Abstract
The prognostic role of the neutrophil-to-lymphocyte ratio (NLR) has been reported in colorectal cancer (CRC); however, its variation and corresponding predicative value in patients undergoing resection remain largely unknown. In the present study, data from 146 patients with CRC were retrospectively collected, optimal cut-off points for preoperative and postoperative low and high NLRs were set, and ΔNLR was calculated. Subsequently, patients were classified into low-low, low-high, high-low and high-high subgroups based on the cut-off points, and their progression-free survival (PFS) was determined. A Cox proportional hazard model was applied to calculate the prognostic value of all factors. The results demonstrated that both preoperative and postoperative NLRs (pre-NLR and post-NLR) but not ΔNLR could predict PFS with optimal cut-off points of 2.39 and 2.96, respectively. For predicting PFS, the pre-NLR had a sensitivity and specificity of 48.80 and 79.50%, respectively, and the post-NLR had a sensitivity and specificity of 63.20 and 56.20%, respectively. Significant differences were identified between low and high pre-NLRs in terms of histological grade (P<0.01) and tumor diameter (P<0.01); however, such differences were only found in terms of age (P<0.01) for low and high post-NLRs. The PFS of patients in the low-low, low-high, high-low and high-high subgroups was 50.30±21.36, 43.67±22.78, 31.06±25.56 and 29.87±24.13 months, respectively, and patients in the high-high subgroup had the worst PFS (P<0.01). Preoperative CEA level, invasive depth, node involvement, distant metastasis and preoperative NLR were independent prognostic factors. In conclusion, a persistently high NLR for patients with CRC undergoing resection was associated with poor prognosis.
Keywords: colorectal cancer, neutrophil-to-lymphocyte ratio, progression-free survival, prognosis, operation
Introduction
Colorectal cancer (CRC) still ranks as one of the leading causes of death for humans, and people are being diagnosed at a younger age (1). Until recently, the search for reliable, inexpensive, and readily available factors that could aid in precise prognostic prediction for patients was underappreciated.
The relationship between cancer and the host inflammatory response has been extensively studied (2,3), and markers of this response have independent prognostic value in patients with a variety of malignancies. It has long been established that cell fractions from peripheral blood, such as neutrophils, lymphocytes, monocytes, and platelets, as well as their ratios, can reflect systematic inflammatory responses, and such values have been applied for prognostic prediction in many malignancies, including lung (4), breast (5), pancreatic (6), and gastric (7) cancers and CRC (8-11). The neutrophil-to-lymphocyte ratio (NLR), which is defined as the ratio of the neutrophil count to the lymphocyte count, has been extensively studied and found to be highly superior when compared to other parameters, such as the platelet-to-lymphocyte ratio, in CRC (12,13). In a systematic review by Malietzis et al (14), a high NLR was found to be associated with significant survival disadvantages with either resection or palliative chemotherapy; an additional two similar systematic reviews supported these results (15,16). Nonetheless, studies have indicated that treatment approaches could affect the counts of neutrophils and lymphocytes (17); in addition, it has been suggested that longitudinal tests of the NLR would be more meaningful for individual patients. However, such reports are still rare.
In this study, we aimed to explore the longitudinal prognostic value of the NLR in patients undergoing resection.
Patients and methods
Patient enrollment
From January 2011 to September 22, 2014, 146 surgically treated cases of colorectal adenocarcinoma (according to the 7th edition of the American Joint Committee on Cancer Staging) were retrospectively collected at Hainan Hospital of the PLA General Hospital. Patients with the following criteria were excluded: i) Age <18 years old; ii) multiple or recurrent malignancies or in situ lesions; iii) a history of previous neoadjuvant therapy; iv) comorbidities with a long-term history of medication use such as hormones; v) detectable complications with an elevated white blood cells beyond the upper limit of the reference (4-10x109/l) including infection, obstruction or acute bleeding before and after the surgery; and vi) the absence of a follow-up date. Clinicopathological parameters, including sex, age, and the carcinoembryonic antigen (CEA) level, were collected before the surgery. In addition, pathological reports for tumor location, histological grade, invasive depth and tumor diameter (cut-off 4 cm) (18) were registered. The postoperative adjuvant therapies were checked. The study followed the Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) (19), was supervised by the ethics committee of Hainan Hospital of the PLA General Hospital (approved ID: 301HLFYLL15), and written informed consent was not obtained because of the retrospective nature of the study.
Determination of the preoperative and postoperative NLR (pre-NLR/post-NLR)
Routine laboratory tests were performed between 6:00 and 9:00 a.m. using peripheral venous blood within 1 month before (preoperative) and at least 7 days after (postoperative) the surgery. The NLR and ΔNLR were calculated as previously reported (20).
Follow-up procedure and definition of progression-free survival (PFS)
The follow-up meetings were conducted by telephone or a visit to the medical records department of the hospital, with intervals of 3-6 months for the first 3 years and 6-12 months for the next 4-5 years. PFS was defined as the interval between the date of operation until the date of first recurrence or death from any cause. The primary study endpoint was the 3-year PFS, as used in a previous study (21), and the last follow-up point occurred in September 2019.
Statistical analysis
All statistical analyses were performed using SPSS 20.0 (SPSS Inc.). Receiver operating characteristic (ROC) curve analysis was used to determine the optimal cut-off value of the pre-NLR/post-NLR for PFS, and the relationships with other clinicopathological parameters were calculated by the χ2 test, Fisher's exact test, or the Mann-Whitney U test when appropriate. Further classification of pre/post-NLR into low-low, low-high, high-low and high-high subgroups was conducted. Kaplan-Meier (K-M) survival curves were applied to compare patients with a low or high NLR before and after the surgery, and significant differences were determined by the log-rank test. Correlation of preoperative and postoperative neutrophil or lymphocyte counts with the NLR or ΔNLR was conducted by Pearson correlation analysis. Finally, univariate and multivariate analyses were conducted by using the Cox proportional hazards model (22). A double-sided P<0.05 was considered statistically significant.
Results
Demographic characteristics and the differences in NLR across various clinicopathological parameters
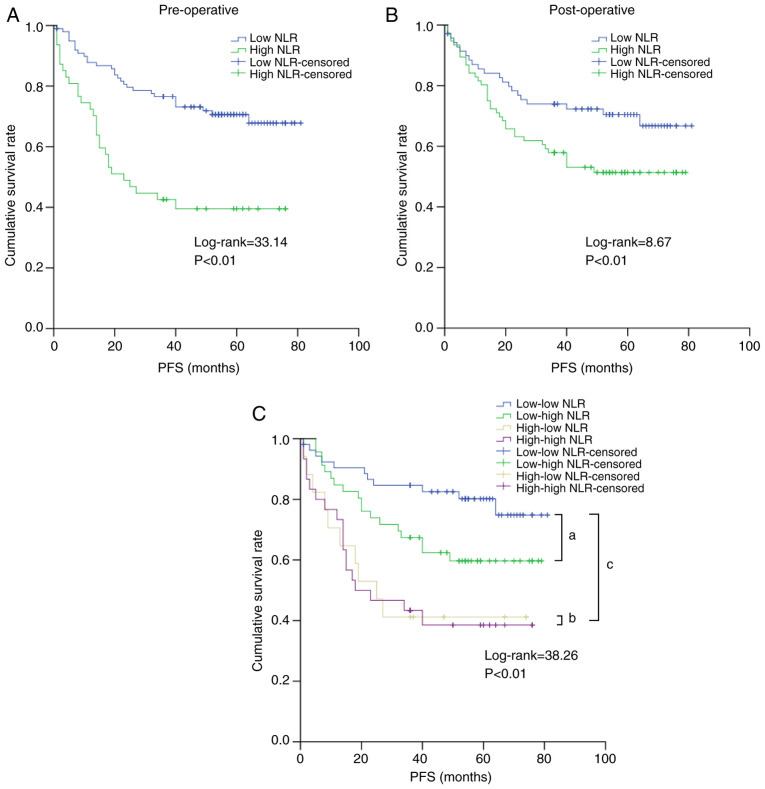
In total, 50 female and 96 male patients were included in the study, and the mean age of the patients was 56.68 years (range: 24-85 years), with a median follow-up time of 41.77 months (range: 1-81 months). As shown in Fig. 1, statistical significance was found regarding the pre/post-NLR predicting the PFS but not the ΔNLR. According to the Youden index, the optimal cut-off values for the pre/post-NLR were 2.39 and 2.96, respectively. For predicting PFS, the pre-NLR had a sensitivity and specificity of 48.80 and 79.50%, respectively, and the post-NLR had a sensitivity and specificity of 63.20 and 56.20%, respectively. Based on these points, patients were then categorized into pre-NLR low or high (<2.39, n=99 or ≥2.39, n=47, respectively) or post-NLR low or high (<2.96, n=70 or ≥2.96, n=76, respectively) subgroups. For the pre-NLR, significant differences were found across various histological grades and tumor diameters; however, for the post-NLR, differences were found only across different ages (Table I).
Figure 1.
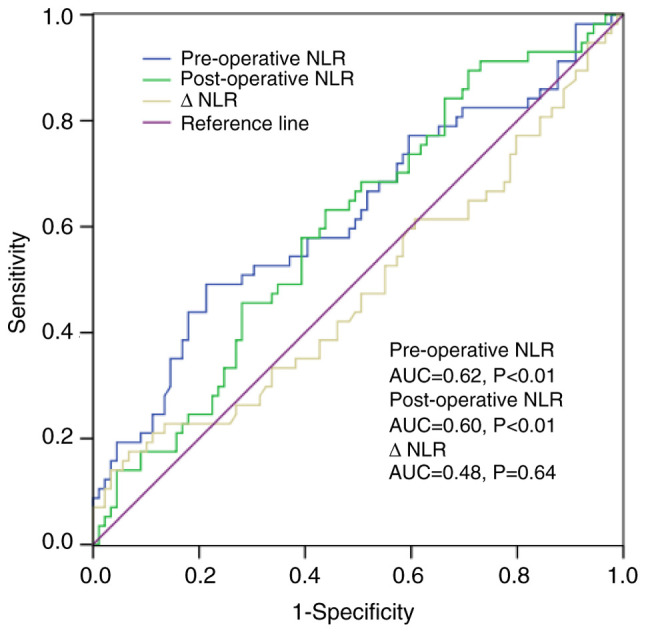
Receiver operating characteristic curve analysis of NLR in the patients. AUC, area under the curve; NLR, neutrophil-to-lymphocyte ratio.
Table I.
Differences in pre-/post-operative NLR for different clinicopathological parameters.
| Pre-operative NLR | Post-operative NLR | |||||
|---|---|---|---|---|---|---|
| Variable | Low, n | High, n | P-value | Low, n | High, n | P-value |
| Age, years | 0.06 | <0.01 | ||||
| <60 | 43 | 26 | 40 | 29 | ||
| ≥60 | 56 | 21 | 30 | 47 | ||
| Sex | 0.27 | 0.63 | ||||
| Female | 36 | 14 | 23 | 27 | ||
| Male | 63 | 33 | 47 | 49 | ||
| Tumor location | 0.80 | 0.71 | ||||
| Right | 26 | 13 | 18 | 21 | ||
| Left | 73 | 34 | 52 | 55 | ||
| Histological grade | <0.01 | 0.07 | ||||
| Well | 2 | 2 | 1 | 3 | ||
| Moderate | 83 | 32 | 59 | 56 | ||
| Poor | 14 | 8 | 10 | 17 | ||
| CEA level | 0.24 | 0.76 | ||||
| Normal | 64 | 27 | 43 | 48 | ||
| Elevated | 35 | 20 | 27 | 28 | ||
| Invasive depth | 0.10 | 0.13 | ||||
| T1+2 | 23 | 7 | 17 | 13 | ||
| T3+4 | 76 | 40 | 53 | 13 | ||
| Tumor diameter, cm | <0.01 | 0.63 | ||||
| <4 | 41 | 9 | 23 | 27 | ||
| ≥4 | 58 | 39 | 47 | 49 | ||
| Node involvement | 0.17 | 0.30 | ||||
| N0 | 59 | 24 | 41 | 41 | ||
| N1+2 | 40 | 23 | 28 | 35 | ||
| Positive nodes numbera | 1.87±3.50 | 3.00±6.12 | 0.25 | 1.90±3.54 | 2.54±5.28 | 0.50 |
| Distant metastasis | 0.11 | 0.06 | ||||
| M0 | 92 | 41 | 66 | 67 | ||
| M1 | 7 | 6 | 4 | 9 | ||
| TNM stages | 0.48 | 0.75 | ||||
| I+II | 57 | 25 | 40 | 42 | ||
| III+IV | 42 | 22 | 30 | 34 | ||
| Adjuvant therapies | <0.01 | 0.11 | ||||
| Received | 54 | 35 | 46 | 43 | ||
| None | 45 | 12 | 24 | 33 | ||
aData are presented as mean ± SD. NLR, neutrophil-to-lymphocyte ratio.
PFS prediction value of the pre/post-NLR
According to K-M analyses, we then examined the PFS prediction value in different pre/post-NLRs. As shown in Fig. 2, patients with a high NLR had inferior PFS both preoperatively and postoperatively. The PFS in the low vs. high NLR groups was 47.22±22.22 vs. 30.30±24.52 months preoperatively and 45.64±23.84 vs. 38.22±24.21 months postoperatively (both P<0.01). After subgroup classification, the PFS for patients in the low-low (n=53), low-high (n=17), high-low (n=46), and high-high (n=30) subgroups was 50.30±21.36, 43.67±22.78, 31.06±25.56 and 29.87±24.13 months, respectively, and those in the high-high subgroup displayed the worst PFS. Compared to patients in the high-low and high-high subgroups, patients in the low-low and low-high subgroups had significantly better PFS.
Figure 2.
Effect of NLR on PFS. (A) Preoperative NLR. (B) Postoperative NLR. (C) Subgroups of NLR. aLog-rank=7.68, P<0.01; bLog-rank=0.02, P=0.90; cLog-rank=31.46, P<0.01. NLR, neutrophil-to-lymphocyte ratio; PFS, progression-free survival.
Univariate and multivariate analyses for the factors correlated with PFS
As shown in Table II, according to the univariate tests, the preoperative CEA level, invasive depth, node involvement, positive node number, distant metastasis, TNM stages, adjuvant therapies and NLR (both preoperative and postoperative) correlated with PFS. When P<0.05 was used as a cut-off in multivariate analysis, the preoperative CEA level, invasive depth, node involvement, distant metastasis and pre-NLR were found to be significantly correlated with PFS.
Table II.
Univariable and multivariable analysis of different parameters for progression-free survival in the patients.
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variable | P-value | HR | 95% CI | P-value | HR | 95% CI |
| Age, years | ||||||
| <60 | Ref. | |||||
| ≥60 | 0.55 | 1.18 | 0.70-1.99 | |||
| Sex | ||||||
| Female | Ref. | |||||
| Male | 0.94 | 0.98 | 0.56-1.70 | |||
| Tumor location | ||||||
| Right | Ref. | |||||
| Left | 0.30 | 1.40 | 0.74-2.65 | |||
| Histological grade | ||||||
| Well | Ref. | |||||
| Moderate + poor | 0.43 | 0.79 | 0.44-1.42 | |||
| CEA level | ||||||
| Normal | Ref. | Ref. | ||||
| Elevated | <0.01 | 3.49 | 2.05-5.95 | <0.01 | 2.77 | 1.58-4.85 |
| Invasive depth | ||||||
| T1+2 | Ref. | |||||
| T3+4 | <0.01 | 5.81 | 1.81-18.58 | |||
| Tumor diameter, cm | ||||||
| <4 | Ref. | |||||
| ≥4 | 0.19 | 1.48 | 0.83-2.63 | |||
| Node involvement | ||||||
| N0 | Ref. | Ref. | ||||
| N1+2 | <0.01 | 3.01 | 1.75-5.16 | <0.01 | 2.40 | 1.38-4.18 |
| Positive nodes number | <0.01 | 1.13 | 1.08-1.17 | |||
| Distant metastasis | ||||||
| M0 | Ref. | Ref. | ||||
| M1 | <0.01 | 7.47 | 3.86-14.45 | <0.01 | 3.56 | 1.78-7.11 |
| TNM stages | ||||||
| I + II | Ref. | |||||
| III + IV | <0.01 | 3.13 | 1.81-5.41 | |||
| Adjuvant therapies | ||||||
| Received | Ref. | |||||
| None | 0.02 | 0.48 | 0.27-0.87 | |||
| Preoperative NLR | ||||||
| <2.39 | Ref. | Ref. | ||||
| ≥2.39 | <0.01 | 2.81 | 1.66-4.74 | <0.01 | 2.78 | 1.61-4.79 |
| Postoperative NLR | ||||||
| <2.96 | Ref. | |||||
| ≥2.96 | 0.04 | 1.75 | 1.02-3.00 | |||
CI, confidence interval; HR, hazard ratio; NLR, neutrophil-to-lymphocyte ratio.
Discussion
In this study, we found that a high NLR, either preoperative or postoperative, was associated with poor prognosis for patients with colorectal cancer (CRC) and that the ΔNLR was less likely to be involved. Overall, patients with a low pre-NLR had different prognoses after surgery, and a significantly prolonged PFS was found in those in the low-low subgroup compared to that in patients in the low-high subgroup; in contrast, patients with a persistently high NLR had poor PFS even if the prognosis changed after the surgery.
It has long been established that a high pretreatment NLR is associated with poor prognosis in CRC, but debates are still unresolved concerning the optimal cut-off points, and there is heterogeneity in the pathological stages used across study samples. In a curative scenario, Malietzis et al (23). studied 506 nonmetastatic patients at a cut-off point of 3 and found that a high NLR was correlated with some negative factors, including older age, higher T and N stages and microvascular invasion, but a high NLR was only an independent prognostic factor for disease-free survival (DFS) and not overall survival (OS). Choi et al (12) performed a study with 549 cases with a cut-off point of 2.6 and found a similar association of a high NLR with older age; they also found that a high NLR was linked to reduced recurrence-free survival. In our study, we identified a high pre-NLR cut-off value of 2.39, which was close to that identified in previous results (24,25). Moreover, this point was maintained even when stage IV disease was excluded (but with different AUC values, data not shown).
The underlying explanation for the association of the pretreatment NLR with the prognosis of CRC remains largely unclear, but part of the reason is that the roles of neutrophils and lymphocytes in cancer are highly complex (26,27). Previous studies have indicated that a high NLR is associated with increased levels of interleukin-6 (IL-6), IL-8, IL-2Ra, hepatocyte growth factor (HGF), macrophage-colony stimulating factor (GM-CSF), and vascular epidermal growth factor (VEGF) (28-30). Some of these cytokines, such as IL-6 and IL-8, play an important role in cancer progression and treatment resistance (28-30). Additionally, a recent study indicated that cancer dissemination can occur at a very early stage in CRC (31); in such a scenario, these early metastatic seeds could also contribute to the high NLR by triggering systemic inflammation. Based on these results, it is plausible that patients with a high NLR-related cytokine profile have cancer cells with specific characteristics, such as enhanced progression or proliferation, which could result in a poor prognosis.
Notably, it has been suggested that longitudinal tests of the NLR are more meaningful for individual patients with CRC; however, related reports are rare. Guo et al (20) explored the prognostic role of the pre-NLR and ΔNLR in 135 patients with CRC and found that both the pre-NLR and ΔNLR were independent factors for OS but not DFS, but the role of the post-NLR was not elucidated. Guthrie et al (32) conducted a study with 206 patients and found that a high pre-NLR and high post-NLR were associated with poor CSS, but only the pre-NLR was an independent prognostic factor. Our results are in line with the study (32). Nonetheless, the post-NLR was previously reported to be an important prognostic factor in many cancers, including gastric cancer (33), hepatocellular carcinoma (34), renal cell carcinoma (35) and CRC (36), but it was noted that it could be affected by many factors. For example, a relatively high post-NLR was associated with surgery-related stress. Stress induced by psychological stressors such as surgery can lead to a high concentration of corticosteroids (37), which can increase neutrophils and impair the functions of lymphocytes (37,38). In contrast, a relatively low post-NLR could be found in patients who underwent adjuvant or first-line chemotherapy (39,40). In our study, patients with a low pre-NLR who shifted to a high post-NLR could present an obviously inferior prognosis when compared to those who maintained a low post-NLR, based on the aforementioned evidence (31,37,38), it is plausible that surgery for these patients could induce a high post-NLR, which could facilitate the outbreak of early micrometastases and the germination of new lesions.
This study has many limitations: First, patients were divided into four sub-groups according to the optimal cut-off points as it was reported in previous studies (41,42), although some other studies reported different taxonomy (43,44), however, the meaning of such classification was greatly attenuated due to the limited sample size in these sub-groups and could result in biased conclusions; second, data relating to other important prognostic factors such as microsatellite instability were not available; and third, more prolonged tests of the post-NLR as well as follow-up would have been required to validate the role of the NLR for the patients.
Overall, our study indicated that a high pre-NLR and high post-NLR predict poor prognosis for patients with colorectal cancer; patients who maintained a low NLR had much better survival.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Medical and Health Science Innovation Project of Sanya (grant nos. 2018YW06 and 2016YW08), the Natural Science Foundation of China (grant no. 81503391) and the Natural Science Foundation Project of Hainan (grant no. 817352).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
BY conceived and designed the study. MC, RX and BY acquired the data. BY analyzed and interpreted the data and drafted the manuscript. MC, RX and BY performed critical revision of the manuscript. BY supervised the study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Medical Ethics Committee of the Hainan Branch of PLA General Hospital (approval no. 301HLFYLL15). Written informed consent was not obtained due to the retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493–e503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 4.Inagaki N, Kibata K, Tamaki T, Shimizu T, Nomura S. Prognostic impact of the mean platelet volume/platelet count ratio in terms of survival in advanced non-small cell lung cancer. Lung Cancer. 2014;83:97–101. doi: 10.1016/j.lungcan.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi H, Abe M, Takumi Y, Hashimoto T, Kobayashi R, Osoegawa A, Miyawaki M, Okamoto T, Sugio K. The prognostic impact of the platelet distribution width-to-platelet count ratio in patients with breast cancer. PLoS One. 2017;12(e0189166) doi: 10.1371/journal.pone.0189166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.An X, Ding PR, Li YH, Wang FH, Shi YX, Wang ZQ, He YJ, Xu RH, Jiang WQ. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers. 2010;15:516–522. doi: 10.3109/1354750X.2010.491557. [DOI] [PubMed] [Google Scholar]
- 7.Murakami Y, Saito H, Shimizu S, Kono Y, Shishido Y, Miyatani K, Matsunaga T, Fukumoto Y, Fujiwara Y. Neutrophil-to-lymphocyte ratio as a prognostic indicator in patients with unresectable gastric cancer. Anticancer Res. 2019;39:2583–2589. doi: 10.21873/anticanres.13381. [DOI] [PubMed] [Google Scholar]
- 8.Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients with colorectal cancer. Br J Cancer. 2013;109:401–407. doi: 10.1038/bjc.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stojkovic Lalosevic M, Pavlovic Markovic A, Stankovic S, Stojkovic M, Dimitrijevic I, Radoman Vujacic I, Lalic D, Milovanovic T, Dumic I, Krivokapic Z. Combined diagnostic efficacy of neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and mean platelet volume (MPV) as biomarkers of systemic inflammation in the diagnosis of colorectal cancer. Dis Markers. 2019;2019(6036979) doi: 10.1155/2019/6036979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milasiene V, Stratilatovas E, Norkiene V, Jonusauskaite R. Lymphocyte subsets in peripheral blood as prognostic factors in colorectal cancer. J Buon. 2005;10:261–264. [PubMed] [Google Scholar]
- 11.Ozawa T, Ishihara S, Kawai K, Kazama S, Yamaguchi H, Sunami E, Kitayama J, Watanabe T. Impact of a lymphocyte to monocyte ratio in stage IV colorectal cancer. J Sur Res. 2015;199:386–392. doi: 10.1016/j.jss.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Choi WJ, Cleghorn MC, Jiang H, Jackson TD, Okrainec A, Quereshy FA. Preoperative neutrophil-to-lymphocyte ratio is a better prognostic serum biomarker than platelet-to-lymphocyte ratio in patients undergoing resection for nonmetastatic colorectal cancer. Ann Surg Oncol. 2015;22 (Suppl 3):S603–S613. doi: 10.1245/s10434-015-4571-7. [DOI] [PubMed] [Google Scholar]
- 13.Song Y, Yang Y, Gao P, Chen X, Yu D, Xu Y, Zhao J, Wang Z. The preoperative neutrophil to lymphocyte ratio is a superior indicator of prognosis compared with other inflammatory biomarkers in resectable colorectal cancer. BMC Cancer. 2017;17(744) doi: 10.1186/s12885-017-3752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malietzis G, Giacometti M, Kennedy RH, Athanasiou T, Aziz O, Jenkins JT. The emerging role of neutrophil to lymphocyte ratio in determining colorectal cancer treatment outcomes: A systematic review and meta-analysis. Ann Surg Oncol. 2014;21:3938–3946. doi: 10.1245/s10434-014-3815-2. [DOI] [PubMed] [Google Scholar]
- 15.Li MX, Liu XM, Zhang XF, Zhang JF, Wang WL, Zhu Y, Dong J, Cheng JW, Liu ZW, Ma L, Lv Y. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: A systematic review and meta-analysis. Int J Cancer. 2014;134:2403–2413. doi: 10.1002/ijc.28536. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Zhao Y, Zheng F. Prognostic significance of elevated preoperative neutrophil-to-lymphocyte ratio for patients with colorectal cancer undergoing curative surgery: A meta-analysis. Medicine (Baltimore) 2019;98(e14126) doi: 10.1097/MD.0000000000014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu-Yuan H, Jing P, Yi-Sheng W, He-Ping P, Hui Y, Chu-Xiong Z, Guo-Jian L, Guo-Qiang W. The impact of chemotherapy-associated neutrophil/lymphocyte counts on prognosis of adjuvant chemotherapy in colorectal cancer. BMC Cancer. 2013;13(177) doi: 10.1186/1471-2407-13-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santullo F, Biondi A, Cananzi FCM, Fico V, Tirelli F, Ricci R, Rizzo G, Coco C, Mattana C, D'Ugo D, et al. Tumor size as a prognostic factor in patients with stage IIa colon cancer. Am J Surg. 2018;15:71–77. doi: 10.1016/j.amjsurg.2017.03.038. [DOI] [PubMed] [Google Scholar]
- 19.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies (remark) J Natl Cancer Inst. 2005;97:1180–1184. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 20.Guo D, Han A, Jing W, Chen D, Jin F, Li M, Kong L, Yu J. Preoperative to postoperative change in neutrophil-to-lymphocyte ratio predict survival in colorectal cancer patients. Future Oncol. 2018;14:1187–1196. doi: 10.2217/fon-2017-0659. [DOI] [PubMed] [Google Scholar]
- 21.Sargent DJ, Wieand HS, Haller DG, Gray R, Benedetti JK, Buyse M, Labianca R, Seitz JF, O'Callaghan CJ, Francini G, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: Individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23:8664–8670. doi: 10.1200/JCO.2005.01.6071. [DOI] [PubMed] [Google Scholar]
- 22.Xing X, Guo J, Wen X, Ding G, Li B, Dong B, Feng Q, Li S, Zhang J, Cheng X, et al. Analysis of PD1, PDL1, PDL2 expression and T cells infiltration in 1014 gastric cancer patients. Oncoimmunology. 2018;7(e1356144) doi: 10.1080/2162402X.2017.1356144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malietzis G, Giacometti M, Askari A, Nachiappan S, Kennedy RH, Faiz OD, Aziz O, Jenkins JT. A preoperative neutrophil to lymphocyte ratio of 3 predicts disease-free survival after curative elective colorectal cancer surgery. Ann Surg. 2014;260:287–292. doi: 10.1097/SLA.0000000000000216. [DOI] [PubMed] [Google Scholar]
- 24.Ozdemir Y, Akin ML, Sucullu I, Balta AZ, Yucel E. Pretreatment neutrophil/lymphocyte ratio as a prognostic aid in colorectal cancer. Asian Pacific J Cancer Prev. 2014;15:2647–2650. doi: 10.7314/apjcp.2014.15.6.2647. [DOI] [PubMed] [Google Scholar]
- 25.Shibutani M, Maeda K, Nagahara H, Noda E, Ohtani H, Nishiguchi Y, Hirakawa K. A high preoperative neutrophil-to-lymphocyte ratio is associated with poor survival in patients with colorectal cancer. Anticancer Res. 2013;33:3291–3294. [PubMed] [Google Scholar]
- 26.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: Neutral no more. Nat Rev Cancer. 2016;16:431–446. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 27.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crusz SM, Balkwill FR. Inflammation and cancer: Advances and new agents. Nat Rev Clin Oncol. 2015;12:584–596. doi: 10.1038/nrclinonc.2015.105. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Hu F, Li G, Li G, Yang X, Liu L, Zhang R, Zhang B, Feng Y. Human colorectal cancer-derived mesenchymal stem cells promote colorectal cancer progression through IL-6/JAK2/STAT3 signaling. Cell Death Dis. 2018;9(25) doi: 10.1038/s41419-017-0176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhai J, Shen J, Xie G, Wu J, He M, Gao L, Zhang Y, Yao X, Shen L. Cancer-associated fibroblasts-derived IL-8 mediates resistance to cisplatin in human gastric cancer. Cancer Lett. 2019;454:37–43. doi: 10.1016/j.canlet.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Hu Z, Ding J, Ma Z, Sun R, Seoane JA, Scott Shaffer J, Suarez CJ, Berghoff AS, Cremolini C, Falcone A, et al. Quantitative evidence for early metastatic seeding in colorectal cancer. Nat Genet. 2019;51:1113–1122. doi: 10.1038/s41588-019-0423-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guthrie GJ, Roxburgh CS, Farhan-Alanie OM, Horgan PG, McMillan DC. Comparison of the prognostic value of longitudinal measurements of systemic inflammation in patients undergoing curative resection of colorectal cancer. Br J Cancer. 2013;109:24–28. doi: 10.1038/bjc.2013.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Min KW, Kwon MJ, Kim DH, Son BK, Kim EK, Oh YH, Wi YC. Persistent elevation of postoperative neutrophil-to-lymphocyte ratio: A better predictor of survival in gastric cancer than elevated preoperative neutrophil-to-lymphocyte ratio. Sci Rep. 2017;7(13967) doi: 10.1038/s41598-017-13969-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dan J, Zhang Y, Peng Z, Huang J, Gao H, Xu L, Chen M. Postoperative neutrophil-to-lymphocyte ratio change predicts survival of patients with small hepatocellular carcinoma undergoing radiofrequency ablation. PLoS One. 2013;8(e58184) doi: 10.1371/journal.pone.0058184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohno Y, Nakashima J, Ohori M, Gondo T, Hatano T, Tachibana M. Followup of neutrophil-to-lymphocyte ratio and recurrence of clear cell renal cell carcinoma. J Urol. 2012;187:411–417. doi: 10.1016/j.juro.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 36.Li Z, Zhao R, Cui Y, Zhou Y, Wu X. The dynamic change of neutrophil to lymphocyte ratio can predict clinical outcome in stage I-III colon cancer. Sci Rep. 2018;8(9453) doi: 10.1038/s41598-018-27896-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5:617–625. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 38.Fukakusa M, Bergeron C, Tulic MK, Fiset PO, Al Dewachi O, Laviolette M, Hamid Q, Chakir J. Oral corticosteroids decrease eosinophil and CC chemokine expression but increase neutrophil, IL-8, and IFN-gamma-inducible protein 10 expression in asthmatic airway mucosa. J Allergy Clin Immunol. 2005;115:280–286. doi: 10.1016/j.jaci.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 39.Yoshino T, Cleary JM, Van Cutsem E, Mayer RJ, Ohtsu A, Shinozaki E, Falcone A, Yamazaki K, Nishina T, Garcia-Carbonero R, et al. Neutropenia and survival outcomes in metastatic colorectal cancer patients treated with trifluridine/tipiracil in the RECOURSE and J003 trials. Ann Oncol. 2020;31:88–95. doi: 10.1016/j.annonc.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu J, Ge XX, Zhu W, Zhi Q, Xu MD, Duan W, Chen K, Gong FR, Tao M, Shou LM, et al. Values of applying white blood cell counts in the prognostic evaluation of resectable colorectal cancer. Mol Med Rep. 2019;19:2330–2340. doi: 10.3892/mmr.2019.9844. [DOI] [PubMed] [Google Scholar]
- 41.Lin GN, Liu PP, Liu DY, Peng JW, Xiao JJ, Xia ZJ. Prognostic significance of the pre-chemotherapy lymphocyte-to-monocyte ratio in patients with previously untreated metastatic colorectal cancer receiving FOLFOX chemotherapy. Chin J Cancer. 2016;35(5) doi: 10.1186/s40880-015-0063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. doi: 10.1007/s11605-020-04523-8. Lin JX, Wang ZK, Huang YQ, Xie JW, Wang JB, Lu J, Chen QY, Lin M, Tu RH, Huang ZN, et al: Dynamic changes in pre- and postoperative levels of inflammatory markers and their effects on the prognosis of patients with gastric cancer. J Gastrointest Surg: Feb 3, 2020 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyatani K, Saito H, Kono Y, Murakami Y, Kuroda H, Matsunaga T, Fukumoto Y, Osaki T, Ashida K, Fujiwara Y. Combined analysis of the pre- and postoperative neutrophil-lymphocyte ratio predicts the outcomes of patients with gastric cancer. Surg Today. 2018;48:300–307. doi: 10.1007/s00595-017-1587-6. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto M, Saito H, Uejima C, Tanio A, Takaya S, Ashida K, Fujiwara Y. Combined pre- and postoperative lymphocyte count accurately predicts outcomes of patients with colorectal cancer. Dig Surg. 2019;36:487–494. doi: 10.1159/000492340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.