Highlights
-
•
The review analyses the role of CAZymes in lignocellulose degradation with focus on the different approaches for improving the enzyme activity.
-
•
Omics based approaches for the analysis of genes, transcripts, proteins and metabolites related to plant degrading mechanism.
-
•
Directed evolution and rational design as approaches for engineering to enhancing the CAZyme properties.
-
•
Microbial engineering of the fungal and microbial strains by adapting techniques like CRISPR/cas9 and use of various expression systems.
-
•
Novel enzymes and cocktail formulation for biorefinery-based application for production of value-added products using low cost plant biomass.
Keywords: CAZyme, Omics, Cell factories, Consolidated bioprocessing, Biorefineries
Abstract
For sustainable growth, concept of biorefineries as recourse to the “fossil derived” energy source is important. Here, the Carbohydrate Active enZymes (CAZymes) play decisive role in generation of biofuels and related sugar-based products utilizing lignocellulose as a carbon source. Given their industrial significance, extensive studies on the evolution of CAZymes have been carried out. Various bacterial and fungal organisms have been scrutinized for the development of CAZymes, where advance techniques for strain enhancement such as CRISPR and analysis of specific expression systems have been deployed. Specific Omic-based techniques along with protein engineering have been adopted to unearth novel CAZymes and improve applicability of existing enzymes. In-Silico computational research and functional annotation of new CAZymes to synergy experiments are being carried out to devise cocktails of enzymes for use in biorefineries. Thus, with the establishment of these technologies, increased diversity of CAZymes with broad span of functions and applications is seen.
1. Introduction
Sustainable development is the concept of continuity and management of accessible resources with search for substitutes, especially for the rapidly depleting fossil-based energy reserves being of prime focus [1]. The ever-expanding world population, estimated to reach 8.5 billion by 2030, and transforming life style (urbanization, industrialization) results in increased energy dissipation [2,3]. This progressively increasing demand for energy cannot be accomplished by conventional non-renewable fossil fuels alone whose huge dependency will make it exhausted in the near future. Other hitches of fossil fuels comprehend environmental hazards by the emission of manifold greenhouse gases, leading to global warming. Furthermore, middle-east countries contribute more than 36 % of the total oil production [4]. In such a scenario, political crisis in these countries will hamper the production and circulation of crude oil worldwide, which eventually upsurges the cost per barrel, leading to haphazard and escalated price every alternate day [5,6]. Biomass is among the preferred resources with competence to meet the challenges of sustainability while addressing environmental issues [7]. The field of biorefineries is based on optimum utilization of plant biomass, which is present in copious amounts and is a prolific source of various complex carbohydrates for generation of renewable source of energy along with far-flung industrially valuable products [8].
Lignocellulose, the utmost component of the plant biomass, consists mostly of cellulose with a composition of approximately 51 %, followed by hemicellulose and lignin, which make up nearly 31 % and 28 %, respectively, with protein, lipid, pectin, soluble sugars and minerals being the minor components [9]. The lignocellulose can be degraded to simple sugars that can be utilized for fabrication of biofuel and innumerable value-added products by using assorted Carbohydrate Active enZymes (CAZymes).
CAZymes are the family of enzymes that works on carbohydrates for their biotransformation i.e. synthesis, metabolism along with their modification and transport [10]. These CAZymes that dominate the diversity of different carbohydrate-based products have been arranged into several classes considering their catalytic activities and similarity of amino acid sequences. The classes include glycoside hydrolases (GHs), carbohydrate esterases (CEs), polysaccharide lyases (PLs), glycosyltransferases (GTs), and auxiliary activities (AA) [11], [12]. To maintain the ever-increasing information about this class of enzymes, an online database system has been created named CAZy (http://www.cazy.org/) [13]. Lately, a new class of CAZyme, lytic polysaccharide monooxygenases (LPMOs), have been identified and classified under the Auxilliary Activity (AA) family. Among these classes, the GH family of CAZymes are the principal enzymes that bring plant biomass degradation. These CAZymes utilises sugar source for the generation of biofuels such as bioethanol and value-added products like biosurfactants and organic acids etc. It is also utilized in fruit juice extraction, bleaching of paper, detergent production and textile processing to name a few [14].
The lignocellulose degradation process has to start with removal of lignin as it imparts structural rigidity to this reserve of carbohydrates, after which cellulose and hemicellulose are released [15]. Although presently available CAZymes are well acknowledged, they have their own constraints in transfiguration of highly recalcitrant cellulose, the most abundant polysaccharide and industrially valuable sugar substrate from lignocellulosic biomass [16]. Furthermore, various strategies available for lignocellulose degradation are highly sensitive to the biomass being used, rendering one strategy for a particular feedstock being ineffective against the other [17]. Thus, effective degradation of lignocellulose requires a range of cocktails of CAZymes acting in a complementary synergistic manner to convert the trapped carbohydrate molecules i.e. the cellulose and hemicellulose, to different products [15]. Moreover, diverse sugar-based products that can be stemmed using these sugar substrates depend on the discovery of a catalyst with coveted properties and fortitude [10].
In nature, various fungi, bacteria, along with decomposer animals enact in the release of carbon trapped in organic matter for upholding of carbon cycle [18]. Their study may help in offering a key to the present-day constraints of available CAZymes [19]. The study of enzyme systems in these organisms can bring new insight into the working mechanism of CAZymes along with novel findings of new unreported enzymes playing weighty role in the decomposition of biomass [20]. The development of various technologies like omics and protein engineering, along with classical methods, has made the discovery, design and selection of CAZymes with high activity and stability possible. These CAZyme are being applied for the production of biofuels along with production of compounds with added values.
2. Omics based approaches aimed at prospection for improved CAZymes
In spite of various research work dedicated to identification of proficient CAZymes producers and genes involved, their preponderance still remains unidentified. Thus, technologies providing an intensive interpretation of such substantial genes and other parameters concerned with CAZyme production are important for their exploitation and biotechnological applications. Number of technologies such as multi-omics measurements, cell sorting, cell isolation, Nuclear Magnetic Resonance (NMR) spectroscopy, direct measurement of enzyme activity, activity-based protein profiling etc., are dedicated towards developing new insights into microbial strategies and functions used to degrade the plant biomass [21]. Nearly 1% of microorganisms in the microbial communities have been estimated to be obtained in pure culture [22]. Thus, 99 % of microorganisms, which can be a potential source for novel enzymes can be overlooked. This might be a significant reason for the development of metaomic approaches for intensive study of novel enzymes such as CAZymes. Various omics approaches are now easily available for different cellular molecules such as proteins, genes, metabolites, RNAs etc. [23], that provide us the opportunity to study and identify functionally significant but uncultivable microorganisms, their genes and mechanisms (Fig. 1).
Fig. 1.
Various Omic approaches applied in the field of biology for detailed study of microorganism for generation of potential CAZymes.
Developing “omics” technology have provided in-depth knowledge of the ability of various plant biomass degrading microorganisms through analysis of their genes, transcripts, proteins and metabolites [24]. This knowledge can be applied for effective production of value-added products. Some important omics technologies that can be applied for prospection of improved CAZymes are discussed.
2.1. Genomics and metagenomics approaches in CAZymes
For genomic studies of CAZyme producing microorganisms, different traditional molecular fingerprinting techniques are routinely used. Such techniques include random amplified polymorphic DNA [25], single-strand-conformation polymorphism [26], ribotyping i.e. 16SrRNA sequencing [27], etc. These traditional techniques, however, are tedious and culture dependent. Hence application of the “omics” technologies provides an escape from these setbacks for studying various producer microorganisms.
Metagenomics explores the genetic materials of different microorganisms isolated directly from environmental samples. It has succour in studying even the unculturable but significant microorganisms [28]. This approach encompasses direct isolation of the DNA without culturing them. This being a culture-independent approach provides serious benefits compared to traditional culture-dependent practices [29]. Pace et al. [30], first isolated and cloned DNA from various environmental samples for the study of unculturable microorganisms. Metagenomic approaches are largely categorized into (i) structural metagenomics, which ascertains the structure of various uncultivated microbes and (ii) functional metagenomics, related to study of genes involved in desired expression. There are a number of metagenomic techniques that can be applied, for example Polymerase chain reaction-denaturing gradient gel electrophoresis (PCR-DGGE). This technique proved to be an efficient metagenomic technology for studying anaerobic CAZymes producing bacteria and archaea in the bioreactor [31]. Likewise, fluorescence in situ hybridization (FISH) technology helps in quantifying the microorganisms involved in biogas production and can be supported by quantitative real-time PCR [32]. Terminal restriction fragment length polymorphism (T-RFLP), has also been harnessed for various studies [33].
Function based metagenomics involves expression of environmental sample DNA in different hosts, using a plasmid for clear examination of specific functions [34]. Number of host are used for this purpose which includes bacteria such as Escherichia coli, Pseudomonas, Xanthomonas etc. E. coli provides several strains that have the ability to harbour mutations. This reduces the DNA degradation (endA) and recombination (recA). The E. coli strains also facilitate the blue/white recombinant screening [35]. In non- E. coli bacterial hosts such as Pseudomonas fluorescens and Xanthomonas campestris, plasmid RK2-based experiments concluded the ability of these hosts to stably maintain very large inserts as per the capacity of parent plasmid [36]. Fungus such as Streptomyces sp. and yeasts [37] such as Saccharomyces, Komagataella sp., Kluyveromyces lactis, Yarrowia lipolytica etc., have also been used as a hosts. Streptomyces does not need plasmid encoded gene products for DNA transfer. In contrast the conjugal transfer of DNA in enteric bacteria requires the plasmid encoded gene products [38]. S. coelicolor A3 and S. lividans have been found to be useful hosts for expression of non-ribosomal peptides, polyketide and deoxysugar biosynthetic gene cluster [38]. Saccharomyces cerevisiae has the ability to survive rough industrial conditions and can secrete eukaryotic active-proteins and has many advantages to be used as a host [39,40]. Screening limitations do not permit study of carbohydrate-active enzymes that degrade lignocelluloses, calling for sequence-based approaches such as “shotgun” approaches [16]. In this approach, unlike traditional 16SrRNA sequencing, where a single genome locus is targeted for amplification, all DNA are cut into fragments and subjected to independent sequencing. This results in reads which are aligned at various genomic locations. Such genomic locations can be sampled from the coding sequences to ultimately identify the specific biological activity expressed by the genome [41]. Illumina’s high sequencing is proficient in generating over one tetra byte of bases per run, while MiSeq produces 15 Gbp per run [16]. Both the sequencing platforms have lower mean error rate (usually<1%) [42]. However, towing to computational requirements, using such methods provides a setback. Pacific Bioscience (PacBio) [43] developed third-generation sequencing technologies scilicet; single-molecule real-time sequencing (SMRT) and Oxford Nanopore’s MinION (MinION being the only portable real time device for DNA sequencing). SMRT is a parallelized single molecule DNA sequencing technique that utilizes a zero-mode waveguide [44]. For CAZyme screening, escalated ORFs and operons are required for downstream screening of the desired enzymes. An assembly stage is particularly important for generating large continuous sequence fragments [24]. For this, numerous assembly algorithms such as IDBA_UD [45], MEGAHIT [46], and metaSPAdes are available [47]. Additionally, longer read sequencing technologies can surmount assembly problem as they typically span the entire ORF.
2.2. Transcriptomics and Meta-transcriptomics approaches
Transcriptomics probes into the transcriptome of an organism which includes all RNA within the population, although mRNA is the principal module for experimental studies. It helps to obtain the entire gene expression profile of all microorganisms present in a natural environment [48]. Functional annotation of genes aids in identifying the gene expressed under metatranscriptomic studies. This provides advantages over metagenomic approaches which focuses on metabolically functional microorganisms and genes lone [49]. Microarray technology has been previously applied for assessment of gene expression profile. Being a novel tool in the field of molecular biology, it is proficient in quantifying thousands of gene transcripts from a given sample. However, this technology which employs several known DNA sequences arrayed in rows and columns in a chip is constrained in its inability to identify novel genes using the universal probes while being time consuming and steep [50]. Transcriptomics analysis helps in identifying active genes and microorganisms involved in lignocelluloses degradation and exploring the marker for controlling industrial production of biogas (value-added product) [51]. The metatranscriptomics approaches can be used for spotting unusual enzyme activity among microbes, studying microbe-microbe interaction among syntrophic microorganisms and many more [52,53].
Metatranscriptomic study of a compost-derived consortia in a medium with sugarcane bagasse as the sole carbon source was conducted. It led to breakthrough of the first exo-1,4-β-xylanase (act on xylo-oligomers) from family 11 of glycoside hydrolase (GH11) [54]. Using approaches of RT-PCR, the microbial origin and expression level of cellulase gene was examined in the rumen of Hu sheep [55]. Metatranscriptomic analysis of rumen microorganisms of adult Holstein cows indicated high quality of non-rRNA reads and the transcripts encoding GHs and some carbohydrate binding molecules [56].
2.3. Proteome and metaproteome based approaches
Proteomics is the characterization of all proteins of an organism whereas, meta-proteomics covers all the proteins obtained from environmental samples. Metaproteomics approaches helps to provide information about functional trait of microorganisms. The analysis usually commences with isolation of proteins from environmental samples which are subjected to fractionation followed by separation via liquid chromatography. The proteins are then subjected to final detection and sequencing by using tandem mass spectrometry (MS/MS) [57]. The two imperative strategies of proteomics that coalesce liquid chromatography with mass spectrometry are top-down and bottom-up. In top-down strategies, proteins are separated by liquid chromatography and then subjected to direct analysis using the tandem mass spectrometry. While bottom-up, also called shotgun, requires additional processing steps. The technique uses trypsin to digest the isolated proteins, which are then separated by liquid chromatography and analysed using mass spectrometry. The approach can be employed for both cultured as well as uncultured microbial isolates [58]. There are certain advantages and disadvantages of both of these approaches against each other. A small and variable fraction of peptide population is obtained in bottom-up proteomics. Hence, only a small percentage of protein sequence is covered. This limited coverage of protein sequence leads to a significant loss of the information relating to Post-Translational Modifications (PTMs) and alternate splice variants [59]. In contrast, top-down approaches deals with MS analysis of un-cleaved intact protein. Thus the labile structural characteristics of proteins are preserved in top-down approaches while they are destroyed in bottom-up MS. The elimination of protein digestion in top-down approaches also saves time. The top-down approach bears some limitations due to the poor solubility and complexity of proteins against the small peptides. These proteins also differ in their solubility under same conditions, thus creating a challenge for top-down proteomics to be considered a robust approach for proteomics study [60]. The small peptides generated in bottom-down approaches are highly soluble under chromatography MS condition.
These approaches can be used for quantification of enzymes and complete metabolic activity of an environmental sample. Irrespective of source organism, proteomics and metaproteomics approaches allows monitoring of all the elemental proteins expressed during the lignocelluloses mass degradation. Thus, these methods can be employed for the detection of catalytic enzymes, pathways and even novel proteins participating in anticipated function [57].
Combination of several omic strategies with metaproteomics has expanded the field view of researchers dedicated towards understanding metabolic pathways involved in plant degradation by different CAZymes [61]. Genome centric metaproteome and metatranscriptome studies were conducted on different anaerobic fungi. The CAZymes profile of these fungi indicated domain-specific specialization, where various endo- and exo- acting enzymes of GH family 5, 6, 8 and 48 were used to target the cellulose structures. GH48 was found to be the most abundant and the other representatives of this family detected also included the dockerin domains. The dockerin domains were associated with cellulosomes of these fungi [62]. Similarly another muti-omic study which included a meta-proteomic and metagenomic analysis of various microorganisms carrying out anaerobic digestion of plant biomass have been done. The results showed that Bacteroidetes is an efficient producer of hydrolases while Firmicutes often produces glycoside hydrolase enzymes which can accomplish degradation of lignocellulosic masses [63].
3. Engineering of CAZyme for improvement of properties
Large spectra of value-added natural products are synthesized using CAZymes with different carbohydrate substrates [64,65]. However, application of CAZymes to fabricate different products is hindered by substrate specificity, restricted activity and stability. Various strategies for excavation of novel enzymes to overcome these issues remains ineffective while consuming a lot of time and money and also being labor intensive [[66], [67], [68]].These factors along with the idea to keep the cost for industrial application low, has bolster different engineering techniques for development of the CAZymes [69]. Different protein engineering methods in complement to computational biology are the techniques currently employed in CAZyme design [10].
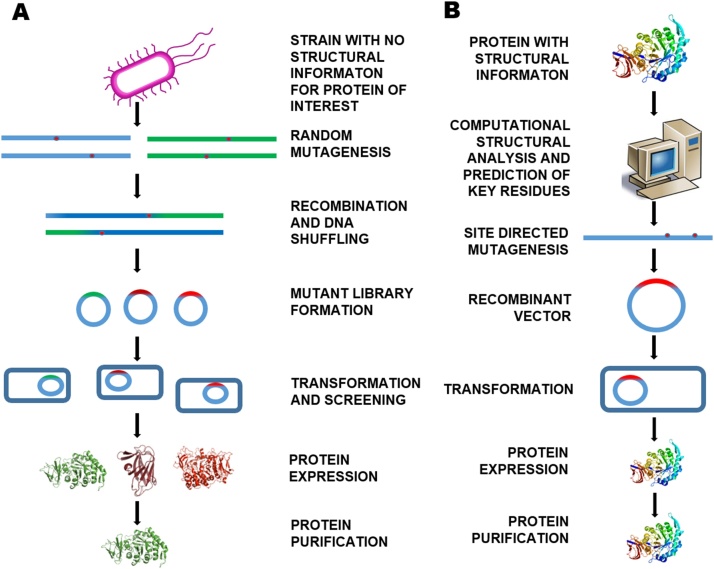
For successful engineering of any protein it requires advance information regarding its amino acid concatenation, structural geometry, active site, subsite and loops forming residues near catalytic core and folds and networks of bonds, along with reaction mechanism [[70], [71], [72]]. Protein engineering is the espoused method for modifying CAZyme for improved activity and stability together with augmentation of the products biosynthesis. It has also helped in improved screening and regulation of gene expression by engineering the sensors for enzymes [73]. The two general approaches for protein engineering involves directed evolution and rational design (Fig. 2) that have advanced over the years for production of different enzymes including the CAZymes [[74], [75], [76]].
Fig. 2.
The different approaches for CAZyme design. (A) Directed evolution uses technique random mutagenesis whereas (B) Rational design is based on a known protein structural information.
3.1. Directed evolution of proteins
Natural selection has engendered diverse number of proteins optimized for their specific biological activities. The natural selection technique can be implemented in a rather controlled manner in the laboratory conditions and the industrially significant enzymes can be further modified [77]. This directed evolution of protein is now commonly practiced for engineering of proteins with minimal structural information which is deficient in case of the methods of rational design. Random mutagenesis technique, using error-prone PCR, DNA shuffling and recombination methods generates large mutant libraries, followed by selection of enzyme with desired properties which is the basic idea of directed evolution [78]. Once the coveted enzyme is identified and expressed in a suitable host, the evolution of high-throughput screening is the major challenge in this method [79].
Several recombination methods have been developed to recombine the variants spawned by error-prone PCR random mutagenesis. The commonly applied recombination technique of Stemmer DNA shuffling method [80] is aided with new techniques like StEP where minuscule annealing and extension generates curtail products which in the next cycle anneals to the parent strand to create recombinants [81]. Random priming is another method where random annealing and extension by polymerase creates recombinants [82]. The homologous genes occurring naturally serves as a database for application of recombination along with directed evolution for modified enzyme performance [83,84].
As the random mutagenesis does not depend on structural information but study of contrast between the three-dimensional make-up of the wild type with the mutant, it also assists in forecasting the effect of further mutations [70]. Innumerous studies, using directed evolution, have been carried out for synthesis of different CAZymes for different functional modification which involves increasing enzyme enantioselectivity [[85], [86], [87]] thermostability [[88], [89], [90]] enzyme activity [78,91,92], increased substrate repertoire [93] and expression of the enzyme [[94], [95], [96]].
In several studies, the technique of error-prone PCR combined with DNA shuffling improved the pH and temperature tolerance while increasing the enzyme production [88], [97]. Using random mutagenesis Bacillus thermocatenulatus lipase showed 17-fold increased substrate specificity for lecithin without any activity lose for tributyrin [98].
Directed evolution has limitation in that only single- or double-point mutations can be screened at a time as including multiple mutations in the mutant library makes it outsize to be screened. For screening of cellulase there is another issue in that crystalline cellulose is to be used and not CMC as increased CMCase decreases the activity on the crystalline form of cellulose [99]. Moreover, the bias nature of transition mutation over transversion is not enough for drastic structural change which is required to bring about appreciable change in the enzyme characteristics [100]. Natural mutation occurs using several mechanisms and can bring about significant change in the enzyme polypeptide backbone which is a challenge to imitate by the process of direct evolution [101]. Moreover, random mutagenesis occurs usually at sites farther than the catalytic pocket which has no effect in the enzyme activity [102].
3.2. Rational design of proteins
Although, the technique of directed evolution has helped in the blooming of various industrially valuable CAZymes, it doesn’t always necessarily generate mutant with increased activity but reverse can also happen. Moreover, labor intensive screening procedure in absence of high throughput screening techniques makes rational design of CAZymes a preferred option for those enzymes with adequate structural information [73]. Here site directed mutagenesis is applied to achieve preconceived amino acid mutation based on the structural and catalytic information of the enzyme of interest [103]. It further gives knowledge of the active site along with the reaction mechanism of the enzymes. These information can be utilized for development of the enzyme itself as well as the other related enzymes for the industrial application [104].
The Endoglucanase Cel12B from thermophilic bacterium Thermotogamaritima, was mutated on the basis of homology modelling followed by structure analysis and site directed mutagenesis. It showed recombinant with increased activity along with enhanced thermostability [105]. Rational design of α-amylase from Alkalimonas amylolytica, generated 5 thermostable mutants along with increase in the alkaline stability and catalytic activities [106], while increased solubility of bacterial laccase from Bacillus sp. HR03 [107]. Using the computational programs CHARMM and the PDB, a computer model with a cellohexose molecule at the binding pocket of the enzyme was created and site-directed mutations with 40 % increase in catalytic activity was achieved in Thermobifida fusca endo/exocellulase Cel9A [108].
Rational design method can also be used to understand the protein-ligand interactions by using techniques of molecular docking and simulations for increasing the catalytic activity and purification of CAZyme with application in affinity column chromatography, bringing down the cost of enzyme production [109]. These techniques give an insight into the catalytic features of the different CAZymes and helps in virtual screening of the CAZymes for their ligands [110,111].
However, numerous failed attempts in rational design techniques has been seen. For example, when rational design was applied to CotA laccase from Bacillus subtilis, the enzyme showed increase redox potential but the catalytic activity subsided by almost four folds [112]. The reasons for such failures maybe attributable to incomplete understanding of the structural and functional aspect of several enzyme. Mostly when homology-based engineering is considered without reference to structural properties of enzymes, the mutation may render enzyme inactive by replacement of strictly conserved amino acid residues that changes the protein conformation without changing targeted enzyme properties [101]. It focuses more on the enthalpy with no consideration for the entropic interaction which might be one of the reasons for failed attempts [113].
The ever-increasing database of crystal structure and 3D information of protein (www.rcsb.org) [114] along with expanding computational and protein modelling tools has promoted rational design to flourish as a technique for evolution of tailored CAZymes for their industrial application. Moreover, development of promethean high-throughput screening techniques also boosts the direct evolution techniques [115]. Both techniques have their own advantages and thus combining both the techniques can be possibly more effective in enhancing the desired enzyme properties [116]. Additionally, newly emerging protein engineering techniques such as nanomaterials-based enzyme immobilization, advanced computational modelling, microenvironment engineering via substrate engineering are being exploited for developing multifunctional enzymes [72].
4. Microbial strain engineering of Filamentous fungi, yeast and bacteria as protein/enzyme cell factories
Bioengineering uses Microbial cell factories as an approach towards developing more efficient industrial grounds. Such approaches require microbial cells as the producing facilities and the process is optimized by metabolic engineering [117].
4.1. Microorganisms for strain engineering
Different bacteria, fungi and yeast have been realized as the natural reservoirs of carbohydrate active enzymes. Such microorganisms can be studied intensively to build a stepping stone towards advancements in biotechnological fields. Some of such microorganisms are discussed below:
4.1.1. Filamentous fungi
The various types of fungi, for instance Ascomycota and Basidiomycota, are capable of producing various suite of CAZymes (Fig. 3). These cascade of various carbohydrate active enzymes are widely considered in the emergent fields of bio-fuels, bio-chemicals, detergents, pulp and paper, textile etc. Large enzyme producers such as Novozymes (Bagsvaerd, Denmark) (https://www.novozymes.com/en), exploits a numbers of ascomycete fungi for production of fungal enzymes cocktail that degrades plant biomass constituents.
Fig. 3.
Ascomycota and Basidiomycota as Microbial Cell Factories (MCFs) for production of wide spectrum of CAZymes.
Various fungi have been found to act against different plant polymers. The genus Pycnoporus is important because of its metabolism pathways that brings about the functionalization of the aromatic compounds of plant biomass to yield upgraded molecules of commercial value [118]. Basidiomycete fungi are potent degrader of cellulose as many species flourish on dead and decaying wood environment rich of cellulose. For this degradation of cellulose they employ various sets of enzymes such as endoglucanase, cellobiohydrolase and β-glucosiadase [119]. Cellobiose being the abundant substrate, the enzymes degrading them are produced by various fungi including several white-rot and brown-rot fungi, the mycorrhizal fungi Pisolithus tinctorius being one potent producer [120].
Reports on CAZymes production by filamentous fungi; Botrytis cineria ATCC 28466, Aspergillus niger ATCC 9029, Schizophyllum commune ATCC 38548, Trichoderma reesei Rut-C30 and Penicillium brasilianum IBT 20888, cultivated on wet-oxidised wheat straw indicated Penicillium brasilianum had highest enzyme (1.34 FPU/mL) activity following enzyme concentration of the culture filtrates by ammonium sulphate [121].Usually ascomycetes have limited activity on lignin polymers but many basidiomycetes fungi possess the ability to degrade these aromatic polymers via production of arrays of enzymes. Soft-rot fungus show the lignin degrading capacity that ranges from minimal to substantial [122].
Experiment on standardized blocks of alder, poplar, and pine woods employing six strains of Graphium, Monodictys, Paecilomyces, Papulospora, Thielaviaterristris and Allescheria, isolated from pulp chip storage piles was conducted. The results showed slow lignin depletion by all the fungi in contrast to the carbohydrate components in case of alder and poplar. Graphium and Papulospora depleted lignin slowly on alder and poplar correspondingly while Monodictys acted on both. Lignin was degraded more rapidly later in case of Papulospora [123].
The production of CAZymes from ustilaginomycetous yeasts has been intensively studied with majority of the enzymes being proficient in degrading xylan, an imperative constituent of hemicellulosic plant cell wall [124]. Pseudozyma hubeiensis pro tem. was shown to produce β-xyloside with highest β-xylosidase activity reported among yeast strains [125]. Similarly, secretome analysis of Kalmonozyma brasiliensisgrown on distinct sources of carbon helped in identifying 20 CAZymes (13 GHs, 3 CEs and 4AAs), which can have significant biotechnological potentials [126]. Two strains of Moesziomyces antarcticuswas shown to produce xylanase of 33 kDa and belonged to GH10 family [127], while M. aphidis strain was shown that the yeast produces amylase that catalyzes endohydrolysis of 1,4-α-D-glycoside linkage in polysaccharides [128]. Pectin degrading activity was identified in Moesziomyces antarcticus, Kalmonozyma fusiformata and Pseudozyma sp. [[129], [130], [131]].
4.1.2. Bacteria
Large number of bacteria have been explored for presence of carbohydrate-active enzymes (CAZymes) for degrading plant biomass and generation of numerous value-added compounds. The cellulolytic bacilli, Clostridium termitidis strain CT1112, identified from the gut of a wood-feeding termite Nasutitermes lujae, showed presence of 355 CAZymes sequences which is higher than the other clostridial species. The bacterium is capable of degrading cellulose, xylan, cellobiose and other sugar, thus be used for biofuel formation with the help of some consolidated bioprocesses [132]. Besides, the mammalian gut is also colonised by microbial communities capable of metabolizing indigestible polysaccharide complex, predominantly the Firmicutes and Bacteroidetes. The Starch utilization system (Sus) has been studied thoroughly for Bacteroides thetaiotamicron [133]. One experiment identified several distinct Sus-like polysaccharide utilization loci (PULs) from bovine rumen microbial communities by means of functional screening of fosmid library [134]. Over 50 distinct Sus-like PULs from bovine rumen microbiome with each locus been allotted phylogenetically to the bacterial phylum Bacteroidetes. Thus the association of the total genomic CAZyme repertoire with Sus-like genes for Bacteroides, was shown [135].
Interests in thermophilic bacteria as a cell catalyst in biofuel and biochemical industries has intensified over the last few years as these bacteria boasts the ability to utilize wide range of carbohydrates including incalcitrant complexes such as cellulose [136]. Studies on Caldicellulosiruptor bescii DSM 6725, indicated that these thermophiles are efficient degrader of unprocessed plant biomass and degrade cellulose and xylan simultaneously [137]. But the degree of substrate conversion was below 20 %, hence leading to a low yield of fermentation product. One of its relative, Caldicellulosirupter saccharolyticus was studied to be co-utilising glucose and xylose [138]. Extensive omics-based analysis of this bacteria showed various features concerned with plant biomass degradation. For instance, genes for multi-functional carbohydrate-active protein that were organised into a single cluster of functional gene, lateral gene transfer were detected. The bacteria produces free acting cellulases in response to polysaccharides [139].
4.2. Engineering of gene expression and regulation related to CAZyme production
Consolidated Bio Processing (CBP) is recognised as prospective advancement for low-cost processing of biomass. Although none of the commonly used microorganisms have all the desired attributes for CBP, they may possess some of the features and their viability can be further increased with genetic recombination [140]. However, enzyme production and substrate hydrolysis must be regulated together with fermentation. Soluble protein maximization is important for CBP [140]. These approaches usually involve engineering the cellulolytic ability into specific industrially important viable microorganisms to facilitate the bioconversion of lignocellulosic plant biomass to biofuels. Some important microorganism used for these purposes includes yeast, Escherichia coli, Zymomonas mobilis. Consolidated bioprocessing of lignocellulose combines four events; (i) Fabrication of saccharolytic enzymes (ii) Hydrolysis of polysaccharides of the pre-treated biomass (iii) Hexose sugars fermentation (iv) Pentose sugars fermentation. Since multiple enzymes are involved for complete degradation of biomass, engineering a proper protein secretion system in modified organism is requisite for expression of several cellulolytic enzymes. CBP approaches usually involves engineering either naturally cellulase producing microorganisms or non-cellulolytic organisms that are modified with high yield products to become significantly cellulolytic [141]. To achieve CBP of amorphous cellulose, Bacillus subtilis 168 strain (non-cellulolytic) was engineered with relevant genes such as ΔalsS and B. Subtilis EG (BsCel5). The modified strain acted on 7 g L−1 of substrate to produce 3.1 g L−1 (+)- lactic acid [142]. While Clostridium cellulolyticum CC-Pmg8 (native-cellulolytic bacterium) was genetically engineered with relevant genes, Z. Mobilis adhll, pdc. The modified bacterium acted upon 50 g L−1 cellulose as substrate to produce 0.83 g L−1 ethanol [143]. One important strategy towards CBP approach is to engineer yeast Saccharomyces cereviciae with several components of cellulolytic system derived from a cellulolytic fungi or bacteria. The recombinant yeast may express cellulases and hemicellulases externally on the cell surface, which can contribute towards realization of CBP. The cell surface engineering when combined with intracellular metabolism engineering, the recombinant yeast may become highly effective showing novel fermentation ability thus more industrially viable [144]. However, the overexpression of cellulase has been also applied in the CBP field. The overexpression of enzyme usually employs techniques such as promoter engineering, gene copy number increase and deficiency of protease. Such approach in S. cereviciae with multi copy number of genes integrated into diploid strain showed a direct production of 7.6 and 7.7 g L−1 ethanol from substrates phosphoric acid-swollen cellulose (PASC) and pre-treated rice straw respectively [145].
4.2.1. Engineering of gene expression in filamentous fungi, yeast and bacteria
Different microorganisms can be subjected to genetic engineering that induces the capability to produce CAZymes. This effectively reduces the dependence on externally added enzymes for conversion of plant biomass to products with utility. Different microorganisms known for producing CAZymes are genetically amended. Ustilago maydis is preloaded with sets of hydrolytic enzymes. For uninterrupted expression of these enzymes, synthetic promoters with constitutive activity were positioned by removing the indigenous promoters [146]. An increased generation of fermentable sugars from xylan, cellobiose and carboxymethyl cellulose was observed. The genetic engineering of Ustilago maydis is important as its polysaccharide hydrolysing enzymes are expressed only during the plant infection.
Using the genomic DNA of strain UM521 as the template, Escherichia coli strain K-12 was used for cloning. Promotor replacement with constitutive active promotor was accomplished with the help of generated storage vector designated pStorI_2−5n (pUMa2326). Ustilago Maydis MB215 promotor replacement mutants were generated via homologous recombination and were obtained via transformation of the progenitor strain MB215. Following these methods and many more the intrinsic CAZymes of smut fungus Ustilago maydis was activated [147].
Expression of a cellulolytic system in S. cereviciae from either fungi (noncomplexed cellulase expression system) or bacteria (non or complexed cellulase expression) has become a predominant strategy for formation of engineered strains having high potential for CBP. A higher amount of ethanol was produced (1.0 g L−1) from PASP (10 g L−1) following co-expression of endoglucanse from T. reesei and β-glucosidase enzyme from Saccharomycopsis fibuligera [148].
Agrobacterium tumefaciens can be used for genetic modifications of an assortment of white-rot fungi via transformation using the T-DNA portion (transfer DNA) of the resident Ti-plasmid [149]. The virulence (vir) gene is responsible for this trans-kingdom transfer. The white-rot fungi Phanerochaete chrysosporium ATCC 32629, Ganoderma sp. RCKK-02, Pleurotussajor-caju, Pycnoporus cinnabarinus, Crinipellis sp. RCK-1 and fungal isolate BHR-UDSC were explored for this Agrobacterium-mediated T-DNA transfer that carries the genes for β-glucuronidase (uidA), green fluorescent protein (gfp) and hygromycin phosphotransferase (hpt). The transformation frequency ranged from 50 to 70% depending upon the fungal isolate. The establishment of steady expression, maintenance and replication of the inserted DNA paved way for expression of the desired trait [149].
Pichia pastoris has become favoured over last decade because of its ability to produce CAZymes. This yeast can be modified by genetic engineering to produce recombinant proteins through application of efficient and stringently regulated promoters [150]. Such engineered cells have very high expression. The production of fungal biomass-degrading recombinant protein using Pichia pastoris, principally aimed at setting up platform for easy screening of the expression enzymes extracellularly by the yeast have been studied. The platform tasks ranged from gene cloning, protein purifications to enzyme activity tests of glycoside hydrolases GH11, GH5 and GH45 [151].
Zymobacter palmae gen. nov., sp. nov. was genetically modified as an efficient ethanol producing bacterium by introducing the E. coli genes expressing xylose catabolic enzymes which included xylose isomerise, xylokinase, transaldolase and transkelolase. [152]. Thermotoga sp. RQ2 was transformed by Caldicellulosiruptor cellulase enzymes for enhanced endoglucanse activity and expressed all the desired enzymes [153].
4.2.2. System for homologous and/or heterologous expression in relevant organism
Heterologous expression of genes denotes expression of complementary DNA (cDNA) or RNA (cRNA) encoding for the desired function from one species to another, such that the cellular machinery of host species carries out the foreign protein expression [154]. Insertion of gene in the host organism for heterologous expression usually requires recombinant DNA technique. On the other hand, homologous expression of genes refers to over-expression of gene in an organism where the gene is naturally found. Such homologous or heterologous expression of genes can be used for increasing the efficiency of the CAZyme producing microorganisms, thus rendering them more viable for industrial and biotechnological applications (Table 1).
Table 1.
Genetic engineering for heterologous expression of the CAZymes.
| Host microorganism | Source Microorganism | Promoter or Genes involved | Enzyme expressed | Yield or enzyme activity | Reference |
|---|---|---|---|---|---|
| Bacterial host | |||||
| Escherichia coli | Erwinia chrysanthemi | CelZ promoter | Endo glucanase | 4−6% of total cell protein | [155] |
| Cellulomonas fimi | PlacUV5 promoter | Exoglucanase | 2−11 times higher yield | [156] | |
| Clostridium acetobutylicum P262 | Native promoter | Endo-1,4-β-d-glucosidase | 75 % in periplasmic space | [157] | |
| Bacteroides succinogenes | Lac promoter | 1,3−1,4-β-D-glucan-4-glucanohydrolyase | 16.7−22.4 mg of sugar equivalent provided per hour per mg of cell protein | [158] | |
| Rhodothermus marinus | BglA gene and lac promoter | Thermostable endoglucanase | 80 % of activity over range of 75−90 °C | [159] | |
| Zymomonas mobilis | Acetobacter xylinum | Native promoter | Endo-β-1,4-glucanase | 75 % in periplasmic space | [160] |
| Acidothermus cellulolyticus | Ptac promoter | Endo-β-1,4-glucanase | ∼20 % extracellular, ∼30 % in periplasm | [161] | |
| Ruminococcus albus | gfo gene | β-glucosidase | 4.7 % extracellular, 61 % in periplasm | [162] | |
| Bacillus subtilis | Native promoter | Endo-β-1,4-glucanase | – | [163] | |
| Filamentous fungi as host | |||||
| Trichoderma reesei | Humicola grisea | Cbh I promoter | xylanase | 0.5 g.L−1 | [164] |
| Chaetomium thermophilum | Cbh I promoter | xylanase | 960 U mg−1 | [165] | |
| Penicillium oxalicum | Pdc promoter | GH11 xylanase | 185653.5 IU mg−1 | [166] | |
| Acrophialophora nainiana | Bh1 promoter xyn6 gene | GH11 xylanase | 172 mgL− 1 | [167] | |
| Aspergillus nidulans | Penicillium purpurogenum | α amylase promoter | Acetoxylan esterase (AXE II) | 27.4 U mg−1 | [168] |
| Chaetomium gracile | Native promoter (cgxA and cgxB genes) | Xylanase (cgxA and cgx B) | High level of activity | [168,169] | |
| Aspergillus niger | AbfA gene | α-l-arabinofuranoside A and B (GH51 and GH54) | 0.16−2.63 U/mL culture medium | [170] | |
| Aspergillus oryzae | Aspergillus oculeatus | XegA gene | Xyloglucanase (GH 12) | Specific activity 260 μmol min−1 mg-1 of protein | [171] |
| Aspergillus pheonius | Msds genes | 1,2-α-mannosidase | Specific activity 3 μmol min−1 mg−1 | [172] | |
| Aspergillus niger D15 | Aspergillus oculeatus | Man1 gene (gdp promoter) | β-mannase | 16,596 nkat min−1 | [173] |
| Yeast as host | |||||
| Saccharomyces cerevisiae | Agaricus bisporous | gdp promoter (gdp gene) | Glyceraldehyde-3 phosphate dehydrogenase | 7.53 g/L of ethanol for 151 g/g orange peel | [173] |
| Saccharomyces cerevisiae | Trichoderma viride | eg3 and bgl1 genes | Endoglucanase and β-glucosidase | 4.63 g/L of ethanol | [174] |
Zymomonas mobilis is an important gram-negative bacterium that differs from other bacteria like Escherichia coli as it performs glucose metabolism by an alternate pathway known as Entner-Doudoroff (E.D.) pathway rather than the conventional glycolytic or EMP pathway. As a consequence of high sugar uptake, lower cellular biomass yield and high alcohol formation, this bacterium is considered to be efficient for its application in biofuel formation. One study investigated the ability of Zymomonas mobilis to express heterologous cellulase extracellularly. The heterologous expression of E1 and GH12 from the bacterium Acidothermus cellulolyticus were investigated where both the cellulolytic enzymes were discerned to be expressed successfully by Zymomonas mobilis as active and soluble enzymes although E1 was expressed at lower levels with GH12 comprising as much as 4.6 % of total cell proteins. The study revealed that notable portions of both the enzymes expressed resided the periplasmic space proving Zymomonas mobilis as an efficient producer of high levels of cellulases thus developing Zymomonas mobilis into an organism for CBP platform [161].
Another important biomass degrading microorganism is Caldicellulosiruptor. To refine the efficiency of Caldicellulosiruptor bescii as a plant degrader, CAZyme from Acidothermus cellulolyticus was introduced to act synergistically with the C. bescii exproteome. The study demonstrated that E1 endo-1,4-β-d-glucanase (GH5) of Acidothermus cellulolyticus acting in concert with CelA, increased the exproteome activity of Caldicellulosiruptor bescii on plant biomass [175].
Inducible or auto-inducible promoters can help in induction of heterologous expression of CAZyme in a rich growth media. For instance, cellulolytic Bacillus subtilis168 when grown in rich growth media containing glucose, tryptone and yeast extract, exhibited a heterologous expression of alkaline cellulase enzyme from Bacillus akibai I-1 under the control of sacB promoter induced by sucrose [176].
4.2.3. Use of CRISPR/cas9 for the construction of improved strains
Clustered regularly interspaced short palindromic repeats (CRISPR) is a DNA sequence family usually derived from bacteriophages and found to be present in the genome of prokaryotic hosts infected previously and used for obliteration of DNA of similar phage in case of next infection. Cas9 also known as CRISPR-associated protein is an enzyme that cleaves complementary sequences of CRISPR sequences. Both CRISPR and cas9 are combined to form technology called CRISPR/cas9 system. CRISPR/cas9 is a simple and a precise method of genetic manipulation adapted from a naturally occurring genome editing system, in which bacteria uses viral genome to make CRISPR sequences after its initial infection with the virus and protect itself from the future infections with the similar viruses using cas9 mechanisms [177].
The filamentous fungi Penicillium subrebescens is one industrially promising cell. This fungus can be subjected to genetic manipulations for strain improvement making it an efficient producer of CAZymes used for generating value-added products from plant biomass. CRISPR-associated RNA-guided DNA endonucleases (cas) technology, was established and demonstrated for the genome editing of this fungus. The ku70 subunit protein encoding ku70 gene, which participate in the non-Homologous End-Joining (NHEJ) pathway was targeted. The generated Penicillium subrebescens NHEJ-deficient strains showed improved capability of homologous recombination [178]. The strains generated following transformation showed no phenotypic alterations to the wild type earning these mutants as parental strains for subsequent transformation events, thus allowing the CRISPR/cas9 system to be used for expansion of repertoire of fungi to contribute at biotechnological and industrial levels [176]. The CRISPR/cas9 approach has some additional advantages like self-replicating plasmids, isolation of cured strains and further use as transformation system.
CRISPR/cas9 system has been used to disrupt target genes in the genome of Ustilago maydis where targeting efficiency with homologous recombination is quite low [179]. Genetic engineering of the baker’s yeast, Saccharomyces cerevisiae, using CRISPR/cas9 method have turned out to be advantageous for genome editing [180].
5. CAZyme application in biorefineries
The main application and necessity for discovery of new CAZymes and engineering of the existing ones is influenced by need of the society for use of the plentiful low-cost plant biomass as a sustainable source of energy in the form of biofuels [16]. According to Administration of Energy Information (EIA) U.S, around 89 % of the total energy utilized in the United States is derived from the non-renewable sources like the fossil fuels with 83 % of the energy being used in electricity generation as of 2018. Their continuous use has the disadvantage of diminishing availability, pollution and increasing price [17]. Here comes the idea of biorefineries, where the production and use of variety of bio-based products as the alternative to the currently used non-conventional products is advocated [181]. Biorefinery offers a sustainable green option to utilize the potential of waste product in optimum way to produce marketable bioproducts and bioenergy, while solving the issue of waste management and the greenhouse gas emissions [182]. The biorefinery based market is predicted to grow globally from $466.6 billion in 2016 to $714.6 billion by 2021 [183].
The biorefineries have been classified based on the source and the products generated of which extensively studied are: Lignocellulose based Biorefinery that uses naturally dry carbon source such as cellulose-containing biomass and wastes; Whole-Crop Biorefinery where crop material such as cereals or maize acts as the substrate and Green Biorefineries with naturally wet biomasses such as green grass, immature cereal as the carbon source [1]. While the whole crop and green biorefineries compete with food and fodder industry for the substrate, the biowaste lignocellulose based biorefineries becomes the ultimate choice for sustainable generation of bioenergy and bioproducts.
The basic steps in lignocellulose based biorefinery involves initial fractionation of the plant material into cellulosic, hemicellulosic and lignin fraction which are hydrolyzed further for releasing their sugar monomers [7]. These sugars are then used in fermentation media for forming various products. However, the major concern of biorefineries is the recalcitrant nature of lignocellulose. The disintegration of lignocellulose structure can be done using the biochemical methods such as the enzymes treatment as well as the physical and chemical methods like pyrolysis. The biochemical method has an advantage over the thermochemical methods in that it converts the carbohydrate into its monomeric form rather than destroying the carbohydrates as in the second case. Moreover, the enzyme technology is preferred as sustainable technology for the saccharification process [184]. Thus, CAZymes are important in biorefineries for degrading complex carbohydrate present in plant lignocellulose to simple sugar for generation of biofuel and value-added products.
5.1. CAZyme cocktails preparation for plant biomass deconstruction
The lignocellulose being recalcitrant requires a complex array of different lytic enzymes i.e the CAZymes which would work in synergy for its depolymerization into copious fermentable sugars [184]. Minimal enzyme system are approaches where instead of cell- based crude enzyme mixture, customized CAZyme formulations can be used when the cellulosic material and pretreatment methods are specified. It has the advantage of requirement of lower enzyme dosage. Studies with enzyme formulation showing better efficiency compared to the crude multi-enzyme have been reported [182].
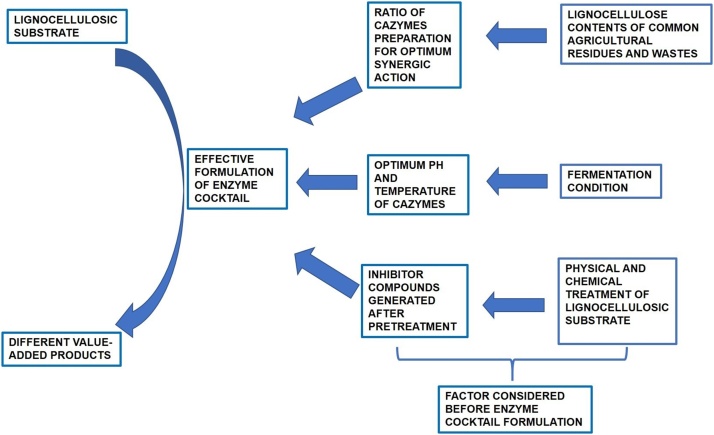
Effective formulation of different potential CAZymes is one of the keys to efficient conversion of lignocellulosic biomass and obtaining maximum yield. The parameters like detailed composition of biomass, pre-treatment methodology, generation of inhibitors, ratio of different CAZymes and their optimum activity conditions are to be considered as illustrated in Fig. 4 [17]. Multifariousness of the plant cell wall is another reason for the inefficiency in using the enzymatic hydrolysis methods [185]. Moreover, inclusion of the accessory enzymes in the cocktail increases the cost, yet has a similar effect to that of the pre-treatment process. Hence for the development of a cost-effective way for lignocellulose degradation, the best approach is the discovery and optimization of minimum content enzyme cocktail [186].
Fig. 4.
Rational for formulation of efficient CAZymes Cocktail.
The classical mechanism for depolymerisation of cellulose includes 1. The Endo-Glucanases (EGs) cleaving at random sites within the polymer, 2. The Cello-Biohydrolases (CBHs) or exoglucanases which exclusively detaches the monomers from both reducing as well as the non-reducing ends and 3. The β-glucosidases that catalyse the reaction of glycosidic bonds hydrolysis along the non-reducing ends with the release of glucose subunits [187].
This classical mechanism of endo-exo hydrolysis has now extended with inclusion of lytic polysaccharide monooxygenases (LPMOs) which acts by cleaving the glycosidic bond in cellulose via oxidation rather than hydrolyzing them [188]. This mechanism of oxidative cleavage has been studies in both the bacterial family 33 of the Carbohydrate Binding Modules (CBM33) as well as fungal protein family 61 Glycoside Hydrolases which have now being reclassified as Auxilliary Activity (AA) family 10 and 9 respectively [189]. Thus, studies regarding these new class of CAZymes and their genes in plant biomass degrading microbiome can lead to new findings and development of novel CAZymes for their application in the biorefineries.
Moreover, additional CAZymes in the form of xylanase, glucuronidase, mannanase etc may be included in the enzyme cocktail for the degradation of the hemicellulose which also adds up to the inaccessibility for cellulose degradation [190]. For the lignin component of the lignocellulose, despite the fact that it can act as the source of the various aromatic compounds, the CAZymes for its depolymerization needs yet to be found as of the current scenario. Some enzymes playing a role in degradation of the precursor structures of lignin have been studied though [191].
Recently lignin degrading enzyme families along with the LPMO families have been brought together under a new class of CAZymes named “Auxiliary Activities" that includes families from ligninolytic enzymes (9) and LPMOs (6). Lignin is found to be integrated together with other polysaccharide component of the plant cell wall and though the ligninolytic enzymes may not directly act on the carbohydrates, they are likely to have a cooperative role in polysaccharide degradation by the classical carbohydrate lytic enzymes [192].
5.2. Novel CAZymes
The currently available commercial enzyme cocktail focuses on degradation of lignocellulosic polymers into pentose and hexose sugar which comprises of the EGs and the CBHs isolated from different aerobic fungal stains like Trichoderma and Aspergillus spp. [188]. Studies of microflora of the rumen and digestive tract of herbivores can provide a breakthrough for unearthing of new CAZymes. The plant biomass has a very short retention time in the gut of these animals, thus putting a constant pressure on resident microorganisms for brisk and efficient utilization of the biomass, hypothesizing selection of enzymes with high activities working synergistically for degradation of lignocellulose.
Studies focused on single enzyme activities leading to development of proprietary multicomponent enzyme preparations when replaced by studies of cocktail formulation have shown to be more encouraging [193].
The composition of currently available commercial cellulase enzyme cocktail formulations have EG I Cel7B, belonging to the glycosyl hydrolase family 7B [GenBank No: M15665] along with CBH I Cel7A of GH family 7A [GenBank No: CAH10320], and CBH II Cel6A of GH family 6A [GenBank No: M16190] of the fugal strain Trichoderma reesei as the minimum requirement [188]. Above 70 % of protein component used for commercial cellulase enzyme mixture comprises of the cellobiohydrolases that processively releases the cellobiose. Though these processive cellobiohydrolases efficiently hydrolyses the crystalline structure of the cellulose, their processivity is restricted by the substrate being intrinsically slow [194]. For the discovery of novel CAZymes, culture dependent technique has the limitation of isolation of these novel enzyme producing organisms from their natural environment which is addressed by culture dependent techniques like the metagenomic approaches whereby the isolation of entire genomic content from a sample escapes the bottleneck of culturing these organisms on growth media [16].
These techniques have already showed their efficiency by being able to discover various new non culturable bacteria possessing polysaccharide degrading abilities [[195], [196], [197]] as well as novel CAZymes [55,198,199] with various roles in polysaccharides degradation abilities which would not have been possible with the classical culture-based methods [200]. Metagenomic study of rumen of Mehsani buffalo and Korean black goat rumen unearthed novel GH26 and KG51 genes respectively which were than expressed and characterized [201,202]. In a similar way novel xylanase was identified and characterized from the metagenomic library of an enrichment culture for rice straw degradation [201].
Rational bioinformatics technique for optimization and synthesis of the target gene leading to production and screening of recombinant protein has helped in discovery of several new undiscovered families with various substrate specificities for their industrial uses [203]. Protein fingerprinting methods then can be used for study of the newly discovered enzymes for the choice of enzyme cocktail to be used and also to know the drawbacks of enzyme cocktails currently available for their use in the industries [204].
Thus, metagenomics along with screening-based approaches help in the discovery as well as increased knowledge of the synergy between the different CAZymes for efficient biomass degeneration [205].
5.3. Value-added products from biosynthetic enzymes in biorefineries
To enhance the economic value of biorefineries, concept of synergy between production of biofuel and value-added product is crucial. The CAZymes can be applied in biorefineries for simple utilization of lignocellulosic sugars for biofuel production, or generation of other polymeric materials along with different organic acids [206]. The carbohydrates have a diverse function and can also act as a substrate, not only for production of biofuels, but also for other different sugar-based products with special focus on the platform chemicals. The lignin demineralising CAZyme that includes laccases, lignin-peroxidases, and manganese peroxidases are used in polymer industries for enzymatic polymerization, oxidative biodegradation of dyes etc [207]. The cellulase are applied in textile industries for dye removal, biostoning, softening of fabric, biopolishing, in the paper and pulp industries for biopulping, biomodification of fibres, deinking. In animal feed industry, the cellulase are used for increased nutritive value and digestibility of forge. As the cellulase can degrade the cell wall of plant pathogens, they are applied in agriculture industry for biopesticide formulations. The cocktail of hemicellulase, cellulase and pectinase is used in vegetable and fruit juice clarification while cellulase along with pectinase is used in extraction of olive oil. The enzyme cocktail of cellulase, hemicellulase and lipases are used during dough making for enhancing flavor, volume and shelf life of the bakery products [208].
Thus, the CAZymes find their use in different areas such as food, textile, health and nutrition [209]. Improved techniques, leading to enhanced understanding of various metabolic pathways and development of different tailormade enzymes and strains, has made possible for the use of CAZymes in production of different value-added products [210].
For industrial application of CAZyme, they should have the properties of better stability under harsh conditions of fermentation environment while efficiently converting the released sugar to final end product. Stain improvement and enzyme engineering strategies such as random mutation and site directed mutagenesis, guided by bioinformatics tools, have been used to attune the properties of the CAZymes [211]. For instance, the thermostability of the endoglucanase Cel8A [95,212,213], exoglucanase Cel48S [214] and β Glucosidase A [96] from Clostridium thermocellum increased upto14-fold when compared with the wild type strain with increased yield in soluble form of glucose using these methods. The substitutive approach for tailor-made cocktails formulations of xylanses and pectinase increased saccharification in bagasse suggesting another strategy for optimization of CAZymes for industrial application [215]. Almost all the commercially available cellulase from dominating enzyme suppliers are the products of random mutagenesis generated derivative of mutant strain RUT-30 of Trichoderma reesei which has excessive cellulase producing properties [216]. Important organisms and the corresponding enzymes system that utilize cheap lignocellulosic substrate to obtain the value-added products has been summarized in Table 2. Mainly the CAZymes themselves are used as commercial products to be used in various industries from food to textile [126,224,227,231].
Table 2.
List of value-added Bio-products generated using CAZymes and their industrial applications.
| Value-added Bio-Products | Lignocellulosic Biomass | Required Enzymes | Organisms | Applications | References | |
|---|---|---|---|---|---|---|
| Biofuels | Biohydrogen | Cellulose | Endoglucanases, Exoglucanases, β-glucosidases | Clostridium termitidis | Automobile industries, electricity generation | [132,217] |
| Biobutanol | Corn stover | Cellulase, Xylanase | Clostridium acetobutylicum | Biofuel and petroleum industries | [218] | |
| Bioethanol | Rice straw, sugar cane bagasse, dried water hyacinth leaves | Cellulase | T. reesei, A. niger | Biofuel, additive in gasoline | [219] | |
| Organic acids | Acetic acid | Crystalline cellulose, corn stover | Cellulases, Hemicellulases | C. thermocellum, C. thermoaceticum | Chemical industries, food industries | [220,221] |
| Citric acid | ||||||
| Gluconic acid | ||||||
| Formic Acid | ||||||
| Levulinic acid | ||||||
| Lactic acid | ||||||
| Sugar alcohol | Arabitol | Glucose, cellulose, wood chips, xylose | Cellulases, Hemicellulases, | Candida guillermondii | Artificial sweetner, prebiotic in food industries | [222] [223], |
| Galactitol | ||||||
| Mannitol | ||||||
| Sorbitol | ||||||
| Xylitol | ||||||
| Secondary metabolites | Lovastatin | Sweet sorghum fiber, wheat bran, corn fiber | Aspergillus terreus | Used in pharmaceutical industries | [224,225] | |
| Cyclosporin | ||||||
| Mycophenolic acid | ||||||
| Other value added products | Maltose | Wheat bran | Amylase | Penicillium decumbens | Sweetener, intravenous sugar supplement | [226,227] |
| Hydroxymethyl furfural (HMF) | Rice straw, sugar cane bagasse, dried water hyacinth leaves | Cellulase | T. reesei, A. niger | Intermediate for different chemicals and products used in pharmaceutical formulas, agriculture, daily products, food additives, and bioenergy | [219,228] | |
| Aromatic aldehydes | Barley straw, grape stalks | Laccases | Trametes versicolor | Flavors, fragrances, and pharmaceutical precursors | [229,230,231] | |
| Chitosan | Grapes pomaces | Pectinase | Aspergillus awamori | Applications in agriculture, medicine, and cosmetics | [232,233] | |
| Xylo oligo-saccharides | Xylose, xylan, cellobiose, glucose | Xylanase | Pseudozyma brasiliensis | As prebiotics, Biofuel production, manufacture of bread, food, and drinks, production of animal feed, paper manufacturing, xylitol production, textile industry, and others | [126,230] | |
| SCP | Cellulose, hemicellulose | Flavobacterium sp., Cellulomonas sp. | Food and feed | [234] | ||
| Xanthan/ EPS | Rice hull, wheat whey | Xanthomonas campestris, Schizophyllum commune | Food, petroleum, and pharmaceuticals | [235,236] | ||
| Vanillin | Lignin | Lignin peroxidase, Manganese peroxidase, Laccase | Pleurotus eryngii, Phanerochaete chrysosporium | Flavoring agents | [237,238,239] | |
6. Future perspective
Discovery of more efficient CAZyme systems driven by studies on previously unexplored environments such as gut microflora of various plant eating animals, termites etc. using more sophisticated omic techniques along with improved engineering techniques will lead to formulation of effective enzymes. This will expand the pool of enzymes available for synergistic enzyme formulations [182]. Development of new high-throughput screening techniques for CAZyme assessment for in vivo optimization of pathway design and in vitro enzyme regio- and stereo- selectivity is the current limitation [10]. Choice of host system along with optimized expression and flux system are crucial in cost- effective production of the CAZymes [182]. Further, use of chemical additives for increased efficiency, binding domains for easy extractions etc will reduce the cost of the enzyme for its application in biorefineries [240,241].
7. Conclusion
The CAZymes are critical from both economic and environmental viewpoint in that they contribute in bioconversion of recalcitrant waste to biofuels and products with added value. The currently available CAZymes have their own set back with production cost being a major driving factor which limits their widespread use as the replacement of the fossil fuels for generation of environment friendly cheap biofuels. The emerging techniques in molecular biology along with introduction of computational biology has led to discovery of various new CAZymes that may be modified further for their industrial application. This review covers various aspects of identifying novel CAZymes and their engineering for boosted performance along with their application in bio-refineries. The profuse techniques discussed here have their own advantages and disadvantages to be considered for their application in the study of the CAZymes. A thrust has been seen in the existing knowledge of various CAZymes especially due to advancement on computation biology, bioinformatics and sequencing techniques. Expectations regarding uncovering and designing tailor made novel CAZymes holds high value in the near future. However, screening is still a major cap in all the different approaches and new technologies needs to be introduced for easy and fast screening of the large data in a cost-effective manner. The multidisciplinary approaches for development of integrated methods holds a promising future in this research field.
Author contributions statement
AKV and DC conceived of the presented idea. AKV, DC and AV discussed the every topics and wrote the review. AKV provided critical feedback, supervised the study and helped in overall shape the review.
Declaration of Competing Interest
The authors declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgement
The authors would like to thank the Department of Microbiology, Sikkim University, for providing the computational infrastructure and central library facilities for procuring references and plagiarism analysis (URKUND: Plagiarism Detection Software). The publication of this article has been facilitated by the grant of a full waiver of publishing fees by Biotechnology Report.
References
- 1.Kamm B., Kamm M. Principles of biorefineries. Appl. Microbiol. Biotechnol. 2010;64:137–145. doi: 10.1007/s00253-003-1537-7. [DOI] [PubMed] [Google Scholar]
- 2.Nations U. World population prospects: The 2015 revision. United Nations Econ. Soc. Aff. 2015;33:1–66. [Google Scholar]
- 3.Bogomolov A., Lepri B., Larcher R., Antonelli F., Pianesi F., Pentland A. Energy consumption prediction using people dynamics derived from cellular network data. EPJ Data Sci. 2016;5:13. doi: 10.1140/epjds/s13688-016-0075-3. [DOI] [Google Scholar]
- 4.Luft G. Dependence on Middle East energy and its impact on global security. In: Stec S., Baraj B., editors. Energy and Environmental Challenges to Security. NATO Science for Peace and Security Series C: Environmental Security. Springer; Dordrecht: 2009. [DOI] [Google Scholar]
- 5.Atabani A.E., Silitonga A.S., Badruddin I.A., Mahlia T.M.I., Masjuki H.H., Mekhilef S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sust. Energ Rev. 2012;16:2070–2093. doi: 10.1016/j.rser.2012.01.003. [DOI] [Google Scholar]
- 6.Zabed H., Sahu J.N., Boyce A.N., Faruq G. Fuel ethanol production from lignocellulosic biomass: an overview on feedstocks and technological approaches. Renew. Sust. Energ Rev. 2016;66:751–774. doi: 10.1016/j.rser.2016.08.038. [DOI] [Google Scholar]
- 7.Fernando S., Adhikari S., Chandrapal C., Murali N. Biorefineries: current status, challenges, and future direction. Energ Fuel. 2006;20:1727–1737. [Google Scholar]
- 8.Carvalheiro F., Duarte L.C., Gírio F.M. Hemicellulose biorefineries: a review on biomass pretreatments. J. Sci. Ind. Res. 2008:849–864. [Google Scholar]
- 9.Pauly M., Keegstra K. Cell‐wall carbohydrates and their modification as a resource for biofuels. Plant J. 2008;54:559–568. doi: 10.1111/j.1365-313x.2008.03463.x. [DOI] [PubMed] [Google Scholar]
- 10.André I., Potocki-Véronèse G., Barbe S., Moulis C., Remaud-Siméon M. CAZyme discovery and design for sweet dreams. Curr. Opin. Chem. Biol. 2014;19:17–24. doi: 10.1016/j.cbpa.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Henrissat B. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 1991;280:309–316. doi: 10.1042/bj2800309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park Y.J., Jeong Y.U., Kong W.S. Genome sequencing and carbohydrate-active enzyme (CAZyme) repertoire of the white rot fungus Flammulinaelastica. Int. J. Mol. Sci. 2018;19:2379. doi: 10.3390/ijms19082379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lombard V., GolacondaRamulu H., Drula E., Coutinho P.M., Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42:D490–D495. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paulino B.N., Pessôa M.G., Molina G., Neto A.A.K., Oliveira J.V., Mano M.C., Pastore G.M. Biotechnological production of value-added compounds by ustilaginomycetous yeasts. Appl. Microbiol. Biotechnol. 2017;101:7789–7809. doi: 10.1007/s00253-017-8516-x. [DOI] [PubMed] [Google Scholar]
- 15.Bredon M., Dittmer J., Noël C., Moumen B., Bouchon D. Lignocellulose degradation at the holobiont level: teamwork in a keystone soil invertebrate. Microbiome. 2018;6:162. doi: 10.1186/s40168-018-0536-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kunath B.J., Bremges A., Weimann A., McHardy A.C., Pope P.B. Protein-Carbohydrate Interactions. Humana Press; New York, NY: 2017. Metagenomics and CAZyme discovery; pp. 255–277. [Google Scholar]
- 17.Merino S.T., Cherry J. Biofuels. Springer; Berlin, Heidelberg: 2007. Progress and challenges in enzyme development for biomass utilization; pp. 95–120. [DOI] [PubMed] [Google Scholar]
- 18.Kuuskeri J., Häkkinen M., Laine P., Smolander O.P., Tamene F., Miettinen S. Time-scale dynamics of proteome and transcriptome of the white-rot fungus Phlebia radiata: growth on spruce wood and decay effect on lignocellulose. Biotechnol. Biofuels. 2016;9:192. doi: 10.1186/s13068-016-0608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.López-Mondéjar R., Zühlke D., Becher D., Riedel K., Baldrian P. Cellulose and hemicellulose decomposition by forest soil bacteria proceeds by the action of structurally variable enzymatic systems. Sci. Rep. 2016;6:25279. doi: 10.1038/srep25279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C., Dong D., Wang H., Müller K., Qin Y., Wang H., Wu W. Metagenomic analysis of microbial consortia enriched from compost: new insights into the role of Actinobacteria in lignocellulose decomposition. Biotechnol. Biofuels. 2016;9:22. doi: 10.1186/s13068-016-0440-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosnow J.J., Anderson L.N., Nair R.N., Baker E.S., Wright A.T. Profiling microbial lignocellulose degradation and utilization by emergent omics technologies. Crit. Rev. Biotechnol. 2017;37(5):626–640. doi: 10.1080/07388551.2016.1209158. [DOI] [PubMed] [Google Scholar]
- 22.Amann R.I., Ludwig W., Schleifer K.H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Mol. Biol. Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raupach M.J., Amann R., Wheeler Q.D., Roos C. The application of “-omics” technologies for the classification and identification of animals. Org. Divers. Evol. 2016;16:1–12. [Google Scholar]
- 24.Jouzani G.S., Sharafi R. Biogas. Springer, Cham; 2018. New “Omics” technologies and biogas production; pp. 419–436. [Google Scholar]
- 25.Dubey S.K., Meena R.K., Sao S., Patel J., Thakur S., Shukla P. Isolation and characterization of cellulose degrading bacteria from biogas slurry and their RAPD profiling. Curr. Res. Microbiol. Biotechnol. 2014;2:416–421. [Google Scholar]
- 26.Delbès C., Moletta R., Godon J.J. Monitoring of activity dynamics of an anaerobic digester bacterial community using 16S rRNA polymerase chain reaction–single‐strand conformation polymorphism analysis. Environ. Microbiol. 2000;2:506–515. doi: 10.1046/j.1462-2920.2000.00132.x. [DOI] [PubMed] [Google Scholar]
- 27.Kröber M., Bekel T., Diaz N.N., Goesmann A., Jaenicke S., Krause L. Phylogenetic characterization of a biogas plant microbial community integrating clone library 16S-rDNA sequences and metagenome sequence data obtained by 454-pyrosequencing. J. Biotechnol. 2009;142:38–49. doi: 10.1016/j.jbiotec.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Nazir A. Review on metagenomics and its applications. Imp. J. Intersd. Res. 2016;2:10. [Google Scholar]
- 29.Gilbert J.A., Dupont C.L. Microbial metagenomics: beyond the genome. Annu. Rev. Mar. Sci. 2011;3:347–371. doi: 10.1146/annurev-marine-120709-142811. [DOI] [PubMed] [Google Scholar]
- 30.Chen Z., Pace U., Heldman J., Shapira A., Lancet D. Isolated frog olfactory cilia: a preparation of dendritic membranes from chemosensory neurons. J. Neurosci. 1986;6:2146–2154. doi: 10.1523/jneurosci.06-08-02146.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Connaughton S., Collins G., O’Flaherty V. Development of microbial community structure and actvity in a high-rate anaerobic bioreactor at 18֯ C. Water Res. 2006;40:1009–1017. doi: 10.1016/j.watres.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 32.Nettmann E., Bergmann I., Pramschüfer S., Mundt K., Plogsties V., Herrmann C., Klocke M. Polyphasic analyses of methanogenic archaeal communities in agricultural biogas plants. Appl. Environ. Microbiol. 2010;76:2540–2548. doi: 10.1128/aem.01423-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deusch S., Tilocca B., Camarinha-Silva A., Seifert J. News in livestock research—use of Omics-technologies to study the microbiota in the gastrointestinal tract of farm animals. Comput. Struct. Biotechnol. J. 2015;13:55–63. doi: 10.1016/j.csbj.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J., Kang L., Liu Z., Yuan S. Gene cloning, heterologous expression and characterization of a Coprinopsiscinerea endo-β-1, 3 (4)-glucanase. Fungal Biol. 2017;121:61–68. doi: 10.1016/j.funbio.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Idalia V.M.N., Bernardo F. Recent Advances on Physiology, Pathogenesis and Biotechnological Applications. In Tech Open; Rijeka, Croatia: 2017. Escherichia coli as a model organism and its application in biotechnology; pp. 253–274. [Google Scholar]
- 36.Aakvik T., Degnes K.F., Dahlsrud R., Schmidt F., Dam R., Yu L. A plasmid RK2-based broad-host-range cloning vector useful for transfer of metagenomic libraries to a variety of bacterial species. FEMS Microbiol. Lett. 2009;296(2):149–158. doi: 10.1111/j.1574-6968.2009.01639.x. [DOI] [PubMed] [Google Scholar]
- 37.Vieira Gomes A.M., Souza Carmo T., Silva Carvalho L., Mendonça Bahia F., Parachin N.S. Comparison of yeasts as hosts for recombinant protein production. Microorganisms. 2018;6(2):38. doi: 10.3390/microorganisms6020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu Z., Hopwood D.A., Khosla C. Directed transfer of large DNA fragments between Streptomyces species. Appl. Environ. Microbiol. 2000;66(5):2274–2277. doi: 10.1128/aem.66.5.2274-2277.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattanovich D., Branduardi P., Dato L., Gasser B., Sauer M., Porro D. Recombinant Gene Expression. Humana Press; Totowa, NJ: 2012. Recombinant protein production in yeasts; pp. 329–358. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen J. Production of biopharmaceutical proteins by yeast: advances through metabolic engineering. Bioengineered. 2013;4(4):207–211. doi: 10.4161/bioe.22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharpton T.J. An introduction to the analysis of shotgun metagenomic data. Front. Plant Sci. 2014;5:209. doi: 10.3389/fpls.2014.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laehnemann D., Borkhardt A., McHardy A.C. Denoising DNA deep sequencing data—high-throughput sequencing errors and their correction. Brief Bioinform. 2016;17:154–179. doi: 10.1093/bib/bbv029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rhoads A., Au K.F. PacBio sequencing and its applications. Genom Proteom. Bioinf. 2015;13(5):278–289. doi: 10.1016/j.gpb.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levene M.J., Korlach J., Turner S.W., Foquet M., Craighead H.G., Webb W.W. Zero-mode waveguides for single-molecule analysis at high concentrations. Science. 2003;299:682–686. doi: 10.1126/science.1079700. [DOI] [PubMed] [Google Scholar]
- 45.Peng Y., Leung H.C., Yiu S.M., Chin F.Y. IDBA-UD: a de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics. 2012;28:1420–1428. doi: 10.1093/bioinformatics/bts174. [DOI] [PubMed] [Google Scholar]
- 46.Li D., Liu C.M., Luo R., Sadakane K., Lam T.W. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics. 2015;31:1674–1676. doi: 10.1093/bioinformatics/btv033. [DOI] [PubMed] [Google Scholar]
- 47.Nurk D., Meleshko A., Korobeynikov P.A. Pevzner. metaSPAdes: a new versatile metagenomic assembler. Genome Res. 2017;27:824–834. doi: 10.1101/gr.213959.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Filiatrault M.J. Progress in prokaryotic transcriptomics. Curr. Opin. Microbiol. 2011;14:579–586. doi: 10.1016/j.mib.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 49.Zakrzewski M., Goesmann A., Jaenicke S., Jünemann S., Eikmeyer F., Szczepanowski R. Profiling of the metabolically active community from a production-scale biogas plant by means of high-throughput metatranscriptome sequencing. J. Biotechnol. 2012;158:248–258. doi: 10.1016/j.jbiotec.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 50.Raghavachari N. Lipoproteins and Cardiovascular Disease. Humana Press; Totowa, NJ: 2013. Microarray technology: basic methodology and application in clinical research for biomarker discovery in vascular diseases; pp. 47–84. [DOI] [PubMed] [Google Scholar]
- 51.Stark L., Giersch T., Wünschiers R. Efficiency of RNA extraction from selected bacteria in the context of biogas production and metatranscriptomics. Anaerobe. 2014;29:85–90. doi: 10.1016/j.anaerobe.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 52.Liu F., Rotaru A.E., Shrestha P.M., Malvankar N.S., Nevin K.P., Lovley D.R. Promoting direct interspecies electron transfer with activated carbon. Energy Environ. Sci. 2012;5(10):8982–8989. [Google Scholar]
- 53.Morita M., Malvankar N.S., Franks A.E., Summers Z.M., Giloteaux L., Rotaru A.E. Potential for direct interspecies electron transfer in methanogenic wastewater digester aggregates. MBio. 2011;2(4) doi: 10.1128/mBio.00159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mello B.L., Alessi A.M., Riaño-Pachón D.M., Deazevedo E.R., Guimarães F.E., Santo M.C.E. Targeted metatranscriptomics of compost-derived consortia reveals a GH11 exerting an unusual exo-1, 4-β-xylanase activity. Biotechnol. Biofuels. 2017;10:254. doi: 10.1186/s13068-017-0944-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He B., Jin S., Cao J., Mi L., Wang J. Metatranscriptomics of the Hu sheep rumen microbiome reveals novel cellulases. Biotechnol. Biofuels. 2019;12:153. doi: 10.1186/s13068-019-1498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dai X., Tian Y., Li J., Su X., Wang X., Zhao S. Metatranscriptomic analyses of plant cell wall polysaccharide degradation by microorganisms in the cow rumen. Appl. Environ. Microbiol. 2015;81:1375–1386. doi: 10.1128/aem.03682-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vanwonterghem I., Jensen P.D., Ho D.P., Batstone D.J., Tyson G.W. Linking microbial community structure, interactions and function in anaerobic digesters using new molecular techniques. Curr. Opin. Biotech. 2014;27:55–64. doi: 10.1016/j.copbio.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 58.Tisdale G. 2016. Protein Found at the Scene of the Crime: The Potential for Using Proteomics for Identification. Available at SSRN 2907742. [Google Scholar]
- 59.Zhang Y., Fonslow B.R., Shan B., Baek M.C., Yates J.R., III Protein analysis by shotgun/bottom-up proteomics. Chem. Rev. 2013;113(4):2343–2394. doi: 10.1021/cr3003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kelleher N.L. 2004. Peer reviewed: Top-down proteomics. (P) [DOI] [Google Scholar]
- 61.Heyer R., Schallert K., Zoun R., Becher B., Saake G., Benndorf D. Challenges and perspectives of metaproteomic data analysis. J. Biotechnol. 2017;261:24–36. doi: 10.1016/j.jbiotec.2017.06.1201. [DOI] [PubMed] [Google Scholar]
- 62.Hagen L.H., Brooke C.G., Shaw C., Norbeck A.D., Piao H., Arntzen M.Ø. Proteome specialization of anaerobic fungi during ruminal degradation of recalcitrant plant fiber. Bio. Rxiv. 2020 doi: 10.1101/2020.01.16.907998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hanreich A., Schimpf U., Zakrzewski M., Schlüter A., Benndorf D., Heyer R. Metagenome and metaproteome analyses of microbial communities in mesophilic biogas-producing anaerobic batch fermentations indicate concerted plant carbohydrate degradation. Syst. Appl. Microbiol. 2013;36:330–338. doi: 10.1016/j.syapm.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 64.Pathak P., Bhardwaj N.K., Singh A.K. Production of crude cellulase and xylanase from Trichoderma harzianum PPDDN10 NFCCI-2925 and its application in photocopier waste paper recycling. Appl. Biochem. Biotechnol. 2014;172:3776–3797. doi: 10.1007/s12010-014-0758-9. [DOI] [PubMed] [Google Scholar]
- 65.Sindhu R., Binod P., Madhavan A., Beevi U.S., Mathew A.K., Abraham A. Molecular improvements in microbial α-amylases for enhanced stability and catalytic efficiency. Bioresour. Technol. 2017;245:1740–1748. doi: 10.3389/fpls.2014.00209. [DOI] [PubMed] [Google Scholar]
- 66.Colin Y., Kintses B., Gielen F., Miton C.M., Fischer G., Mohamed M.F. Ultrahigh-throughput discovery of promiscuous enzymes by picodroplet functional metagenomics. Nat. Commun. 2015;6:1–12. doi: 10.1038/ncomms10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mak W.S., Tran S., Marcheschi R., Bertolani S., Thompson J., Baker D. Integrative genomic mining for enzyme function to enable engineering of a non-natural biosynthetic pathway. Nat. Commun. 2015;6:1–10. doi: 10.1038/ncomms10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sévin D.C., Fuhrer T., Zamboni N., Sauer U. Nontargeted in vitro metabolomics for high-throughput identification of novel enzymes in Escherichia coli. Nat. Methods. 2017;14:187. doi: 10.1038/nmeth.4103. [DOI] [PubMed] [Google Scholar]
- 69.Chen R., Dou J. Biofuels and bio-based chemicals from lignocellulose: metabolic engineering strategies in strain development. Biotechnol. Lett. 2016;38:213–221. doi: 10.1007/s10529-015-1976-0. [DOI] [PubMed] [Google Scholar]
- 70.Svensson B., Sogaard M. Protein engineering of amylases. Biochem. Soc. Trans. 1992;20:34–42. doi: 10.1042/bst0200034. [DOI] [PubMed] [Google Scholar]
- 71.Chuankhayan P., Hsieh C.Y., Huang Y.C., Hsieh Y.Y., Guan H.H., Hsieh Y.C. Crystal structures of Aspergillus japonicus fructosyltransferase complex with donor/acceptor substrates reveal complete subsites in the active site for catalysis. J. Biol. Chem. 2010;285(30):23251–23264. doi: 10.1074/jbc.M110.113027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parra-Saldivar R., Ramirez-Mendoza R.A., Sharma A., Oza G., Zavala-Yoe R., Iqbal H.M. Biomass, Biofuels. Biochemicals Elsevier; 2020. Robust enzymes designing for efficient biocatalysis; pp. 49–63. [Google Scholar]
- 73.Li C., Zhang R., Wang J., Wilson L.M., Yan Y. Protein engineering for improving and diversifying natural product biosynthesis. Trends Biotechnol. 2020 doi: 10.1016/j.tibtech.2019.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen R. A general strategy for enzyme engineering. Trends Biotechnol. 1999;17:344–345. doi: 10.1016/s0167-7799(99)01324-4. [DOI] [PubMed] [Google Scholar]
- 75.Cherry J.R., Fidantsef A.L. Directed evolution of industrial enzymes: an update. Curr. Opin. Biotechnol. 2003;14:438–443. doi: 10.1016/s0958-1669(03)00099-5. [DOI] [PubMed] [Google Scholar]
- 76.Wohlfahrt G., Pellikka T., Boer H., Teeri T.T., Koivula A. Probing pH-dependent functional elements in proteins: modification of carboxylic acid pairs in cellobiohydrolase Cel6A. Biochemistry. 2003;42:10095–10103. doi: 10.1021/bi034954o. [DOI] [PubMed] [Google Scholar]
- 77.Arnold F.H. Design by directed evolution. Acc. Chem. Res. 1998;31:125–131. doi: 10.1021/ar960017f. [DOI] [Google Scholar]
- 78.McCarthy J.K., Uzelac A., Davis D.F., Eveleigh D.E. Improved catalytic efficiency and active site modification of 1, 4-β-D-glucan glucohydrolase a from Thermotoga neapolitana by directed evolution. J. Biol. Chem. 2004;279:11495–11502. doi: 10.1074/jbc.m305642200. [DOI] [PubMed] [Google Scholar]
- 79.Arnold F.H., Volkov A.A. Directed evolution of biocatalysts. Curr. Opin. Chem. Biol. 1999;3:54–59. doi: 10.1016/s1367-5931(99)80010-6. [DOI] [PubMed] [Google Scholar]
- 80.Stemmer W.P. Rapid evolution of a protein in vitro by DNA shuffling. Nature. 1994;370:389–391. doi: 10.1038/370389a0. [DOI] [PubMed] [Google Scholar]
- 81.Zhao H., Giver L., Shao Z., Affholter J.A., Arnold F.H. Molecular evolution by staggered extension process (StEP) in vitro recombination. Nat. Biotechnol. 1998;16:258–261. doi: 10.1038/nbt0398-258. [DOI] [PubMed] [Google Scholar]
- 82.Shao Z., Zhao H., Giver L., Arnold F.H. Random-priming in vitro recombination: an effective tool for directed evolution. Nucleic Acids Res. 1998;26:681–683. doi: 10.1093/nar/26.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Crameri A., Raillard S.A., Bermudez E., Stemmer W.P. DNA shuffling of a family of genes from diverse species accelerates directed evolution. Nature. 1998;391:288–291. doi: 10.1038/34663. [DOI] [PubMed] [Google Scholar]
- 84.Kumamaru T., Suenaga H., Mitsuoka M., Watanabe T., Furukawa K. Enhanced degradation of polychlorinated biphenyls by directed evolution of biphenyl dioxygenase. Nat. Biotechno. 1998;16:663–666. doi: 10.1038/nbt0798-663. [DOI] [PubMed] [Google Scholar]
- 85.Reetz M.T., Zonta A., Schimossek K., Jaeger K.E., Liebeton K. Creation of enantioselective biocatalysts for organic chemistry by in vitro evolution. Angew. Chem. Int. Ed. Engl. 1997;36:2830–2832. [Google Scholar]
- 86.Bornscheuer U.T., Altenbuchner J., Meyer H.H. Directed evolution of an esterase for the stereoselective resolution of a key intermediate in the synthesis of epothilones. Biotechnol. Bioeng. 1998;58:554–559. doi: 10.1002/(sici)1097-0290(19980605)58:5%3C554::aid-bit12%3E3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 87.Proba K., WoÈrn A., Honegger A., PluÈckthun A. Antibody scFv fragments without disulfide bonds, made by molecular evolution. J. Mol. Biol. 1998;275:245–253. doi: 10.1006/jmbi.1997.1457. [DOI] [PubMed] [Google Scholar]
- 88.Lin L., Meng X., Liu P., Hong Y., Wu G., Huang X. Improved catalytic efficiency of Endo-β-1, 4-glucanase from Bacillus subtilis BME-15 by directed evolution. Appl. Microbiol. Biotechnol. 2009;82:671. doi: 10.1007/s00253-008-1789-3. [DOI] [PubMed] [Google Scholar]
- 89.Nakazawa H., Okada K., Onodera T., Ogasawara W., Okada H., Morikawa Y. Directed evolution of endoglucanase III (Cel12A) from Trichoderma reesei. Appl. Microbiol. Biotechnol. 2009;83:649–657. doi: 10.1007/s00253-009-1901-3. [DOI] [PubMed] [Google Scholar]
- 90.Adesioye F.A., Makhalanyane T.P., Vikram S., Sewell B.T., Schubert W.D., Cowan D.A. Structural characterization and directed evolution of a novel acetyl xylan esterase reveals thermostability determinants of the carbohydrate esterase 7 family. Appl Environ Microbial. 2018;84 doi: 10.1128/aem.02695-17. e02695-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.J. S. Okkels. Method for preparing polypeptide variants. US patent (1997): 97-09664.
- 92.C. C. Fuglsang, J. S. Okkels, D. A. Petersen, S. A. Patkar, M. Thellersen, A. Svendsen. U.S. Patent No. 7,157,262. Washington, DC: U.S. Patent and Trademark Office (2007).
- 93.Kim Y.W., Lee S.S., Warren R.A.J., Withers S.G. Directed evolution of a glycosynthase from Agrobacterium sp. Increases its catalytic activity dramatically and expands its substrate repertoire. J. Biol. Chem. 2004;279:42787–42793. doi: 10.1074/jbc.m406890200. [DOI] [PubMed] [Google Scholar]
- 94.Bulter T., Alcalde M., Sieber V., Meinhold P., Schlachtbauer C., Arnold F.H. Functional expression of a fungal laccase in Saccharomyces cerevisiae by directed evolution. Appl. Environ. Microbiol. 2003;69:987–995. doi: 10.1128/aem.69.2.987-995.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Anbar M., Gul O., Lamed R., Sezerman U.O., Bayer E.A. Improved thermostability of Clostridium thermocellum endoglucanase Cel8A by using consensus-guided mutagenesis. Appl. Environ. Microbiol. 2012;78:3458–3464. doi: 10.1128/AEM.07985-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoav S., Stern J., Salama-Alber O., Frolow F., Anbar M., Karpol A. Directed evolution of Clostridium thermocellum β-Glucosidase a towards enhanced thermostability. Int. J. Mol. Sci. 2019;20:4701. doi: 10.3390/ijms20194701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang T., Liu X., Yu Q., Zhang X., Qu Y., Gao P., Wang T. Directed evolution for engineering pH profile of endoglucanase III from Trichoderma reesei. Biomol. Eng. 2005;22:89–94. doi: 10.1016/j.bioeng.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 98.Kauffmann I., Schmidt-Dannert C. Conversion of Bacillus thermocatenulatus lipase into an efficient phospholipase with increased activity towards long-chain fatty acyl substrates by directed evolution and rational design. Protein Eng. 2001;14:919–928. doi: 10.1093/protein/14.11.919. [DOI] [PubMed] [Google Scholar]
- 99.Wilson D.B. Cellulases and biofuels. Curr. Opin. Biotech. 2009;20:295–299. doi: 10.1016/j.copbio.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 100.Matsumura I. 2000. IBC’s Fifth Annual World Congress on Enzyme Technologies. Las Vegas. [Google Scholar]
- 101.Chen R. Enzyme engineering: rational redesign versus directed evolution. Trends Biotechnol. 2001;19:13–14. doi: 10.1016/s0167-7799(00)01522-5. [DOI] [PubMed] [Google Scholar]
- 102.Horsman G.P., Liu A.M., Henke E., Bornscheuer U.T., Kazlauskas R.J. Mutations in distant residues moderately increase the enantioselectivity of Pseudomonas fluorescens esterase towards methyl 3bromo‐2‐methylpropanoate and ethyl 3phenylbutyrate. Chem. Eur. J. 2003;9:1933–1939. doi: 10.1002/chem.200204551. [DOI] [PubMed] [Google Scholar]
- 103.Chen R., Greer A., Dean A.M. Redesigning secondary structure to invert coenzyme specificity in isopropylmalate dehydrogenase. Proc. Natl. Acad. Sci. 1996;93:12171–12176. doi: 10.1073/pnas.93.22.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kirst H.A., Yeh W.K., editors. Enzyme Technologies for Pharmaceutical and Biotechnological Applications. CRC Press; 2001. [Google Scholar]
- 105.Zhang J., Shi H., Xu L., Zhu X., Li X. Site-directed mutagenesis of a hyperthermophilic endoglucanase Cel12B from Thermotoga maritima based on rational design. PLoS One. 2015;10 doi: 10.1371/journal.pone.0133824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Deng Z., Yang H., Shin H.D., Li J., Liu L. Structure-based rational design and introduction of arginines on the surface of an alkaline α-amylase from Alkalimonas amylolytica for improved thermostability. Appl. Microbiol. Biotechnol. 2014;98:8937–8945. doi: 10.1007/s00253-014-5790-8. [DOI] [PubMed] [Google Scholar]
- 107.Rasekh B., Khajeh K., Ranjbar B., Mollania N., Almasinia B., Tirandaz H. Protein engineering of laccase to enhance its activity and stability in the presence of organic solvents. Eng. Life Sci. 2014;14:442–448. [Google Scholar]
- 108.Escovar-Kousen J.M., Wilson D., Irwin D. Integration of computer modeling and initial studies of site-directed mutagenesis to improve cellulase activity on Cel9A from Thermobifidafusca. Appl. Biochem. Biotech. 2004;113:287. doi: 10.1385/abab:113:1-3:287. [DOI] [PubMed] [Google Scholar]
- 109.Liu F.F., Wang T., Dong X.Y., Sun Y. Rational design of affinity peptide ligand by flexible docking simulation. J. Chromatogr. A. 2007;1146:41–50. doi: 10.1016/j.chroma.2007.01.130. [DOI] [PubMed] [Google Scholar]
- 110.Samaei‐Daryan S., Goliaei B., Ebrahim‐Habibi A. Characterization of surface binding sites in glycoside hydrolases: a computational study. J. Mol. Recognit. 2017;30:e2624. doi: 10.1002/jmr.2624. [DOI] [PubMed] [Google Scholar]
- 111.Lyu Q., Zhang K., Zhu Q., Li Z., Liu Y., E Structural and biochemical characterization of a multidomain alginate lyase reveals a novel role of CBM32 in CAZymes. BBA-Gen Subjects. 2018;1862:1862–1869. doi: 10.1016/j.bbagen.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 112.Durao P., Bento I., Fernandes A.T., Melo E.P., Lindley P.F., Martins L.O. Perturbations of the T1 copper site in the CotA laccase from Bacillus subtilis: structural, biochemical, enzymatic and stability studies. J. Biol. Inorg. Chem. 2006;11:514. doi: 10.1007/s00775-006-0102-0. [DOI] [PubMed] [Google Scholar]
- 113.Ottosson J., Rotticci‐Mulder J.C., Rotticci D., Hult K. Rational design of enantioselective enzymes requires considerations of entropy. Protein Sci. 2001;10:1769–1774. doi: 10.1110/ps.13501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Berman H.M., Westbrook J., Feng Z., Gilliland G., Bhat T.N., Weissig H., Shindyalov I.N., Bourne P.E. The protein data bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Georgiou G., DeWitt N. Enzyme beauty. Nat. Biotechnol. 1999;17:1161–1162. doi: 10.1038/70701. [DOI] [PubMed] [Google Scholar]
- 116.Altamirano M.M., Blackburn J.M., Aguayo C., Fersht A.R. Directed evolution of new catalytic activity using the α/β-barrel scaffold. Nature. 2000;403:617–622. doi: 10.1038/35001001. [DOI] [PubMed] [Google Scholar]
- 117.Villaverde A. 2010. Nanotechnology, Bionanotechnology and Microbial Cell Factories; p. 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Falconnier B., Lapierre C., Lesage-Meessen L., Yonnet G., Brunerie P., Colonna-Ceccaldi B. Vanillin as a product of ferulic acid biotransformation by the white-rot fungus Pycnoporus cinnabarinus I-937: identification of metabolic pathways. J. Biotechnol. 1994;37:123–132. [Google Scholar]
- 119.Baldrian P., Valášková V. Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol. Rev. 2008;32:501–521. doi: 10.1111/j.1574-6976.2008.00106.x. [DOI] [PubMed] [Google Scholar]
- 120.Cao W., Crawford D.L. Carbon nutrition and hydrolytic and cellulolytic activities in the ectomycorrhizal fungus Pisolithus tinctorius. Can. J. Microbiol. 1993;39(5):529–535. [Google Scholar]
- 121.Thygesen A., Thomsen A.B., Schmidt A.S., Jørgensen H., Ahring B.K., Olsson L. Production of cellulose and hemicellulose-degrading enzymes by filamentous fungi cultivated on wet-oxidised wheat straw. Enzyme. Microb. Tech. 2003;32:606–615. [Google Scholar]
- 122.Crawford D.L., Crawford R.L. Microbial degradation of lignin. Enzyme Microb. Technol. 1980;2:11–22. [Google Scholar]
- 123.Eslyn W.E., Kirk T.K., Effland M.J. Changes in the chemical composition of wood caused by six soft-rot fungi. Phytopathology. 1975;65:473–476. [Google Scholar]
- 124.Kumar V., Satyanarayana T. Generation of xylooligosaccharides from microwave irradiated agroresidues using recombinant thermo-alkali-stable endoxylanase of the polyextremophilic bacterium Bacillus halodurans expressed in Pichia pastoris. Bioresour. Technol. 2015;179:382–389. doi: 10.1016/j.biortech.2014.12.049. [DOI] [PubMed] [Google Scholar]
- 125.Mhetras N., Liddell S., Gokhale D. Purification and characterization of an extracellular β-xylosidase from Pseudozyma hubeiensis NCIM 3574 (PhXyl), an unexplored yeast. Amb. Exp. 2016;6:73. doi: 10.1186/s13568-016-0243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.KaupertNeto A.A., Borin G.P., Goldman G.H., Damásio A.R.D.L., Oliveira J.V.D.C. Insights into the plant polysaccharide degradation potential of the xylanolytic yeast Pseudozyma brasiliensis. FEMS Yeast Res. 2016;16 doi: 10.1093/femsyr/fov117. [DOI] [PubMed] [Google Scholar]
- 127.Watanabe T., Suzuki K., Sato I., Morita T., Koike H., Shinozaki Y., Ueda H., Koitabashi M., Kitamoto H. Simultaneous bioethanol distillery wastewater treatment and xylanase production by the phyllosphere yeast Pseudozyma antarctica GB-4(0) AMB. Exp. 2015;5:1. doi: 10.1186/s13568-015-0121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hirose E., Izawa N., Adachi J., Mori S., Mase T. Purification, characterization and application of α-amylase from Pseudozyma aphidis I-8. J. Appl. Glycosci. 2009;56 doi: 10.5458/jag.56.207. pp. 207–124. [DOI] [Google Scholar]
- 129.Buzzini P., Martini A. Extracellular enzymatic activity profiles in yeast and yeast‐like strains isolated from tropical environments. J. Appl. Microbiol. 2002;93:1020–1025. doi: 10.1046/j.1365-2672.2002.01783.x. [DOI] [PubMed] [Google Scholar]
- 130.Trindade R.C., Resende M.A., Silva C.M., Rosa C.A. Yeasts associated with fresh and frozen pulps of Brazilian tropical fruits. Syst. Appl. Microbiol. 2002;25:294–300. doi: 10.1078/0723-2020-00089. [DOI] [PubMed] [Google Scholar]
- 131.Oliveira R.Q., Rosa C.A., Uetanabaro A.P.T., Azeredo A., Neto A.G., Assis S.A. Polygalacturonase secreted by yeasts from Brazilian semi-arid environments. Int. J. Food Sci. Nutr. 2009;60:72–80. doi: 10.1080/09637480802534517. [DOI] [PubMed] [Google Scholar]
- 132.Munir R.I., Schellenberg J., Henrissat B., Verbeke T.J., Sparling R., Levin D.B. Comparative analysis of carbohydrate active enzymes in Clostridium termitidis CT1112 reveals complex carbohydrate degradation ability. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.D’Elia J.N., Salyers A.A. Effect of regulatory protein levels on utilization of starch by Bacteroides thetaiotaomicron. J. Bacteriol. 1996;178:7180–7186. doi: 10.1128/jb.178.24.7180-7186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rosewarne C.P., Pope P.B., Cheung J.L., Morrison M. Analysis of the bovine rumen microbiome reveals a diversity of Sus-like polysaccharide utilization loci from the bacterial phylum Bacteroidetes. J. Ind. Microbiol. Biotechnol. 2014;41:601–606. doi: 10.1007/s10295-013-1395-y. [DOI] [PubMed] [Google Scholar]
- 135.Martens E.C., Lowe E.C., Chiang H., Pudlo N.A., Wu M., McNulty N.P. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol. 2011;9 doi: 10.1371/journal.pbio.1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Blumer-Schuette S.E., Brown S.D., Sander K.B., Bayer E.A., Kataeva I., Zurawski J.V. Thermophilic lignocellulose deconstruction. FEMS Microbiol. Rev. 2014;38(3):393–448. doi: 10.1111/1574-6976.12044. [DOI] [PubMed] [Google Scholar]
- 137.Yang S.J., Kataeva I., Hamilton-Brehm S.D., Engle N.L., Tschaplinski T.J., Doeppke C. Efficient degradation of lignocellulosic plant biomass, without pretreatment, by the thermophilic anaerobe “Anaerocellum thermophilum” DSM 6725. Appl. Environ. Microbiol. 2009;75(14):4762–4769. doi: 10.1128/AEM.00236-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.van de Werken H.J., Verhaart M.R., VanFossen A.L., Willquist K., Lewis D.L., Nichols J.D. Hydrogenomics of the extremely thermophilic bacterium Caldicellulosiruptor saccharolyticus. Appl. Environ. Microbiol. 2008;74(21):6720–6729. doi: 10.1128/AEM.00968-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Blumer-Schuette S.E., Ozdemir I., Mistry D., Lucas S., Lapidus A., Cheng J.F. Complete genome sequences for the anaerobic, extremely thermophilic plant biomass-degrading bacteria Caldicellulosiruptor hydrothermalis, Caldicellulosiruptor kristjanssonii, Caldicellulosiruptor kronotskyensis, Caldicellulosiruptor owensensis, and Caldicellulosiruptor lactoaceticus. J. Bacteriol. 2011;193:1483–1484. doi: 10.1128/jb.01515-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lynd L.R., Van Zyl W.H., McBride J.E., Laser M. Consolidated bioprocessing of cellulosic biomass: an update. Curr. Opin. Biotech. 2005;16:577–583. doi: 10.1016/j.copbio.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 141.Hasunuma T., Okazaki F., Okai N., Hara K.Y., Ishii J., Kondo A. A. Review of enzymes and microbes for lignocellulosic biorefinery and the possibility of their application to consolidated bioprocessing technology. Bioresour. Technol. 2013;135:513–522. doi: 10.1016/j.biortech.2012.10.047. [DOI] [PubMed] [Google Scholar]
- 142.Zhang X.Z., Sathitsuksanoh N., Zhu Z., Zhang Y.H.P. One-step production of lactate from cellulose as the sole carbon source without any other organic nutrient by recombinant cellulolytic Bacillus subtilis. Metab. Eng. 2011;13:364–372. doi: 10.1016/j.ymben.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 143.Guedon E., Desvaux M., Petitdemange H. Improvement of cellulolytic properties of Clostridium cellulolyticum by metabolic engineering. Appl. Environ. Microbiol. 2002;68:53–58. doi: 10.1128/aem.68.1.53-58.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hasunuma T., Kondo A. Development of yeast cell factories for consolidated bioprocessing of lignocellulose to bioethanol through cell surface engineering. Biotechnol. Adv. 2012;30:1207–1218. doi: 10.1016/j.biotechadv.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 145.Yamada R., Tanaka T., Ogino C., Kondo A. Gene copy number and polyploidy on products formation in yeast. Appl. Microbiol. Biotechnol. 2010;88:849–857. doi: 10.1007/s00253-010-2850-6. [DOI] [PubMed] [Google Scholar]
- 146.Geiser E., Reindl M., Blank L.M., Feldbrügge M., Wierckx N., Schipper K. Activating intrinsic carbohydrate-active enzymes of the smut fungus Ustilago maydis for the degradation of plant cell wall components. Appl. Environ. Microbiol. 2016;82:5174–5185. doi: 10.1128/aem.00713-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Geiser E., Przybilla S.K., Friedrich A., Buckel W., Wierckx N., Blank L.M., Bölker M. Ustilago maydis produces itaconic acid via the unusual intermediate trans‐aconitate. Microb. Biotechnol. 2016;9:116–126. doi: 10.1111/1751-7915.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Den Haan R., Rose S.H., Lynd L.R., van Zyl W.H. Hydrolysis and fermentation of amorphous cellulose by recombinant Saccharomyces cerevisiae. Metab. Eng. 2007;9:87–94. doi: 10.1016/j.ymben.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 149.Sharma K.K., Kuhad R.C. Genetic transformation of lignin degrading fungi facilitated by Agrobacterium tumefaciens. BMC Biotechnol. 2011;10:67. doi: 10.1186/1472-6750-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cregg J.M., Tolstorukov I., Kusari A., Sunga J., Madden K., Chappell T. Vol. 463. Academic Press; 2009. pp. 169–189. (Expression in the Yeast Pichia pastoris. In Methods in Enzymology). [DOI] [PubMed] [Google Scholar]
- 151.Haon M., Grisel S., Navarro D., Gruet A., Berrin J.G., Bignon C. Recombinant protein production facility for fungal biomass-degrading enzymes using the yeast Pichia pastoris. Front. Microbiol. 2015;6:1002. doi: 10.3389/fmicb.2015.01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yanase H., Sato D., Yamamoto K., Matsuda S., Yamamoto S., Okamoto K. Genetic engineering of Zymobacter palmae for production of ethanol from xylose. Appl. Environ. Microbiol. 2007;73:2592–2599. doi: 10.1128/aem.02302-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Xu H. Bowling Green State University; 2015. Genetic Modification of Thermotoga to Degrade Cellulose. Doctoral dissertation. [Google Scholar]
- 154.Olins P.O., Lee S.C. Recent advances in heterologous gene expression in Escherichia coli. Curr. Opin. Biotech. 1993;4(5):520–525. doi: 10.1016/0958-1669(93)90071-4. [DOI] [PubMed] [Google Scholar]
- 155.Zhou S., Yomano L.P., Saleh A.Z., Davis F.C., Aldrich H.C., Ingram L.O. Enhancement of Expression and Apparent Secretion of Erwinia chrysanthemi Endoglucanase (Encoded bycelZ) in Escherichia coli. B. Appl. Environ. Microbiol. 1999;65:2439–2445. doi: 10.1128/aem.65.6.2439-2445.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lam T.L., Wong R.S., Wong W.K.R. Enhancement of extracellular production of a Cellulomonas fimiexo glucanase in Escherichia coli by the reduction of promoter strength. Enzyme Microb. Technol. 1997;20:482–488. doi: 10.1016/s0141-0229(96)00203-7. [DOI] [PubMed] [Google Scholar]
- 157.Zappe H., Jones D.T., Woods D.R. Cloning and expression of Clostridium acetobutylicum endoglucanase, cellobiase and amino acid biosynthesis genes in Escherichia coli. Microbiology. 1986;132:1367–1372. doi: 10.1099/00221287-132-5-1367. [DOI] [PubMed] [Google Scholar]
- 158.Irvin J.E., Teather R.M. Cloning and expression of a Bacteroides succinogenes mixed-linkage beta-glucanase (1, 3-1, 4-beta-D-glucan 4-glucanohydrolase) gene in Escherichia coli. Appl. Environ. Microbiol. 1988;54:2672–2676. doi: 10.1128/aem.54.11.2672-2676.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Spilliaert R., Hreggvidsson G.O., Kristjansson J.K., Eggertsson G., Palsdottir A. Cloning and Sequencing of a Rhodothermus marinus Gene, bglA, coding for a Thermostable β-Glucanase and its Expression in Escherichia coli. Eur. J. Biochem. 1994;224:923–930. doi: 10.1111/j.1432-1033.1994.00923.x. [DOI] [PubMed] [Google Scholar]
- 160.Okamoto T., Yamano S., Ikeaga H., Nakamura K. Cloning of the Acetobacter xylinum cellulase gene and its expression in Escherichia coli and Zymomonas mobilis. Appl. Microbiol. Biotechnol. 1994;42:563–568. doi: 10.1007/bf00173921. [DOI] [PubMed] [Google Scholar]
- 161.Linger J.G., Adney W.S., Darzins A. Heterologous expression and extracellular secretion of cellulolytic enzymes by Zymomonas mobilis. Appl. Environ. Microbiol. 2010;76:6360–6369. doi: 10.1128/aem.00230-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yanase H., Nozaki K., Okamoto K. Ethanol production from cellulosic materials by genetically engineered Zymomonas mobilis. Biotechnol. Lett. 2005;27:259–263. doi: 10.1007/s10529-004-8295-1. [DOI] [PubMed] [Google Scholar]
- 163.Yoon K.H., Park S.H., Pack M.Y. Transfer of Bacillus subtilis endo-β-1, 4-glucanase gene into Zymomonas anaerobia. Biotechnol. Lett. 1988;10:213–216. [Google Scholar]
- 164.De Faria F.P., Te’o V.S.J., Bergquist P.L., Azevedo M.O., Nevalainen K.M.H. Expression and processing of a major xylanase (XYN2) from the thermophilic fungus Humicolagrisea var. Thermoidea in Trichoderma reesei. Lett. Appl. Microbiol. 2002;34:119–123. doi: 10.1046/j.1472-765x.2002.01057.x. [DOI] [PubMed] [Google Scholar]
- 165.Mäntylä A., Paloheimo M., Hakola S., Lindberg E., Leskinen S., Kallio J. Production in Trichoderma reesei of three xylanases from Chaetomium thermophilum: a recombinant thermoxylanase for biobleaching of kraft pulp. Appl. Microbiol. Biotechnol. 2007;76:377–386. doi: 10.1007/s00253-007-1020-y. [DOI] [PubMed] [Google Scholar]
- 166.Wang J., Mai G., Liu G., Yu S. Molecular cloning and heterologous expression of an acid-stable endoxylanase gene from Penicillium oxalicum in Trichoderma reesei. J. Microbiol. Biotechnol. 2013;23:251–259. doi: 10.4014/jmb.1208.08030. [DOI] [PubMed] [Google Scholar]
- 167.Salles B.C., Te’o V.S., Gibbs M.D., Bergquist P.L., Edivaldo Filho X.F., Ximenes E.A., Nevalainen K.H. Identification of two novel xylanase-encoding genes (xyn5 and xyn6) from Acrophialophora nainiana and heterologous expression of xyn6 in Trichoderma reesei. Biotechnol. Lett. 2007;29:1195–1201. doi: 10.1007/s10529-007-9380-z. [DOI] [PubMed] [Google Scholar]
- 168.Su X., Schmitz G., Zhang M., Mackie R.I., Cann I.K. Heterologous gene expression in filamentous fungi. Adv Appl Microb. 2012;81:1–61. doi: 10.1016/b978-0-12-394382-8.00001-0. Academic Press. [DOI] [PubMed] [Google Scholar]
- 169.Yoshino S., Oishi M., Moriyama R., Kato M., Tsukagoshi N. Two family G xylanase genes from Chaetomium gracile and their expression in Aspergillus nidulans. Curr. Genet. 1995;29:73–80. doi: 10.1007/bf00313196. [DOI] [PubMed] [Google Scholar]
- 170.Flipphi M.J., Visser J., van der Veen P., de Graaff L.H. Cloning of the Aspergillus niger gene encoding α-L-arabinofuranosidase A. Appl. Microbiol. Biotechnol. 1993;39:335–340. doi: 10.1007/bf00192088. [DOI] [PubMed] [Google Scholar]
- 171.Pauly M., Andersen L.N., Kauppinen S., Kofod L.V., York W.S., Albersheim P., Darvill A. A xyloglucan-specific endo-β-1, 4-glucanase from Aspergillus aculeatus: expression cloning in yeast, purification and characterization of the recombinant enzyme. Glycobiology. 1999;9:93–100. doi: 10.1093/glycob/9.1.93. [DOI] [PubMed] [Google Scholar]
- 172.Ichishima E., Taya N., Ikeguchi M., Chiba Y., Nakamura M., Kawataba C. Molecular and enzymic properties of recombinant 1, 2-α-mannosidase from Aspergillus saitoi overexpressed in Aspergillus oryzae cells. Biochem. J. 1999;339:589–597. [PMC free article] [PubMed] [Google Scholar]
- 173.van Zyl P.J., Moodley V., Rose S.H., Roth R.L., Van Zyl W.H. Production of the Aspergillus aculeatus endo-1, 4-β-mannanase in Aspergillus niger. J Ind Microbiol Biot. 2009;36:611–617. doi: 10.1007/s10295-009-0551-x. [DOI] [PubMed] [Google Scholar]
- 174.Gong Y., Tang G., Wang M., Li J., Xiao W., Lin J., Liu Z. Direct fermentation of amorphous cellulose to ethanol by engineered Saccharomyces cerevisiae coexpressing Trichoderma viride EG3 and BGL1. J. Gen. Appl. Microbiol. 2014;60:198–206. doi: 10.2323/jgam.60.198. [DOI] [PubMed] [Google Scholar]
- 175.Kim S.K., Chung D., Himmel M.E., Bomble Y.J., Westpheling J. In vivo synergistic activity of a CAZyme cassette from Acidothermus cellulolyticus significantly improves the cellulolytic activity of the C. bescii exoproteome. Biotechnol. Bioeng. 2017;114:2474–2480. doi: 10.1002/bit.26366. [DOI] [PubMed] [Google Scholar]
- 176.Liu S.L., Du K. Enhanced expression of an endoglucanase in Bacillus subtilis by using the sucrose-inducible sacB promoter and improved properties of the recombinant enzyme. Protein Expres. Purif. 2012;83:164–168. doi: 10.1016/j.pep.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 177.Ceasar S.A., Rajan V., Prykhozhij S.V., Berman J.N., Ignacimuthu S. Insert, remove or replace: a highly advanced genome editing system using CRISPR/Cas9. BBA-Mol. Cell Res. 2016;1863(9):2333–2344. doi: 10.1016/j.bbamcr.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 178.Salazar-Cerezo S., Kun R.S., de Vries R.P., Garrigues S. CRISPR/Cas9 technology enables the development of the filamentous ascomycete fungus Penicillium subrubescens as a new industrial enzyme producer. Enzyme Microb. Tech. 2020;133:109463. doi: 10.1016/j.enzmictec.2019.109463. [DOI] [PubMed] [Google Scholar]
- 179.Schuster M., Schweizer G., Reissmann S., Kahmann R. Genome editing in Ustilago maydis using the CRISPR–Cas system. Fungal Genet. Biol. 2016;89:3–9. doi: 10.1016/j.fgb.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 180.Stovicek V., Borodina I., Forster J. CRISPR–Cas system enables fast and simple genome editing of industrial Saccharomyces cerevisiae strains. Metab. Eng. Commun. 2015;2:13–22. doi: 10.1016/j.meteno.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.FitzPatrick M., Champagne P., Cunningham M.F., Whitney R.A. A biorefinery processing perspective: treatment of lignocellulosic materials for the production of value-added products. Bioresour. Technol. 2010;101:8915–8922. doi: 10.1016/j.biortech.2010.06.125. [DOI] [PubMed] [Google Scholar]
- 182.Champreda V., Mhuantong W., Lekakarn H., Bunterngsook B., Kanokratana P., Zhao X.Q. Designing cellulolytic enzyme systems for biorefinery: from nature to application. J. Biosci. Bioeng. 2019 doi: 10.1016/j.jbiosc.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 183.Gobina E. BCC Research Report EGY054C. 2016. Biorefinery technologies: global markets. [Google Scholar]
- 184.Horn S.J., Vaaje-Kolstad G., Westereng B., Eijsink V. Novel enzymes for the degradation of cellulose. Biotechnol. Biofuels. 2012;5:45. doi: 10.1186/1754-6834-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chundawat S.P., Beckham G.T., Himmel M.E., Dale B.E. Deconstruction of lignocellulosic biomass to fuels and chemicals. Annu. Rev. Chem. Biomol. Eng. 2011;2:121–145. doi: 10.1146/annurev-chembioeng-061010-114205. [DOI] [PubMed] [Google Scholar]
- 186.Meyer A.S., Rosgaard L., Sørensen H.R. The minimal enzyme cocktail concept for biomass processing. J. Cereal Sci. 2009;50:337–344. [Google Scholar]
- 187.Gao D., Haarmeyer C., Balan V., Whitehead T.A., Dale B.E., Chundawat S.P. Lignin triggers irreversible cellulase loss during pretreated lignocellulosic biomass saccharification. Biotechnol. Biofuels. 2014;7:175. doi: 10.1186/s13068-014-0175-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Foreman P.K., Brown D., Dankmeyer L., Dean R., Diener S., Dunn-Coleman N.S. Transcriptional regulation of biomass-degrading enzymes in the filamentous fungus Trichoderma reesei. J. Biol. Chem. 2003;278:31988–31997. doi: 10.1074/jbc.m304750200. [DOI] [PubMed] [Google Scholar]
- 189.Vaaje-Kolstad G., Westereng B., Horn S.J., Liu Z., Zhai H., Sørlie M., Eijsink V.G. An oxidative enzyme boosting the enzymatic conversion of recalcitrant polysaccharides. Science. 2010;330:219–222. doi: 10.1126/science.1192231. [DOI] [PubMed] [Google Scholar]
- 190.Várnai A., Huikko L., Pere J., Siika-Aho M., Viikari L. Synergistic action of xylanase and mannanase improves the total hydrolysis of softwood. Bioresour. Technol. 2011;102:9096–9104. doi: 10.1016/j.biortech.2011.06.059. [DOI] [PubMed] [Google Scholar]
- 191.Pollegioni L., Tonin F., Rosini E. Lignin‐degrading enzymes. FEBS J. 2015;282:1190–1213. doi: 10.1111/febs.13224. [DOI] [PubMed] [Google Scholar]
- 192.Levasseur A., Drula E., Lombard V., Coutinho P.M., Henrissat B. Expansion of the enzymatic repertoire of the CAZy database to integrate auxiliary redox enzymes. Biotechnol. Biofuels. 2013;6:41. doi: 10.1186/1754-6834-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Morrison J.M., Elshahed M.S., Youssef N.H. Defined enzyme cocktail from the anaerobic fungus Orpinomyces sp. Strain C1A effectively releases sugars from pretreated corn stover and switchgrass. Sci. Rep. 2016;6:1–12. doi: 10.1038/srep29217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kurašin M., Väljamäe P. Processivity of cellobiohydrolases is limited by the substrate. J. Biol. Chem. 2011;286:169–177. doi: 10.1074/jbc.m110.161059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hess M., Sczyrba A., Egan R., Kim T.W., Chokhawala H., Schroth G. Metagenomic discovery of biomass-degrading genes and genomes from cow rumen. Science. 2011;331:463–467. doi: 10.1126/science.1200387. [DOI] [PubMed] [Google Scholar]
- 196.Pope P.B., Mackenzie A.K., Gregor I., Smith W., Sundset M.A., McHardy A.C. Metagenomics of the Svalbard reindeer rumen microbiome reveals abundance of polysaccharide utilization loci. PLoS One. 2012;7 doi: 10.1371/journal.pone.0038571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Naas A.E., Mackenzie A.K., Mravec J., Schückel J., Willats W.G.T., Eijsink V.G.H., Pope P.B. Do rumen Bacteroidetes utilize an alternative mechanism for cellulose degradation? M Bio. 2014;5:e01401–01414. doi: 10.1128/mbio.01401-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li L.L., McCorkle S.R., Monchy S., Taghavi S., van der Lelie D. Bioprospecting metagenomes: glycosyl hydrolases for converting biomass. Biotechnol. Biofuels. 2009;2:10. doi: 10.1186/1754-6834-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lewin A., Zhou J., Pham V.T.T., Haugen T., El Zeiny M., Aarstad O. Novel archaeal thermostable cellulases from an oil reservoir metagenome. AMB Exp. 2017;7:1–17. doi: 10.1186/s13568-017-0485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ufarté L., Bozonnet S., Laville E., Cecchini D.A., Pizzut-Serin S., Jacquiod S. Microbial Environmental Genomics (MEG) Humana Press; New York, NY: 2016. Functional metagenomics: construction and high-throughput screening of fosmid libraries for discovery of novel carbohydrate-active enzymes; pp. 257–271. [DOI] [PubMed] [Google Scholar]
- 201.Mo X.C., Chen C.L., Pang H., Feng Y., Feng J.X. Identification and characterization of a novel xylanase derived from a rice straw degrading enrichment culture. Appl. Microbiol. Biotechnol. 2010;87:2137–2146. doi: 10.1007/s00253-010-2712-2. [DOI] [PubMed] [Google Scholar]
- 202.Patel A.B., Patel A.K., Shah M.P., Parikh I.K., Joshi C.G. Isolation and characterization of novel multifunctional recombinant family 26 glycoside hydrolase from Mehsani buffalo rumen metagenome. Biotechnol. Appl. Biochem. 2016;63:257–265. doi: 10.1002/bab.1358. [DOI] [PubMed] [Google Scholar]
- 203.Helbert W., Poulet L., Drouillard S., Mathieu S., Loiodice M., Couturier M. Discovery of novel carbohydrate-active enzymes through the rational exploration of the protein sequences space. Proc. Natl. Acad. Sci. 2019;116:6063–6068. doi: 10.1073/pnas.1815791116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cattaneo C., Spertino S., Boatti L., Icardi S., Cavaletto M. Protein fingerprinting in the choice of cellulase cocktails for the conversion of lignocellulosic biomass. Anal. Methods. 2014;6:4046–4055. [Google Scholar]
- 205.Wilkens C., Busk P.K., Pilgaard B., Zhang W.J., Nielsen K.L., Nielsen P.H., Lange L. Diversity of microbial carbohydrate-active enzymes in Danish anaerobic digesters fed with wastewater treatment sludge. Biotechnol. Biofuels. 2017;10:158. doi: 10.1186/s13068-017-0840-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhang Y.H.P. Reviving the carbohydrate economy via multi-product lignocellulose biorefineries. J. Ind. Microbiol. Biotechnol. 2008;35:367–375. doi: 10.1007/s10295-007-0293-6. [DOI] [PubMed] [Google Scholar]
- 207.Yadav M., Yadav H.S. Applications of ligninolytic enzymes to pollutants, wastewater, dyes, soil, coal, paper and polymers. Environ. Chem. Lett. 2015;13:309–318. [Google Scholar]
- 208.Jayasekara S., Ratnayake R. Intechopen; 2019. Microbial Cellulases: An overview and applications in cellulose. [DOI] [Google Scholar]
- 209.Garron M.L., Henrissat B. The continuing expansion of CAZymes and their families. Curr. Opin. Chem. Biol. 2019;53:82–87. doi: 10.1016/j.cbpa.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 210.Gupta V.K., Kubicek C.P., Berrin J.G., Wilson D.W., Couturier M., Berlin A. Fungal enzymes for bio-products from sustainable and waste biomass. Trends Biochem. Sci. 2016;41:633–645. doi: 10.1016/j.tibs.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 211.Kries H., Blomberg R., Hilvert D. De novo enzymes by computational design. Curr. Opin. Chem. Biol. 2013;17:221–228. doi: 10.1016/j.cbpa.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 212.Anbar M., Lamed R., Bayer E.A. Thermostability enhancement of Clostridium thermocellum cellulosomal endoglucanase Cel8A by a single glycine substitution. Chem. Cat. Chem. 2010;2:997–1003. [Google Scholar]
- 213.Yi Z.L., Pei X.Q., Wu Z.L. Introduction of glycine and proline residues onto protein surface increases the thermostability of endoglucanase CelA from Clostridium thermocellum. Bioresour. Technol. 2011;102:3636–3638. doi: 10.1016/j.biortech.2010.11.043. [DOI] [PubMed] [Google Scholar]
- 214.Smith M.A., Rentmeister A., Snow C.D., Wu T., Farrow M.F., Mingardon F., Arnold F.H. A diverse set of family 48 bacterial glycoside hydrolase cellulases created by structure‐guided recombination. FEBS J. 2012;279:4453–4465. doi: 10.1111/febs.12032. [DOI] [PubMed] [Google Scholar]
- 215.Thite V.S., Nerurkar A.S. Crude xylanases and pectinases from Bacillus spp. Along with commercial cellulase formulate an efficient tailor-made cocktail for sugarcane bagasse saccharification. Bioenergy Res. 2019:1–15. [Google Scholar]
- 216.Peterson R., Nevalainen H. Trichoderma reesei RUT-C30–thirty years of strain improvement. Microbiology. 2012;158:58–68. doi: 10.1099/mic.0.054031-0. [DOI] [PubMed] [Google Scholar]
- 217.Gomez-Flores M., Nakhla G., Hafez H. Microbial kinetics of Clostridium termitidis on cellobiose and glucose for biohydrogen production. Biotechnol. Lett. 2015;37:1965–1971. doi: 10.1007/s10529-015-1891-4. [DOI] [PubMed] [Google Scholar]
- 218.Wang L., Chen H. Increased fermentability of enzymatically hydrolyzed steam-exploded corn stover for butanol production by removal of fermentation inhibitors. Process Biochem. 2011;46:604–607. [Google Scholar]
- 219.Sukumaran R.K., Singhania R.R., Mathew G.M., Pandey A. Cellulase production using biomass feed stock and its application in lignocellulose saccharification for bio-ethanol production. Renew. Energy. 2009;34:421–424. [Google Scholar]
- 220.Datta R. Acidogenic fermentation of lignocellulose–acid yield and conversion of components. Biotechnol. Bioeng. 1981;23:2167–2170. [Google Scholar]
- 221.Raman B., McKeown C.K., Rodriguez M., Brown S.D., Mielenz J.R. Transcriptomic analysis of Clostridium thermocellum ATCC 27405 cellulose fermentation. BMC Microbiol. 2011;11:134. doi: 10.1186/1471-2180-11-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Meyrial V., Delgenes J.P., Moletta R., Navarro J.M. Xylitol production from D-xylose by Candida guillermondii: fermentation behaviour. Biotechnol. Lett. 1991;13:281–286. [Google Scholar]
- 223.Yamaguchi A., Sato O., Mimura N., Hirosaki Y., Kobayashi H., Fukuoka A. Direct production of sugar alcohols from wood chips using supported platinum catalysts in water. Catal. Commun. 2014;54:22–26. [Google Scholar]
- 224.Szakács G., Morovján G., Tengerdy R.P. Production of lovastatin by a wild strain of Aspergillus terreus. Biotechnol. Lett. 1998;20:411–415. [Google Scholar]
- 225.Tengerdy R.P., Szakacs G. Bioconversion of lignocellulose in solid substrate fermentation. Biochem. Eng. J. 2003;13:169–179. [Google Scholar]
- 226.Aiyer P.V. Amylases and their applications. Afr. J. Biotechnol. 2005;4 [Google Scholar]
- 227.Sun X., Liu Z., Qu Y., Li X. The effects of wheat bran composition on the production of biomass-hydrolyzing enzymes by Penicillium decumbens. Biotechnol Fuels Chem Humana Press. 2007:239–248. doi: 10.1007/s12010-007-8049-3. [DOI] [PubMed] [Google Scholar]
- 228.Haldar D., Purkait M.K. Lignocellulosic conversion into value-added products: a review. Process Biochem. 2020;89:110–133. [Google Scholar]
- 229.Fritz-Langhals E., Kunath B. Synthesis of aromatic aldehydes by laccase-mediator assisted oxidation. Tetrahedron Lett. 1998;39:5955–5956. [Google Scholar]
- 230.Vazquez M.J., Alonso J.L., Domınguez H., Parajo J.C. Xylooligosaccharides: manufacture and applications. Trends Food Sci Tech. 2000;11:387–393. [Google Scholar]
- 231.Moldes D., Lorenzo M., Sanromán M.A. Different proportions of laccase isoenzymes produced by submerged cultures of Trametes versicolor grown on lignocellulosic wastes. Biotechnol. Lett. 2004;26:327–330. doi: 10.1023/b:bile.0000015452.40213.bf. [DOI] [PubMed] [Google Scholar]
- 232.Kittur F.S., Kumar A.B.V., Tharanathan R.N. Low molecular weight chitosans—preparation by depolymerization with Aspergillus niger pectinase, and characterization. Carbohydr. Res. 2003;338:1283–1290. doi: 10.1016/s0008-6215(03)00175-7. [DOI] [PubMed] [Google Scholar]
- 233.Botella C., Diaz A., De Ory I., Webb C., Blandino A. Xylanase and pectinase production by Aspergillus awamori on grape pomace in solid state fermentation. Process Biochem. 2007;42:98–101. [Google Scholar]
- 234.Nasseri A.T., Rasoul-Amini S., Morowvat M.H., 2Ghasemi Y. Single cell protein: production and process. Am. J. Food. Technol. 2011;6:103–116. [Google Scholar]
- 235.Savvides A.L., Katsifas E.A., Hatzinikolaou D.G., Karagouni A.D. Xanthan production by Xanthomonas campestris using whey permeate medium. World J. Microb. Biotechnol. 2012;28:2759–2764. doi: 10.1007/s11274-012-1087-1. [DOI] [PubMed] [Google Scholar]
- 236.Öner E.T. Pretreatment Techniques for Biofuels and Biorefineries. Springer; Berlin, Heidelberg: 2013. Microbial production of extracellular polysaccharides from biomass; pp. 35–56. [Google Scholar]
- 237.Tien M., Kirk T.K. Lignin-degrading enzyme from the hymenomycete Phanerochaete chrysosporium Burds. Science. 1983;221:661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]
- 238.Martínez A.T., Camarero S., Gutiérrez A., Bocchini P., Galletti G.C. Studies on wheat lignin degradation by Pleurotus species using analytical pyrolysis. J Anal Appl Pyrol. 2001;58:401–411. [Google Scholar]
- 239.Kaur B., Chakraborty D. Biotechnological and molecular approaches for vanillin production: a review. Appl. Biochem. Biotechnol. 2013;169:1353–1372. doi: 10.1007/s12010-012-0066-1. [DOI] [PubMed] [Google Scholar]
- 240.Li Y., Sun Z., Ge X., Zhang J. Effects of lignin and surfactant on adsorption and hydrolysis of cellulases on cellulose. Biotechnol. Biofuels. 2016;9:20. doi: 10.1186/s13068-016-0434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Jia J., Zhang W., Yang Z., Yang X., Wang N., Yu X. Novel magnetic cross-linked cellulase aggregates with a potential application in lignocellulosic biomass bioconversion. Molecules. 2017;22:269. doi: 10.3390/molecules22020269. [DOI] [PMC free article] [PubMed] [Google Scholar]