Abstract
Electroactive bacteria could perform bi-directional extracellular electron transfer (EET) to exchange electrons and energy with extracellular environments, thus playing a central role in microbial electro-fermentation (EF) process. Unbalanced fermentation and microbial electrosynthesis are the main pathways to produce value-added chemicals and biofuels. However, the low efficiency of the bi-directional EET is a dominating bottleneck in these processes. In this review, we firstly demonstrate the main bi-directional EET mechanisms during EF, including the direct EET and the shuttle-mediated EET. Then, we review representative milestones and progresses in unbalanced fermentation via anode outward EET and microbial electrosynthesis via inward EET based on these two EET mechanisms in detail. Furthermore, we summarize the main synthetic biology strategies in improving the bi-directional EET and target products synthesis, thus to enhance the efficiencies in unbalanced fermentation and microbial electrosynthesis. Lastly, a perspective on the applications of microbial electro-fermentation is provided.
Keywords: Unbalanced fermentation, Microbial electrosynthesis, Extracellular electron transfer, Synthetic biology
1. Introduction
Electroactive bacteria (EAB), including exoelectrogens and electrotrophs, could perform bidirectional electron transfer (BET) to exchange electrons and energy with environments, thus playing the central role in bioelectrochemical systems (BES). A conventional BES generally consists of anode, cathode and electroactive bacteria cells. BESs have been used in two ways including microbial fuel cells (MFCs) and microbial electrolysis cells (MECs) for current generation [1,2], value-added chemicals production [3,4], hydrogen production [5], and water desalination [6], etc.
Conventional fermentation usually produces chemicals that accompanied with formation of many by-products in order to achieve intracellular redox balance, and the accumulation or lack of intracellular reducing equivalents would block metabolic flow towards the product or cause thermodynamic limits [7]. Electro-fermentation (EF), including unbalanced fermentation and microbial electrosynthesis, has been proposed as a novel fermentation process, which could use electron transfer to promote substance metabolism via changing the intracellular redox state, such as the oxidation reduction potential (ORP) and the NADH/NAD+ ratio [7].
Electrons could be provided by either the microorganisms or the electrodes to overcome the metabolic limitations in anodic or cathodic EF, respectively. The oxidized product is generated when the electrode serves as the electron acceptor in anodic EF processes, which is referred as unbalanced fermentation (UF). Conversely, the electrode supplies electrons to achieve the conversion of extracellular electrons into intracellular reducing power to drive the biosynthesis of reduced products, which is named as cathodic electro EF or microbial electrosynthesis (MES). MES could generate value-added chemicals such as biofuels by reduction of greenhouse gas CO2, which is potentially a sustainable biotechnology to decrease the CO2 emission [3,8].
The bidirectional electron transfer plays a crucial role in EF process because electrons are the fundamental driving force of metabolic flux [9]. In this review, we firstly demonstrate the main EET mechanisms during EF, (i) direct EET based on cytochromes, nanowires, and other redox proteins and (ii) shuttle-mediated EET including self-secreted molecules (e.g., flavins, phenazines, and so on), exogenous electron transfer mediators (e.g., neutral red, methyl viologen, and so on) and primary metabolites (e.g., H2, formate, etc.) (Fig. 1). Then, we show representative applications of EF including UF and MES in various microorganisms based on different mechanisms of electron transfer. Finally, we summarize the main strategies based on synthetic biology or genetic engineering to promote the EF and provide future prospective for potential applications of EF.
Fig. 1.
Mechanisms of bidirectional extracellular electron transfer (EET) during the electro-fermentation process, including cytochromes, nanowires, and other redox proteins (such as ferredoxins) based direct electron transfer and redox mediators or primary metabolites mediated electron transfer. (A) Electrons generated by intracellular metabolism in the cytoplasm of microbes are transferred to the anode for oxidized chemicals production. Both c-type cytochromes and conductive pili (nanowires) have the ability to transfer electrons directly to extracellular electron acceptors. Electron mediators and artificial mediators can shuttle between electrode and microorganisms and exchange electrons between them through their own redox transformation. (B) Microbes uptake electrons from the cathode for reduced chemicals production. Both c-type cytochromes and ferredoxins have the ability to acquire electrons directly from extracellular electron donors. Primary metabolites and artificial mediators can shuttle between electrode and microorganisms and exchange electrons between them through their own redox transformation. RFox: oxidized riboflavin; RFred: reduced riboflavin; MVox: oxidized methyl viologen; MVred: reduced methyl viologen; NRox: oxidized neutral red; NRred: reduced neutral red.
2. The mechanisms of EET
The EET of electroactive bacteria allows microbes to drive their metabolism through interactions with minerals or electrodes. It has been found that most EABs can transfer electrons either directly or mediately between the interior and the exterior environments.
2.1. Direct EET
Direct EET can occur through a direct contact between the electroactive bacteria and an electrode interface or metals without involvement of any dissociative redox compounds. Direct EET can be achieved by cytochromes, nanowires and other redox proteins in different electroactive microorganisms, which will be reviewed, respectively.
2.1.1. Multi-heme cytochromes
Cell-surface exposed multi-heme cytochromes are the most prevalent proteins in EET pathways being responsible for both electrons deliver and uptake. The model metal-reducing bacterium Shewanella oneidensis and Geobacter sulfurreducens utilize transmembrane c-type cytochrome system to transfer electrons. In S. oneidensis, electrons are derived from carbon oxidation and entered the quinone pool, which could be oxidized by inner membrane-anchored dehydrogenase CymA. Then CymA delivers electrons to span a ternary MtrABC complex directly through FccA and STC in the periplasm [10]. Analogously, quinols are catalyzed to obtain electrons by cytochromes ImcH and CbcL in Geobacter sp, then the periplasmic cytochrome PpcA captures and transports electrons to the OmaB-OmbB-OmcB cytochromes complex. Some metal-oxidizing bacteria could obtain electrons through their member-bound cytochromes. The phototrophic Fe(II)-oxidizing bacterium Rhodopseudomonas palustris TIE-1 can use Fe(II) as an electron donor to fix CO2 through PioABC complex [11]. PioA and PioB oxidize Fe (II) extracellularly to obtain electrons, which were then transferred across the outer membrane to the high-potential iron protein PioC in periplasm [12]. Similarly, depending on the MtoABD cytochrome proteins, the metal-oxidizing bacterium Sideroxydans lithotrophicus ES-1 can capture electrons from extracellular Fe(II) to enable the autotrophic growth [13]. In this process, MtoA directly oxidizes Fe(II) to capture electrons, and the periplasmic cytochrome MtoD transfers electrons to CymA, providing energy for intracellular metabolism [14].
2.1.2. Nanowires
On mineral or electrode surfaces, electrons can be transported by respiring in biofilm. However, only on the biofilm layer next to these surfaces, electroactive bacteria can directly contact with the minerals or electrodes. G. sulfurreducens could produce a type IV pili composed by PilA protein that is response for the long-distance (>10 μm) direct electron transfer to solid metals and electrodes at far distances from cells [15]. However, a recent study based on cryoelectron microscopy indicated that the polymerized hexa-heme cytochromes OmcS are the real conduits for long-distance electron transport rather than pilA [16]. For S. oneidensis, the nanowires are extensions of the outer membranes that could perform direct EET via cytochromes MtrABC and OmcA [17].
2.1.3. Other redox proteins
Many other redox proteins in various microorganisms could also perform direct EET. Some acetogens and methanogens could use membrane-bound NADH: ferredoxin oxidoreductase complexes to promote electron transport, which are composed of redox-driven ion pumps to generate proton gradients [[18], [19], [20]]. The methanogenic archaeon Methanococcus maripaludis could produce surface-associated hydrogenases and formate dehydrogenases to mediate direct electron uptake [21].
2.2. Shuttle-mediated EET
Some redox-active chemical compounds, named as “electron shuttles” or “cofactors”, could enable indirect electron transport from the intracellular insoluble metals or electrodes in EABs. These diffusible redox compounds mainly include (1) cell self-excreted the small redox molecules; (2) artificial redox mediators such as neutral red and methyl viologen; (3) primary metabolites such as H2 and formate.
2.2.1. Self-excreted small molecules of cells
In Shewanella sp., flavin mononucleotide (FMN) and riboflavin (RF) can be secreted and used to transport electrons to metal oxides or the anodes [2]. FMN or RF obtain electrons from c-type cytochromes, deliver it to the surface of anodes, and then could be recharged by outer membrane cytochromes in turn. Recent studies have shown that MR-1 used self-secreted flavins not only as electron shuttles, but also as redox cofactors bound to cytochromes which led to a more rapid kinetics with a 103–105-fold enhancement than as shuttles [22,23]. Likewise, other cell self-excreted small molecules such as pyocyanin (PYO), phenazine-1-carboxylate (PCA), phenazine-1-carboxamide (PCN) and 1-hydroxyphenazine (1-OHPHZ) have been reported in Pseudomonas sp [10].
2.2.2. Artificial redox mediators
Besides the above mediators, there are other artificial redox mediators, such as neutral red, methyl viologen, anthraquinone-2, 6-disulfonate (AQDS), potassium ferricyanide and artificial-synthesized phospholipid polymers can mediate the EET [18,24]. Although these redox mediators play analogous roles in the EET, the mechanisms might be different to some extent. The diverse in mechanisms can be owed to many factors, such as their molecular space structures, polarities, and abilities to dissociate.
2.2.3. Primary metabolites
Furthermore, electrons can be transferred by primary metabolites H2 and formate, which are electrochemically consumed or produced by microorganisms to realize a variation of mediated electron transfer [24]. Both could be generated at electrodes and used as electron donors in MES systems [25,26]. For acetogens or methanogens, H2 is one of their preferred electron donors for autotrophic growth [27,28].
3. Unbalanced fermentation (Anodic electro-fermentation)
The use of microbial fermentation process to achieve chemicals production is extremely attractive due to its mild production conditions and less environmental pollution [24]. However, low output and product purity limit its further development. In recent years, the combination of electrochemical systems and fermentation has brought exciting progress. In the unbalanced fermentation, microorganisms maintain intracellular redox balance and achieve continuous as well as efficient chemicals production by transferring excess electrons to the electrodes, which is an unlimited electron acceptor [[29], [30], [31]]. Genetic modification of microbial chassis could further improve the efficiency of EF. The representative examples of AEF in recent years are summarized in Table 1. Based on the mechanisms of electron transfer, unbalanced fermentation can be divided into direct EET-based-based type and shuttle-mediated EET-based type, as discussed in the following.
Table 1.
Representative examples of chemicals synthesis via anodic electro-fermentations (AEF) and microbial electrosynthesis (MES).
| Process | Microbial organism | Substrate | Product | Mechanisms of EET | Genetic modification of host | Title/Yield/Productivity | Reference |
|---|---|---|---|---|---|---|---|
| AEF | |||||||
| Shewanella oneidensis | Glycerol | Ethanol; Acetate |
Direct electron transfer | Introduction of glycerol utilization module from Escherichia coli and ethanol production module from Zymomonas mobilis | Ethanol titer of 1.28 ± 0.02 g L-1, yield of (52 ± 4) %; Acetate titer of 0.29 ± 0.08 g L-1, yield of (13 ± 6) % |
[36] | |
| Shewanella oneidensis | Glucose | Acetate | Direct electron transfer | Introduction of galactose permease (galP) and glucose kinase (glk) genes from Escherichia coli | No | [33] | |
| Shewanella oneidensis | Lactate | Acetoin | Direct electron transfer | Deletion of prophages in genome; Introduction of acetolactate synthase and acetolactate decarboxylase from Bacillus subtilis; Knockout of the acetate kinase (ackA) and phosphotransacetylase (pta) genes |
Acetoin production rate of 0.91 mgh−1, yield of 52%, titer of 0.24 g L-1 | [29] | |
| Pseudomonas putida | Glucose | 2-ketogluconic acid | Direct electron transfer | Overexpression of periplasmic glucose dehydrogenase GCD | 2-ketogluconic acid production rate of 0.25 ± 0.02 mmol gCDW−1 h−1 | [34] | |
| Klebsiella pneumoniae | Glycerol | Acetate; 3-HP; 1,3-PDO |
Direct electron transfer | No | Acetate titer of 21.7 mM; 3-HP titer of 7.6 mM; 1,3-PDO titer of 45.5 mM |
[35] | |
| Escherichia coli | Lactate | Acetate; Ethanol |
Direct electron transfer | Introduction of the Mtr pathway of Shewanella oneidensis MR-1 | Acetate production rate of 0.038 mM day−1; Ethanol titer of 40 ± 3 μM | [39] | |
|
Clostridium cellobioparum; Geobacter sulfurreducens |
Glycerol | Ethanol | Direct electron transfer | Adaptive evolution of Clostridium cellobioparum | Ethanol titer of 10 g L-1 | [40] | |
|
Cellulomonas uda; Geobacter sulfurreducens |
Cellobiose | Ethanol | Direct electron transfer | Adaptive evolution and deleted hydrogenase gene of Geobacter sulfurreducens | No | [41] | |
| Ralstonia eutropha | Fructose | PHB | PMF-mediated electron transfer | No | No | [42] | |
| Klebsiella pneumoniae | Glycerol | 3-HP | 2-hydroxy-1,4-naphthoquinone (HNQ)-mediated electron transfer | Overexpression of aldehyde dehydrogenase (AldH) | 3-HP titer of 21.5 ± 2.2 mM | [43] | |
| Escherichia coli | Glucose | Acetoin | Methylene blue-mediated electron transfer | Deletion of the genes that encoding for enzymes of central reactions (ΔfrdA-D ΔadhE ΔldhA Δpta–ack); Introduction of the genes for the acetolactate synthase (alsS) and the acetolactate decarboxylase (alsD) and c-type cytochromes from Shewanella oneidensis | Production of 0.79 mol acetoin per mol glucose | [31] | |
|
Escherichia coli; Methanobacterium formicicum |
Glycerol | Ethanol; Acetate |
Methylene blue-mediated electron transfer | Introduction of c-type cytochromes CymA, MtrA and STC from Shewanella oneidensis | Ethanol production rate of 12.12 ± 1.70 mgh−1, yield of (35 ± 5) %, titer of 55.25 ± 7.76 g L-1; Acetate production rate of 8.94 ± 0.52 mgh−1, yield of (20 ± 1) %, titer of 40.75 ± 2.37 g L-1 | [45] | |
| Pseudomonas putida F1 | Glucose | 2-Keto-gluconate | Seven different mediators-based mediated electron transfer | No | 2-Keto-gluconate production rate of 1.75 ± 0.33 mgh−1, yield of (90 ± 2) %, titer of 1.47 ± 0.27 g L-1 | [44] | |
|
Corynebacterium glutamicum Zymomonas mobilis |
Glucose Glucose |
l-lysine; Ethanol |
Ferricyanide-mediated electron transfer; Methylene blue, neutral red, methyl naphthoquinone, 1,4- riboflavin, tempol, humic acid, and butanedisulfonate-mediated electron transfer |
Feedback-deregulated mutant Overexpression of redox-related genes ZMO0899, ZMO1116, and ZMO1885 |
l-lysine titer of 2.9 Mm, production rate of 0.2 mmol L−1h−1 Bioelectricity generation 2.0 mWm−2; Ethanol titer ~42.5 g L-1 |
[30] |
|
| MES |
|||||||
| Geobacter sulfurreducens | CO2; Succinate | Glycerol | Direct electron transfer | No | Glycerol titer of 8.7 ± 0.3 mM | [47] | |
| Sporomusa ovate | CO2 | Acetate | Direct electron transfer | No | No | [48] | |
| Clostridium pasteurianum DSM 525 | Glucose; Glycerol | Butanol; 1,3-propandiol | Direct electron transfer | No | Butanol titer of 1.00 ± 0.20 g L-1 from glucose; 1,3-propandiol titer of 4.74 g L-1 from glycerol |
[49] | |
| Shewanella oneidensis MR-1 | Acetoin | 2,3-butanediol | Direct electron transfer | Heterologous expression of a light-driven proton pump (PR) and butanediol dehydrogenase (Bdh); Knockout of hydrogenase gene ΔhyaBΔhydA |
2,3-butanediol titer of 0.03 mM | [52] | |
| Shewanella oneidensis MR-1 | CO2; Fumarate; Pyruvate | H2; Lactate; Formate; Succinate |
Methyl viologen-mediated electron transfer | No | Accumulated 242 ± 24 nmol of H2; ~9100 nmol lactate (93% yield); ~1600 nmol of formate (16% yield); ~8300 nmol succinate (70% yield) |
[53] | |
| Clostridium pasteurianum | Glycerol | 1,3‐propanediol; n‐butanol | Neutral red and brilliant blue-mediated electron transfer | No | BuOH yields of 0.35 mol mol-1 glycerol in NR-mediated BES; 1,3‐PDO yields of 0.41 mol mol-1 glycerol in BB-mediated BES |
[54] | |
| Escherichia coli | Acetophenone | (R)-1-phenylethanol | Methyl viologen-mediated electron transfer | Genetically introduction of cytochromes MtrA, CymA, and STC and heme exporter proteins ccmA-H from Shewanella oneidensis; Heterogenous expression of alcohol dehydrogenase from Lactobacillus brevis |
(R)-1-phenylethanol yield of (39.4 ± 5.7) % | [55] | |
| Escherichia coli | Glucose | Succinate | Neutral red-mediated electron transfer | Heterogenous expression of mtrABC, fccA and cymA from Shewanella oneidensis MR-1 | Succinate yield of 1.10 mol mol-1 glucose | [57] | |
| Saccharomyces cerevisiae | Dhea | 7α–OH–DHEA | Neutral red and 7α-hydroxylase-mediated electron transfer | Heterogenous expression of 7α-hydroxylase | 7α–OH–DHEA titer of 288.6 ± 7.8 mg L−1 | [56] | |
| Ralstonia eutropha | CO2 | Isopropanol | H2-mediated electron transfer | No | Isopropanol titer of 216 mgL−1 | [59] | |
| Ralstonia eutropha | CO2 | PHB; Isopropanol; C4 and C5 alcohols |
H2-mediated electron transfer | No | PHB titer of ~700 mgL−1; Isopropanol titer of ~600 mgL−1; C4+C5alcohols titer of ~220 mgL−1 |
[60] | |
| Xanthobacter autotrophicus | N2 and H2O | NH3 | H2-mediated electron transfer | No | NH3 concentration of ~0.8 mM | [61] | |
|
Sporomusa ovate; Methanococcus maripaludis |
CO2 | Acetate; CH4 |
H2-mediated electron transfer | No | Acetate titer of 0.2–0.3 mM; CH4 titer of 0.2–0.3 mM; |
[62] | |
| Sporomusa ovate | CO2 | Acetate | H2-mediated electron transfer | No | Acetate titer of 6.4 ± 1.1 g L−1 | [64] | |
| Ralstonia eutropha | CO2 | 3-methyl-1-butanol (3 MB); Isobutanol | Formate-mediated electron transfer | Introduction of genes alsS, ilvC, ilvD, kivd, and yqhD; Knockout of PHB synthesis gene cluster (phaC1, phaA and phaB1) |
3 MB and isobutanol titer of 140 mgL−1 | [25] | |
| Ralstonia eutropha | CO2 | PHB | Formate and neutral red-mediated electron transfer | Heterologous expression of the ribulose-1,5-bisphosphate carboxylase (Rubisco) from Synechococcus elongatus PCC7942 | PHB titer of 485 ± 13 mgL−1 | [65] | |
3.1. Direct EET-based unbalanced fermentation
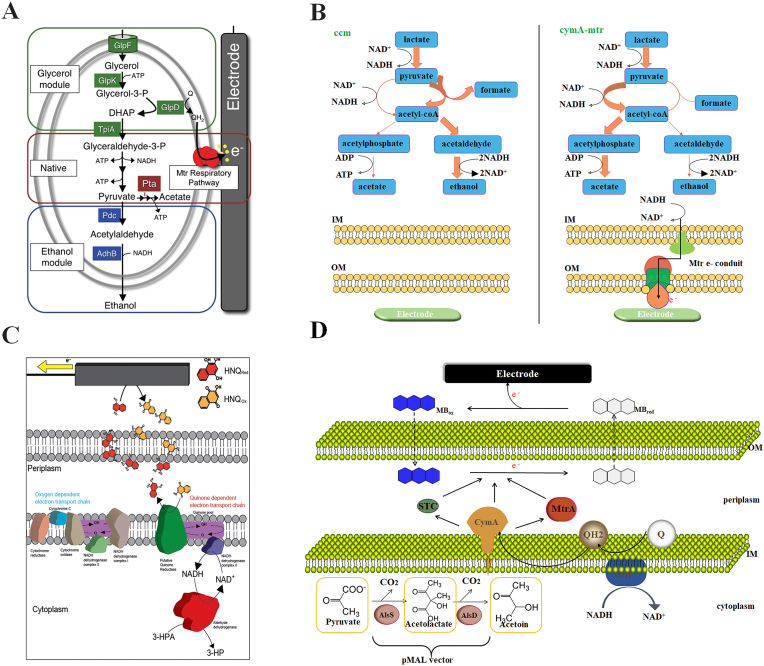
The model electroactivity microorganisms, such as Shewanella and Geobacter, can directly contact with electrode to release excess electrons generated by intracellular metabolism, so the intracellular redox conditions of them are easily to be maintained [32,33]. Introduction of synthetic biology strategies makes it possible to achieve more elaborate regulation of microbial redox metabolism pathways and expand the bacterial species suitable for electro-fermentation [31,34,35]. Flynn et al. built a modified ethanol fermentation S. oneidensis that could produce ethanol from glycerol without many byproducts through electrode-driven alteration of metabolism flux [36]. First, the heterologous glycerol utilization and ethanol production modules were introduced in S. oneidensis, then a key gene pta in the acetate synthesis pathway was knocked out. By providing the anode as an electron sink, high titer and pure ethanol production was achieved through eliminating redox constraints and shifting other unbalanced reactions (Fig. 2A). Nakagawa et al. introduced galactose permease (galP) and glucose kinase (glk) genes of E. coli into S. oneidensis to produce acetate in electrochemical bioreactor [33]. Bursac et al. made a series of genetic modifications to the acetoin production from lactate in S. oneidensis [29]. Similar works were performed in Pseudomonas putida and Klebsiella pneumoniae L17 [34,35]. It is worth mentioning that electrode-based respiration could drive metabolic pathway shift through decoupling of the electron and carbon balances, which could enhance their industrial application as a potential fermentation strain.
Fig. 2.
Unbalanced fermentation (anodic electro-fermentation) for the production of chemicals based on direct EET (A and B) and shuttle-mediated EET (C and D). (A) Metabolic modules added to S. oneidensis to enable electrode-dependent conversion of glycerol to ethanol [36]. (B) Electron transfer through the Mtr electron conduit alters metabolism to higher oxidation product in E. coli[39]. (C) HNQ mediated 3-hydroxypropionic acid (3-HP) production in engineered K. pneumoniae[43]. (D) Methylene blue (MB)-mediated acetoin production in engineered E. coli[31].
The non-model electroactivity microorganisms such as E. coli and Sacharomyces cerevisiae could play a crucial role in the fermentation process due to their robustness and mature gene manipulation tools. However, they are not ideal for anodic electro-fermentation because they could not naturally perform EET [32]. EET components from S. oneidensis have been previously introduced into E. coli to endow it with electroactivity [37,38]. TerAvest et al. enhanced the electrons transfer ability of E. coli by introducing the c-type cytochromes MtrA, CymA, and STC from S. oneidensis MR-1, which can improve its performance in unbalanced fermentation systems [39]. Based on this system they obtained 8-fold higher current constantly without affecting the ability of substrate utilization and bacterial viability. The introduction of Mtr module adjusted the metabolic flow, improved redox balance and shifted metabolism to oxidation product acetate (Fig. 2B).
Microbial consortium is another strategy to significantly improve the efficiency of unbalanced fermentation. Speers et al. developed a microbial electrolysis cell (MEC) driven by the fermentation bacterium Clostridium cellobioparum and the exoelectrogen G. sulfurreducens for the production of ethanol from glycerol [40]. Their research highlighted the potential of consortia in unbalanced fermentation. They further constructed a single-chamber microbial electrolysis cells (SCMEC) containing Cellulomonas uda and G. sulfurreducens to produce ethanol through cellobiose electro-fermentation, which gained maximum titer and productivity [41]. Cellulomonas uda was used to utilize cellobiose to produce ethanol, then the by-products lactate and acetate could be removed by G. sulfurreducens and provide electrons to maintenance electrochemical systems. After adaptive evolution and the deletion of hydrogenase gene of G. sulfurreducens strains, the rates of substrate consumption and product formation were further increased.
3.2. Shuttle-mediated EET-based unbalanced fermentation
The introduction of various exogenous electron transfer mediators could accelerate the electron flow and promote the generation of target products. Nishio et al. enhanced the polyhydroxybutyrate (PHB) production rate by 60% in Ralstonia eutropha electrochemical system through addition of poly (2-methacryloyloxyethyl phosphorylcholine-co-vinyl-ferrocene) (PMF), which is a biocompatible mediator [42]. Kim et al. constructed a BES to produce 3-Hydroxypropionic acid (3-HP) from glycerol in engineered K. pneumoniae [43]. In this BES, 2-hydroxy-1,4-naphthoquinone (HNQ) could enhance electron transfer between the electrode and overexpressed aldehyde dehydrogenase (AldH), resulting in a 1.7-fold higher productivity (Fig. 2C). Lai et al. took K3 [Fe(CN)6] and [Co(bpy)3](ClO4)2 as a shuttle in a BES to produce 2-keto-gluconate from glucose under anaerobic conditions in P. putida F1 [44]. Förster et al. achieved electrode-assisted acetoin production from substrate glucose of E. coli by introduction of EET pathway of S. oneidensis and addition of the soluble redox mediator methylene blue [31]. Firstly, the genes of the anaerobic carbon metabolism were deleted to avoid extra NADH consuming and accumulate pyruvate. Then acetoin synthesis genes alsS and alsD from Bacillus subtilis were heterologous expressed to synthesis acetoin (Fig. 2D). Sturm-Richter et al. developed a co-culture electrocatalysis system of Methanobacterium formicicum and E. coli to produce ethanol and acetate from glycerol [45]. Vassilev et al. also achieved an anaerobic l-lysine production in a BES with Corynebacterium glutamicum lysC mutant as the chassis [30]. Ferricyanide was added as an artificial electron mediator to accelerate EET process and achieve anoxic respiration, thus realizing the l-lysine titer of 2.9 mM at rates of 0.2 mmol L−1h−1 under anaerobic conditions. Geng et al. recently introduced Tween 80 into a Zymomonas mobilis-inoculated bioelectrochemical system and accelerated the electricity generation and ethanol production simultaneously. Tween 80 was used as a surfactant to enhance the permeability cell membranes and transport of various electron shuttles across cell membranes [46].
4. Microbial electrosynthesis (Cathodic electro-fermentation)
Microbial electrosynthesis technology could realize the conversion of electrons to reducing equivalents for reduction of CO2 into chemicals. The recent studies of MES in various hosts are listed in Table 1. In MES systems, the mechanisms of microbial cells consuming electrons for their metabolism or chemical synthesis also consist of two categories of EET: direct contact-based EET and shuttle-mediated EET based on exogenous redox compounds or primary metabolites.
4.1. Direct EET-based microbial electrosynthesis
The mechanisms of delivering cathodic electrons by direct EET were rather poorly understood before G. sulfurreducens was proved to draw electrons from polarized stainless steel cathodes to reduce fumarate [47], which was the first reported study on the CO2 reduction in Geobacter and provided a landmark for microbial electrosynthesis. Then, a wide variety of electrotrophs were reported. Nevin et al. found that nickel nanowires anchored-graphite electrode could deliver electrons directly to S. ovata for acetate production [48], which achieved a conversion rate of 82%. Choi et al. showed that Clostridium pasteurianum DSM 525 could direct transfer electrons from cathode to achieve metabolic shift [49]. Two NADH-consuming pathways including butanol from glucose and 1,3-propandiol from glycerol were promoted, both of which were electron-dense metabolites (Fig. 3A). In the S. oneidensis strain, the reverse Mtr-pathway was feasible for fumarate reduction and electrosynthesis [50], and the potential mechanism of electron uptake from cathode was further studied by Annette et al. [51]. Recently, Tefft and TerAvest realized electrode-driven acetoin reduction in S. oneidensis MR-1 via inward electron transfer [52]. In this system, the thermodynamically unfavorable function of NADH dehydrogenase was overcome by proton-motive force generated from light. The electrons from electrode were transport through Mtr complex, quinone pool and NADH, sequentially to reduce acetoin by heterologous butanediol dehydrogenase (Bdh). Further deletion of hydrogenase genes (ΔhyaBΔhydA) removed the competitive electron sink and improved 2,3-butanediol accumulation (Fig. 3B).
Fig. 3.
Examples of microbial electrosynthesis (cathodic electro-fermentation) based on direct EET (A and B) and shuttle-mediated EET (C, D, E, and F). (A) Electricity-driven metabolic shift of C. pasteurianum for butanol production from glucose and 1,3-propandiol production from glycerol [49]. (B) 2,3-butanediol production in a hydrogenase-deficient S. oneidensis using native Mtr proteins and exogenous light-driven proton pump (proteorhodopsin) for NADH accumulation [52]. (C) Different effects of neutral red (NR) and the barely studied redox mediator brilliant blue (BB) on the growth and product formation of C. pasteurianum grown on glycerol in a newly developed bioelectrochemical system [54]. (D) Chiral alcohol (R)-1-phenylethanol production from acetophenone in engineered E. coli by using methyl viologen (MV) as mediator to achieve EET [55]. (E) Hydrogen-driven microbial electrosynthesis from CO2 to acetate and methane in S. ovate and M. maripaludis via highly biocompatible transition-metal-based cathodes, respectively [62]. (F) Formate dehydrogenase (FDH)-assisted electrosynthesis of (3-hydroxybutyrate) (PHB) in R. eutropha, which overexpressed the ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) [65]. Formate reduced from CO2 and NR both served as the electron carriers to transfer electrons derived from cathodes into R. eutropha. PEM: proton exchange membrane; Rnf: a membrane-bound NADH:ferredoxin oxidoreductase; PEC: periplasmic electron carriers (FccA, CctA); OM: outer membrane; IM: inner membrane; LED: green light source; BBox: oxidized brilliant blue; BBred: reduced brilliant blue; NRox: oxidized neutral red; NRred: reduced neutral red; MVox: oxidized methyl viologen; MVred: reduced methyl viologen; LbADH: alcohol dehydrogenase from L. brevis; MtrA, STC and CymA: proteins of the electron transfer pathway in S. oneidensis MR-1.
4.2. Shuttle-mediated EET-based microbial electrosynthesis
Electrically redox mediators could be applied as electron transporters (either electron donors or acceptors) between electrodes and cells for the regeneration of reducing power and enhance the efficiency of electrosynthesis. Rowe et al. found that methyl viologen (MV) shuttled electrons from water-soluble abiotic photosensitizers into S. oneidensis MR-1 to support target reactions including H2 evolution, CO2 reduction, succinate and lactate production [53]. Utesch et al. reported a newly developed bioelectrochemical system of C. pasteurianum grown on glycerol by adding artificial mediators neutral red (NR) and brilliant blue (BB) with different electrochemical properties [54]. The addition of NR leaded to metabolic shifts in BES and increased the n‐butanol yield by as high as 33%, while BB preferred to enhance the formation of 1,3‐propanediol (Fig. 3C). Furthermore, the addition of exogenous redox mediators could achieve the electrosynthesis in the model microorganisms E. coli and Saccharomyces cerevisiae with insufficient direct EET [[55], [56], [57]]. Mayr et al. demonstrated a chiral alcohol biocatalytic platform based on chassis E. coli, which heterogenous expressed alcohol dehydrogenase from Lactobacillus brevis [55]. They used MV for the regeneration of NADPH, which could cross the outer cell membrane of E. coli (Fig. 3D). Zhang et al. constructed an efficient 7α-hydroxylation bioelectrocatalytic system in the recombinant Saccharomyces cerevisiae by incorporated electron shuttle NR-mediated EET and achieved an electricity-assisted cofactors recharge [56]. Cytochrome P450 monooxygenase utilized electrons derived from both electrodes and the oxidation of glucose to catalyze hydroxylation of steroids. Eventually, the 7α–OH–DHEA yield reached 2.4-fold of its counterpart in the absence of EET.
In addition to artificially additional electron shuttles, small metabolites H2 and formate can also serve as electron carriers for microorganisms. In cathodes, water is split to H2 by a catalyst, then generated H2 would be consumed by the hydrogenases of autotrophic microorganisms to facilitate the bio-reduction of CO2 [58]. Torella et al. reported an integrated electrochemical system for isopropanol production in R. eutropha by using earth-abundant metals cobalt phosphate (Co-Pi) as catalyst [59]. Further development of this biocompatible inorganic–biological hybrid system achieved synthesis of liquid fusel alcohols in R. eutropha [60] and synthesis of NH3 in Xanthobacter autotrophicus [61] by Liu and co-workers. Kracke et al. demonstrated microbial CO2 reduction to C1 and C2 compounds by using hydrogen, which produced in situ with non-precious metal (CoP, MoS2, and NiMo) cathodes, as mediator to accelerate bio-cathodes electron supply speed [62]. The homoacetogenic bacterium S. ovata and methanogenic archaeon Methanococcus maripaludis, which could metabolize CO2 and H2, were introduced to investigate production of liquid chemical acetate and gaseous hydrocarbon CH4, respectively (Fig. 3E) and achieved almost 100% of coulombic efficiencies. However, the low solubility of H2 in water may decreased the efficiency of electron transfer flow from water-splitting to CO2 fixation [63]. To alleviate this bottleneck, Rodrigues et al. recently introduced a biocompatible perfluorocarbon nanoemulsion as a H2 carrier and improved faradaic efficiency of CO2 reduction in S. ovata [64]. Besides, formate could be applied as a soluble and safe replacement for H2 in MES systems, which could be oxidized to CO2 in turn and generate NADH. Li and coworkers firstly demonstrated a formate-mediated MES in R. eutropha to produce higher alcohols [25]. Chen and colleagues constructed a formate dehydrogenase (FDH)-assisted R. eutropha MES for production of PHB [65]. Formate was converted from CO2 through FDH, and served as the both electron carrier and carbon source for CO2 fixation and PHB synthesis. Meanwhile, NR was applied to facilitate the regeneration of extracellular NADH (Fig. 3F). Eventually, the titer of PHB in genetically engineered R. eutropha was increased to 485 ± 13 mgL−1.
5. Conclusions and perspectives
Microbial electro-fermentation (EF) possesses great promise for value-added chemicals and biofuels production [66]. However, the productivities and efficiencies achieved by above systems is still too low to afford industrial scale-up production [67]. The main bottlenecks of EF are the insufficient EET rate, the lack of available gene editing tools to engineer metabolic pathways of target products, and electrode materials and operation of the bio-electrochemical reactors, which all together limited the scale-up applications of EF. For electroactive microorganisms, due to the limited knowledge on EET mechanisms and the absence of available genetic tools, it is usually difficult to further enhance the EET rate [17]. Furthermore, exploration of efficient genetic modification tools to engineer target product synthesis pathways in these non-model electroactive microorganisms is also a challenging problem. On the other hand, for most non-electroactive organisms, due to the lack of electron transfer pathway, they are not able to directly deliver or uptake electrons, which severely limited their applications in anodic electro-fermentation or cathodic electro-fermentation. Although addition of redox mediators or surfactants could promote indirect EET and regenerate intracellular reduce equivalents [46,65], their inhibition on the cell growth would be hazard for practical applications. Lastly, to achieve efficient coupling of inward electrons with cell growth and metabolic pathways is a bottleneck in the development of EF.
Recently, combining electro-fermentation with metabolic engineering have becoming an open attractive approach for biosynthesis [67]. Therefore, there is a core requirement for improving the performance of EF through synthetic biology strategies. Synthetic biology tools offer many opportunities to promote the bidirectional EET rates and increase the production of target chemicals. The reduced equivalent balancing and regeneration is a critical issue as the insufficient supply of reduced equivalent would hinder or even end the process of EF. Intracellular NAD (H/+) and redox state (NADH/NAD+ ratio) are the sources of EET process. Modular strategies to increase intracellular NAD (H/+) pool and the ratio of NADH/NAD+ could significantly enhance the current production of exoelectrogens [68,69]. Other strategies including heterogeneous overexpression of porins to improve cell membrane permeability [70,71], increasing the synthesis of endogenous redox mediators [2,71], expanding the substrate utilization spectrum to accelerate substrate utilization [36,72], and modification of genes related to biofilm formation [73] could promote EET in electroactive microorganisms. On the other hand, heterologous introduction of electron transfer modules and related genes in cytochrome maturation system in traditional fermentation microorganisms which inability of EET would be a promising strategy to promote the EF [31,55]. Apart from EET, engineering of target compound synthesis pathways could further enhance efficiency of EF [74]. Synthetic biology strategies including overexpression of key enzymes [43] and interference or deletion of competitive pathways [31] could redirect metabolic flow and improve the target chemical production. Furthermore, the application of customized consortium will achieve fine-tuning of metabolic pathways and enhance chemical production [40,41].
In summary, synthesis biology provides a significant solution to enhance the performance of EF via engineering of EET and metabolic pathways.
Furthermore, the scaling up of EF is limited by electrode materials, the structure and operation of bioelectrochemical reactors. On the one hand, ideal electrode materials should have a biocompatible surface required for cell-electrode attachment, adequate specific surface area, strong chemical stability (including corrosion resistance), excellent mechanical strength, high conductivity, a scalable and manufacturable system, and low cost [7,18]. On the other hand, technical parameters and obstacles such as the electrical internal resistances of lab-scale reactors, temperature, pH and salinity ranges of operational environment should be optimized for feasible industrial applications [75].
Author contributions
ZG and HY contributed equally to the work. ZG and HY drafted the manuscript; JZ, FL, and HS critically revised the manuscript.
Acknowledgments
This research was supported by the National Key Research and Development Program of China (No. 2018YFA0901300), and Independent Innovation Fund of Tianjin University (0903065070, 0903065083; 0903065084).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Feng Li, Email: messilifeng@163.com.
Hao Song, Email: hsong@tju.edu.cn.
References
- 1.Venkata Mohan S., Velvizhi G., Annie Modestra J., Srikanth S. Microbial fuel cell: critical factors regulating bio-catalyzed electrochemical process and recent advancements. Renew Sustain Energy Rev. 2014;40:779–797. doi: 10.1016/j.rser.2014.07.109. [DOI] [Google Scholar]
- 2.Lin T., Ding W., Sun L., Wang L., Liu C., Song H. Engineered Shewanella oneidensis -reduced graphene oxide biohybrid with enhanced biosynthesis and transport of flavins enabled a highest bioelectricity output in microbial fuel cells. Nanomater Energy. 2018;50:639–648. doi: 10.1016/j.nanoen.2018.05.072. [DOI] [Google Scholar]
- 3.Prevoteau A., Carvajal-Arroyo J.M., Ganigue R., Rabaey K. Microbial electrosynthesis from CO2: forever a promise? Curr Opin Biotechnol. 2019;62:48–57. doi: 10.1016/j.copbio.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Rabaey K., Rozendal R.A. Microbial electrosynthesis – revisiting the electrical route for microbial production. Nat Rev Microbiol. 2010;8(10):706–716. doi: 10.1038/nrmicro2422. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y., Angelidaki I. Microbial electrolysis cells turning to be versatile technology: recent advances and future challenges. Water Res. 2014;56:11–25. doi: 10.1016/j.watres.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 6.Sevda S., Yuan H., He Z., Abu-Reesh I.M. Microbial desalination cells as a versatile technology: functions, optimization and prospective. Desalination. 2015;371:9–17. doi: 10.1016/j.desal.2015.05.021. [DOI] [Google Scholar]
- 7.Moscoviz R., Toledo-Alarcón J., Trably E., Bernet N. Electro-fermentation: how to drive fermentation using electrochemical systems. Trends Biotechnol. 2016;34(11):856–865. doi: 10.1016/j.tibtech.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Vassilev I., Hernandez P.A., Batlle-Vilanova P., Freguia S., Krömer J.O., Keller J., Ledezma P., Virdis B. Microbial electrosynthesis of isobutyric, butyric, caproic acids, and corresponding alcohols from carbon dioxide. ACS Sustainable Chem Eng. 2018;6(7):8485–8493. doi: 10.1021/acssuschemeng.8b00739. [DOI] [Google Scholar]
- 9.Jiang Y., May H.D., Lu L., Liang P., Huang X., Ren Z. Carbon dioxide and organic waste valorization by microbial electrosynthesis and electro-fermentation. Water Res. 2019;149:42–55. doi: 10.1016/j.watres.2018.10.092. [DOI] [PubMed] [Google Scholar]
- 10.Liu X., Shi L., Gu J. Microbial electrocatalysis: redox mediators responsible for extracellular electron transfer. Biotechnol Adv. 2018;36(7):1815–1827. doi: 10.1016/j.biotechadv.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Guzman M.S., Rengasamy K., Binkley M.M., Jones C., Ranaivoarisoa T.O., Singh R., Fike D.A., Meacham J.M., Bose A. Phototrophic extracellular electron uptake is linked to carbon dioxide fixation in the bacterium Rhodopseudomonas palustris. Nat Commun. 2019;10(1):1355. doi: 10.1038/s41467-019-09377-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bose A., Gardel E.J., Vidoudez C., Parra E.A., Girguis P.R. Electron uptake by iron-oxidizing phototrophic bacteria. Nat Commun. 2014;5(1):3391. doi: 10.1038/ncomms4391. [DOI] [PubMed] [Google Scholar]
- 13.Shi L., Rosso K.M., Zachara J.M., Fredrickson J.K. Mtr extracellular electron-transfer pathways in Fe(III)-reducing or Fe(II)-oxidizing bacteria: a genomic perspective. Biochem Soc Trans. 2012;40(6):1261–1267. doi: 10.1042/BST20120098. [DOI] [PubMed] [Google Scholar]
- 14.Beckwith C.R., Edwards M.J., Lawes M., Shi L., Butt J.N., Richardson D.J., Clarke T.A. Characterization of MtoD from Sideroxydans lithotrophicus: a cytochrome c electron shuttle used in lithoautotrophic growth. Front Microbiol. 2015;6:332. doi: 10.3389/fmicb.2015.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan Y., Adhikari R.Y., Malvankar N.S., Ward J.E., Nevin K.P., Woodard T.L., Smith J.A., Snoeyenbos-West O.L., Franks A.E., Tuominen M.T., Lovley D.R. The low conductivity of Geobacter uraniireducens pili suggests a diversity of extracellular electron transfer mechanisms in the genus Geobacter. Front Microbiol. 2016;7:980. doi: 10.3389/fmicb.2016.00980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang F., Gu Y., O'Brien J.P., Yi S.M., Yalcin S.E., Srikanth V., Shen C., Vu D., Ing N.L., Hochbaum A.I., Egelman E.H., Malvankar N.S. Structure of microbial nanowires reveals stacked hemes that transport electrons over micrometers. Cell. 2019;177(2):361–410. doi: 10.1016/j.cell.2019.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar A., Hsu L.H.-H., Kavanagh P., Barrière F., Lens P.N.L., Lapinsonnière L., Lienhard V.J.H., Schröder U., Jiang X., Leech D. The ins and outs of microorganism – electrode electron transfer reactions. Nat Rev Chem. 2017;1(3) doi: 10.1038/s41570-017-0024. [DOI] [Google Scholar]
- 18.Chen H., Dong F., Minteer S.D. The progress and outlook of bioelectrocatalysis for the production of chemicals, fuels and materials. Nat Catal. 2020;3(3):225–244. doi: 10.1038/s41929-019-0408-2. [DOI] [Google Scholar]
- 19.Schuchmann K., Müller V. Autotrophy at the thermodynamic limit of life: a model for energy conservation in acetogenic bacteria. Nat Rev Microbiol. 2014;12(12):809–821. doi: 10.1038/nrmicro3365. [DOI] [PubMed] [Google Scholar]
- 20.Kracke F., Vassilev I., Krömer J.O. Microbial electron transport and energy conservation – the foundation for optimizing bioelectrochemical systems. Front Microbiol. 2015;6:575. doi: 10.3389/fmicb.2015.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deutzmann J.S., Sahin M., Spormann A.M. Extracellular enzymes facilitate electron uptake in biocorrosion and bioelectrosynthesis. mBio. 2015;6(2) doi: 10.1128/mBio.00496-15. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okamoto A., Hashimoto K., Nealson K.H., Nakamura R. Rate enhancement of bacterial extracellular electron transport involves bound flavin semiquinones. Proc Natl Acad Sci U S A. 2013;110(19):7856. doi: 10.1073/pnas.1220823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breuer M., Rosso Kevin M., Blumberger J. Flavin binding to the deca-heme cytochrome MtrC: insights from computational molecular simulation. Biophys J. 2015;109(12):2614–2624. doi: 10.1016/j.bpj.2015.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X., Yu X. Enhancement of butanol production: from biocatalysis to bioelectrocatalysis. ACS Energy Lett. 2020:867–878. doi: 10.1021/acsenergylett.9b02596. [DOI] [Google Scholar]
- 25.Li H., Opgenorth P.H., Wernick D.G., Rogers S., Wu T., Higashide W., Malati P., Huo Y., Cho K.M., Liao J. Integrated electromicrobial conversion of CO2 to higher alcohols. Science. 2012;335(6076):1596. doi: 10.1126/science.1217643. [DOI] [PubMed] [Google Scholar]
- 26.Blanchet E., Duquenne F., Rafrafi Y., Etcheverry L., Erable B., Bergel A. Importance of the hydrogen route in up-scaling electrosynthesis for microbial CO2 reduction. Energy Environ Sci. 2015;8(12):3731–3744. doi: 10.1039/c5ee03088a. [DOI] [Google Scholar]
- 27.Villano M., Aulenta F., Ciucci C., Ferri T., Giuliano A., Majone M. Bioelectrochemical reduction of CO2 to CH4 via direct and indirect extracellular electron transfer by a hydrogenophilic methanogenic culture. Bioresour Technol. 2010;101(9):3085–3090. doi: 10.1016/j.biortech.2009.12.077. [DOI] [PubMed] [Google Scholar]
- 28.Marshall C.W., Ross D.E., Fichot E.B., Norman R.S., May H.D. Electrosynthesis of commodity chemicals by an autotrophic microbial community. Appl Environ Microbiol. 2012;78(23):8412–8420. doi: 10.1128/AEM.02401-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bursac T., Gralnick J.A., Gescher J. Acetoin production via unbalanced fermentation in Shewanella oneidensis. Biotechnol Bioeng. 2017;114(6):1283–1289. doi: 10.1002/bit.26243. [DOI] [PubMed] [Google Scholar]
- 30.Vassilev I., Giesselmann G., Schwechheimer S.K., Wittmann C., Virdis B., Kromer J.O. Anodic electro-fermentation: anaerobic production of L-Lysine by recombinant Corynebacterium glutamicum. Biotechnol Bioeng. 2018;115(6):1499–1508. doi: 10.1002/bit.26562. [DOI] [PubMed] [Google Scholar]
- 31.Forster A.H., Beblawy S., Golitsch F., Gescher J. Electrode-assisted acetoin production in a metabolically engineered Escherichia coli strain. Biotechnol Biofuels. 2017;10:65. doi: 10.1186/s13068-017-0745-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuda S., Liu H., Kouzuma A., Watanabe K., Hashimoto K., Nakanishi S. Electrochemical gating of tricarboxylic acid cycle in electricity-producing bacterial cells of Shewanella. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0072901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakagawa G., Kouzuma A., Hirose A., Kasai T., Yoshida G., Watanabe K. Metabolic characteristics of a glucose-utilizing Shewanella oneidensis strain grown under electrode-respiring conditions. PLoS One. 2015;10(9) doi: 10.1371/journal.pone.0138813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu S., Lai B., Plan M.R., Hodson M.P., Lestari E.A., Song H., Krömer J.O. Improved performance of Pseudomonas putida in a bioelectrochemical system through overexpression of periplasmic glucose dehydrogenase. Biotechnol Bioeng. 2018;115(1):145–155. doi: 10.1002/bit.26433. [DOI] [PubMed] [Google Scholar]
- 35.Kim M.Y., Kim C., Ainala S.K., Bae H., Jeon B.-H., Park S., Kim J.R. Metabolic shift of Klebsiella pneumoniae L17 by electrode-based electron transfer using glycerol in a microbial fuel cell. Bioelectrochemistry. 2019;125:1–7. doi: 10.1016/j.bioelechem.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Flynn J.M., Ross D.E., Hunt K.A., Bond D.R., Gralnick J.A. Enabling unbalanced fermentations by using engineered electrode-interfaced bacteria. Microbiology. 2010;1(5) doi: 10.1128/mBio.00190-10. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jensen H.M., Albers A.E., Malley K.R., Londer Y.Y., Cohen B.E., Helms B.A., Weigele P., Groves J.T., Ajo-Franklin C.M. Engineering of a synthetic electron conduit in living cells. Proc Natl Acad Sci U S A. 2010;107(45):19213–19218. doi: 10.1073/pnas.1009645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldbeck C.P., Jensen H.M., TerAvest M.A., Beedle N., Appling Y., Hepler M., Cambray G., Mutalik V., Angenent L.T., Ajo-Franklin C.M. Tuning promoter strengths for improved synthesis and function of electron conduits in Escherichia coli. ACS Synth Biol. 2013;2(3):150–159. doi: 10.1021/sb300119v. [DOI] [PubMed] [Google Scholar]
- 39.TerAvest M.A., Zajdel T.J., Ajo-Franklin C.M. The Mtr pathway of Shewanella oneidensis MR-1 couples substrate utilization to current production in Escherichia coli. ChemElectroChem. 2014;1(11):1874–1879. doi: 10.1002/celc.201402194. [DOI] [Google Scholar]
- 40.Speers A.M., Young J.M., Reguera G. Fermentation of glycerol into ethanol in a microbial electrolysis cell driven by a customized consortium. Environ Sci Technol. 2014;48(11):6350–6358. doi: 10.1021/es500690a. [DOI] [PubMed] [Google Scholar]
- 41.Awate B., Steidl R.J., Hamlischer T., Reguera G. Stimulation of electro-fermentation in single-chamber microbial electrolysis cells driven by genetically engineered anode biofilms. J Power Sources. 2017;356:510–518. doi: 10.1016/j.jpowsour.2017.02.053. [DOI] [Google Scholar]
- 42.Nishio K., Kimoto Y., Song J., Konno T., Ishihara K., Kato S., Hashimoto K., Nakanishi S. Extracellular electron transfer enhances polyhydroxybutyrate productivity in Ralstonia eutropha. Environ Sci Technol Lett. 2013;1(1):40–43. doi: 10.1021/ez400085b. [DOI] [Google Scholar]
- 43.Kim C., Kim M.Y., Michie I., Jeon B.H., Premier G.C., Park S., Kim J.R. Anodic electro-fermentation of 3-hydroxypropionic acid from glycerol by recombinant Klebsiella pneumoniae L17 in a bioelectrochemical system. Biotechnol Biofuels. 2017;10:199. doi: 10.1186/s13068-017-0886-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai B., Yu S., Bernhardt P.V., Rabaey K., Virdis B., Kromer J.O. Anoxic metabolism and biochemical production in Pseudomonas putida F1 driven by a bioelectrochemical system. Biotechnol Biofuels. 2016;9:39. doi: 10.1186/s13068-016-0452-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sturm-Richter K., Golitsch F., Sturm G., Kipf E., Dittrich A., Beblawy S., Kerzenmacher S., Gescher J. Unbalanced fermentation of glycerol in Escherichia coli via heterologous production of an electron transport chain and electrode interaction in microbial electrochemical cells. Bioresour Technol. 2015;186:89–96. doi: 10.1016/j.biortech.2015.02.116. [DOI] [PubMed] [Google Scholar]
- 46.Geng B., Cao L., Li F., Song H., Liu C., Zhao X., Bai F. Potential of Zymomonas mobilis as an electricity producer in ethanol production. Biotechnol Biofuels. 2020;13:36. doi: 10.1186/s13068-020-01672-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soussan L., Riess J., Erable B., Delia M.-L., Bergel A. Electrochemical reduction of CO2 catalysed by Geobacter sulfurreducens grown on polarized stainless steel cathodes. Electrochem Commun. 2013;28:27–30. doi: 10.1016/j.elecom.2012.11.033. [DOI] [Google Scholar]
- 48.Nevin K.P., Woodard T.L., Franks A.E., Summers Z.M., Lovley D.R. Microbial electrosynthesis: feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds. Microbiology. 2010;1(2) doi: 10.1128/mBio.00103-10. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi O., Kim T., Woo H.M., Um Y. Electricity-driven metabolic shift through direct electron uptake by electroactive heterotroph Clostridium pasteurianum. Sci Rep. 2014;4(1):6961. doi: 10.1038/srep06961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ross D.E., Flynn J.M., Baron D.B., Gralnick J.A., Bond D.R. Towards electrosynthesis in Shewanella: energetics of reversing the mtr pathway for reductive metabolism. PLoS One. 2011;6(2) doi: 10.1371/journal.pone.0016649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rowe A.R., Rajeev P., Jain A., Pirbadian S., Okamoto A., Gralnick J.A., El-Naggar M.Y., Nealson K.H. Tracking electron uptake from a cathode into Shewanella cells: implications for energy acquisition from solid-substrate electron donors. mBio. 2018;9(1) doi: 10.1128/mBio.02203-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tefft N.M., TerAvest M.A. Reversing an extracellular electron transfer pathway for electrode-driven acetoin reduction. ACS Synth Biol. 2019;8(7):1590–1600. doi: 10.1021/acssynbio.8b00498. [DOI] [PubMed] [Google Scholar]
- 53.Rowe S.F., Le Gall G., Ainsworth E.V., Davies J.A., Lockwood C.W.J., Shi L., Elliston A., Roberts I.N., Waldron K.W., Richardson D.J., Clarke T.A., Jeuken L.J.C., Reisner E., Butt J.N. Light-driven H2 evolution and C═C or C═O bond hydrogenation by Shewanella oneidensis: a versatile strategy for photocatalysis by nonphotosynthetic microorganisms. ACS Catal. 2017;7(11):7558–7566. doi: 10.1021/acscatal.7b02736. [DOI] [Google Scholar]
- 54.Utesch T., Sabra W., Prescher C., Baur J., Arbter P., Zeng A. Enhanced electron transfer of different mediators for strictly opposite shifting of metabolism in Clostridium pasteurianum grown on glycerol in a new electrochemical bioreactor. Biotechnol Bioeng. 2019;116(7):1627–1643. doi: 10.1002/bit.26963. [DOI] [PubMed] [Google Scholar]
- 55.Mayr J.C., Grosch J.H., Hartmann L., Rosa L.F.M., Spiess A.C., Harnisch F. Resting Escherichia coli as chassis for microbial electrosynthesis: production of chiral alcohols. ChemSusChem. 2019;12(8):1631–1634. doi: 10.1002/cssc.201900413. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Z., Li F., Cao Y., Tian Y., Li J., Zong Y., Song H. Electricity-driven 7α-hydroxylation of a steroid catalyzed by a cytochrome P450 monooxygenase in engineered yeast. Catal Sci Technol. 2019;9(18):4877–4887. doi: 10.1039/c9cy01288e. [DOI] [Google Scholar]
- 57.Wu Z., Wang J., Liu J., Wang Y., Bi C., Zhang X. Engineering an electroactive Escherichia coli for the microbial electrosynthesis of succinate from glucose and CO2. Microb Cell Factories. 2019;18(1):15. doi: 10.1186/s12934-019-1067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lubitz W., Ogata H., Rudiger O., Reijerse E. Hydrogenases Chem Rev. 2014;114(8):4081–5048. doi: 10.1021/cr4005814. [DOI] [PubMed] [Google Scholar]
- 59.Torella J.P., Gagliardi C.J., Chen J.S., Bediako D.K., Colon B., Way J.C., Silver P.A., Nocera D.G. Efficient solar-to-fuels production from a hybrid microbial-water-splitting catalyst system. Proc Natl Acad Sci U S A. 2015;112(8):2337–2342. doi: 10.1073/pnas.1424872112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu C., Colón B.C., Ziesack M., Silver P.A., Nocera D.G. Water splitting-biosynthetic system with CO₂ reduction efficiencies exceeding photosynthesis. Science. 2016;352(6290):1210–1213. doi: 10.1126/science.aaf5039. [DOI] [PubMed] [Google Scholar]
- 61.Liu C., Sakimoto K.K., Colón B.C., Silver P.A., Nocera D.G. Ambient nitrogen reduction cycle using a hybrid inorganic–biological system. Proc Natl Acad Sci U S A. 2017;114(25):6450–6455. doi: 10.1073/pnas.1706371114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kracke F., Wong A.B., Maegaard K., Deutzmann J.S., Hubert M.A., Hahn C., Jaramillo T.F., Spormann A.M. Robust and biocompatible catalysts for efficient hydrogen-driven microbial electrosynthesis. Commun Chem. 2019;2:45. doi: 10.1038/s42004-019-0145-0. [DOI] [Google Scholar]
- 63.Liu C., Nangle S.N., Colon B.C., Silver P.A., Nocera D.G. (13)C-labeling the carbon-fixation pathway of a highly efficient artificial photosynthetic system. Faraday Discuss. 2017;198:529–537. doi: 10.1039/c6fd00231e. [DOI] [PubMed] [Google Scholar]
- 64.Rodrigues R.M., Guan X., Iñiguez J.A., Estabrook D.A., Chapman J.O., Huang S., Sletten E.M., Liu C. Perfluorocarbon nanoemulsion promotes the delivery of reducing equivalents for electricity-driven microbial CO2 reduction. Nat Catal. 2019;2(5):407–414. doi: 10.1038/s41929-019-0264-0. [DOI] [Google Scholar]
- 65.Chen X., Cao Y., Li F., Tian Y., Song H. Enzyme-assisted microbial electrosynthesis of poly(3-hydroxybutyrate) via CO2 bioreduction by engineered Ralstonia eutropha. ACS Catal. 2018;8(5):4429–4437. doi: 10.1021/acscatal.8b00226. [DOI] [Google Scholar]
- 66.Rosenbaum M.A., Berger C., Schmitz S., Uhlig R. Microbial Electrosynthesis I: pure and defined mixed culture engineering. Adv Biochem Eng Biotechnol. 2019;167:181–202. doi: 10.1007/10_2017_17. [DOI] [PubMed] [Google Scholar]
- 67.Schievano A., Pepé Sciarria T., Vanbroekhoven K., De Wever H., Puig S., Andersen S.J., Rabaey K., Pant D. Electro-Fermentation – merging electrochemistry with fermentation in industrial applications. Trends Biotechnol. 2016;34(11):866–878. doi: 10.1016/j.tibtech.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 68.Li F., Li Y., Sun L., Chen X., An X., Yin C., Cao Y., Wu H., Song H. Modular engineering intracellular NADH regeneration boosts extracellular electron transfer of Shewanella oneidensis MR-1. ACS Synth Biol. 2018;7(3):885–895. doi: 10.1021/acssynbio.7b00390. [DOI] [PubMed] [Google Scholar]
- 69.Li F., Li Y., Cao Y., Wang L., Liu C., Shi L., Song H. Modular engineering to increase intracellular NAD(H/(+)) promotes rate of extracellular electron transfer of Shewanella oneidensis. Nat Commun. 2018;9(1):3637. doi: 10.1038/s41467-018-05995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yong Y., Yu Y., Yang Y., Liu J., Wang J., Song H. Enhancement of extracellular electron transfer and bioelectricity output by synthetic porin. Biotechnol Bioeng. 2013;110(2):408–416. doi: 10.1002/bit.24732. [DOI] [PubMed] [Google Scholar]
- 71.Yang Y., Ding Y., Hu Y., Cao B., Rice S.A., Kjelleberg S., Song H. Enhancing bidirectional electron transfer of Shewanella oneidensis by a synthetic flavin pathway. ACS Synth Biol. 2015;4(7):815–823. doi: 10.1021/sb500331x. [DOI] [PubMed] [Google Scholar]
- 72.Li F., Li Y., Sun L., Li X., Yin C., An X., Chen X., Tian Y., Song H. Engineering Shewanella oneidensis enables xylose-fed microbial fuel cell. Biotechnol Biofuels. 2017;10:196. doi: 10.1186/s13068-017-0881-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu T., Yu Y., Deng X., Ng C., Cao B., Wang J., Rice S.A., Kjelleberg S., Song H. Enhanced Shewanella biofilm promotes bioelectricity generation. Biotechnol Bioeng. 2015;112(10):2051–2059. doi: 10.1002/bit.25624. [DOI] [PubMed] [Google Scholar]
- 74.Kracke F., Lai B., Yu S., Krömer J.O. Balancing cellular redox metabolism in microbial electrosynthesis and electro fermentation – a chance for metabolic engineering. Metab Eng. 2018;45:109–120. doi: 10.1016/j.ymben.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 75.Harnisch F., Rosa L.F., Kracke F., Virdis B., Kromer J.O. Electrifying white biotechnology: engineering and economic potential of electricity-driven bio-production. ChemSusChem. 2015;8(5):758–766. doi: 10.1002/cssc.201402736. [DOI] [PubMed] [Google Scholar]