Abstract
Nanotechnology has been widely developed to improve the solubility of active pharmaceutical ingredients. Co-crystal discovery has also taken much attention in drug design and development. A combination of the two techniques generates “nano-co-crystallization”, a new approach to obtaining the superior character of drugs. Previously, a new diclofenac-proline co-crystal (DPC) arrangement has been reported. The present research attempted to develop a nano-diclofenac-proline-co-crystal (NDPC) and to evaluate its formation kinetics, and dissolution-diffusion improvements. Both top-down and bottom-up methods optimized nano-co-crystal production. The top-down technique was used through the wet milling procedure and neat grinding procedures, while the bottom-up technique was performed through the globule inversion phase and fast evaporation assisted microwaving. The NDPCs obtained were then characterized by dynamic light scattering, binocular microscope, scanning electron microscopy, transmission electron microscopy, differential scanning calorimetry, powder x-ray diffractometry, and Fourier transform infrared spectrophotometry. The kinetics of NDPC formation was determined based on the difference of microwaving versus the co-crystal yield, which was analyzed using Fourier transform infrared spectroscopy. Dissolution was tested by type 2 apparatus, and diffusion was tested using Franz diffusion cells. The bottom-up method by fast evaporation assisted microwaving provided the best nano-co-crystal with a mean diameter of 598.2 ± 63.2 nm and a polydispersity index of 0.278 ± 0.062. Nano-co-crystal formation kinetic, which was evaluated by FTIR, indicated to follow first order. Finally, NDPC showed the superior dissolution and diffusion profile than conventional-DPC. In this study, we demonstrate a promising alternative for improving the dissolution and diffusion of the drug by nano-co-crystallization.
Keywords: Diclofenac acid, L-proline, Co-crystal, Nano-co-crystal, Dissolution, Diffusion, Materials science, Nanomaterials, Materials characterization, Materials chemistry, Chemistry, Analytical chemistry, Organic chemistry, Physical chemistry, Supramolecular chemistry, Pharmaceutical chemistry
Diclofenac acid; L-proline; Co-crystal; Nano-co-crystal; Dissolution; diffusion; Materials Science; Nanomaterials; Materials Characterization; Materials Chemistry; Chemistry; Analytical chemistry; Organic chemistry; Physical chemistry; Supramolecular Chemistry; Pharmaceutical chemistry.
1. Introduction
Diclofenac is one of the most successful non-steroidal anti-inflammatory drug (NSAID) drugs marketed and has good pharmacological effects [1]. It is prescribed as an anti-inflammatory and local pain and also used to treat arthritis and soft injuries [2]. Diclofenac is a class II of the Biopharmaceutics Classification System (BCS) drug that has high permeability but low solubility; hence, it is not available in acid form but mainly available in its salt forms such as diclofenac sodium or diclofenac potassium [3]. Many existing drugs have good pharmacological activity, but their work is limited by solubility and dissolution problems, including one is diclofenac [4]. Good solubility is something that drugs need to have because the drug requires certain solubility to get better bioavailability in the body.
Several modifications have been reported to improve the solubility of diclofenac. One was the formation of diclofenac acid - proline, which succeeded in increasing diclofenac acid solubility up to 7.6 folds [5]. Co-crystal is defined as a mixture of two compounds with a stoichiometric equimolar ratio that interacts via hydrogen bonding or other weak forces [6, 7].
Various research developments have been carried out to overcome various solubility problems, and nanocrystalline formation is one of the desired methods. When a co-crystal is made on the nanoscale (nano-co-crystal), the solubility and dissolution rate can increase even further. This increase is not only due to the structure of the co-crystal but also because of its small size [8]. Modification by nano-co-crystal formation has been developed and used to optimize solubility as a solution to drug formulation, increase dissolution, and improve the absorption of drugs [9, 10, 11].
Nanonization means reducing the size of microparticles to the nano size, i.e., less than 1000 nm [2]. Smaller particle size will increase the surface area to increase solubility, dissolution rate, and bioavailability [10, 11, 12, 13, 14, 15, 16]. In addition, the advantages of the nano-particle size are high adhesion, applicability through all route administration, increased stability, and reduced dose requirements [9]. However, there is a limitation on the method for class II BCS drugs due to their polarity. Besides, the stability of co-crystals depends on the molecular structure of the starting compounds, so that not all nanonization produces nano-co-crystals with good quality and needs appropriate optimization methods [15, 17].
There are three approaches used in nano-co-crystal production, i.e., the bottom-up and top-down methods and a combination of the two [18, 19, 20, 21, 22]. The top-down process reduces the size of crystal particles to nano-size using shear forces or mechanical energy [23, 24, 25]. The bottom-up method is based on the formation crystal by dissolving the substance in a solvent and then allowing precipitation through evaporation or adding an anti-solvent [26, 27]. Lately, it has been found that microwaving can also be used as a nano-powder synthesis tool, especially for nanomaterials [28]. Microwaving has also been reportedly used as a method for co-crystal formation [29].
Several characterization techniques were needed to confirm the properties of nano-co-crystal. Particle size characterization was carried out by non-imaging technique dynamic light scattering (DLS) using particle size analyzer (PSA) instrument; the particle shape and morphology was carried out by imaging technique binocular microscope, scanning electron microscopy (SEM), and transmission electron microscopy (TEM) which can also confirm the particle size. Surface properties measured by zeta potential. The solid properties of nano-co-crystals were characterized by Fourier transform infrared (FTIR), powder x-ray diffraction (PXRD), and thermal analysis differential scanning calorimetry (DSC). The evaluation of physicochemical properties was tested by dissolution and diffusion studies to determine the advantages.
This study aimed to determine whether DPC can be produced on the nanoscale while maintaining the stability of the intermolecular bonds. The resulting NDPC was characterized and the dissolution and diffusion profiles of NDPC were tested and compared to those of regular-sized DPC. To the best of our knowledge, there have been no studies that have focused on the production of nano-co-crystal diclofenac-acid L-proline.
2. Materials and methods
2.1. Materials
Diclofenac sodium >99% (Merck, Tokyo, Japan), L-proline (Merck, Tokyo, Japan), hydrochloric acid/HCl (Merck, Berlin, Germany), potassium bromide Fourier-transform infrared spectroscopy (FTIR) grade (Merck, Tokyo, Japan), acetone, methanol, toluene, sodium chloride, PVP, ethanol, ethyl acetate, potassium hydrogen phosphate/KH2PO4, sodium hydroxide/NaOH, coconut oil, oleic acid, white vaseline, cholesterol, stearic acid, squalene, liquid paraffin, palmitic acid, olive oil, and potassium nitrate. All materials and solvents were purchased from Sigma-Aldrich, Merck, Indonesia.
2.2. Preparation of co-crystal and nano-co-crystal
2.2.1. Production of diclofenac acid-proline co-crystal (DPC)
Diclofenac acid-proline with stoichiometric molar ration (1:1) was mixed and ground for about 30 min while five drops ethanol were added to the mixture while grinding.
2.2.2. Optimization of diclofenac acid-proline nano-co-crystal production
The production of NDPC was developed by the top-down and bottom-up methods. The top-down method was performed by the wet milling and neat grinding procedures. For wet milling, 500 mg of DPC was placed in a mortar and mixed with PVP 5% (in ethanol) and 5% of tween-80 (in ethanol) while milling. Another preparation without tween-80 solution also was made for comparison. The almost clear mixture was put in a beaker glass and sonicated for 5, 10, 15, 20, and 30 min at 30 °C. Each resulting sonication at different times was evaluated by PSA (Beckman Coulter Delsa™ Nano C Particle Analyzer, Malvern, Southborough, MA).
For the neat grinding procedure, 1 g DPC was ground for about 8 h while adding several drops of ethanol 96%. At each h nano-co-crystal was sampled and then dissolved in sodium lauryl sulfate 0.1% w/w. The sample then was evaluated by PSA.
The bottom-up method was done by anti-solvent, globule inverse phase system, and fast evaporation procedure. For the anti-solvent procedure, PVP 5% (in ethanol 90%) was sonicated (WiseClean WUC-D06H, Gangwon-do, South Korea) and 250 mg DPC was dissolved in ethanol 96% and injected to PVP solution while sonicating for about 10 min. Then, it was dropped with 2 mL ethanol 50% while sonicating again for 15 min for the inversion phase process. The resulting mixture was evaluated by particle size analyzer.
For the inverse globule inverse phase, in a three-necked separable flask equipped with magnetic stirrer, thermometer, and dropping funnel, 40 mL cyclohexane was placed and heated on a water bath 50 °C. Then 50 mg DPC was dissolved with 2 mL methanol pro analysis, placed in dropping funnel, and dropped to the cyclohexane followed by mechanical stirring 1600 rpm for 5 min. The resulting mixture was sonicated for about 10 min at 30 °C, and then at room temperature; the globule size was measured by particle size analyzer. The procedure was repeated for mixing and sonicating times of 10, 15, 20 and 30 min.
For the fast evaporation procedure, diclofenac acid - L-proline (1:1) was diluted in ethanol, sonicated, filled in a petri dish, and placed in a freezer to gain the supersaturating condition, before being recrystallized rapidly with a magnetic stirrer at 350 rpm and heat of 40 °C; this process was repeated three times. Then it was microwaved (Microwave SHARP, R-230R(S), Tokyo, Japan) for about 10 min. The resulting nano-co-crystal was evaluated by PSA.
2.3. Kinetics of nano-co-crystallization during microwaving
After the fast evaporation procedure, the sample was microwaved at a power of 776W. It was sampled every minute until the sample melted. The sample was then analyzed quantitatively using FTIR. The AUC (Area under the Curve) of the derived spectra at the selected wavelength was measured to determine the kinetics of nano-co-crystal formation.
2.4. Characterization of NDPC by FTIR, PXRD, and DSC
NDPC and DPC were characterized by FTIR using potassium bromide pellet method. The FTIR instrument used was an infrared spectrophotometer (Jasco FT/IR 4200 type A, Tokyo, Japan); the range was set from 4000 cm−1 to 400 cm−1 with a resolution of 16 cm−1.
For PXRD analysis, the sample was ground and placed between Mylar films prior to analysis by the PXRD instrument. The PXRD instrument included a Smartlab X-ray diffractometer (Rigaku Corporation, Tokyo, Japan). The pattern was collected from 2θ = 2°–40° at 25 °C at a scan rate of 0.01° and 3°/min, using a Cu-Kα source at 45 kV and 200 mA.
DSC characterization used a Thermo plus DSC 8230L (Rigaku Corporation, Tokyo, Japan). The sample was heated at a rate of 10°/min from 25 to 300 °C under a nitrogen purge at 100 mL/min.
2.5. Particle size and surface analysis
The average particle size, polydispersity index, and zeta potential of the nano-co-crystals were determined by Beckman Coulter Delsa™ Nano C Particle Analyzer (Malvern, Southborough, MA) at 25 °C with angle 165°. All samples were diluted with sodium lauryl sulfate (SLS) 0.1% w/w solution before analysis and averaged over a set of three measurements.
The samples were put on object glass and viewed using a binocular microscope. The co-crystal morphology was observed using a VSZ-107BN (Jakarta, Indonesia) microscope with a magnification of 100 times.
Morphological evaluation was captured by SEM (JEOL JSM-IT300, Tokyo, Japan) at the accelerating energy of 15.0 kV and magnification 10,000:1. The sample was coated first with carbon coating during preparation using JEOL EC-32010CC Carbon Coater. Morphological evaluation was also performed using transmission electron microscopy TEM HT7700 (Hitachi, Japan) at 80 kV. The sample was dispersed in the solvent, and one drop of the sample was put into the carbon support film on copper before putting it into the instrument.
2.6. Dissolution study
A dissolution test was performed using dissolution tester type-2 Guoming RC-1 (Tianjin, China), carried out for the co-crystal and nano-co-crystal samples in three media, i.e., gastric condition (pH 1.2) and intestinal conditions (pH 6.8 and pH 7.4). Then the absorbance was measured using a diode array spectrophotometer HP/Agilent 8453 (California, USA) at λ = 276 nm.
The pH 1.2 buffer medium was prepared from 0.1 M HCl with CO2-free distilled water. The pH 6.8 buffer medium was prepared from 50 mL of 0.2 M KH2PO4 with 22.4 mL 0.2 M NaOH. Meanwhile, the pH 7.4 medium was prepared from 50 mL of 0.2 M KH2PO4 with 41.7 mL 0.2 M NaOH solution. Both phosphate-buffered media were added by 200 mL CO2-free distilled water and adjusted until to the targeted pH.
2.7. Diffusion study
A Franz's diffusion cell with the sample compartment area of 1.5 cm was prepared by School of Pharmacy, Bandung Institute of Technology, Indonesia. The artificial membrane was made from Whatman paper grade 1 (pore's diameter = 11 μm). Whatman papers were cut into circle shapes with a diameter adjusted to the sample plate of diffusion cell dimension and then were impregnated with Spangler's synthetic sebum. This artificial sebum was consisted of (w/w): coconut oil 15%, 15% oleic acid, 15% white vaseline, 5% cholesterol, 5% stearic acid, 5% squalene, 10% liquid paraffin, 10% palmitic acid, and 20% olive oil. All ingredients were melted, and then the Whatman papers were inserted into the liquid for 15 min. Next, the Whatman papers were lifted and placed between the two dry papers so that the Spangler's sebum liquid excess could be absorbed, and then it was dried for one night (12h). To ensure the uniformity of membranes, each artificial membrane was weighed before and after being impregnated. The papers with weight differences of 61 ± 3.01 mg were chosen (based on the mean ± standard deviation from the calculation of 50 membrane's weight, P = 0.05).
The diffusion test was carried out also in three media, gastric conditions (pH 1.2), intestinal conditions (pH 6.8 and pH 7.4), as that was used in the dissolution test. The Franz diffusion cell was set with a speed of 30 rpm at 37 °C for 8 h using Pump Pro (Watson-Marlow, Wilmington, MA). The test substance was sampled at 1, 2, 3, 4, 5, 6, 7, 8 h, and then the absorbance was measured using a diode array spectrophotometer HP/Agilent 8453 (California, USA) at λ = 276 nm.
3. Results and discussion
3.1. Optimization of nano-co-crystal production
Diclofenac acid nanocrystals were successfully produced and exhibited a nano-sized crystal of 279 nm [30]. However, NDPC production has never been developed before. Diclofenac acid is a non-polar compound and soluble in non-polar solvents. In contrast, L-proline is a polar amino acid zwitterion that is soluble in polar solvents such as water [31].
Optimization was done through the top-down and bottom-up methods. Top-down nano-co-crystal production was carried out through wet milling and neat grinding procedures. In the wet milling procedure, PVP K30 was used as a stabilizer. PVP K30 has been successfully used in the production of nanocrystals and nanosuspensions as stabilizers [32, 33]. The wet milling procedure produced a particle size of 515.5 nm with a polydispersity index (PI) of 0.215 after 30 min of milling.
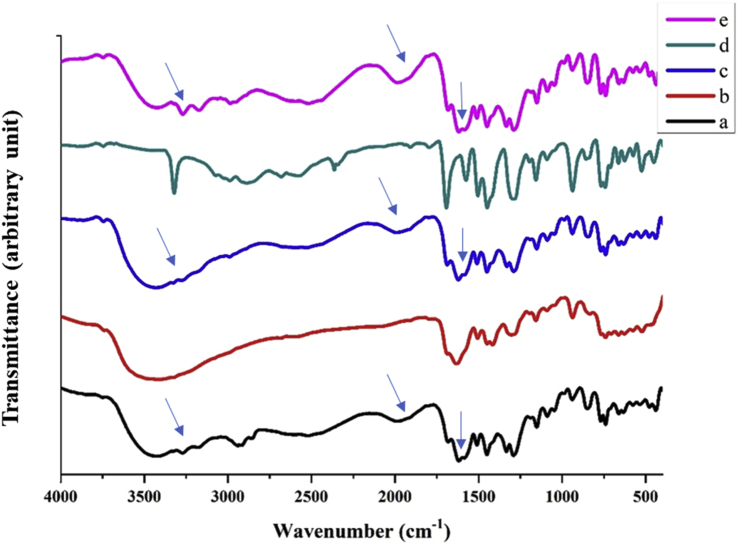
However, as seen from Figure 1b, the wet milling procedure did not have the same FTIR spectra as DPC (Figure 1a), meaning that the nano-co-crystal formed as fractions between diclofenac acid and proline crystals. The distinctive bands of co-crystals are ticked by a blue arrow on the wavelength of 3270-3170 cm−1, 1605 cm−1, and 1994 cm−1 (Figure 3) [5, 29]. Disintegration happened due to solvation, as well as the milling process. Diclofenac and L-proline have very different polarity, so it was not easy to produce stable co-crystal DPC when nano-sized. When the component of a co-crystal has a widely different solubility in aqueous solutions, it leads to the disintegration of the co-crystal [34, 35].
Figure 1.
Crystalline properties optimization of a) DPC; b) NDPC wet milling procedure; c) NDPC from neat grinding; d) NDPC from globule inversion phase procedure; e) NDPC from fast evaporation procedure. Blue arrow ticked the distinctive bands of cocrystal on 3270, 3170, 1994, and 1606 cm−1.
Figure 3.
FTIR spectra of a) the physical mixture diclofenac acid and L-proline; b) DPC; c) NDPC before microwaving; d) NDPC after microwaving.
The neat grinding procedure produced the smallest particle size after grinding for 6 h with particle sizes of 857.9 nm and a polydispersity index of 0.353. After 6 h, the particle size enlarged due to particle aggregation. Based on Figure 1c, the crystalline properties of this procedure show the properties’ similarity to DPC (Figure 1a) as the starting material. The neat grinding procedure produced a pure co-crystal because there was no dissolution process to separate each co-crystal component. However, this procedure was considered inadequate to produce the expected nano-size co-crystal. Nano-co-crystal production has been reported with the neat grinding procedure conducted by Hong et al. in 2015, but produced different particle sizes, from nano-to micro-size, due to co-crystal aggregation; this also had a negative impact on size uniformity [36, 37]. Also, the limitation of the top-down method is that the crystallinity decreases become unstable [38].
While the bottom-up method was carried out through phase inversion globules and fast evaporation assisted by microwaving, the phase inversion globule procedure provided a globular size of 310.3 nm and PI of 0.316. The particle size of NDPC was smaller than the globule because the particles were inside the globule. However, based on Figure 1d, this procedure also yielded nanocrystalline properties that were different from those of DPC (Figure 1a). This fraction also happened because of solvation and the rapid mixing process. The bottom-up procedure was done to reduce the agglomeration that occurred in the top-down method, but it was challenging to select the best solvents to maintain co-crystal stability considering the difference in solubility between the components is very different [36, 39].
The fast evaporation procedure produced nanocrystals with a size of 598.2 nm and a PI of 0.278. This procedure was assisted by microwaving and delivering a consistent nano-co-crystal with the same profile as DPC (Figure 1e) with an optimum co-crystal peak and relatively fixed particle size.
In this process, ethanol was used, and due to the dissolution and rapid stirring process in this procedure, the resulting nano-co-crystal also underwent separation. Therefore, a new process was carried out by microwaving the sample at a 776 W power to help bind the intermolecular interaction in the co-crystal; microwave energy gives molecular rotation, so the interaction between co-crystal components was easier and faster [29].
This microwaving method has also often been used in the process of making nanomaterials, but drug development using the microwaving process still does not exist [28]. It has been reported that chemical reactions by microwaving heating technology represent sustainable “green” chemistry by utilizing safer solvents and reaction conditions, preventing the waste of products because only a small amount of solvent is required for this process, and minimizing the time of the reaction [28, 29]. The optimization data resulted in Table 1.
Table 1.
Optimization procedure NDPC production.
| Procedure | Diameter size (nm) | PI | Crystalline properties |
|---|---|---|---|
| Wet milling | 515.5 | 0.215 | Physical mixture |
| Neat grinding | 857.9 | 0.353 | Co-crystal |
| Phase inversion globule | 310.0 | 0.316 | Physical mixture |
| Fast evaporation + microwaving | 598.2 | 0.278 | Co-crystal |
3.2. Kinetics of nano-co-crystallization during microwaving
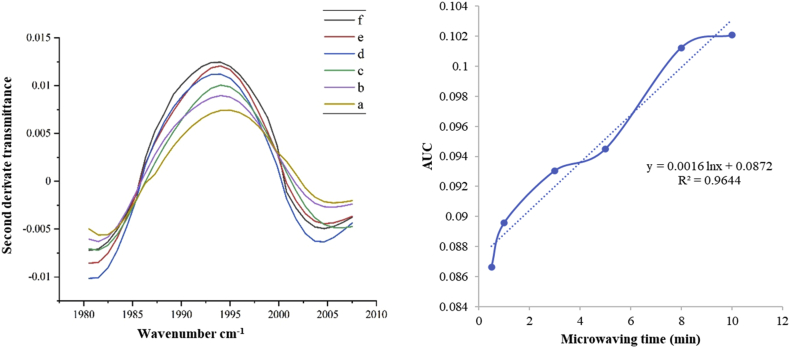
As seen in Figure 2, the absorbance of the co-crystal peak showed an increase in the AUC value of derived absorbance of the co-crystal peak with microwaving time until 12 min. The increase in AUC can be seen in Figure 2 (left). The kinetic study of NDPC shows that the optimum microwaving time was about at 8–12 min, indicated by the highest intensity of the AUC at the distinctive co-crystal peak. The selected range of wavelength number was 1980 cm−1 to 2007 cm−1 and exhibited the best logarithmic line equation, y = 0.0016 ln x + 0.0872, with a correlation coefficient of 0.9644. Nano-co-crystal formation kinetics followed the first order kinetics (Figure 2 - right).
Figure 2.
Second derivate FTIR transmittance spectra of NDPC during microwaving time of a) 30 s; b) 1 min; c) 3 min; d) 5 min; e) 8 min; f) 10 min (left); regression line obtained from the data (right).
3.3. Analysis of NDPC by FTIR, PXRD, and DSC
The nano-co-crystal before microwaving had the same FTIR spectra as the physical mixture, i.e., it presented diclofenac acid at 3324 cm−1 (O–H functional group) and L-proline peaks at 1619 cm−1 (C=O functional group) rather than the co-crystal peak. NDPC after microwaving showed the same distinctive peak as the co-crystal at wavenumbers 3270 cm−1, 3170 cm−1, 1994 cm−1, and 1605 cm−1 (Figure 3) [5,29].
These band changes represented the stretching of the new hydrogen bonds that formed in both co-crystals and nano-co-crystals with fast evaporation assisted by microwaving.
Figure 4 shows that NDPC after microwaving has the identical PXRD distinctive peak as DPC at 2θ = 4.40°, 9.73°, 11.58°, 13.23°, 14.47°, 19.52°, 20.5°, and 25.50° [5, 29]. On the other hand, the NDPC diffractogram before microwaving shows diclofenac peaks at 2θ = 10.71°, 13.45°, 18.84°, 24.40°, and L-proline peaks at 15.2°, 17.8°, which indicates disintegration of the co-crystal as they were identical to the physical mixture diffractogram [5, 29].
Figure 4.
PXRD diffractogram of a) the physical mixture diclofenac acid and L-proline; b) DPC; c) NDPC before microwaving; d) NDPC after microwaving.
The DSC profile is used to confirm the resulting nano-co-crystal component. The DSC profile for the DPC, NDPC before and after microwaving are presented in Figure 5. All had the same melting point at ~155 °C, confirmed in our previous report [5, 29]. The data proved that both the co-crystal and nano-co-crystal had a similar crystal structure.
Figure 5.
DSC of a) diclofenac acid; b) L-proline; c) NDPC before microwaving; d) DPC; e) NDPC after microwaving.
3.4. Particle size analysis and zeta potential
The fast evaporation assisted by microwaving exhibited a mean NDPC particle size of 598.2 ± 63.2 nm and a poly dispersion index (PDI) of 0.278 ± 0.062. The PDI value indicated the good distribution of the particle size. As for coarse co-crystal powder, the particle size was found to be 4–14 μm. The zeta potential value for NDPC in the SLS solution was -66.0 mV, indicating a stable particle that did not undergo agglomeration. However, the result of this negative charge can also be due to the addition of SLS as a dispersing solution because the SLS gives a negative charge to the zeta potential [40].
3.5. Surface morphology
Binocular microscope, combined with camera visualization, is used for imaging the form of the co-crystal of DPC itself. The SEM results in Figure 6b show NDPC within the nano-range. The smallest size width was 400 nm and length 400 nm, while the biggest size width was 400 nm and length 1000 nm. The TEM test results based on Figures 6c and 6d show images of the nano-co-crystal in the globule as a carrier and the nano-co-crystal outside the globule. Figure 6 also shows that the smallest nano-co-crystal size was around 100 nm in width and 350 nm in length. This result shows DPC to be within the nano-range and proper distribution.
Figure 6.
Image of a) binocular microscope of DPC (100x); b) SEM of NDPC; c) TEM of NDPC within globule, d) TEM of NDPC outside the globule. NDPC was produced by fast evaporation assisted by the microwaving method.
3.6. Dissolution test
The dissolution profiles of the co-crystal and nano-co-crystal of diclofenac acid-proline are presented in Figure 7. The data represents the percentage of drug release of the co-crystal and nano-co-crystal in each medium at 60 min. It can be seen in Figure 7a and Table 2 that the dissolution test results at pH 1.2 medium showed an increase in the number of substances dissolved in the nano-size range. The increase was not too different, i.e., from 3.08 ± 0.58% to 4.09 ± 0.68%. Diclofenac acid was difficult to dissolve in acidic solvents because there is no protonation process [41].
Figure 7.
Dissolution profiles of DPC and NDPC in medium of aqueous a) pH 1.2 buffer; b) pH 6.8 phosphate buffer; c) pH 7.4 phosphate buffer. Data are expressed as mean ± standard deviation (n = 3).
Table 2.
Drug release of NDPC compared to DPC (n = 3).
| Sample | Percentage of drug release after 60 min (%) in medium |
||
|---|---|---|---|
| pH 1.2 buffer | pH 6.8 buffer | pH 7.4 buffer | |
| DPC | 3.08 ± 0.58 | 85.09 ± 0.16 | 42.55 ± 0.25 |
| NDPC | 4.09 ± 0.68 | 97.00 ± 0.25 | 100.57 ± 0.10 |
The dissolution test results in the pH 6.8 phosphate buffer medium (Figure 7b) showed an increase in the amount of dissolution for NDPC, which was 97.00 ± 0.25% compared to DPC of 85.09 ± 0.16%. The dissolution test results in the pH 7.4 phosphate buffer medium (Figure 7c) showed that there was also an increase in the dissolution percentage from 42.55 ± 0.25% to 100.57 ± 0.10% for DPC and NDPC, respectively. This result indicates a 1.32-fold increase in the NDPC drug release in pH 1.2 buffer, 1.14-fold in pH 6.8 buffer, and 2.46-fold in pH 7.4 buffer. The dissolution study show the enhancement of the drug release, because nano-sized particles have a large surface area in contact with the medium, thereby increasing the solubility, can enhance the dissolution rate and solubility of an insoluble drug [42, 43]. The percentage of drug dissolution with DPC did not reach the same level as with NDPC because the nano-co-crystal had higher saturation solubility [43].
3.7. Diffusion test
The result of the diffusion test in pH 1.2, 6.8, and 7.4 buffer media displayed the increase of percentage drug diffused of NDPC compared to micro-size DPC. As shown in Figure 8 and Table 3, the result indicated that NDPC diffused by 1.64-fold in pH 1.2, 2.06-fold in pH 6.8, and 1.71-fold in pH 7.4. NDPC showed a higher percentage than micro-size DPC. This enhancement also was caused by the smaller particle sizes obtained by nano-co-crystal formation.
Figure 8.
Diffusion profiles of DPC and NDPC (n = 3).
Table 3.
Drug diffused of NDPC compared to DPC (n = 3).
| Sample | Percentage of drug diffuse after 8h (%) in medium |
||
|---|---|---|---|
| pH 1.2 buffer | pH 6.8 buffer | pH 7.4 buffer | |
| DPC | 3.30 ± 0.10 | 17.85 ± 0.44 | 57.04 ± 1.92 |
| NDPC | 5.44 ± 0.20 | 36.67 ± 0.71 | 97.59 ± 1.23 |
4. Conclusion
NDPC, with the nano-size and a stable co-crystal structure of diclofenac – proline, was successfully produced using the fast evaporation assisted by the microwaving method. This new nano-co-crystal showed the enhancement of the drug released and drug diffused percentages compared to the DPC coarse powder.
Declarations
Author contribution statement
I. Nugrahani and W.N. Auli: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research was supported by the Collaboration Overseas Research Program, No. 026/WCU-ITB/LL/2019, Institute of Research and Community Service, Bandung Institute of Technology, Indonesia.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Castellari C., Ottani S. Two monoclinic forms of diclofenac acid. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1997;53(6):794–797. [Google Scholar]
- 2.Pireddu R., Sinico C., Ennas G., Marongiu F., Muzzalupo R., Lai F., Fadda A.M. Novel nanosized formulations of two diclofenac acid polymorphs to improve topical bioavailability. Eur. J. Pharmaceut. Sci. 2015;77:208–215. doi: 10.1016/j.ejps.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Goh C.F., Lane M.E. Formulation of diclofenac for dermal delivery. Int. J. Pharm. 2014;473(1-2):607–616. doi: 10.1016/j.ijpharm.2014.07.052. [DOI] [PubMed] [Google Scholar]
- 4.Peltonen L., Hirvonen J. Physicochemical characterization of nano- and microparticles. Curr. Nanosci. 2008;4(1):38–43. [Google Scholar]
- 5.Nugrahani I., Utami D., Ibrahim S., Prasetya Y.P., Uekusa H. Zwitterionic co-crystal of diclofenac and L-proline: structure determination, solubility, kinetics of co-crystallization, and stability study. Eur. J. Pharmaceut. Sci. 2018;117:168–176. doi: 10.1016/j.ejps.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Remenar J.F., Morissette S.L., Peterson M.L., Moulton B., MacPhee J.M., Guzmán H.R. Crystal engineering of novel co-crystals of a triazole drug with 1,4-dicarboxylic acids. J. Am. Chem. Soc. 2003;125(28):8456–8457. doi: 10.1021/ja035776p. [DOI] [PubMed] [Google Scholar]
- 7.Brittain H.G. Pharmaceutical co-crystals: the coming wave of new drug substances. J. Pharmacol. Sci. 2013;102(2):311–317. doi: 10.1002/jps.23402. [DOI] [PubMed] [Google Scholar]
- 8.Peltonen L. Practical guidelines for the characterization and quality control of pure drug nanoparticles and nano-cocrystals in the pharmaceutical industry. Adv. Drug Deliv. Rev. 2018;131:101–115. doi: 10.1016/j.addr.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Kaur J., Kumar S. Development of nanocrystal formulation with improved dissolution. J. Drug Deliv. Therapeut. 2018;8(5):118–129. [Google Scholar]
- 10.Armentano I., Bitinis N., Fortunati E., Mattioli S., Rescignano N., Verdejo R., Lopez-Manchado M.A., Kenny J.M. Multifunctional nanostructured PLA materials for packaging and tissue engineering. Prog. Polym. Sci. 2013;38(10-11):1720–1747. [Google Scholar]
- 11.Junyaprasert V.B., Morakul B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. J. Pharm. Sci. Asian. 2015;10(1):13–23. [Google Scholar]
- 12.Chen H., Khemtong C., Yang X., Chang X., Gao J. Nanonization strategies for poorly water-soluble drugs. Drug Discov. Today. 2011;662(7-8):1–7. doi: 10.1016/j.drudis.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Gatoo M.A., Naseem S., Arfat M.Y., Dar A.M., Qasim K., Zubair S. Physicochemical properties of nanomaterials: implication in associated toxic manifestations. BioMed Res. Int. 2014;2014:1–8. doi: 10.1155/2014/498420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patravale V.B., Date A.A., Kulkarni R.M. Nanosuspensions: a promising drug delivery strategy. J. Pharm. Pharmacol. 2004;56(7):827–840. doi: 10.1211/0022357023691. [DOI] [PubMed] [Google Scholar]
- 15.Yan D., Bučar D.K., Delori A., Patel B., Lloyd G.O., Jones W., Duan X. Ultrasound-assisted construction of halogen-bonded nanosized co-crystals that exhibit thermosensitive luminescence. Chem. Eur J. 2013;19(25):8213–8219. doi: 10.1002/chem.201203810. [DOI] [PubMed] [Google Scholar]
- 16.Yan D. Micro-/nanostructured multicomponent molecular materials: design, assembly, and functionality. Chem. Eur J. 2015;21(13):4880–4896. doi: 10.1002/chem.201405456. [DOI] [PubMed] [Google Scholar]
- 17.Gigliobianco M.R., Casadidio C., Censi R., Martino P.D. Nanocrystals of poorly soluble drugs: drug bioavailability and physicochemical stability. Pharmaceutics. 2018;10(3):134. doi: 10.3390/pharmaceutics10030134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shegokar R., Müller R.H. Nanocrystals: industrially feasible multifunctional formulation technology for poorly soluble active. Int. J. Pharm. 2010;399(1-2):129–139. doi: 10.1016/j.ijpharm.2010.07.044. [DOI] [PubMed] [Google Scholar]
- 19.Waard H.d., Frijlink H.W., Hinrichs W.L.J. Bottom-up preparation techniques for nanocrystals of lipophilic drugs. Pharm. Res. (N. Y.) 2011;28(5):1220–1223. doi: 10.1007/s11095-010-0323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabinow B.E. Nanosuspensions in drug delivery. Nat. Rev. Drug Discov. 2004;3(9):785–796. doi: 10.1038/nrd1494. [DOI] [PubMed] [Google Scholar]
- 21.Ranjan S., Dasgupta N., Chakraborty A.R., Samuel S.M., Ramalingan C., Shanker R., Kumar A. Nanoscience and nanotechnologies in food industries: opportunities and research trends. J. Nano Res. 2014;16(6):2464. [Google Scholar]
- 22.Bailey M.M., Berkland C.J. Nanoparticle formulations in pulmonary drug delivery. Med. Res. Rev. 2009;29(1):196–212. doi: 10.1002/med.20140. [DOI] [PubMed] [Google Scholar]
- 23.Salazar J., Ghanem A., Müller R.H., Möschwitzer J.P. Nanocrystals: comparison of the size reduction effectiveness of a novel combinative method with conventional top-down approaches. Eur. J. Pharm. Biopharm. 2012;81(1):82–90. doi: 10.1016/j.ejpb.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Biswas A., Bayer I.S., Biris A.S., Wang T., Dervishi E., Faupel F. Advances in top-down and bottom-up surface nanofabrication: techniques, applications & future prospect. Adv. Colloid Interface Sci. 2012;170(1-2):2–27. doi: 10.1016/j.cis.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Friščić T., Jones W. Recent Advances in understanding the mechanism of co-crystal formation via grinding. Cryst. Growth. 2009;9(3):1621–1637. [Google Scholar]
- 26.Dickinson P.A., Howells S.W., Kellaway I.W. Novel nanoparticles for pulmonary drug administration. J. Drug Target. 2001;9(4):295–302. doi: 10.3109/10611860108997937. [DOI] [PubMed] [Google Scholar]
- 27.Chen X., Young T.J., Sarkari M., Williams R.O., 3rd., Johnston K.P. Preparation of cyclosporine nanoparticles by evaporative precipitation into aqueous solution. Int. J. Pharm. 2002;242(1-2):3–14. doi: 10.1016/s0378-5173(02)00147-3. [DOI] [PubMed] [Google Scholar]
- 28.Dabrowska S., Chudoba T., Wojnarowicz J., Łojkowski W. Current trends in the development of microwaving reactors for the synthesis of nanomaterials in laboratories and industries: a review. Crystals. 2018;379(8):1–26. [Google Scholar]
- 29.Nugrahani I., Utami D., Ayuningtyas L., Garmana A.N., Oktaviary R. New preparation method using microwave, kinetics, in vitro dissolution- diffusion, and anti-inflammatory study of diclofenac- proline co–crystal. Chemistry. 2019;4(45):13396–13403. [Google Scholar]
- 30.Pireddu R., Caddeo C., Valenti D., Marongiu F., Scano A., Ennas G., Lai F., Fadda A.M., Sinico C. Diclofenac acid nanocrystals as an effective strategy to reduce in vivo skin inflammation by improving dermal drug bioavailability. Colloids Surf. B Biointerfaces. 2018;143:64–70. doi: 10.1016/j.colsurfb.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 31.Jensen K., Lobmann K., Rades T., Grohganz H. Improving co-amorphous drug formulations by the addition of the highly water soluble amino acid, proline. Pharm. Times. 2014;6(3):416–435. doi: 10.3390/pharmaceutics6030416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bastami Z., Taheri A., Soltanpour S. Formulation, optimization and characterization of gemfibrozil nanocrystals prepared by wet milling technique. Asian J. Pharm. 2015;9(1):19–22. [Google Scholar]
- 33.Ma J., Yang Y., Sun Y., Sun J. Optimization, characterization and in vitro/vivo evaluation of azilsartan nanocrystals. Asian J. Pharm. Sci. 2017;12(4):344–352. doi: 10.1016/j.ajps.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung H.R., Kwon E., Oikawa H., Kasai H., Nakanishi H. Effect of solvent on organic nanocrystal growth using the reprecipitation method. J. Cryst. Growth. 2006;294(2):459–463. [Google Scholar]
- 35.Karashima M., Kimoto K., Yamamoto K., Kojima T., Ikeda Y. A novel solubilization technique for poorly soluble drugs through the integration of nanocrystal and co-crystal technologies. Eur. J. Pharm. Biopharm. 2016;107:142–150. doi: 10.1016/j.ejpb.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Liu M., Hong C., Li G., Ma P., Xie Y. The Generation of myricetin–nicotinamide nanococrystals by top down and bottom up technologies. Nanotechnology. 2016;27(39):395601. doi: 10.1088/0957-4484/27/39/395601. [DOI] [PubMed] [Google Scholar]
- 37.Hong C., Xie Y., Yao Y., Li G., Yuan X., Shen H. A novel strategy for pharmaceutical co-crystal generation without knowledge of stoichiometric ratio: myricetin co-crystals and a ternary phase diagram. Pharm. Res. (N. Y.) 2015;32(1):47–60. doi: 10.1007/s11095-014-1443-y. [DOI] [PubMed] [Google Scholar]
- 38.Van Eerdenbrugh B., Van den Mooter G., Augustijns P. Top-down production of drug nanocrystals: nanosuspension stabilization, miniaturization and transformation into solid products. Int. J. Pharm. 2008;364(1):64–75. doi: 10.1016/j.ijpharm.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 39.Huang Y., Li J.M., Lai Z.H., Wu J., Lu T.B., Chen J.M. Phenazopyridine-phthalimide nano-cocrystal: release rate and oral bioavailability enhancement. Eur. J. Pharmaceut. Sci. 2017;109:581–586. doi: 10.1016/j.ejps.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 40.Ochi M., Kawachi T., Toita E., Hashimoto I., Yuminoki K., Onoue S., Hashimoto N. Development of nanocrystal formulation of meloxicam with improved dissolution and pharmacokinetic behaviors. Int. J. Pharm. 2014;474(1-2):151–156. doi: 10.1016/j.ijpharm.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 41.Streubel A., Siepmann J., Dashevsky A., Bodmeier R. pH-independent release of a weakly basic drug from water-insoluble and -soluble matrix tablets. J. Contr. Release. 2000;67(1):101–110. doi: 10.1016/s0168-3659(00)00200-5. [DOI] [PubMed] [Google Scholar]
- 42.Nagarwal R.C., Kumar R., Dhanawat M., Das N., Pandir J.K. Nanocrystal technology in the delivery of poorly soluble drugs: an overview. Curr. Drug Deliv. 2011;8(4):398–406. doi: 10.2174/156720111795767988. [DOI] [PubMed] [Google Scholar]
- 43.Müller R.H., Junghanns J.A.H. Nanocrystal technology, drug delivery and clinical applications. Int. J. Nanomed. 2008;3(3):295–309. doi: 10.2147/ijn.s595. [DOI] [PMC free article] [PubMed] [Google Scholar]