Abstract
Malaria is a longstanding public health problem in sub-Saharan Africa, while arthropod-borne viruses (arboviruses) like dengue and chikungunya cause an underrecognized burden of disease. While many human and environmental drivers affect the dynamics of vector-borne diseases, here we argue that the direct effects of warming temperatures are likely to promote greater environmental suitability for dengue and other arboviruses transmitted by Aedes aegypti while reducing the suitability for malaria transmitted by Anopheles gambiae. Environmentally-driven changes in disease dynamics will no doubt be complex and heterogeneous, but given that current public efforts are targeted to malaria control, we encourage the public health community to consider Aedes aeypgti and dengue, chikungunya, and other arboviruses as potential emerging public health threats in sub-Saharan Africa.
Keywords: Africa, malaria, Anopheles gambiae, Aedes aegypti, dengue virus, chikungunya virus, Zika virus, temperature, climate change, transmission
The global health community has expressed growing concern that climate change will alter the distribution and burden of vector-borne diseases, potentially reversing the gains of control programs and expanding the threat of emerging diseases (1–4). Malaria still imposes a major burden of morbidity and mortality in sub-Saharan Africa (228 million cases and 405,000 deaths in 2018), despite recent intensive control efforts that have succeeded in reducing transmission in many locations (5–7). At the same time, many other vector-borne diseases, including Rift Valley fever, dengue, chikungunya, yellow fever, Zika, o’nyong’nyong, West Nile, leishmaniasis, river blindness, and African sleeping sickness, circulate regularly in humans, wildlife, and livestock in sub-Saharan Africa, although their burden is less well characterized (8–12). For example, over 27,000 cases of arbovirus infections transmitted by Aedes vectors have been reported in West Africa since 2007 (13). It is now well established that temperature has nonlinear effects on vector-borne disease transmission, and that different mosquito and parasite species differ in this response, resulting in differences in their thermal optima and limits (2,4,14–19). As a result, the direction and magnitude of the effects of climate change on transmission of specific vector-borne diseases will differ across geographic regions.
In this Personal View, we summarize and visualize published data to make the case that climate change, in conjunction with urbanization, is likely to drive a shift in most of sub-Saharan Africa from climates most suitable for malaria transmission by rural Anopheles spp. mosquitoes to climates more suitable for transmission of dengue and other arboviruses by Aedes aegypti mosquitoes, with major consequences for public health and disease control strategies. Specifically, we draw from three lines of evidence: transmission models fit from laboratory thermal performance data; independent data on human infection; and widespread existing distributions of Aedes aegypti, dengue, and chikungunya in sub-Saharan Africa. While the drivers of vector-borne disease dynamics are multifaceted and include human mobility, rainfall and water storage practices, urbanization, and others, the increasing temperature suitability for arbovirus transmission merits attention from the global health community, in tandem with ongoing efforts toward malaria control. Therefore, although we cannot conclusively predict changes in disease incidence based on temperature alone, we argue that the effects of temperature change will promote arbovirus transmission and increasingly limit malaria transmission by rural vector species in much of sub-Saharan Africa, acting in concert with urbanization and other changes.
Transmission models
Climate change will affect vector-borne disease transmission because temperature affects vector population size, survival, biting, pathogen incubation rates, and vector competence, with potential additional effects via rainfall and humidity (15–17,20). The physiological effects of temperature on the vector and pathogen traits that drive transmission are well-established from laboratory experiments and field studies (20–24). Both ectotherm physiology theory and data from a wide variety of ectotherm taxa and traits demonstrate that the thermal responses of development, survival, and reproduction are often unimodal, peaking at intermediate temperatures and declining at both low and high temperatures (25–27). Laboratory experiments confirm that these nonlinear thermal responses are pervasive across mosquito and pathogen taxa and traits (15–17,21,28–30). We previously developed temperature-dependent R0 models that incorporate empirically measured effects of temperature on mosquito biting rate, immature survival probability, immature development rate, adult lifespan, fecundity, vector competence (probability of becoming infectious following exposure to an infectious blood meal), and parasite development rate, and in turn on mosquito population size, for malaria transmitted by Anopheles gambiae (and, where data were not available, from traits derived from other Anopheles spp.) (15,31) and for dengue, chikungunya, and Zika transmitted by Aedes aegypti (16,17). Because the Ae. aegypti temperature-dependent R0 relationships were very similar for all three viruses (16,17), we hereafter focus on results from the dengue model. For both malaria and arboviruses, vector and parasite traits and R0 peak at intermediate temperatures and are suppressed at both low and high temperatures (2,15–18,28). The thermal optima and ranges for transmission vary by vector and parasite species: malaria transmission by Anopheles gambiae peaks at 25°C, while arbovirus transmission by Aedes aegypti peaks at 29°C (15–17,31) (Fig. 1: lines). Multiple vector and parasite traits contribute to differences in the thermal response of transmission across species (32).
Figure 1. Malarial and non-malarial fever among Kenyan children from 2014–2018 versus temperature, overlaid on basic reproduction number curves for malaria and dengue.
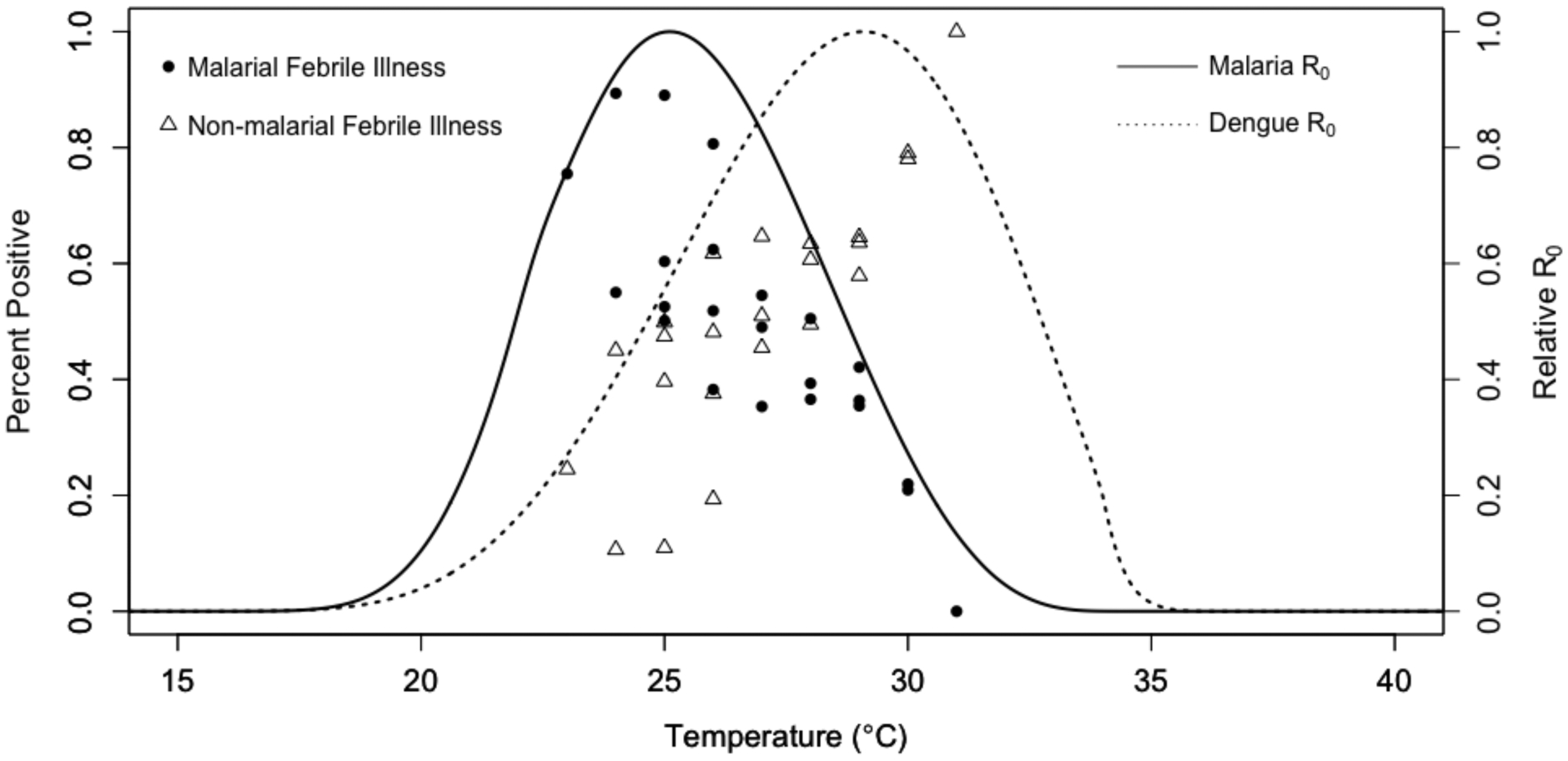
Points represent proportion of children with positive malaria smears (filled circles) and proportion of children with non-malarial fever (open triangles) over temperature. Land surface temperatures at each participant visit were calculated as 30-day mean temperatures lagged by one month (the time window in which we expect temperature to affect transmission), specific to each of the four clinic sites. Proportions were calculated at 1°C intervals of temperature (x-axis) at each of the four different outpatient clinic sites in western and coastal Kenya where children with undifferentiated fever were recruited, for up to four points per temperature bin (12,33–36). Lines represent predicted basic reproduction number (R0, rescaled to range from zero to one) for malaria (solid line) and dengue (dashed line) as a function of temperature from ecological models based on laboratory mosquito and parasite data (15–17). For methods detail, see Supplementary Materials, pages 2–3.
Independent data on human infection indicate potential for shifts in disease burden
Field data from both mosquito-based metrics of transmission risk (e.g., entomological inoculation rate) (15,37) and in human incidence and at local and continental scales (16,36) strongly support nonlinear effects of temperature on transmission predicted from laboratory studies and mathematical models. Recent work from our cohort study of febrile children in four villages in Kenya showed a unimodal relationship between blood smear positivity for malaria and temperature, with a peak at 25°C (30-day average temperature, lagged by one month: the time scale at which we expect temperature to affect transmission) and a sharp decline in smear positivity above the optimum temperature (Fig. 1; filled circles) (36). This result strongly supports the independently predicted 25°C optimum from the temperature-dependent malaria R0 model (Fig. 1; solid line) (15). In the same study, non-malarial fever, much of which is caused by dengue and chikungunya, increased with temperature throughout the observed temperature range, supporting the relatively warm thermal optimum of dengue (Fig. 1; dashed line and open triangles). As further evidence for the physiological constraints on malaria and arbovirus transmission, previous studies supported the thermal optima predicted from mechanistic models. First, a study of dengue in 20 cities in Colombia showed a unimodal relationship between incidence and weekly average temperature (multiple time windows and lags were explored) that peaked at a mean temperature of 28°C (38), supporting the model-predicted optimum for dengue transmission of 29°C (16). Second, the predicted unimodal effects of temperature on transmission, peaking at 25°C for malaria and 29°C for dengue, chikungunya, and Zika viruses (also transmitted by Aedes aegypti), are supported by continental-scale data on entomological inoculation rate in Africa and human incidence in the Americas, respectively (15–17).
Shifting climate suitability for malaria and Aedes aegypti-transmitted viruses
As climate change leads to warming temperatures, the intermediate thermal optima for vector transmission have two immediate implications. First, for all vector-borne diseases, climate change will drive increases in some regions and decreases in others, depending on current and future local climates relative to the optimum and thermal limits for disease transmission. Second, the relative suitability for different vector-borne diseases will shift: the climate may simultaneously become more suitable for some diseases and less suitable for others. In regions where temperatures are regularly between 25–29°C, including much of sub-Saharan Africa, a warming climate will become less suitable for malaria but more suitable for dengue, chikungunya, and other arboviruses transmitted by Ae. aegypti (Fig. 2). Specifically, the highest density of people exposed to high temperature suitability for transmission (the ‘risk hotspot’) for malaria is projected to shift toward higher elevations such as the Albertine Rift region and higher latitudes in Southern Africa (Fig. 2A–C, red circles). Meanwhile, the risk hotspot for dengue, chikungunya, and other Aedes aegypti-transmitted arboviruses is predicted to expand from West Africa throughout sub-Saharan Africa (Fig. 2D–F).
Figure 2.
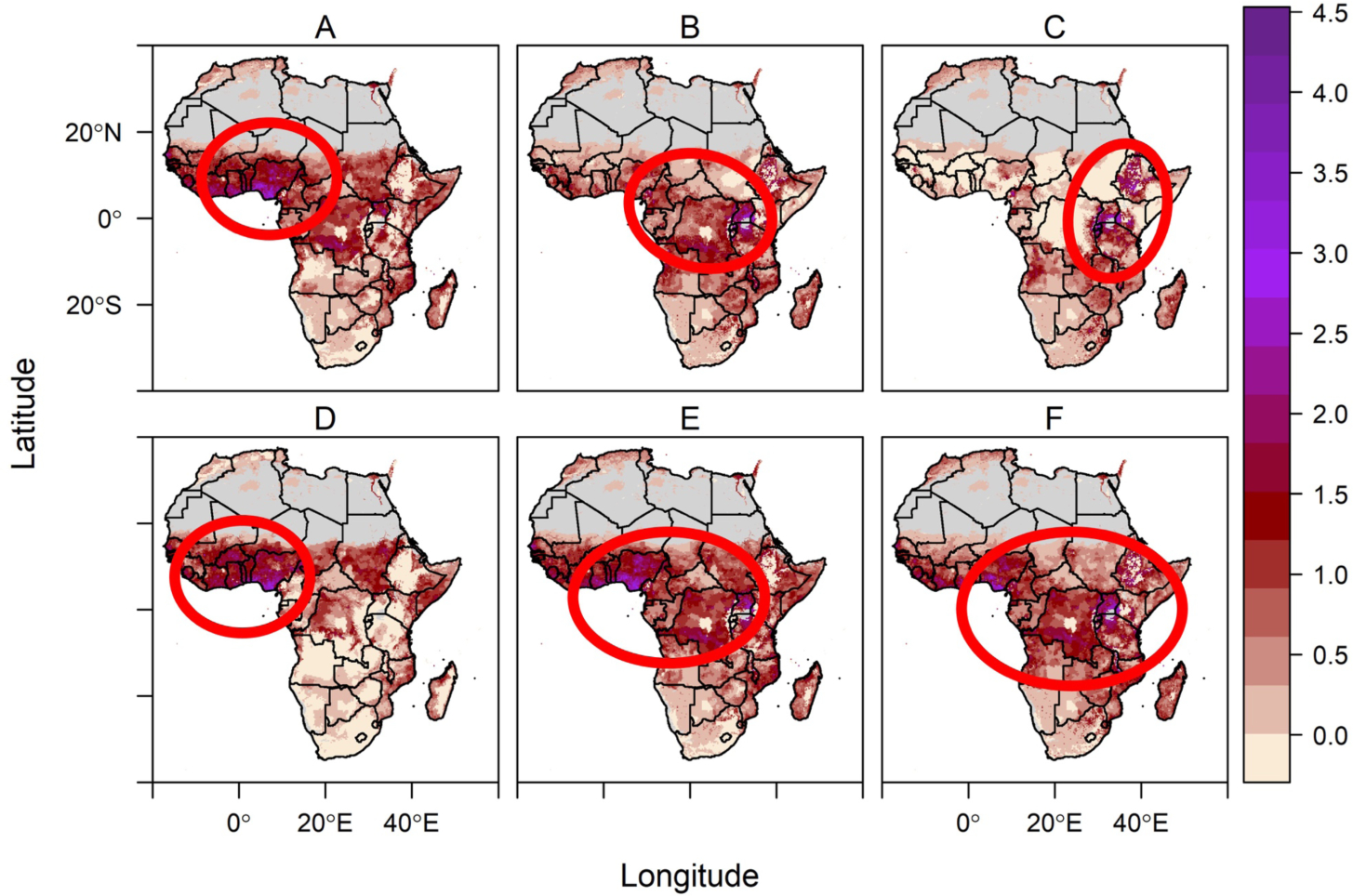
Temperature-driven malaria risk hotspot (red circles; top row [A-C]) shifts to high elevations in East Africa while Aedes aegypti-transmitted arbovirus risk hotspot (red circles; bottom row [D-F]) expands throughout sub-Saharan Africa from current (left column [A, D]) to 2050 (middle column [B, E]) to 2080 (right column [C, F]). Color scale indicates the number of months per year predicted to have highly suitable (relative R0 > 0.5) temperatures for transmission, multiplied by population density (log(1 + population density)), for a scaled index of person-months of high risk for transmission. Temperature suitability for transmission is based on the upper 50th percentile of relative R0 from temperature-dependent R0 models (15,16). All climate projections are based on the business as usual climate scenario RCP 8.5, using the HadGEM2-ES General Circulation Model. The red circles indicating hotspots are shown to ease visualization of the areas of highest person-months of risk. An aridity mask (gray) blocks out regions that are too dry for malaria transmission (39). This figure is intended to illustrate one possible scenario of temperature-driven risk, rather than making a specific prediction about future disease burden, which additionally depends on moisture availability, human population growth and mobility, and other factors. For Methods details, see Supplementary Materials, pages 4–8.
In conjunction with climate change, urbanization is driving widespread changes in habitat, microclimate, and human populations and is occurring more rapidly in sub-Saharan Africa than anywhere else in the world (though these transitions can be complex and diverse) (40,41). Urbanization affects vector-borne disease transmission by altering the availability of vector breeding habitat and contact with humans. Aedes aegypti mosquitoes breed in human-made container habitats such as discarded tires, cans, buckets, and water storage containers, all of which increase in density in urban areas but are also present in villages (10,42,43). By contrast, Anopheles gambiae and some other African malaria vectors breed in naturally occurring pools of water, which are more common in rural areas, although malaria transmission can also occur in cities (44,45). In addition to affecting breeding habitat, urban areas form ‘heat islands’ with microclimates that are several degrees warmer than surrounding vegetated areas, which can directly influence vector development and survival (46) and may benefit warmer-adapted Aedes aegypti over Anopheles gambiae mosquitoes. Urbanization may therefore act synergistically with warming climate to promote the shift from Anopheles-transmitted malaria to Aedes-transmitted arboviruses in sub-Saharan Africa.
Widespread distribution of Aedes aegypti and arboviruses in sub-Saharan Africa
Although shifts in climate suitability do not necessarily translate into a shift in disease burden from malaria to dengue and other arboviruses in sub-Saharan Africa, mounting evidence supports this hypothesis. First, for expansions in transmission to occur, Aedes aegypti mosquitoes and arboviruses must be present in the region. Growing evidence suggests that the vectors and arboviruses are already widespread and under-recognized in sub-Saharan Africa, in part because of misdiagnosis and a public health focus on malaria and Anopheles vectors (Table 1) (10,13,33–35,42,47–53). For example, recent arbovirus surveillance work in Kenya in regions of high malaria endemicity (Fig. 1) showed that ~10–20% of febrile children were positive for dengue virus infection for much of the year (Fig. 3A), and that Aedes aegypti mosquito vectors were abundant in and around households year-round (Fig. 3B; see Supplementary Materials, page 3, for Methods). These data suggest ongoing endemic transmission of dengue in at least four geographically distinct Kenyan populations. Recently, large chikungunya epidemics have also occurred in Mombasa, Mandera, and Lamu, Kenya (54–56) and in the Kassala state of Sudan, where heavy rains flooded a major river, sparking an outbreak (57). Growing evidence suggests that both endemic and epidemic transmission of dengue, chikungunya, and other Aedes-transmitted arboviruses regularly occurs in sub-Saharan Africa, though it may be undiagnosed or misdiagnosed as malaria (Table 1) (13,33,58–61). At the same time that the arbovirus threat is increasingly recognized in sub-Saharan Africa, malaria has declined dramatically in the last two decades (5–7). While the drivers of this decline are undoubtedly complex, and much has been attributed to the success of malaria control programs, it is also possible that some of the decline results from decreasing climate suitability due to increasing temperature. The extent to which warming temperatures have already reduced malaria transmission remains to be assessed because few have recognized that the optimum for malaria transmission is as low as 25°C (62).
Table 1.
Evidence for Aedes aegypti vectors, arbovirus transmission, and over-diagnosis of malaria across sub-Saharan Africa.
| Location | Evidence | Reference |
|---|---|---|
| Kenya (western) | Dengue infection in children | (33) |
| Kenya (coastal) | Dengue and West Nile virus transmission in children and adults | (63) |
| Kenya | Acute flavivirus and alphavirus infection in children | (35) |
| Kenya | Serological evidence of arboviral infection in children | (51) |
| Kenya (coastal) | O’nyong Nyong virus and chikungunya virus transmission | (52) |
| Kenya (western) | O’nyong Nyong virus and chikungunya virus transmission | (64) |
| Kenya | Chikungunya infection in febrile children | (53) |
| Kenya (Mombasa) | Chikungunya outbreak | (54) |
| Kenya | Aedes aegypti breeding sites in rural and urban, coastal and western locations | (43) |
| Tanzania | Severe febrile illness and overdiagnosis of malaria | (49) |
| Tanzania | Rift Valley Fever and alphavirus seroepidemiology | (12) |
| Uganda (rural) | Febrile patients and overdiagnosis of malaria | (48) |
| Uganda (Zika Forest) | Arbovirus serology in endemic population | (61) |
| East African Community Region | Arbovirus infection | (11) |
| Cameroon | Flavivirus seroepidemiology | (12) |
| Cameroon | Aedes aegypti and Ae. albopictus present | (65) |
| Cameroon | Aedes aegypti and Ae. albopictus present | (66) |
| Central African Republic | Aedes aegypti and Ae. albopictus present | (67) |
| Mozambique | Dengue, chikungunya, Rift Valley fever, West Nile, and Zika virus seroepidemiology | (12) |
| Cote d’Ivoire (southeast) | Aedes mosquitoes present in an arbovirus-endemic setting | (42) |
| Sierra Leone | Rift Valley Fever virus, flaviviruses, and alphaviruses | (68) |
| West Africa | Expansion of DENV-3 | (60) |
| West Africa | Dengue, chikungunya, and Zika outbreaks and Aedes aegypti and Ae. albopictus presence | (13) |
| Africa | Dengue virus infection | (59) |
| Africa | Overdiagnosis and co-morbidity of severe malaria | (50) |
Figure 3. High rates of dengue virus infection in febrile children (A) and consistently high abundance of Aedes aegypti mosquitoes (B) in four villages in Kenya suggests that arboviruses are an underrecognized public health burden.
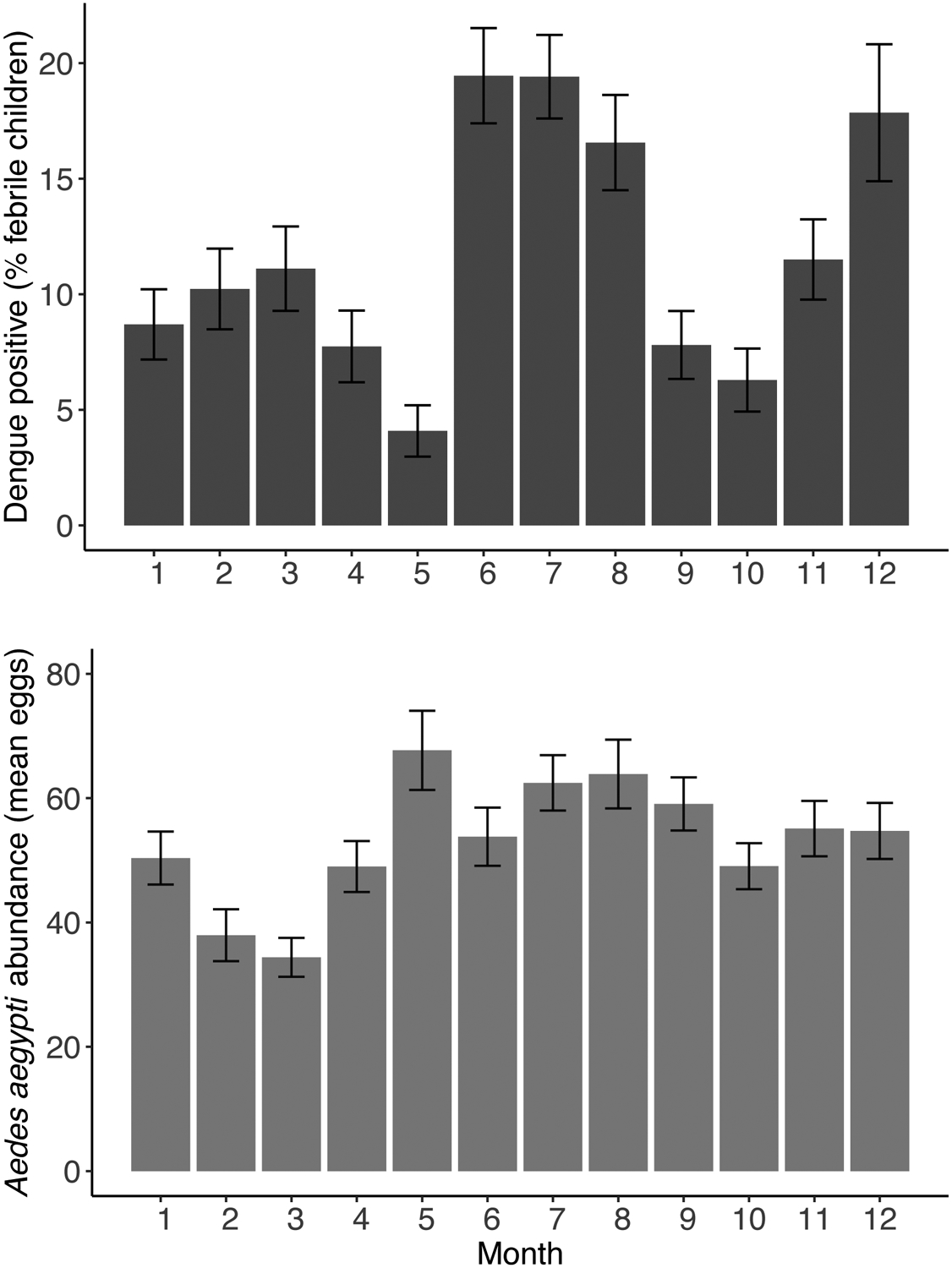
The rates of dengue positivity (A) are measured as the percentage of children <18 years of age with undifferentiated febrile illness attending outpatient care who tested positive by PCR or IgG ELISA for dengue virus infection (69). Data were compiled from four different clinics in western and coastal Kenya during each calendar month between 2014 and 2018. Aedes aegypti abundance (B) was measured as the monthly average number of Aedes aegypti eggs per household recovered from ovitraps placed in and around houses. Error bars indicate standard errors of the mean. For Methods, see Supplementary Materials, page 3.
Discussion
The degree to which changes in climate suitability for transmission translate into changes in the landscape of disease depends on other factors that shape disease dynamics, including pathogen exposure history, housing type, vector control and public health efforts, rainfall, and human mobility (70–73). Exposure history is particularly important because newly occurring transmission in naïve populations may more sharply increase the burden of disease than increases in already endemic populations with some acquired immunity (74,75). Therefore, even if climate change leads to geographic shifts rather than net increases in populations at risk of disease (Fig. 2), these shifts are not neutral from a public health perspective, and may be disruptive to populations, healthcare systems, and economies that have not historically experienced either malaria or arboviral diseases. Within endemic regions, the interannual variability and seasonality of transmission could further change in response to changing rainy seasons and their interaction with temperature (72,76,77). At the same time, changes in demography, population growth, migration, and socioeconomic conditions may mitigate the impacts of climate change on vector-borne disease dynamics (70,78,79). However, climate places limits on where transmission can and cannot occur regardless of population characteristics and may exacerbate effects of changing social vulnerability to disease.
Mosquitoes and parasites are not static threats but evolving organisms that respond to ecological conditions and selective pressures imposed by their changing environments. The potential for mosquitoes to adapt to warming temperatures by increasing their thermal optima and limits remains unknown (80). Mosquitoes quickly and repeatedly evolve resistance to insecticides when vector control programs impose strong selective pressure (81). However, temperature-driven selection on mosquitoes may not align with selection on the parasites they transmit. At warm temperatures, mosquito longevity is the major limitation on transmission because short pathogen incubation periods and frequent biting cannot overcome declining mosquito lifespans to sustain transmission (4,15,31). But even short-lived mosquitoes may achieve high fitness at warm temperatures if rapid development and high fecundity outweigh the cost of shorter lifespans. As a result, selection may not lead to increased mosquito survival at high temperatures, and therefore evolution may not rescue vector transmission as temperatures exceed current thermal optima.
Even if temperatures become warm enough to drive existing populations extinct or to suppress their ability to transmit disease, warmer-adapted mosquitoes (including Aedes aegypti and Anopheles stephensi, an urban malaria vector in India (82)) could invade and replace current An. gambiae populations transmitting malaria in Africa. Incipient speciation has already occurred in Africa in Ae. aegypti subtypes (83,84) and in the An. gambiae species complex (85–88), suggesting that both can adapt to changing ecological conditions including urbanization and, potentially, climate. Aedes albopictus, another arbovirus vector, is also present in some regions of Africa, and where it co-occurs with Ae. aegypti it can be competitively dominant (65–67). Temperature-dependent R0 models suggest that Ae. albopictus has a cooler thermal optimum (26°C) and upper thermal limit (32°C) than Ae. aegypti, which could limit the expansion and transmission potential of this species under warming climates (16,89). Climate-driven ecological and evolutionary changes in mosquito communities that might alter the direct physiological effects of temperature are therefore highly uncertain.
Although many aspects of the changing environmental and population landscapes that shape disease transmission remain unknown, we have outlined three lines of evidence suggesting that climate change, in concert with urbanization, will drive a shift in disease transmission in sub-Saharan Africa from malaria to arboviruses like dengue and chikungunya in the next few decades (Fig. 2). First, temperature-dependent transmission models predict increased suitability for Aedes-transmitted arboviruses and decreased suitability for malaria (Fig. 1) (15,16). Second, large-scale entomological and human disease data and local human incidence data provide evidence that warming temperatures above thermal optima drive declines in transmission (Fig. 1) (15,16). Third, at the same time that malaria is declining in much of sub-Saharan Africa, arboviruses and Aedes aegypti already pose an underrecognized public health burden, which could expand under increased climate suitability (Figs. 2–3; Table 1).
Malaria has already declined precipitously in much of Central and South America and the Caribbean in the last three decades at the same time that dengue, chikungunya, and Zika have exploded to cause half a million to >2 million cases per year (www.paho.org) (90–92). The drivers of these disease trends are almost certainly complex and multivariate, including multiple aspects of environmental and human population change. Nonetheless, the correspondence between shifting temperature suitability predicted from laboratory data and models and the observed shifts from malaria to dengue and other arboviruses is striking. For example, in a country-scale analysis of arbovirus transmission in Latin America and the Caribbean from 2014–2016, weekly mean temperatures averaged 25.6°C across the region (range in weekly average temperature from 21.5 – 28.7°C across countries),(16) spanning the range where malaria transmission peaks and begins to decline while arbovirus transmission increases steeply with temperature (Fig. 1).
Disease control strategies that are effective against malaria—such as long-lasting insecticide-treated bednets, indoor residual spraying, and artemisinin combination therapy—are ineffective against dengue, which uses the day-biting and container-breeding Aedes aegypti mosquito as its primary vector (93,94) and currently has no specific drug therapy or broadly effective vaccine available (the development and roll-out of the Sanofi-Pasteur dengue vaccine has had mixed results (95,96)). A shift from malaria to dengue in sub-Saharan Africa would therefore require public health efforts to retool to control an ecologically different vector and pathogen, a shift that has already taken place throughout much of the Americas. In particular, the development of accurate point-of-care diagnostics for dengue and chikungunya viruses and community-based vector control will be increasingly important for targeted care and prevention of arboviruses (13,34,35,53). While malaria eradication efforts remain critical, given the year-round circulation of dengue and chikungunya and abundance of Aedes spp. mosquitoes in Africa, public health efforts should also prepare for a potentially growing threat of arboviral disease in Africa.
Supplementary Material
Search strategy and selection criteria.
This Personal View primarily summarizes evidence from our own work and that of collaborators. The references in Table 1 were selected based on our own reading of the peer-reviewed literature in English, suggestions from two anonymous reviewers, and Google Scholar searches of “arbovirus,” “dengue,” “chikungunya,” “Aedes aegypti,” or “Aedes albopictus,” and “Africa” performed in May 2020. They were chosen based on providing some evidence of arbovirus or vector presence in Africa, and are not an exhaustive list.
Key Messages.
Malaria transmission by Anopheles gambiae peaks at 25°C while dengue transmission by Aedes aegypti peaks at 29°C, based on mechanistic transmission models parameterized and validated with laboratory and field data. Warming temperatures in the tropics are expected to favor transmission of dengue over malaria.
Independent data on human infections of malaria and dengue support the predicted nonlinear effect of temperature on disease incidence. In tropical regions, where temperatures regularly hover around 25°C, warmer temperatures correspond to a decrease in malaria incidence and an increase dengue and chikungunya incidence.
Dengue, chikungunya, and their Aedes aegypti mosquito vector are already widespread but under-recognized in Africa, based on studies of vector abundance, human serology, and acute infections from across Africa. As climate suitability increases for arboviruses, these diseases could expand and overtake the public health burden of malaria.
While malaria control efforts remain critical, arbovirus control through increased surveillance and testing capacity and vector control of container-breeding, day-biting Aedes aegypti is a critical emerging public health need in Africa. Testing and diagnostic capacity for arboviruses, as well as awareness of vector ecology and exposure risk, lag well behind that of malaria in most of sub-Saharan Africa, where climate change is expected to promote dengue and other arboviruses.
Acknowledgements
This paper is dedicated to Drew Gilmour, who was born to EAM while the paper was under revision: may this work inspire action to mitigate climate change and to reduce the burden of disease, especially in children, in your lifetime. ADL was funded by the National Institutes of Health (R01 AI102918). MMS was supported by a T32 Epidemiology Training grant and a Stephen Bechtel Endowed Postdoctoral Fellowship from the Maternal Child Health Research Institute. EAM and SJR were supported by the National Science Foundation (DEB-1518681; https://nsf.gov/). EAM was supported by the National Institutes of Health (1R35GM133439-01). EAM was supported by the Helman Scholarship, and the Terman Fellowship. EAM, ADL, and JMC were also supported by the Stanford Woods Institute for the Environment (https://woods.stanford.edu/research/environmental-venture- projects). SJR was funded by NSF (DEB-1641145) and a CDC grant 1U01CK000510-01: Southeastern Regional Center of Excellence in Vector-Borne Diseases: the Gateway Program. This publication was supported by the Cooperative Agreement Number above from the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention.
Footnotes
Declaration of Interests: The authors declare no conflicts of interest.
References
- 1.Rogers DJ, Randolph SE. Climate change and vector-borne diseases. Adv Parasitol. 2006;62:345–81. [DOI] [PubMed] [Google Scholar]
- 2.Liu-Helmersson J, Stenlund H, Wilder-Smith A, Rocklöv J. Vectorial capacity of Aedes aegypti: effects of temperature and implications for global dengue epidemic potential. PLoS ONE. 2014. March 6;9(3):e89783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altizer S, Ostfeld RS, Johnson PTJ, Kutz S, Harvell CD. Climate Change and Infectious Diseases: From Evidence to a Predictive Framework. Science. 2013. August 2;341(6145):514–9. [DOI] [PubMed] [Google Scholar]
- 4.Parham P, Michael E. Modeling the Effects of Weather and Climate Change on Malaria Transmission. Environ Health Perspect. 2010;118(5):620–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015. September 16;526:207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith DL, Cohen JM, Chiyaka C, Johnston G, Gething PW, Gosling R, et al. A sticky situation: the unexpected stability of malaria elimination. Philos Trans R Soc B Biol Sci. 2013. August 5;368(1623):20120145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. WHO | World Malaria Report 2018 [Internet]. WHO. 2018. [cited 2019 Mar 9]. Available from: http://www.who.int/malaria/media/world-malaria-report-2018/en/
- 8.Simo FBN, Bigna JJ, Kenmoe S, Ndangang MS, Temfack E, Moundipa PF, et al. Dengue virus infection in people residing in Africa: a systematic review and meta-analysis of prevalence studies. Sci Rep. 2019. Sep 20;9(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Outbreak Summaries | Rift Valley Fever | CDC [Internet]. 2020. [cited 2020 May 18]. Available from: https://www.cdc.gov/vhf/rvf/outbreaks/summaries.html
- 10.Weetman D, Kamgang B, Badolo A, Moyes C, Shearer F, Coulibaly M, et al. Aedes Mosquitoes and Aedes-Borne Arboviruses in Africa: Current and Future Threats. Int J Environ Res Public Health. 2018. January 28;15(2):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyaruaba R, Mwaliko C, Mwau M, Mousa S, Wei H. Arboviruses in the East African Community partner states: a review of medically important mosquito-borne Arboviruses. Pathog Glob Health. 2019. July 4;113(5):209–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gudo ES, Ali S, António VS, Chelene IR, Chongo I, Demanou M, et al. Seroepidemiological Studies of Arboviruses in Africa In: Hilgenfeld R, Vasudevan SG, editors. Dengue and Zika: Control and Antiviral Treatment Strategies [Internet]. Singapore: Springer Singapore; 2018. [cited 2019 Oct 30]. p. 361–71. (Advances in Experimental Medicine and Biology). Available from: 10.1007/978-981-10-8727-1_25 [DOI] [PubMed] [Google Scholar]
- 13.Buchwald AG, Hayden MH, Dadzie SK, Paull SH, Carlton EJ. Aedes-borne disease outbreaks in West Africa: A call for enhanced surveillance. Acta Trop. 2020. May 19;105468. [DOI] [PubMed] [Google Scholar]
- 14.Lafferty KD. The ecology of climate change and infectious diseases. Ecology. 2009. April;90(4):888–900. [DOI] [PubMed] [Google Scholar]
- 15.Mordecai EA, Paaijmans KP, Johnson LR, Balzer C, Ben-Horin T, de Moor E, et al. Optimal temperature for malaria transmission is dramatically lower than previously predicted. Ecol Lett. 2013;16(1):22–30. [DOI] [PubMed] [Google Scholar]
- 16.Mordecai EA, Cohen JM, Evans MV, Gudapati P, Johnson LR, Lippi CA, et al. Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLoS Negl Trop Dis. 2017. April 27;11(4):e0005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tesla B, Demakovsky LR, Mordecai EA, Ryan SJ, Bonds MH, Ngonghala CN, et al. Temperature drives Zika virus transmission: evidence from empirical and mathematical models. Proc R Soc B. 2018. August 15;285(1884):20180795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansson MA, Powers AM, Pesik N, Cohen NJ, Staples JE. Nowcasting the spread of chikungunya virus in the Americas. PLoS ONE. 2014. August 11;9(8):e104915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perkins TA, Metcalf CJE, Grenfell BT, Tatem AJ. Estimating drivers of autochthonous transmission of chikungunya virus in its invasion of the Americas. PLoS Curr [Internet]. 2015. February 10 [cited 2015 Aug 17];7 Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4339250/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart Ibarra AM, Ryan SJ, Beltrán E, Mejía R, Silva M, Muñoz Á. Dengue vector dynamics (Aedes aegypti) influenced by climate and social factors in Ecuador: implications for targeted control. PLoS ONE. 2013. November 12;8(11):e78263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delatte H, Gimonneau G, Triboire A, Fontenille D. Influence of temperature on immature development, survival, longevity, fecundity, and gonotrophic cycles of Aedes albopictus, vector of chikungunya and dengue in the Indian Ocean. J Med Entomol. 2009. January 1;46(1):33–41. [DOI] [PubMed] [Google Scholar]
- 22.Li R, Xu L, Bjørnstad ON, Liu K, Song T, Chen A, et al. Climate-driven variation in mosquito density predicts the spatiotemporal dynamics of dengue. Proc Natl Acad Sci. 2019. February 26;116(9):3624–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyd MF. Studies on Plasmodium vivax. 2. the influence of temperature on the duration of the extrinsic incubation period. Am J Epidemiol. 1932. November 1;16(3):851–3. [Google Scholar]
- 24.Tjaden NB, Thomas SM, Fischer D, Beierkuhnlein C. Extrinsic incubation period of dengue: knowledge, backlog, and applications of temperature dependence. PLoS Negl Trop Dis. 2013. June 27;7(6):e2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angilletta MJ. Thermal Adaptation: A Theoretical and Empirical Synthesis. Oxford University Press; 2009. 305 p. [Google Scholar]
- 26.Kingsolver JG. The Well-Temperatured Biologist: (American Society of Naturalists Presidential Address). Am Nat. 2009;174(6):755–68. [DOI] [PubMed] [Google Scholar]
- 27.Huey RB, Berrigan D. Temperature, demography, and ectotherm fitness. Am Nat. 2001;158(2):204–10. [DOI] [PubMed] [Google Scholar]
- 28.Shocket MS, Ryan SJ, Mordecai EA. Temperature explains broad patterns of Ross River virus transmission. eLife. 2018;7:e37762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bayoh MN. Studies on the development and survival of anopheles gambiae sensu stricto at various temperatures and relative humidities [Internet] [Doctoral]. Durham University; 2001. [cited 2014 Oct 15]. Available from: http://etheses.dur.ac.uk/4952/ [Google Scholar]
- 30.Bayoh M, Lindsay S. Temperature-related duration of aquatic stages of the Afrotropical malaria vector mosquito Anopheles gambiae in the laboratory. Med Vet Entomol. 2004;18(2):174–9. [DOI] [PubMed] [Google Scholar]
- 31.Johnson LR, Ben-Horin T, Lafferty KD, McNally A, Mordecai E, Paaijmans KP, et al. Understanding uncertainty in temperature effects on vector-borne disease: a Bayesian approach. Ecology. 2015. January 1;96(1):203–13. [DOI] [PubMed] [Google Scholar]
- 32.Mordecai EA, Caldwell JM, Grossman MK, Lippi CA, Johnson LR, Neira M, et al. Thermal biology of mosquito-borne disease. Ecol Lett [Internet]. 2019. [cited 2019 Jul 16];0(0). Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/ele.13335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vu DM, Mutai N, Heath CJ, Vulule JM, Mutuku FM, Ndenga BA, et al. Unrecognized Dengue Virus Infections in Children, Western Kenya, 2014–2015. Emerg Infect Dis. 2017. November;23(11):1915–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hooft AM, Ripp K, Ndenga B, Mutuku F, Vu D, Baltzell K, et al. Principles, practices and knowledge of clinicians when assessing febrile children: a qualitative study in Kenya. Malar J. 2017. September 20;16(1):381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hortion J, Mutuku FM, Eyherabide AL, Vu DM, Boothroyd DB, Grossi-Soyster EN, et al. Acute Flavivirus and Alphavirus Infections among Children in Two Different Areas of Kenya, 2015. Am J Trop Med Hyg. 2019. January 9;100(1):170–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah MM, Krystosik AR, Ndenga BA, Mutuku FM, Caldwell JM, Otuka V, et al. Malaria smear positivity among Kenyan children peaks at intermediate temperatures as predicted by ecological models. Parasit Vectors. 2019. Jun 6;12(1):288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hay SI, Rogers DJ, Toomer JF, Snow RW. Annual Plasmodium falciparum entomological inoculation rates (EIR) across Africa: literature survey, internet access and review. Trans R Soc Trop Med Hyg. 2000. March;94(2):113–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peña-García VH, Triana-Chávez O, Arboleda-Sánchez S. Estimating Effects of Temperature on Dengue Transmission in Colombian Cities. Ann Glob Health [Internet]. 2017. October 25 [cited 2017 Nov 10];0(0). Available from: http://www.annalsofglobalhealth.org/article/S2214-9996(17)30663-X/abstract [DOI] [PubMed] [Google Scholar]
- 39.Ryan SJ, McNally A, Johnson LR, Mordecai EA, Ben-Horin T, Paaijmans K, et al. Mapping Physiological Suitability Limits for Malaria in Africa Under Climate Change. Vector-Borne Zoonotic Dis. 2015. November 18;15(12):718–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Njoh AJ. Urbanization and development in sub-Saharan Africa. Cities. 2003. June 1;20(3):167–74. [Google Scholar]
- 41.Tiffen M Transition in Sub-Saharan Africa: Agriculture, Urbanization and Income Growth. World Dev. 2003. August 1;31(8):1343–66. [Google Scholar]
- 42.Zahouli JBZ, Koudou BG, Müller P, Malone D, Tano Y, Utzinger J. Urbanization is a main driver for the larval ecology of Aedes mosquitoes in arbovirus-endemic settings in southeastern Côte d’Ivoire. PLoS Negl Trop Dis. 2017. July 13;11(7):e0005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ngugi HN, Mutuku FM, Ndenga BA, Musunzaji PS, Mbakaya JO, Aswani P, et al. Characterization and productivity profiles of Aedes aegypti (L.) breeding habitats across rural and urban landscapes in western and coastal Kenya. Parasit Vectors. 2017. July 12;10(1):331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donnelly MJ, McCall P, Lengeler C, Bates I, D’Alessandro U, Barnish G, et al. Malaria and urbanization in sub-Saharan Africa. Malar J. 2005. February 18;4(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keiser J, Utzinger J, Castro MCD, Smith TA, Tanner M, Singer BH. URBANIZATION IN SUB-SAHARAN AFRICA AND IMPLICATION FOR MALARIA CONTROL. Am J Trop Med Hyg. 2004. August 1;71(2_suppl):118–27. [PubMed] [Google Scholar]
- 46.Murdock CC, Evans MV, McClanahan TD, Miazgowicz KL, Tesla B. Fine-scale variation in microclimate across an urban landscape shapes variation in mosquito population dynamics and the potential of Aedes albopictus to transmit arboviral disease. PLoS Negl Trop Dis. 2017. May 30;11(5):e0005640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stoler J, al Dashti R, Anto F, Fobil JN, Awandare GA. Deconstructing “malaria”: West Africa as the next front for dengue fever surveillance and control. Acta Trop. 2014. June 1;134:58–65. [DOI] [PubMed] [Google Scholar]
- 48.Ghai RR, Thurber MI, El Bakry A, Chapman CA, Goldberg TL. Multi-method assessment of patients with febrile illness reveals over-diagnosis of malaria in rural Uganda. Malar J. 2016. September 7;15(1):460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reyburn H, Mbatia R, Drakeley C, Carneiro I, Mwakasungula E, Mwerinde O, et al. Overdiagnosis of malaria in patients with severe febrile illness in Tanzania: a prospective study. BMJ. 2004. November 18;329(7476):1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gwer S, Newton CRJC, Berkley JA. Over-Diagnosis and Co-Morbidity of Severe Malaria in African Children: A Guide for Clinicians. Am J Trop Med Hyg. 2007. December 1;77(6_Suppl):6–13. [PMC free article] [PubMed] [Google Scholar]
- 51.Sutherland LJ, Cash AA, Huang Y-JS, Sang RC, Malhotra I, Moormann AM, et al. Serologic Evidence of Arboviral Infections among Humans in Kenya. Am J Trop Med Hyg. 2011. July 1;85(1):158–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.LaBeaud AD, Banda T, Brichard J, Muchiri EM, Mungai PL, Mutuku FM, et al. High Rates of O’Nyong Nyong and Chikungunya Virus Transmission in Coastal Kenya. PLoS Negl Trop Dis. 2015. February 6;9(2):e0003436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waggoner J, Brichard J, Mutuku F, Ndenga B, Heath CJ, Mohamed-Hadley A, et al. Malaria and Chikungunya Detected Using Molecular Diagnostics Among Febrile Kenyan Children. Open Forum Infect Dis [Internet]. 2017. July 1 [cited 2019 Mar 13];4(3). Available from: https://academic.oup.com/ofid/article/4/3/ofx110/3858096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.World Health Organization. WHO | Chikungunya – Mombasa, Kenya [Internet]. WHO. 2018. [cited 2019 Mar 9]. Available from: http://www.who.int/csr/don/27-february-2018-chikungunya-kenya/en/
- 55.Berry IM, Eyase F, Pollett S, Konongoi LS, Figueroa K, Ofula V, et al. Recent outbreaks of chikungunya virus (CHIKV) in Africa and Asia are driven by a variant carrying mutations associated with increased fitness for Aedes aegypti. bioRxiv. 2018. July 20;373316. [Google Scholar]
- 56.Sergon K, Njuguna C, Kalani R, Ofula V, Onyango C, Konongoi LS, et al. Seroprevalence of Chikungunya Virus (CHIKV) Infection on Lamu Island, Kenya, October 2004. Am J Trop Med Hyg. 2008. February 1;78(2):333–7. [PubMed] [Google Scholar]
- 57.Abdelaziz K Sudan reports outbreak of mosquito-borne Chikungunya disease in… Reuters [Internet]. 2018. September 25 [cited 2019 Jan 18]; Available from: https://www.reuters.com/article/us-sudan-health-chikungunya-idUSKCN1M52MB [Google Scholar]
- 58.Fokam EB, Levai LD, Guzman H, Amelia PA, Titanji VPK, Tesh RB, et al. Silent circulation of arboviruses in Cameroon. East Afr Med J. 2010. January 1;87(6):262–268–268. [DOI] [PubMed] [Google Scholar]
- 59.Amarasinghe A, Kuritsky JN, Letson GW, Margolis HS. Dengue Virus Infection in Africa. Emerg Infect Dis. 2011. August;17(8):1349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Franco L, Caro AD, Carletti F, Vapalahti O, Renaudat C, Zeller H, et al. Recent expansion of dengue virus serotype 3 in West Africa. Eurosurveillance. 2010. February 18;15(7):19490. [PubMed] [Google Scholar]
- 61.Demina AV, Lutwama JJ, Hertz T, Lobel L. Assessing the serological antibody repertoire to Flaviviruses in the endemic population of the Zika forest in Uganda. J Immunol. 2017. May 1;198(1 Supplement):122.12–122.12. [Google Scholar]
- 62.Yamana TK, Bomblies A, Eltahir EAB. Climate change unlikely to increase malaria burden in West Africa. Nat Clim Change. 2016. November;6(11):1009–13. [Google Scholar]
- 63.Vu DM, Banda T, Teng CY, Heimbaugh C, Muchiri EM, Mungai PL, et al. Dengue and West Nile Virus Transmission in Children and Adults in Coastal Kenya. Am J Trop Med Hyg. 2017. January 11;96(1):141–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kamau KK, Magoma GN, Kwallah A ole, Syengo CK, Mwau M. Seroprevalence of chikungunya fever virus and O’nyong Nyong fever virus among febrile patients visiting selected hospitals in 2011–2012 Trans Nzoia County, Kenya. 2018;
- 65.Simard F, Nchoutpouen E, Toto JC, Fontenille D. Geographic Distribution and Breeding Site Preference of Aedes albopictus and Aedes aegypti (Diptera: Culicidae) in Cameroon, Central Africa. J Med Entomol. 2005. September 1;42(5):726–31. [DOI] [PubMed] [Google Scholar]
- 66.Tedjou AN, Kamgang B, Yougang AP, Njiokou F, Wondji CS. Update on the geographical distribution and prevalence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae), two major arbovirus vectors in Cameroon. PLoS Negl Trop Dis. 2019. March 18;13(3):e0007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kamgang B, Ngoagouni C, Manirakiza A, Nakouné E, Paupy C, Kazanji M. Temporal Patterns of Abundance of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) and Mitochondrial DNA Analysis of Ae. albopictus in the Central African Republic. PLoS Negl Trop Dis [Internet]. 2013. December 12 [cited 2020 May 15];7(12). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3861192/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Hearn AE, Voorhees MA, Fetterer DP, Wauquier N, Coomber MR, Bangura J, et al. Serosurveillance of viral pathogens circulating in West Africa. Virol J. 2016. October 3;13(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992. March 1;30(3):545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wesolowski A, Qureshi T, Boni MF, Sundsøy PR, Johansson MA, Rasheed SB, et al. Impact of human mobility on the emergence of dengue epidemics in Pakistan. Proc Natl Acad Sci. 2015. September 8;201504964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salje H, Cummings DAT, Rodriguez-Barraquer I, Katzelnick LC, Lessler J, Klungthong C, et al. Reconstruction of antibody dynamics and infection histories to evaluate dengue risk. Nature. 2018. May;557(7707):719–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stewart-Ibarra AM, Lowe R. Climate and non-climate drivers of dengue epidemics in southern coastal Ecuador. Am J Trop Med Hyg. 2013;88(5):971–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johansson MA, Cummings DA, Glass GE. Multiyear climate variability and dengue—El Nino southern oscillation, weather, and dengue incidence in Puerto Rico, Mexico, and Thailand: a longitudinal data analysis. PLoS Med. 2009;6(11):e1000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rodriguez-Barraquer I, Cordeiro MT, Braga C, Souza WV de, Marques ET, Cummings DAT. From Re-Emergence to Hyperendemicity: The Natural History of the Dengue Epidemic in Brazil. PLoS Negl Trop Dis. 2011. January 4;5(1):e935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patz JA, Reisen WK. Immunology, climate change and vector-borne diseases. Trends Immunol. 2001. April 1;22(4):171–2. [DOI] [PubMed] [Google Scholar]
- 76.Mabaso MLH, Craig M, Ross A, Smith T. Environmental predictors of the seasonality of malaria transmission in Africa: the challenge. Am J Trop Med Hyg. 2007. January 1;76(1):33–8. [PubMed] [Google Scholar]
- 77.Anyamba A, Chretien J-P, Britch SC, Soebiyanto RP, Small JL, Jepsen R, et al. Global Disease Outbreaks Associated with the 2015–2016 El Niño Event. Sci Rep. 2019. February 13;9(1):1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gething PW, Smith DL, Patil AP, Tatem AJ, Snow RW, Hay SI. Climate change and the global malaria recession. Nature. 2010. May 20;465(7296):342–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tompkins AM, Caporaso L. Assessment of malaria transmission changes in Africa, due to the climate impact of land use change using Coupled Model Intercomparison Project Phase 5 earth system models. Geospatial Health [Internet]. 2016. March 31 [cited 2019 Mar 9]; Available from: https://geospatialhealth.net/index.php/gh/article/view/380 [DOI] [PubMed] [Google Scholar]
- 80.Sternberg ED, Thomas MB. Local adaptation to temperature and the implications for vector-borne diseases. Trends Parasitol. 2014. March;30(3):115–22. [DOI] [PubMed] [Google Scholar]
- 81.Liu N Insecticide Resistance in Mosquitoes: Impact, Mechanisms, and Research Directions. Annu Rev Entomol. 2015;60(1):537–59. [DOI] [PubMed] [Google Scholar]
- 82.Shapiro LLM, Whitehead SA, Thomas MB. Quantifying the effects of temperature on mosquito and parasite traits that determine the transmission potential of human malaria. PLOS Biol. 2017. October 16;15(10):e2003489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Powell JR. Mosquitoes on the move. Science. 2016. November 25;354(6315):971–2. [DOI] [PubMed] [Google Scholar]
- 84.Rose NH, Sylla M, Badolo A, Lutomiah J, Ayala D, Aribodor OB, et al. Climate and urbanization drive mosquito preference for humans. bioRxiv. 2020. February 13;2020.02.12.939041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Costantini C, Ayala D, Guelbeogo WM, Pombi M, Some CY, Bassole IH, et al. Living at the edge: biogeographic patterns of habitat segregation conform to speciation by niche expansion in Anopheles gambiae. BMC Ecol. 2009. May 21;9(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simard F, Ayala D, Kamdem GC, Pombi M, Etouna J, Ose K, et al. Ecological niche partitioning between Anopheles gambiae molecular forms in Cameroon: the ecological side of speciation. BMC Ecol. 2009. May 21;9(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kamdem C, Tene Fossog B, Simard F, Etouna J, Ndo C, Kengne P, et al. Anthropogenic Habitat Disturbance and Ecological Divergence between Incipient Species of the Malaria Mosquito Anopheles gambiae. PLoS ONE [Internet]. 2012. June 22 [cited 2020 May 15];7(6). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3382172/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Djamouko-Djonkam L, Mounchili-Ndam S, Kala-Chouakeu N, Nana-Ndjangwo SM, Kopya E, Sonhafouo-Chiana N, et al. Spatial distribution of Anopheles gambiae sensu lato larvae in the urban environment of Yaoundé, Cameroon. Infect Dis Poverty. 2019. October 9;8(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ryan SJ, Carlson CJ, Mordecai EA, Johnson LR. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl Trop Dis. 2019. March 28;13(3):e0007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Benelli G, Mehlhorn H. Declining malaria, rising of dengue and Zika virus: insights for mosquito vector control. Parasitol Res. 2016. May 1;115(5):1747–54. [DOI] [PubMed] [Google Scholar]
- 91.Carter KH, Singh P, Mujica OJ, Escalada RP, Ade MP, Castellanos LG, et al. Malaria in the Americas: Trends from 1959 to 2011. Am J Trop Med Hyg. 2015. February 4;92(2):302–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002. February 1;10(2):100–3. [DOI] [PubMed] [Google Scholar]
- 93.Erlanger TE, Keiser J, Utzinger J. Effect of dengue vector control interventions on entomological parameters in developing countries: a systematic review and meta-analysis. Med Vet Entomol. 2008;22(3):203–21. [DOI] [PubMed] [Google Scholar]
- 94.Spiegel JM, Bonet M, Ibarra A-M, Pagliccia N, Ouellette V, Yassi A. Social and environmental determinants of Aedes aegypti infestation in Central Havana: results of a case–control study nested in an integrated dengue surveillance programme in Cuba. Trop Med Int Health. 2007;12(4):503–10. [DOI] [PubMed] [Google Scholar]
- 95.Ferguson NM, Rodríguez-Barraquer I, Dorigatti I, Mier-y-Teran-Romero L, Laydon DJ, Cummings DAT. Benefits and risks of the Sanofi-Pasteur dengue vaccine: Modeling optimal deployment. Science. 2016. September 2;353(6303):1033–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Flasche S, Jit M, Rodríguez-Barraquer I, Coudeville L, Recker M, Koelle K, et al. The Long-Term Safety, Public Health Impact, and Cost-Effectiveness of Routine Vaccination with a Recombinant, Live-Attenuated Dengue Vaccine (Dengvaxia): A Model Comparison Study. PLOS Med. 2016. November 29;13(11):e1002181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


