Abstract
Data on characteristics of clinical trials with probiotics, registered at ClinicalTrials.gov were retrieved from the registry . Data on the registration process itself (moment of registration of the trial in relation to date of inclusion of first participant, data of completion of the study) as well as the number of participants were analysed. A trend analysis (over the period 2000-2020) was made of the number of participants in clinical trials with probiotics on gastrointestinal conditions and diseases. A comparison was made between conditions and diseases studied in completed clinical trials with those trials not yet recruiting participants. The data can be used and reused for assessment of the quality of clinical studies with probiotics and to identify future trends in applications of probiotics.
Keywords: Probiotics, Clinical trials, Registration, ClinicalTrials.gov
Specifications Table
| Subject | nutrition |
| Specific subject area | Registration of clinical studies with probiotics |
| Type of data | Tables Graphs |
| How data were acquired | Data on clinical studies with probiotics were obtained from ClinicalTrials.gov (https://clinicaltrials.gov/). Raw data were retrieved and ordered by TMGD, data were checked by GTR). |
| Data format | Raw Analysed |
| Parameters for data collection | The parameters which were collected include characteristics of the registration themselves such as the NCT registration number, title of the study, date of registration. Additional parameters collected include the number of participants, their age category, gender, health status, the underlying disease or condition (e.g. digestive system disease), nature of the study (intervention, observational), product (species, strain), dosage, route of administration, frequency, duration, primary outcome measurement (health effect), duration of the intervention/study, start and end date of inclusion, sponsors, and location (country, city). |
| Description of data collection | Data were collected from ClinicalTrial.gov. The dosage of the probiotic product, species and strain identification, route of administration, frequency, duration of the intervention, primary outcome measurement (health effect), and duration of the intervention were recorded from the individual study information provided. The current functionality of the ClinicalTrials.gov database allows the user to download information on the other studied parameters (such as starting date of inclusion, and number of participants) directly from the website. |
| Data source location | Institution: University College Roosevelt City/Town/Region: Middelburg Country: The Netherlands Latitude: 51.49907, Longitude: 3.61104 Primary data sources: https://clinicaltrials.gov/https://clinicaltrials.gov/ct2/results?cond=probiotics&term=&cntry=&state=&city=&dist= |
| Data accessibility | With the article |
| Related research article | Dronkers TMG, Ouwehand AC, Rijkers GT. Global analysis of clinical trials with probiotics. Heliyon. 2020;6(7):e04467. Published 2020 Jul 17. doi:10.1016/j.heliyon.2020.e04467 |
Value of the Data
-
•
The data on clinical trials with probiotics are important to gain more insight into registration procedures, the clinical conditions addressed, as well as the major characteristics, including size of the study (number of participants), and duration of the study [1,2].
-
•
Both (clinical) researchers as well as producers of probiotics can benefit from these data [3,4,5].
-
•
The data on registered studies which are recruiting participants give insight in the future applications of probiotics and the organisation and design of appropriate clinical studies [6,7].
1. Data Description
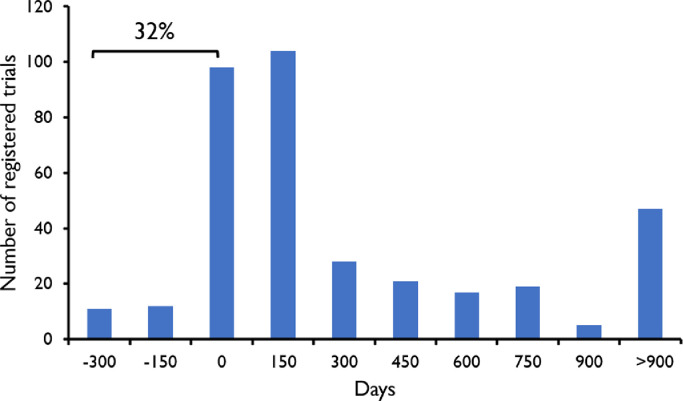
This data article contains tabulated information on characteristics of clinical studies with probiotics. The registration data of a clinical trial with probiotics in the ClinicalTrials.gov registry in relation to the date of inclusion of the first participant, as well as in relation to the completion of the study is shown in Figs. 1 and 2, respectively.
Fig. 1.
Timing of registration of clinical trials with probiotics. X-axis shows the difference (in days) between the date of registration of the clinical trial and the date of inclusion of the first participants. Positive numbers indicate that the trial was registered after completion of the study. Day 0 indicates the moment of inclusion of the first participant. 32% of the trials were registered before inclusion of the first participant.
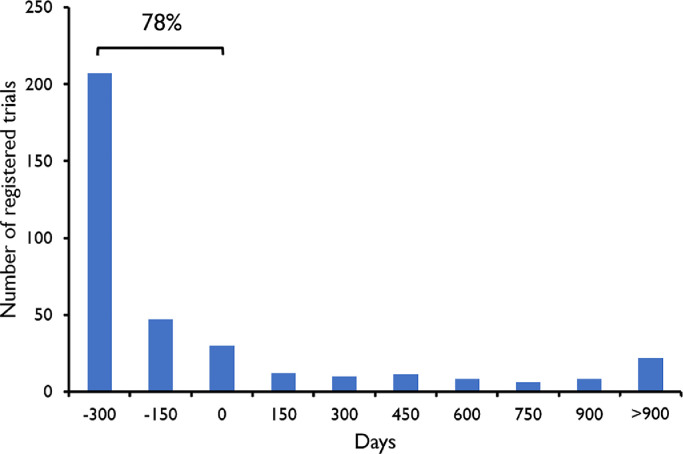
Fig. 2.
Timing of registration of clinical trials with probiotics. X-axis shows the difference (in days) between the date of registration of the clinical trial and the date of completion of the study. Positive numbers indicate that the trial was registered after completion of the study. Day 0 indicates the moment of inclusion of the first participant. 78% of the trials were registered before completion of the study.
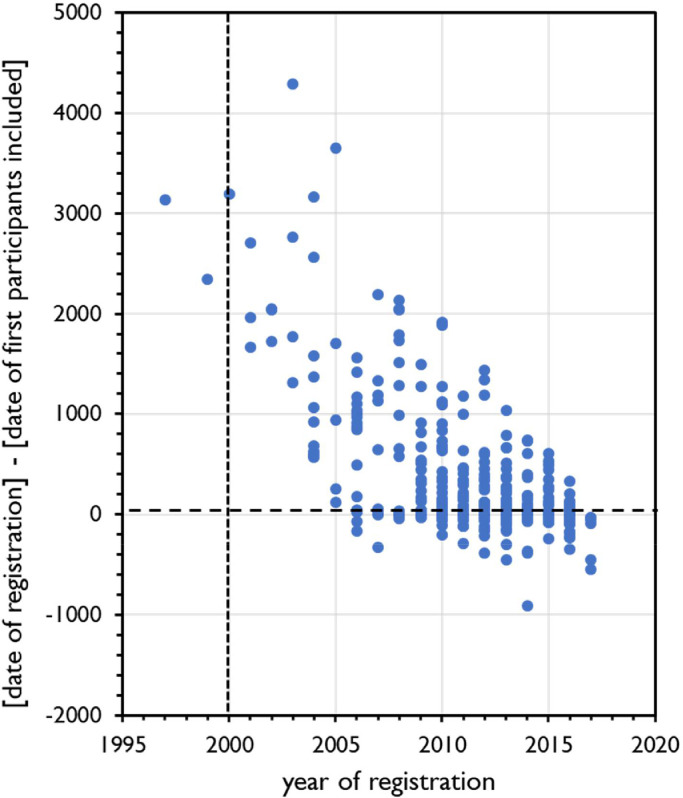
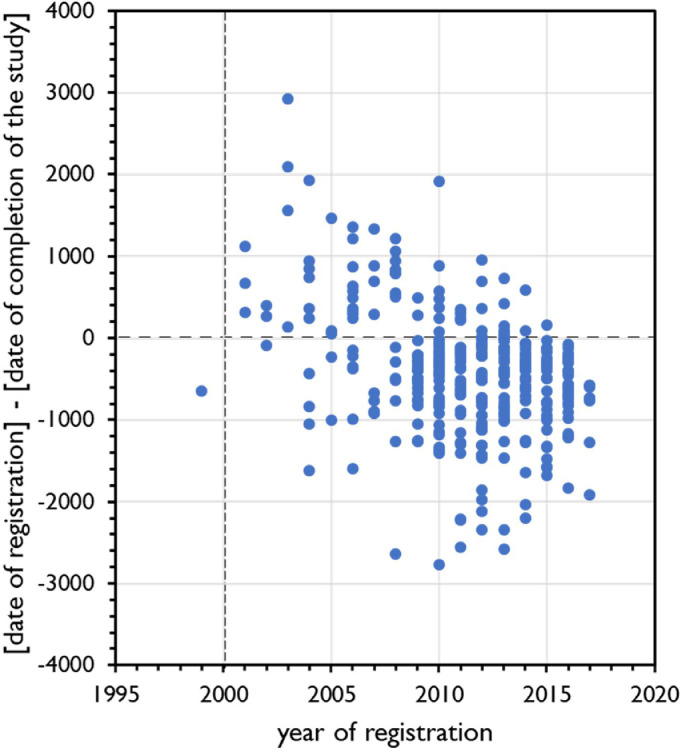
Figs. 3 and 4 show the trend over the years 1997-2017 of the moment of registration of a clinical trial in relation to inclusion of the first participant (Fig. 3) and in relation to completion of the study (Fig. 4).
Fig. 3.
Trend analysis 1997-2017 of the moment of registration of clinical trials with probiotics. Year of registration at ClinicalTrials.gov on the X-axis. Y-axis shows the difference (in days) between the date of registration of the clinical trial and the date of inclusion of the first participant. Positive numbers indicate that the trial was registered after inclusion of the first participant. Broken vertical line indicates the year of introduction of ClinicalTrials.gov. Regression analysis R2=0.155101, F=63.88351, significance F=1.96E-14.
Fig. 4.
Trend analysis 1999-2017 of the moment of registration of clinical trials with probiotics. Year of registration at ClinicalTrials.gov on the X-axis. Y-axis shows the difference (in days) between the date of registration of the clinical trial and the date of completion of the study. Positive numbers indicate that the trial was registered after completion of the study. Broken vertical line indicates the year of introduction of ClinicalTrials.gov. Regression analysis R2=0.1485531, F=60.7159, significance F=7.68E-14.
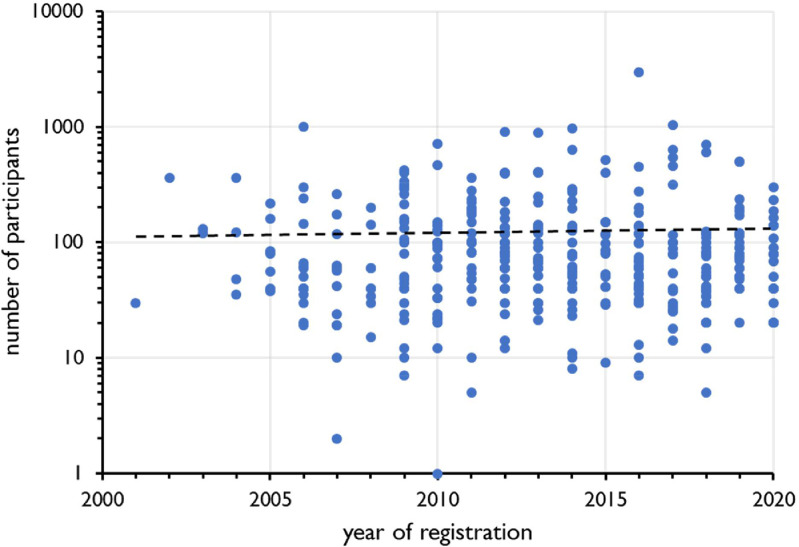
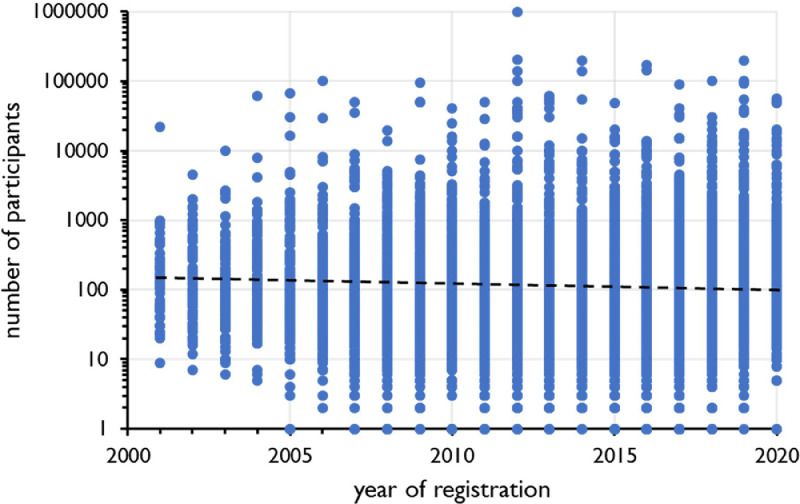
The median number of participants included in a clinical trial with probiotics is 74, which is not significantly different from that of 71 participants in all clinical studies registered at ClinicalTrials.gov [1]. Fig. 5 shows the trend analysis over the years 2000-2020 of the number of participants included in clinical studies with probiotics for gastrointestinal conditions. Fig. 6 shows a comparable analysis for 10,000 of the 17,000 clinical trials for gastrointestinal disorders registered at ClinicalTrials.gov during the same period in total.
Fig. 5.
Trend analysis of the number of participants in clinical trials with probiotics on gastrointestinal conditions (n=349) over the period 2000-2020. Year of registration at ClinicalTrials.gov on the X-axis, number of participants on the Y-axis. Broken line represents regression line. R2=0.00045882, F=0.159284, significance F=0.690061812.
Fig. 6.
Trend analysis of the number of participants in clinical trials on gastrointestinal conditions (n=9731) over the period 2000-2020. Year of registration at ClinicalTrials.gov on the X-axis, number of participants on the Y-axis. Broken line represents regression line. R2=0,000104368, F=1,01550609, significance F=0,313612416.
The 15 most frequent conditions and diseases studied in clinical trials with probiotics are shown in Table 1. A comparison is made between completed studies and studies which are registered but are not (yet) recruiting participants. The data show which conditions and diseases are addressed in current ongoing studies with probiotics.
Table 1.
Conditions and diseases studied in clinical trials with probiotics.
| Completed studies | N=826 |
Not yet recruiting | N=139 | ||||
|---|---|---|---|---|---|---|---|
| Rank | Condition | N | % | Condition | N | % | P |
| 1 | Digestive System Diseases | 169 | 20,5 | Communicable Diseases | 26 | 18,7 | |
| 2 | Gastrointestinal Diseases | 169 | 20,5 | Infection | 26 | 18,7 | |
| 3 | Communicable Diseases | 164 | 19,9 | Digestive System Diseases | 21 | 15,1 | |
| 4 | Infection | 164 | 19,9 | Gastrointestinal Diseases | 21 | 15,1 | |
| 5 | Digestive | 110 | 13,3 | Immune System Diseases | 12 | 8,6 | |
| 6 | Intestinal Diseases | 102 | 12,4 | Mental Disorders | 12 | 8,6 | 0.0009 |
| 7 | Syndrome | 97 | 11,8 | Digestive | 12 | 8,6 | |
| 8 | Diarrhea | 74 | 9,0 | Diarrhea | 11 | 7,9 | |
| 9 | Respiratory Tract Diseases | 72 | 8,7 | Hypersensitivity | 11 | 7,9 | |
| 10 | Metabolic Diseases | 70 | 8,5 | Metabolic Diseases | 11 | 7,9 | |
| 11 | Gastroenteritis | 65 | 7,9 | Psychotic Disorders | 11 | 7,9 | 0.0024 |
| 12 | Colonic Diseases | 64 | 7,8 | Intestinal Diseases | 10 | 7,2 | |
| 13 | Immune System Diseases | 64 | 7,8 | Skin Diseases | 9 | 6,5 | 0.0236 |
| 14 | Respiratory Tract Infections | 60 | 7,3 | Syndrome | 9 | 6,5 | |
| 15 | Hypersensitivity | 58 | 7,0 | Body weight | 8 | 5,8 | |
The ClinicalTrials.gov repository lists 1514 studies for probiotics (assessed on July 29, 2020), of which 826 are completed and 139 are not (yet) recruiting. Studies are ranked by the frequency of the condition or disease studied. Condition and disease identifiers are assigned by ClinicalTrials.gov and a single study can address several conditions. The number of studies (N) and percentage (%) is indicated. Significance of differences between completed studies and the not yet recruiting studies in terms of the conditions studies was determined by Fisher's exact Chi-square. P values lower than 0.05 are indicated.
2. Experimental design, materials and methods
All data were collected from ClinicalTrial.gov, a database of privately and publicly funded clinical studies conducted around the world, maintained by the U.S. National Library of Medicine. “probiotics” was used as search term in the field “other terms” on the landing page of the website. The relevant parameters from the clinical studies were either collected manually or downloaded from the website as an XML file (downloaded on July 27 2020). The maximum number of studies that can be downloaded is 10,000. For studies with probiotics this is (more than) enough; for a comparison with all clinical studies on gastrointestinal conditions, the first 10,000 studies (listed in order of relevance by ClinicalTrials.gov) was selected from over 17,000 listed.
The analysis of frequencies and calculation of number of days was done in Excel 2016 (Microsoft Corporation, Redmond, Washington, United States). Statistical significance of differences between groups was calculated by Chi-square using the Analysis Toolpack add-in of Excel. The same toolpack was also used for regression analysis.
Ethics statement
This work did not involve the use of human subjects or animal experiments.
Declaration of Competing Interest
(1) The work for this article was co-funded by the International Probiotics Association (internationalprobiotics.org). (2) G.T. Rijkers and A.C. Ouwehand are members of the Scientific Advisory Board of the International Probiotics Association (http://internationalprobiotics.org/). A.C. Ouwehand is an employee of DuPont Nutrition and BioSciences, which manufactures and markets probiotics. (3) The authors declare that, apart from what is stated above, they have no competing financial interests or personal relationships which have, or could be perceived to have, influenced the work reported in this article.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.dib.2020.106269.
Appendix. Supplementary materials
References
- 1.Dronkers TMG, Ouwehand AC, Rijkers GT. Global analysis of clinical trials with probiotics. Heliyon. 2020;6(7):e04467. doi: 10.1016/j.heliyon.2020.e04467. Published 2020 Jul 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dronkers TMG, Krist L, Van Overveld FJ, Rijkers GT. The ascent of the blessed: regulatory issues on health effects and health claims for probiotics in Europe and the rest of the world. Benef. Microbes. 2018;9(5):717–723. doi: 10.3920/BM2017.0196. [DOI] [PubMed] [Google Scholar]
- 3.Laws G, Kemp R. Probiotics and health: understanding probiotic trials. N. Z. Med. J. 2019;132(1498):90–96. Published 2019 Jul 12. [PubMed] [Google Scholar]
- 4.Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019;25(5):716–729. doi: 10.1038/s41591-019-0439-x. [DOI] [PubMed] [Google Scholar]
- 5.Lee ES, Song EJ, Nam YD, Lee SY. Probiotics in human health and disease: from nutribiotics to pharmabiotics. J. Microbiol. 2018;56(11):773–782. doi: 10.1007/s12275-018-8293-y. [DOI] [PubMed] [Google Scholar]
- 6.Kane L, Kinzel J. The effects of probiotics on mood and emotion. JAAPA. 2018;31(5):1–3. doi: 10.1097/01.JAA.0000532122.07789.f0. [DOI] [PubMed] [Google Scholar]
- 7.Roudsari MR, Karimi R, Sohrabvandi S, Mortazavian AM. Health effects of probiotics on the skin. Crit. Rev. Food Sci. Nutr. 2015;55(9):1219–1240. doi: 10.1080/10408398.2012.680078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.