Highlights
-
•
Meckel’s diverticulum (MD) is the most common type of vitelline duct remnant (VDR), but MD adenocarcinoma is rare.
-
•
We reported a case of MD adenocarcinoma accompanied with the umbilical side of VDR and the huge cystic lesion.
-
•
The huge cystic lesion was bloated by tumor components filling up the duct due to cancer progression.
-
•
The recognition of tumor development of the embryonic remnant origin is considered important for the treatment of this adenocarcinoma.
Keywords: Meckel’s diverticulum adenocarcinoma, Vitelline duct remnant, Huge cystic lesion, Case report
Abstract
Introduction
Vitelline duct remnant (VDR) is a rare abnormality of the primitive yolk sac, and Meckel's diverticulum (MD) is the most common type. MD is a congenital small intestinal diverticulum that leaves the ileal side of vitelline duct, and MD adenocarcinoma is extremely rare.
Presentation of case
A 49 year-old-man with abdominal mass was diagnosed as a huge pelvic tumor. We resected this tumor together with the invading ileum and the ileocecum. On histopathological and immunohistochemical analysis, tumor was diagnosed as adenocarcinoma and originated from the digestive tract. Considering that the cord extending from the umbilicus was connected to the tumor and that the tumor invaded the terminal ileum, we made a diagnosis of MD adenocarcinoma accompanied with the umbilical side of VDR and the huge cystic lesion bloated by tumor components filling up the duct due to cancer progression.
Discussion
The reported occurrence of MD tumors is 0.5%–3.2%. The incidence of adenocarcinoma is 21.7% for the malignant tumors in MD. It is likely that a highly advanced local invasion and lymph node metastases are involved and that the prognosis of this adenocarcinoma is poor. There is no recommended chemotherapeutic regimen for MD adenocarcinoma. It is expected that cases should be accumulated in the future for the development of a more optimally recommended regimen.
Conclusion
Although the incidence of our case is extremely rare, the recognition of tumor development of the embryonic remnant origin is considered important for the treatment of this adenocarcinoma.
1. Introduction
Vitelline duct provides nutrition to early developing embryo. The duct provides a communication between primitive yolk sac on ventral side of the embryo and midgut loop through umbilicus [1]. It gradually attenuates and involutes from the terminal part of ileum by 5th to 9th week of gestational period [2]. When the duct that should normally disappear is left behind, various malformations occur, and these are defined as VDR. VDR is a rare abnormality of the primitive yolk sac, and MD is the most common type, accounting for 67% of cases. MD is a congenital small intestinal diverticulum that leaves the ileal side of vitelline duct and it occurs in approximately 2% of the general population [2,3]. MD adenocarcinoma is extremely rare [4,5]. Herein, we report a case of it accompanied with the umbilical side of VDR and the huge cystic lesion.
This case report has been reported in accordance with the SCARE Criteria [6].
2. Presentation of case
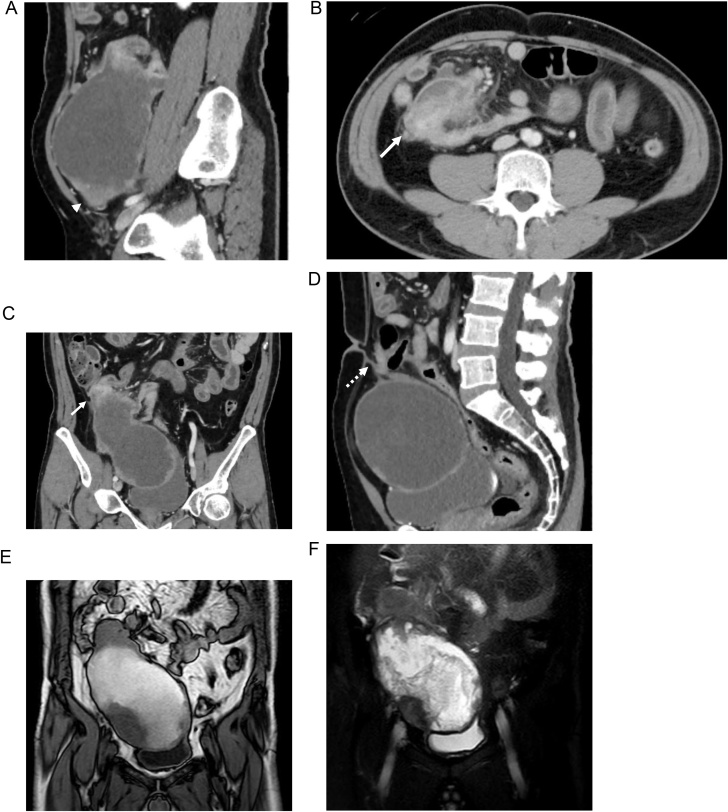
A 49 year-old-man visited our hospital with abdominal bloating and lower abdominal mass for 2 years duration. He had no significant medical history and no family history. A fist-sized mass was palpated on his lower right abdomen. Abdominal computed tomography (CT) showed a huge, 17-cm pelvic tumor with a mixture of cystic and subcapsular parenchymal lesions, which had spooled up the terminal ileum (Fig. 1A–C). Simultaneously, a cord-like material leading to a tumor from the umbilical region was observed (Fig. 1D). On abdominal magnetic resonance imaging (MRI), the cystic tumor lumen exhibited a partition-wall structure, and both T1/T2-weighted images showed low- and high-intensity signals for the parenchymal lesion and the liquid component, respectively; furthermore, the tumor had a mosaic-like pattern (Fig. 1E and F). Total colonoscopy revealed no lesions in the colon; however, the ileal lumen on the ileocecal valve side was highly edematous, and the endoscope did not pass through. Therefore, we were unable to perform a biopsy of the tumor. Chest CT showed no specific findings such as lung metastases.
Fig. 1.
Abdominal CT showed a huge, 17-cm pelvic tumor with a mixture of cystic and subcapsular parenchymal lesions (arrowhead), which had spooled up the terminal ileum (arrow) (A-C). A cord-like material leading to a tumor from the umbilical region was observed (dotted arrow) (D). Abdominal MRI showed the cystic tumor lumen exhibited a partition-wall structure, and both T1 (E)/T2 (F)-weighted images showed low- and high-intensity signals for the parenchymal lesion and the liquid component, respectively; furthermore, the tumor had a mosaic-like pattern.
Accordingly, we diagnosed the lesion as a huge pelvic tumor, possibly originated from the terminal ileum and connected to the umbilicus.
Because of the obstruction of the terminal ileum due to tumor progression, we performed a semi-urgently laparotomy. Intraoperative findings showed a cord covered with preperitoneal adipose tissue between the huge cystic tumor and the umbilicus (Fig. 2A). The localized tumor extensively adhered and spread over the right urinary bladder and on the surface of the right retroperitoneum. Tumor detachment from the urinary bladder and retroperitoneal tissue was possible. However, tumor detachment from the ileum, which was considered to be in a tumor-invaded region 60 cm from the ileocecal area, was impossible. Therefore, we resected this huge cystic tumor together with the invading ileum and the ileocecum (Fig. 2B).
Fig. 2.
Intraoperative findings showed a cord covered with preperitoneal adipose tissue between the huge cystic tumor and the umbilicus (A). Schema of the tumor localization and resection site of the specimen (B).
The tumor was 22 × 13.6 × 12.5 cm in size (Fig. 3A) and invaded the ileum (Fig. 3B). The cystic lesion was surrounded by a fibrous cystic wall and filled with ocherous porridge-like contents (Fig. 3C). In invaded ileal regions, the tumor seemed to be sub-totally exposed to the mucosal surface over the lumen of the ileum, causing obstruction. However, the tumor growth itself appeared not from the ileal mucosa but from the lower part of the ileal mucosa or outside the ileal lumen to induce proliferation and invasion, which, ultimately, induced bowel obstruction (Fig. 3D).
Fig. 3.
Specimen findings (A: ventral side/B: dorsal side). The tumor was 22 × 13.6 × 12.5 cm in size, and the tumor invaded ileum (B: dotted arrow). The cystic lesion was surrounded by a fibrous cystic wall and filled with ocherous porridge-like contents (C). In invaded ileal regions, the tumor seemed to be sub-totally exposed to the mucosal surface over the lumen of the ileum, causing obstruction (D). However, the tumor growth itself appeared not from the ileal mucosa but from the lower part of the ileal mucosa or outside the ileal lumen to induce proliferation and invasion (D: arrowheads).
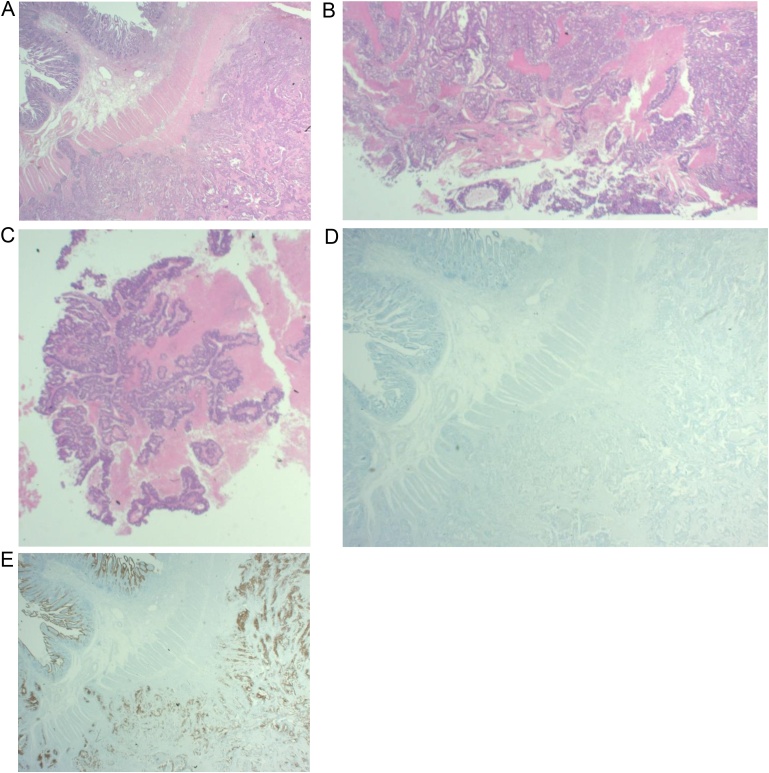
On histopathological examination, obstructed ileal lesions, cystic wall lesions and porridge-like contents were all diagnosed as moderately differentiated tubular adenocarcinoma (Fig. 4A–C), and the immunohistochemical analysis with cytokeratin 7(-)/cytokeratin 20(++) confirmed that it was the adenocarcinoma originated from the digestive tract (Fig. 4D and E). The ileal mesenteric lymph node metastases were positive. As for the retrieval cord connecting the tumor from the umbilicus, stumps were missing during specimen fixation; hence, histopathological examination was impossible.
Fig. 4.
On histopathological examination, obstructed ileal lesions (A), cystic wall lesions(B) and porridge-like contents (C) were all diagnosed as moderately differentiated tubular adenocarcinoma. The immunohistochemical analysis with cytokeratin 7(-) (D)/cytokeratin 20(++) (E) confirmed that it was the adenocarcinoma originated from the digestive tract.
Considering that the cord extending from the umbilicus was connected to the tumor and that the tumor invaded the terminal ileum, we made a diagnosis of MD adenocarcinoma accompanied with the umbilical side of VDR and the huge cystic lesion bloated by tumor components filling up the duct due to cancer progression.
The postoperative course was uneventful and his abdominal symptoms also disappeared. He was discharged on the 12th day after surgery and postoperative adjuvant chemotherapy was performed for 6 months postoperation without complications. However, 4 months after the administration, the recurrence of multiple para-superior mesenteric artery lymph nodes and peritoneal dissemination appeared; therefore, the patient is currently receiving a second-line chemotherapy regimen.
3. Discussion
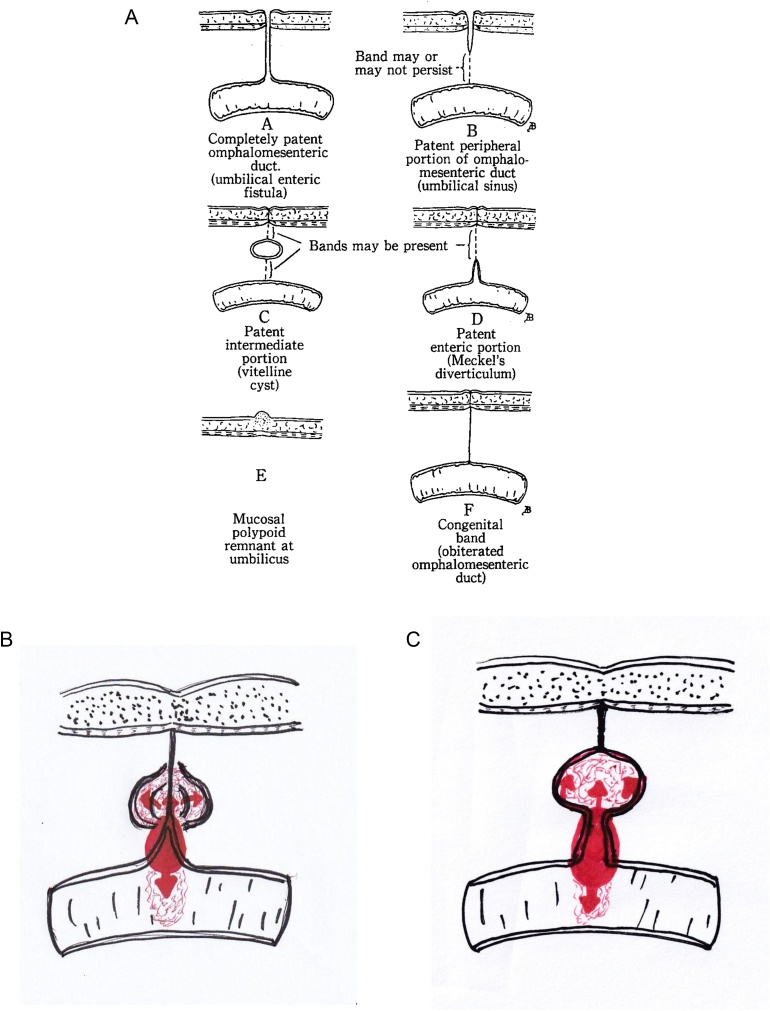
VDR is classified by FOX [7] (Fig. 5A). The most common type of VDR is the D type (known as MD, a remnant of the intestinal side), accounting for 67% of cases, followed by other rare types [2,3]. In our case, VDR was of the D type; the MD adenocarcinoma led to not only the ileal side but also the formation of a huge cystic lesion by spreading in VDR of the umbilicus side (Fig. 5B). However, we did not rule out the possibility of the C type with a congenital residual cyst in the VDR, in this situation, it is considered to be the mixed type involving C and D (Fig. 5C). A literature review revealed no reports of MD adenocarcinoma with an umbilical side of VDR or a huge cystic lesions; our case is therefore considered to be the first one. As for the E type, which occurs in the abdominal wall of the umbilicus, only two cases have been reported [8,9].
Fig. 5.
The classification of VDR (FOX, 1951) (A). VDR in our case was of the D type; the MD adenocarcinoma led to not only the ileal side but also the formation of a huge cystic lesion by spreading in VDR of the umbilicus side (B). However, we did not rule out the possibility of the C type with a congenital residual cyst in the VDR, in this situation, it is considered to be the mixed type involving C and D (C).
MD tumors are rare, and the reported occurrence is 0.5%–3.2% [10,11]. Although there are several reports of carcinoid, gastrointestinal stroma tumor, sarcoma, and lymphoma [5], the incidence of adenocarcinoma is 21.7% for the malignant tumors in MD.
The early diagnosis of MD adenocarcinoma is difficult because of its obscure anatomical characteristics. Therefore, it is often discovered only after the patients have advanced cancer with clinical symptoms include abdominal mass, abdominal pain, and so on; bowel obstruction symptoms often occur, as noted in our case.
In diagnostic imaging, CT or MRI shows a small intestine tumor or a pelvic tumor, but detailed local diagnoses and definitive diagnosis of MD adenocarcinoma is considered difficult. In our case, CT and MRI showed a huge pelvic tumor involving the terminal ileum and a cord connecting the tumor to the umbilicus. At this stage, we diagnosed a huge pelvic tumor that was strongly suggestive of being malignant; this tumor was connected to the umbilicus by a cord and involved the terminal ileum. However, no clear diagnosis of VDR or MD adenocarcinoma was made.
Postoperatively, macroscopic findings of the specimen revealed the presence of a cord that connects the main tumor to the umbilicus and provided evidence that the tumor involved the ileum with bowel obstruction, and then, we speculated the possibility of MD-occurring tumor with VDR.
Finally, because histopathological examination and immunochemical analysis confirmed that the adenocarcinoma was originated from the digestive tract, we diagnosed this tumor as MD adenocarcinoma accompanied with the umbilical side of VDR and the huge cystic lesion, at Stage IIIB(T4N2M0) according to the Union for International Cancer Control (UICC) classification [12].
The chief treatment option is surgical resection of the tumor, but there is no definite view on the operative procedure. However, if curative surgical resection is possible, long-term survival can be expected; hence, partial resection of the small intestine that secures a sufficient resection margin on the oral and anal sides is recommended, and in some cases, ileocecal resection is necessary. Simultaneously, regional lymph nodes dissection may be appropriate.
Regarding the prognosis of MD adenocarcinoma, it is likely that a highly advanced local invasion and lymph node metastases are involved and that the prognosis is poor [4,5,13]. In our case, the recurrence of multiple para-superior mesenteric artery lymph nodes and peritoneal dissemination appeared. However, both of them were distant metastases, and the postoperative recurrence would not have been avoided even with a longer resection of the ileum or the ascending colon.
Currently, there is no recommended chemotherapeutic regimen for MD adenocarcinoma. When MD adenocarcinoma is classified as ileal cancer, the chemotherapeutic regimen for ileal cancer in Japan is generally performed according to “the Japanese Society for Cancer of the Colon and Rectum Guidelines for the Treatment of Colorectal Cancer” [14]. Accordingly, the patient received adjuvant chemotherapy with the “Capecitabine + Oxaliplatin” regimen, but the recurrence appeared. Therefore, second-line chemotherapy with the “S-1 + Irinotecan + Bevacizumab” regimen is currently underway. There are only a few reports on chemotherapy for MD adenocarcinoma [15,16]. It is expected that cases should be accumulated in the future for the development of a more optimally recommended regimen.
4. Conclusion
We reported a case of MD adenocarcinoma accompanied with the umbilical side of VDR and the huge cystic lesion that seemed to have been formed by filling tumor components inside VDR. Although the incidence of our case is extremely rare, the recognition of tumor development of the embryonic remnant origin is considered important for the treatment of this adenocarcinoma with surgery and/or chemotherapy.
Declaration of Competing Interest
The authors report no conflicts of interest.
Funding
This report has not received any funding.
Ethical approval
This is a case report and it did not require ethical approval from ethics committee according to our institution.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Conception and design of the study: MT.
Performed the surgery and perioperative management on the patient: MT, YM, KI.
Data acquisition: MT.
Drafting and revising of the article: MT, YM, KI, HT.
Final approval of the version to be submitted: MT, YM, KI, HT.
Registration of research studies
NA.
Guarantor
Masahiro Tawada, MD.
Provenance and peer review
Not commissioned, externally peer-reviewed.
References
- 1.Vane D.W., West K.W., Grosfeld J.L. Vitelline duct anomalies. Experience with 217 childhood cases. Arch. Surg. 1987;122:542–547. doi: 10.1001/archsurg.1987.01400170048007. [DOI] [PubMed] [Google Scholar]
- 2.Moore T.C. Omphalomesenteric duct malformations. Semin. Pediatr. Surg. 1996;5:116–123. [PubMed] [Google Scholar]
- 3.Coetzee T. Clinical anatomy of the umbilicus. S. Afr. Med. J. 1980;57:463–466. [PubMed] [Google Scholar]
- 4.Kusumoto H., Yoshitake H., Mochida K. Adenocarcinoma in Meckel’s diverticulum: report of a case and review of 30 cases in the English and Japanese literature. Am. J. Gastroenterol. 1992;87:910–913. [PubMed] [Google Scholar]
- 5.Kabir S.A., Raza S.A., Kabir S.I. Malignant neoplasms of Meckel’s diverticulum; an evidence based review. Ann. Med. Surg. 2019;43:75–81. doi: 10.1016/j.amsu.2019.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agha R.A., Borrelli M.R., Farwana R. The SCARE 2018 statement: updating consensus surgical case report (SCARE) guidelines. Int. J. Surg. 2018;60:132–136. doi: 10.1016/j.ijsu.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 7.Fox P.F. Uncommon umbilical anomalies in children. Surg. Gynecol. Obset. 1951;92:95–100. [PubMed] [Google Scholar]
- 8.Glazer G. Primary adenocarcinoma arising in a vitello-intestinal duct remnant at the umbilicus. Br. J. Surg. 1973;60:247–249. doi: 10.1002/bjs.1800600323. [DOI] [PubMed] [Google Scholar]
- 9.Lei L., Deisch J.K. Serous cystadenocarcinoma arising in presumed vitelline duct remnant: a case report and implications in the management of cancer of unknown primary. Case Rep. Pathol. 2016 doi: 10.1155/2016/4365217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi M., Takeuchi S., Awazu S. Meckel’s diverticulum. Investigation of 600 patients in Japanese literature. Am. J. Surg. 1978;136:247–249. doi: 10.1016/0002-9610(78)90238-6. [DOI] [PubMed] [Google Scholar]
- 11.Yahchouchy E.K., Marano A.F., Etienne J.C. Meckel’s Diverticulum. J. Am. Coll. Surg. 2001;192:658–662. doi: 10.1016/s1072-7515(01)00817-1. [DOI] [PubMed] [Google Scholar]
- 12.Brierley J., Gospodarowicz M.K., Wittekind C. 8th ed. John Wiley & Sons Inc; Oxford: 2017. TNM Classification of Malignant Tumours. [Google Scholar]
- 13.Sujit V.S., Nitin B., Rajiv P. Krukenberg tumor: metastasis of Meckel’s diverticula adenocarcinoma to ovaries. J. Nippon. Med. Sch. 2009;76:96–102. doi: 10.1272/jnms.76.96. [DOI] [PubMed] [Google Scholar]
- 14.Hashiguchi Y., Muro K., Saito Y. Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2020;25:1–42. doi: 10.1007/s10147-019-01485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Y., Yang X., Ye Y. Adenocarcinoma located at a Meckel’s diverticulum: a case report and literature review. J. Cancer Res. Ther. 2017;13:878–881. doi: 10.4103/jcrt.JCRT_124_17. [DOI] [PubMed] [Google Scholar]
- 16.Bahesh E.E., Abell B.M., Sugarbaker P.H. Peritoneal metastases from malignant degeneration of ectopic gastric epithelium in Meckel’s diverticulum: a case report. Int. J. Surg. Case Rep. 2019;61:305–308. doi: 10.1016/j.ijscr.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]