response (increase in NaF uptake and decrease in PSA, N = 13). The 13 patients showing increasing week 6 SUVtotal and decreasing week 8 PSA also showed decreasing SUVtotal at the week 12, indicating the majority (93%) of patients flaring in SUVtotal showed signs of improvement in PSA.
A previous report of patients with mCRPC receiving abiraterone defined MDP bone scan flare as a progressing MDP bone scan 3 months after initiating treatment accompanied by a 50% decrease in PSA, followed by subsequent improvement in MDP bone scans 3 months later [1]. Bone flare was identified in 10 of 23 evaluable patients (44%) [1]. This incidence rate is likely lower than patients showing NaF flare (61%) in the present study as a result of later imaging and poorer detection sensitivity of MDP bone scans that limits the detection of bone flare. Separately, flare has also been detected on CT images in a small proportion of mCRPC patients (3/39) receiving CYP17-inhibiting therapies at week 12 of treatment [17]. Changes in average HU across bone lesions were not correlated to changes in any global SUV metric in the present study (Spearman’s ρ = −0.02, 0.01, and 0.06 for SUVmean, SUVtotal, and SUVmax, respectively); however, the week 6 timepoint may be too early to detect CT flare.
In an attempt to separate men experiencing bone flare from men showing disease progression, PCWG2 defined MDP bone scan progression as at least two new confirmed bone lesions on the first scan, followed by two new confirmed lesions at the next assessment timepoint (2 + 2 rule) [18], ensuring only truly progressing patients would be considered for termination of treatment. Here we have reported stable lesion numbers across timepoints, suggesting that the development of new lesions was not characteristic of NaF flare in this cohort of patients. Within the subset of 14 NaF flaring patients, median changes in lesion number at week 6 was −2.2% (range −33 to 100%). Moreover, change in lesion number between baseline and week 6 scans was not predictive of progression-free survival (HR = 1.02, p = 0.9). As has been suggested in the past [19], newly detected MDP bone scan lesion in patients showing signs of bone flare may have been present on baseline scans but below the detectability threshold. In addition, automated methods allow for a more complete and objective analysis compared to manual observation.
In this study, an increase in SUVmean at week 6 correlated with prolonged PFS (HR = 0.58, p = 0.02), with increases in SUVmax and SUVtotal showing similar trends though not significant. Multivariable analysis revealed an increase in SUVmean at week 6 and a decrease in PSA at week 8 were independent predictors of prolonged PFS (HR = 0.57, p = 0.02; HR = 1.97, p = 0.03, respectively). Thus, as has been suggested in previous studies, a bone flare response to therapy may be indicative of a healing reaction within boney lesions [2–4], representing treatment success rather than disease progression. It has been shown that patient level NaF PET SUV metrics measured at week 12 are better indicators of PFS than measurements taken at week 6 [10]. This supports the notion that response assessment at week 6 is unreliable due to this bone flare response [5]. Flare was shown to be subsiding at the week 12 timepoint, although increases in SUV metrics did not consistently return to levels present at baseline or below.
Reports of flare in mCRPC patients receiving enzalutamide have been discordant: de Giorgi et al. [7] reported no signs of bone flare using 18F-Fluorocholine PET/CT, while Ning et al. [20] confirmed the presence of bone flare using MDP bone scans. In the present study, no enzalutamide patients showed signs of NaF flare. Similar to the report by de Giorgi et al., this may be due to the small patient cohort receiving enzalutamide (six patients) and inclusion of pre-treated patients (response rates for those receiving first-line CRPC therapy may differ from those receiving second-line therapy or greater). Thus, we cannot reliably confirm whether week 6 NaF flare is characteristic of only mCRPC patients receiving androgen-biosynthesis inhibitors (abiraterone, orteronel) as opposed to androgen-signaling pathway inhibitors (enzalutamide).
Limitations of this study include lack of long-term follow-up for a full determination of NaF flare beyond 12 weeks. In addition, the definition of flare included any changes in NaF, regardless of test–retest limits of agreement (LOA). For a more comprehensive definition of flare, more patients are needed to account for these test–retest LOA. It should also be noted that the endpoint utilized in this study was a composite endpoint and should be considered exploratory [10]. Lastly, further examination of bone flare in mCRPC patients receiving orteronel and enzalutamide is needed as only four and six patients receiving this drug, respectively, received NaF PET/CT scans at all three timepoints.
To conclude, results in this study show the promise of using automated methods to detect and semi-quantitatively analyze NaF PET/CT bone flare at early imaging timepoints in patients with mCRPC receiving CYP17A1-inhibiting therapies. The presence of a flare phenomenon must be accounted for when determining imaging schedules for patients receiving therapy for mCRPC. This is especially important in evaluating emerging mCRPC treatments and imaging modalities for assessing treatment response, as the exact cause, timing, and impact of bone flare on individual patients is still unknown.
Supplementary Material
Acknowledgements
We would like to thank the patients who volunteered their time, imaging technologists for data acquisition, and Christine Jaskowiak for her assistance. We would also like to thank the University of Wisconsin Carbone Cancer Center. This study was supported by the Prostate Cancer Foundation (PCF) through the PCF Creativity Award (Liu, Jeraj) and PCF Mazzone Challenge Award (Jeraj, Liu), and conducted within the Prostate Cancer Clinical Trials Consortium (PCCTC). Additional support provided by the United States Department of Defense Prostate Cancer Research Program (W81XWH-17-0020)
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1038/s41391-018-0110-5) contains supplementary material, which is available to authorized users.
Conflict of interest Funding from this work received from the Prostate Cancer Foundation. RJ and GL are cofounders of AIQ Solutions. The remaining authors declare that they have no conflict of interest.
References
- 1.Ryan CJ, Shah S, Eleni E, Smith MR, Taplin M-E, Bubley GJ, et al. Phase II Study of Abiraterone Acetate Plus Prednisone in Chemotherapy-Naive Metastatic Castration-Resistant Prostate Cancer Demonstrating Radiographic Flare Discordant With Serologic Measures of Response. Clin Cancer Res. 2011;17:4854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong AJ, Kaboteh R, Carducci MA, Damber JE, Stadler WM, Hansen M, et al. Assessment of the bone scan index in a randomized placebo-controlled trial of tasquinimod in men with metastatic castration-resistant prostate cancer (mCRPC). Urol Oncol. 2014;32:1308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Sullivan GJ, Carty FL, Cronin CG. Imaging of bone metastasis: an update. World J Radiol. 2015;7:202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauerle T, Semmler W. Imaging response to systemic therapy for bone metastases. Eur Radiol. 2009;19:2495–507. [DOI] [PubMed] [Google Scholar]
- 5.Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34:1402–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman RE, Mashiter G, Whitake rKB, Moss DW, Rubens RD, Fogelman I. Bone scan flare predicts successful systemic therapy for bone metastases. J Nucl Med. 1988;29:1354–9. [PubMed] [Google Scholar]
- 7.de Giorgi U, Paola C, Emanuela S, Vincenza C, Luca BS, Cecilia M, et al. 18F-Fluorocholine PET/CT for early response assessment in patients with metastatic castration-resistant prostate cancer treated with enzalutamide. Eur J Nucl Med Mol Imaging. 2015;42:1337–8. [DOI] [PubMed] [Google Scholar]
- 8.Massard C, Fizazi K. Targeting continued androgen receptor signaling in prostate cancer. Clin Cancer Res. 2011;17:3876–83. [DOI] [PubMed] [Google Scholar]
- 9.Morisson C, Jeraj R, Liu G. Imaging of castration-resistant prostrate cancer: development of imaging response biomarkers. Curr Opin Urol. 2013;23:230–6. [DOI] [PubMed] [Google Scholar]
- 10.Harmon SA, Perk T, Lin C, Eickhoff J, Choyke PL, Dahut WL, et al. Quantitative Assessment of Early [18F]Sodium Fluoride Positron Emission Tomography/Computed Tomography Response to Treatment in Men With Metastatic Prostate Cancer to Bone. J Clin Oncol. 2017;35:2829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apolo AB, Lindenberg L, Shih JH, Mena E, Kim JW, Park JC, et al. Prospective Study Evaluating Na18F PET/CT in Predicting Clinical Outcomes and Survival in Advanced Prostate Cancer. J Nucl Med. 2016;57:886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin C, Bradshaw T, Perk T, Harmon S, Eickhoff J, Jallow N, et al. Repeatability of Quantitative 18F-NaF PET: A Multicenter Study. J Nucl Med. 2016;57:1872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rohren EM, Etchebehere EC, Araujo JC, Hobbs BP, Swanston NM, Everding M, et al. Determination of skeletal tumor burden on (18)F-fluoride PET/CT. J Nucl Med. 2015;56:1507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yip S, Perk T, Jeraj R. Development and evaluation of an articulated registration algorithm for human skeleton registration. Phys Med Biol. 2014;59:1485–99. [DOI] [PubMed] [Google Scholar]
- 15.Yip S, Jeraj R. Use of articulated registration for response assessment of individual metastatic bone lesions. Phys Med Biol. 2014;59:1501–14. [DOI] [PubMed] [Google Scholar]
- 16.Rosner B, Glynn RJ, Lee ML. The Wilcoxon signed rank test for paired comparisons of clustered data. Biometrics. 2006;62:185–92. [DOI] [PubMed] [Google Scholar]
- 17.Messiou C, Cook G, Reid AH, Attard G, Dearnaley D, de Bono JS, et al. The CT flare response of metastatic bone disease in prostate cancer. Acta Radiol. 2011;52:557–61. [DOI] [PubMed] [Google Scholar]
- 18.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossleigh MA, Byrne MJ, Whitney P, Reynolds PM. The Assessment of Response to Therapy of Bone Metastases in Breast Cancer. Aust N Z J Med. 1984;14:19–22. [DOI] [PubMed] [Google Scholar]
- 20.Ning YM, Chen C, Maher VE, Xu JX, Kim G, Pazdur R. Tumor progression versus bone scan “flare” in new lesions detected on early bone scans in patients with chemo-naïve metastatic castration resistant prostate cancer (mCRPC) treated with placebo or enzalutamide. J Clin Oncol. 2016;34(2_suppl):305–305. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


