Abstract
Density functional theory (DFT) free energy data and the reaction mechanism of the rhodium(triphenylphosphine)carbonyl-2,4-dioxo-3-pentyl-4-hydroxybenzoate plus iodomethane reaction are presented. The rhodium(I) reactant is a simplified model of the rhodium(I) of the rhodium(triphenylphosphine)carbonyl-2,4-dioxo-3-pentyl-4-decanyloxybenzoate plus iodomethane reaction (full model), presented in the related research article “Rhodium(triphenylphosphine)carbonyl-2,4-dioxo-3-pentyl-4-decanyloxybenzoate: A DFT study of Oxidative Addition and Methyl Migration” [1]. The goal is to illustrate that DFT calculations of a simplified model give the same information regarding the reaction scheme and free energy data as for the full model, while it requires much less computational resources to obtain the data. Furthermore the reaction scheme of the simplified model are in agreement with experimental observation of the full model [2].
Keywords: Rhodium, Oxidative addition, DFT, CO insertion, Methyl migration
Specifications Table
| Subject | Physical and Theoretical Chemistry |
| Specific subject area | DFT calculations of chemical reaction kinetics. |
| Type of data | Image Graph Figure |
| How data were acquired | Electronic structure calculations, using the Amsterdam Density Functional (ADF) 2018 programme. |
| Data format | Raw Analyzed |
| Parameters for data collection | Default criteria for calculations as implemented in the ADF 2018 programme were used, with the implicit solvent model COSMO, the BP86 functional with D3 dispersion correction and all electron STO- ZORA/TZP basis set, computing analytical frequencies. |
| Description of data collection | Data were collected from DFT output files. |
| Data source location | High-Performance Computor, University of the Free State, Nelson Mandela Street, Bloemfontein, South Africa. High-Performance Computor (Stallo), UiT – The Arctic University of Norway, Tromsø N-9037, Norway. |
| Data accessibility | With the article. |
| Related research article | M.M. Conradie, Rhodium(triphenylphosphine)carbonyl-2,4-dioxo-3-pentyl-4-decanyloxybenzoate: A DFT study of Oxidative Addition and Methyl Migration, Inorganica Chim. Acta. (2020) In Press. https://doi.org/10.1016/j.ica.2020.119954 |
Value of the Data
-
•
Oxidative addition reactions has application in catalysis, such as the Monsanto process, where a rhodium catalyst reacts with methyl iodide. Free energy data and understanding of related reactions between rhodium and methyl iodide may lead to the development of more efficient catalysts.
-
•
Data of free energy involved in model catalytic cycles is vital for industry, while theoretical chemists may use the data for the design of alternative cheaper catalysts.
-
•
Comparison of the free energy data of a simplified model reaction mechanism versus the full model reaction mechanism, suggest that the simplified rhodium complex should have a similar experimental behaviour as the full large experimental rhodium molecule.
-
•
Free energy data of a simplified model reaction show in this case that the same information may be obtained by a simplified reactant as by a full large reactant, saving on computational resources.
1. Data Description
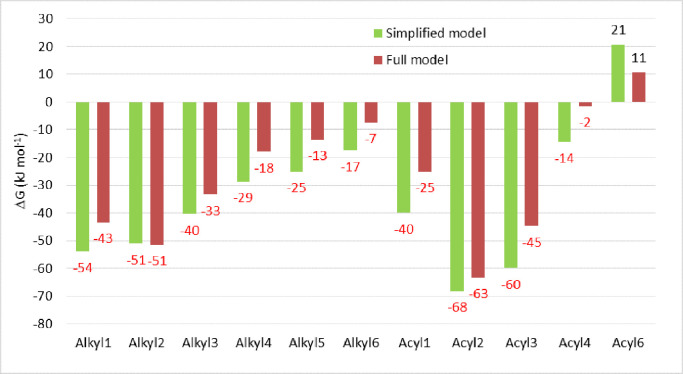
This data article provide free energies and the reaction mechanism of the [Rh(CH3COC(HOC6H4COO)COCH3)(CO)(PPh3)] + CH3I reaction, a simplified model system of the [Rh(CH3COC(C10H21OC6H4COO)COCH3)(CO)(PPh3)] + CH3I reaction described in the related research article [1], see Fig. 1 for the BP86-D3 optimized geometries of the simplified and full experimental rhodium(I) reactant (CH3I = methyliodide). Both the full and simplified reactant are of the type [Rh(β-diketonato)(CO)(PPh3)] of which various experimental [3], [4], [5], [6] and some theoretical studies [7], [8], [9] are available, however none of the other published [Rh(β-diketonato)(CO)(PPh3)] studies had a R3 substituent on the C3 position of the β-diketone H3C-CO-CHR3-CO-CH3. The free energies of the different rhodium(III) reaction products of the simplified and full experimental system shown in Fig. 2, are compared in Fig. 3. The same reaction scheme is obtained for the simplified model as for the full experimental model, see Scheme 1, that are in agreement with experimental observation of the full model [2]. The free energies involved in the transition states and different reaction products of the simplified and full experimental system are compared in Fig. 4. Tables with the free energy data as well as the optimized geometries are provided as supporting information. Free energy data of the full model is from the related research article [1].
Fig. 1.
DFT BP86-D3 optimized geometry of Rh(I) of the full experimental [1,2] and simplified model. Color code (online version): Rh (dark red), O (red), C (black), H (white), P (orange). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Geometries of the different Rh(III) products of the Rh(I) + CH3I reaction. Each product shown also has an enantiomer (mirror image). R3 = (HOC6H4COO) for the simplified model of this study and R3 = (C10H21OC6H4COO) for the full model from [1].
Fig. 3.
Relative BP86-D3 calculated free energies ΔG (kJ mol−1) of the different Rh(III)-alkyl and Rh(III)-acyl reaction products of the Rh(I) + CH3I reaction for the full experimental model [1,2] and the simplified model. G(Rh(I) + CH3I) is taken as zero. Acyl5 converged to acyl2. 1 eV = 96.485 kJ mol−1.
Scheme 1.
Schematic presentation of the DFT calculated different reaction steps of the Rh(I) + CH3I reaction. The movement of the applicable atoms in the transition states is indicated with red arrows. From reference [1] with stylistic changes. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 4.
Relative free energies ΔG (kJ mol−1) of the reactants, transition states and products of the Rh(I) + CH3I reaction for the full experimental model [1,2] and the simplified model. G(Rh(I) + CH3I) is taken as zero. 1 eV = 96.485 kJ mol−1.
2. Experimental design, materials and methods
DFT calculations were done as described in the related research article [1], namely the ADF 2018 (Amsterdam Density Functional) programme [10] with the BP86 functional, the Grimme's D3 dispersion correction and all electron STO- ZORA/TZP basis set as implemented in ADF. Solvent effects were taken into account in all calculations using the implicit COSMO model of solvation with methanol (ε0 = 32.6) as solvent. Analytical frequency analyses have been performed on all the solvent-optimized geometries to verify minima, TS geometries and to obtain free energies G. The geometries of the different molecules was constructed, using ChemCraft [11]. ChemCraft was also used to visualize the optimized geometries obtained. Free energies were obtained from the ADF output files, looking for “Gibbs free energy”. Example input files, as well as the optmized geometries are provided as supporting information.
Ethics statement
This work does not require any ethical statement.
Declaration of Competing Interest
The author declares that there is no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The author acknowledge financial support from the South African National Research Foundation (Grant numbers 108960). The CHPC of South Africa, the High Performance Computing facility of the UFS and the Norwegian Supercomputing Program (UNINETT Sigma2, Grant no. NN9684K) are acknowledged for computer time.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.dib.2020.106253.
Appendix. Supplementary materials
References
- 1.Conradie M.M. Rhodium(triphenylphosphine)carbonyl-2,4-dioxo-3-pentyl-4-decanyloxybenzoate: a DFT study of oxidative addition and methyl migration. Inorganica Chim. Acta. 2020 doi: 10.1016/j.ica.2020.119954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stuurman N.F., Buitendach B.E., Twigge L., Swarts P.J., Conradie J. Rhodium(triphenylphosphine)carbonyl-2,4-dioxo-3-pentyl-4-decanyloxybenzoate: synthesis, electrochemistry and oxidative addition kinetics. New J. Chem. 2018;42:4121–4132. doi: 10.1039/C7NJ05039A. [DOI] [Google Scholar]
- 3.Conradie J., Lamprecht G.J., Roodt A., Swarts J.C. Kinetic study of the oxidative addition reaction between methyl iodide and [Rh(FcCOCHCOCF3)(CO)(PPh3)]: Structure of [Rh(FcCOCHCOCF3)(CO)(PPh3)(CH3)(I)] Polyhedron. 2007;26:5075–5087. doi: 10.1016/j.poly.2007.07.004. [DOI] [Google Scholar]
- 4.Stuurman N.F., Conradie J. Iodomethane oxidative addition and CO migratory insertion in monocarbonylphosphine complexes of the type [Rh((C6H5)COCHCO((CH2)nCH3))(CO)(PPh3)]: Steric and electronic effects. J. Organomet. Chem. 2009;694:259–268. doi: 10.1016/j.jorganchem.2008.10.040. [DOI] [Google Scholar]
- 5.Basson S.S., Leipoldt J.G., Nel J.T. The oxidative addition of methyl iodide to β-diketonecarbonyltriphenylphosphinerhodium(I) complexes. Inorg. Chim. Acta. 1984;84:167–172. doi: 10.1016/S0020-1693(00)82403-2. [DOI] [Google Scholar]
- 6.Varshavsky Y.S., Cherkasova T.G., Buzina N.A., Bresler L.S. Spectral characteristics of products formed by reaction between Rhacac(PPh3)(CO) and methyl iodide. J. Organomet. Chem. 1994;464:239–245. doi: 10.1016/0022-328X(94)87280-5. [DOI] [Google Scholar]
- 7.Conradie J. Density functional theory calculations of Rh-β-diketonato complexes. Dalton Trans. 2015;44:1503–1515. doi: 10.1039/C4DT02268H. [DOI] [PubMed] [Google Scholar]
- 8.Conradie M.M., Conradie J. Methyl iodide oxidative addition to [Rh(acac)(CO)(PPh3)]: an experimental and theoretical study of the stereochemistry of the products and the reaction mechanism. Dalton Trans. 2011;40:8226–8237. doi: 10.1039/c1dt10271k. [DOI] [PubMed] [Google Scholar]
- 9.Conradie M.M., Conradie J. Methyl iodide oxidative addition to rhodium(I) complexes: a DFT and NMR study of [Rh(FcCOCHCOCF3)(CO)(PPh3)] and the rhodium(III) reaction products. S. Afr. J. Chem. 2008;61:102–111. [Google Scholar]
- 10.te Velde G., Bickelhaupt F.M., Baerends E.J., Fonseca Guerra C., van Gisbergen S.J.A., Snijders J.G., Ziegler T. Chemistry with ADF. J. Comput. Chem. 2001;22:931–967. doi: 10.1002/jcc.1056. [DOI] [Google Scholar]
- 11.CHEMCRAFT, (n.d.). http://www.chemcraftprog.com/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.