Abstract
Background:
There are limited data on population-wide assessment of cost in Crohn’s disease (CD) and ulcerative colitis (UC).
Aim:
To estimate the societal cost of actively treated CD and UC in Sweden
Methods:
We identified 10,117 prevalent CD and 19,762 prevalent UC patients, aged ≥18 years on January 1, 2014 and 4,028 adult incident CD cases and 8,659 adult incident UC cases (2010-2013) from Swedish Patient Register. Each case was matched to 5 population comparators. Health care costs were calculated from medications, outpatient visits, hospitalizations, and surgery. Cost of productivity losses was derived from disability pension and sick leave.
Results:
The mean annual societal costs per working age patient (18-64y) with CD and UC were $22,813 (vs $7,533 per comparator) and $14,136 (vs $7,351 per comparator), respectively. In patients aged ≥ 65y, the mean annual costs of CD and UC were $9,726 and $8,072 vs $3,875 and $4,016 per comparator, respectively. The majority of cost for both CD (56%) and UC (59%) patients originated from productivity losses. Higher societal cost of working age CD patients as compared to UC patients was related to greater utilization of anti-TNF (22.2% vs 7.4%) and increased annual disability pension (44 days vs 25 days). Among incident CD and UC patients, the mean total cost over the first year per patient was over 3 times higher than comparators.
Conclusion:
In Sweden, the societal cost of incident and prevalent CD and UC patients was consistently 2 to 3 times higher than the general population.
INTRODUCTION:
Crohn’s disease (CD) and ulcerative colitis (UC), collectively known as inflammatory bowel disease (IBD), are chronic inflammatory diseases of the gastrointestinal tract with rising global incidence1 resulting in substantial morbidity2, health care costs, and loss of productivity3, 4. In parallel, biologic therapies, particularly those that target tumor necrosis factor-alpha (TNF-α) have been increasingly used for treatment of IBD due to their superior efficacy to older conventional medical therapies including 5-aminosalicylic acid (5-ASA), corticosteroids, thiopurines and methotrexate5, 6. Although these treatments have been shown to improve quality of life and work-related productivity7, it is expected that their broader use is also associated with rise in direct health cost expenditures. Therefore, there is increasingly a need for comprehensive assessment of societal cost of IBD. Such studies could provide guidance for developing cost-saving interventions.
Previous studies of cost in patients with IBD have suffered from a number of drawbacks. First, some studies have extrapolated cost from short-term data available in randomized controlled trials or derived their estimates from a single center or region which may not reflect the real-world costs or be a representative sample8–11 . Second, most studies have assessed short-term costs without considering the indirect and long-term health-care and productivity costs, including rates of hospitalizations, surgery, work loss, and disability12, 13. Third, there are significant heterogeneities in measurement of health care cost stemming from incomplete assessment of components of cost and lack of third party data on these expenditures, which could significantly minimize the risk of measurement errors and recall bias. This is best demonstrated in a recent systematic review of annual indirect cost, as measured by work loss and disability, in IBD which found values ranging from $159 to $14,13614. Such significant heterogeneity limits our ability to fully assess the economic burden of IBD. Additionally, differences in healthcare systems may account for heterogeneities observed in cost, which also highlights the need for comprehensive country-specific analyses of cost. Lastly, very few studies have compared the cost of IBD patients to those of the general population, which could provide a more clear picture of the relative societal cost of IBD.
We therefore sought to comprehensively estimate total societal cost of CD and UC in a nationwide study in Sweden where all cost components were derived from population-based registers with near complete coverage. Specifically, we aimed to estimate the annual and monthly costs related to work loss, hospitalizations, prescription medications use, and surgery in register-identified incident and prevalent CD and UC patients in relation to the corresponding costs in the general population comparators. As the components of cost vary according to age of diagnosis, we stratified all analyses by working adults (defined here as age 18-64 years) and older adults (defined here as age ≥ 65 years).
METHODS:
The Swedish Patient Register:
The Swedish healthcare system is tax-funded and offers universal access including prescription coverage above an annual threshold of 2200 SEK or $264. In 2014, the health care system provided for 9.7 million residents in Sweden, 5.7 million of whom were working age adults. The Swedish National Board of Health and Welfare has collected individual-level data on hospital discharges on a countywide level since 1964 (nationwide since 1987)15. Each record, organized according to an individual's personal identity number16, includes date of birth, sex, dates of hospital admission, hospital department, and discharge diagnoses (including surgical procedures), coded according to the International Classification of Diseases. In 2001, this register was expanded to include non-primary outpatient care17.
Register Sources:
Incident and prevalent cases of CD and UC were identified from the Swedish Patient Register (1964-2015), the Swedish Quality Register for Inflammatory Bowel Disease (SWIBREG) (1995-2015), and the Prescribed Drug Register (2005-2015). Each register has been described previously18, 19. Sick leave and disability pension data were retrieved from the Social Insurance Agency, while hospital care and surgery, and non-infusion drug use were retrieved from the National Patient Register and Prescribed Drug Register, respectively. Lastly, use of infusion-based biologic therapy was identified through data from National Patient Register, the Prescribed Drug Register, and SWIBREG.
Cohort Assembly:
Prevalent Cohort:
All adult prevalent cases defined as having ≥ 2 CD (ICD9: ‘555’, ICD10: “K50’) or UC ( ICD9: ‘556’, ICD10: ‘K51’) visits, age ≥ 18 years, and receiving medical therapy in the form of 5-ASA, corticosteroids, immunomodulators (thiopurines and methotrexate), or anti-TNF therapy on January 1, 2014 were identified from the Swedish National Patient Register and SWIBREG (Figure 1). Other inclusion and exclusion criteria are described in Figure 1. All prevalent cases were followed from January 1, 2014 until December 31, 2014.
Figure 1:
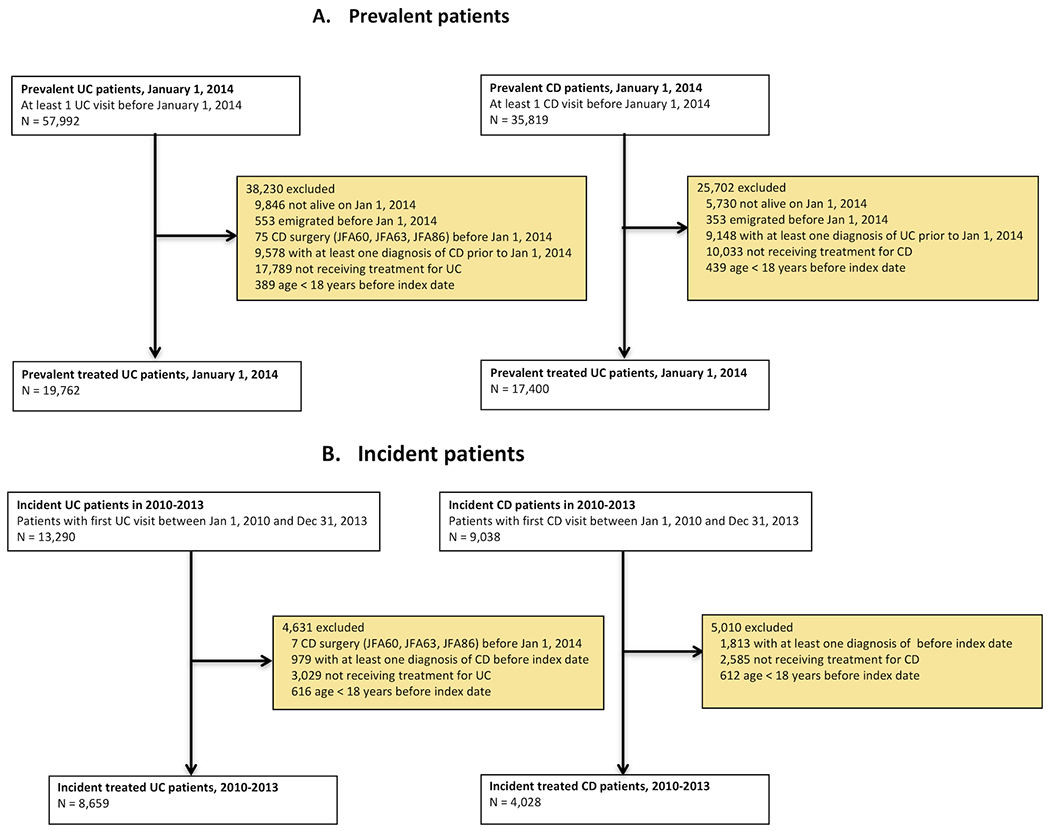
Flow chart of eligible patients
Incident Cohort:
Incident adult cases were defined as patients ≥ 18 years with ≥ 1 inpatient or outpatient encounters with primary or secondary diagnosis of CD or UC and receiving medical therapy in the form of 5-ASA, corticosteroids, immunomodulators (thiopurines and methotrexate), or anti-TNF therapy from January 1, 2010 until December 31st, 2013 (Figure 1). This time period was selected to ensure capturing of cost related to anti-TNF therapy in CD and UC (European Medicines Agency granted approval of infliximab as the first anti-TNF treatment for IBD in March of 2006). All incident cases were followed from one year before to one year after the time of initial diagnosis in 2010-2013.
Matched General Population Comparators
To compare the cost of incident and prevalent CD and UC patients to the general population, up to five comparators from the Total Population Register were matched to each case according to age, sex, county, and education (ascertained at the time of eligibility for inclusion in both incident and prevalent cases and comparators)20, 21. Linkage of all register sources were conducted using each individual's personal identity number.
Calculation of Costs:
All costs were estimated over the follow up time and were converted to USD (1 USD = 6.86 SEK in 2015; current conversion in 2020 is 10.42 SEK).
Healthcare Costs:
Costs for non-primary outpatient care visits (up to three primary diagnostic codes), hospital admission days, and surgery were calculated using the diagnosis-related group codes in 2015, estimated by using the weighted average of costs per diagnostic related group (https://www.socialstyrelsen.se/en/statistics-and-data/statistics/). Medications were broadly categorized into IBD-related medications which included 5-ASA, corticosteroids, immunomodulators (thiopurines and methotrexate), anti-TNF therapy and non-IBD related medications which were labeled as other medications. Costs of non-infusion prescribed medications for IBD were directly derived from the Prescribed Drug Register and adjusted to 2015 using the inflation index for drugs in Sweden. Infliximab use and dosage were collected from SWIBREG and the National Patient Register. Data on dosage registered in SWIBREG were turned into costs by using the 2015 drug costs in Sweden (https://www.tlv.se/in-english.html). While no data on dosage are recorded in the National Patient Register, we used dosage information from SWIBREG, stratified on men and women, to estimate dosage and costs for the identified infusions in the National Patient Register.
Productivity loss:
The Swedish social insurance system provides compensation for sick leave and disability pension. Sick leave is compensated if it exceeds 2 weeks at which point the episode is registered in the Social Insurance Agency Database. The employer is responsible for payment related to days 2-14 unless the employee is in need of recurrent short term leaves or have had a recent sick leave (within 4 days). Temporary compensation for sick leave for up to one year is provided. Disability pension is provided if work performance is reduced by at least 25% and is usually followed by one year of sick leave. In Sweden, the typical age of retirement is between 63 and 67 years with the most common age of retirement being 65 years. Therefore, we did not calculate productivity loss for individuals older than 65 years.
Work loss was estimated using the human capital approach, which took into consideration the number of accumulated days of sick leave and disability pension for each episode22. The productivity loss was then derived by multiplying the accumulated work loss days with the average salary in Sweden in 2015 (32,000 SEK/month = 4,665 USD). (https://www.scb.se/en/finding-statistics). All employers in Sweden pay 31.42% of the gross salary in social security contributions consisting of charges for pensions (10.21%), health insurance (3.55%) and other social benefits.
Total societal cost:
Total societal cost was estimated by summing costs related to healthcare and productivity loss.
Statistical Analysis:
Consistent with our prior analyses23, 24, values of cost are reported in means as it’s widely thought to be the most informative representation of cost and resource utilization. However, since cost is not normally distributed, median values are additionally reported. Since the age of retirement in Sweden is generally around 65 years, cost analyses were separated according to age groups,18-64 years and ≥ 65 years, with work loss only assessed in the former group. We also separated cost estimates according to type of medication use, defined as 5-ASA, immunomodulators use, and use of biologic therapy. Patients on combination of 5-ASA and an immunomodulator were placed in the immunomodulator group while patients on anti-TNF and 5-ASA or immunomodulator were placed in anti-TNF group. Lastly, in the incident cohort, monthly cost was estimated from one year prior to identification as an incident case to one year after to examine changes in the cost around the time of diagnosis. Confidence intervals for cost differences between patients and general population comparators were estimated using non-parametric bootstrapping. All data were analyzed using SAS version 9.4 (Cary, NC).
Study Approval and Patient and Public Involvement:
The study was approved by the Stockholm Ethics Review Board. Informed consent was waived by the board since the study was strictly register-based. In this register-based study patients or public were not directly involved in the design, recruitment or conduct of the study.
RESULTS:
Our study population included 10,117 and 19,762 actively treated prevalent CD and UC patients, aged ≥18 years identified from the Swedish National Patient Register on January 1, 2014 (Table 1, Supplementary Tables 1–2). Similarly, we identified 4,028 and 7,540 adult incident CD and UC cases undergoing treatment between 2010 and 2013, respectively (Table 1, Supplementary Tables 1–2). Baseline characteristics of all CD and UC patients according to prevalent or incident status at the time of their eligibility for our study are reported in Table 1. The average age at study entry was 50 years in prevalent cases and 46 years in incident cases (i.e. age of diagnosis) with a similar proportion of men and women (49% women for prevalent cases and 51% for incident cases). Nearly 41% of prevalent CD and UC cases were on immunomodulators or anti-TNF therapy compared to 9% of incident cases at one month after diagnosis.
Table 1:
Characteristics of prevalent and incident Crohn’s disease (CD) and ulcerative colitis (UC) patients∆
| Variable | Prevalent treated CD and UC patients† (2014) | Incident treated CD and UC patients†(2010-2013) | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall N = 29,879 | Anti-TNF N = 2,393 | Immunomodulator N = 8,387 | 5-ASA N = 14,484 | Matched comparators N = 146,934 | Overall N =12,687 | Matched comparators N =63,097 | ||
| Disease type, n (%) | ||||||||
| Crohn’s disease | 10,117 (33.9%) | 1,531 (64.0%) | 4,279 (51.0%) | 2,054 (14.2%) | 49,468 (33.7%) | 4, 028 (31.7%) | 20,055 (31.8%) | |
| Ulcerative colitis | 19,762 (66.1%) | 862 (36.0%) | 4,108 (49.0%) | 12,430 (85.8%) | 97,466 (66.3%) | 8,659 (68.3%) | 43,042 (68.2%) | |
| Female, n (%) | 14,532 (48.6%) | 1,016 (42.5%) | 3,847 (45.9%) | 7,150 (49.4%) | 71,498 (48.7%) | 6,482 (51.1%) | 32,254 (51.1%) | |
| Age (years) | ||||||||
| Mean (SD) | 50.1 (17.6) | 41.4 (15.2) | 46.3 (16.9) | 51.1 (17.4) | 50.1 (17.5) | 45.6 (19.1) | 45.6 (19.1) | |
| Median (25th-75th) | 49.7 (35.7-64.2) | 39.6 (28.2-52.1) | 45.6 (32.1-59.9) | 50.8 (37.1-65.0) | 49.7 (35.6-64.1) | 42.8 (28.5-61.6) | 42.8 (28.5-61.6) | |
| Range (min-max) | 18.0-100.6 | 18.0-83.9 | 18.0-95.8 | 18.0-100.6 | 18.0-99.7 | 18.0-100.6 | 18.0-100.9 | |
| 18-64 years, n (%) | 22,294 (74.6%) | 2,155 (90.1%) | 6,867 (81.9%) | 10,603 (73.2%) | 109,980 (74.8%) | 9,937 (78.3%) | 49,467 (78.4%) | |
| ≥ 65 years, n (%) | 7,585 (25.4%) | 238 (9.9%) | 1,520 (18.1%) | 3,881 (26.8%) | 36,954 (25.2%) | 2,750 (21.7%) | 13,630 (21.6%) | |
| Level of education, n (%) | ||||||||
| ≤9y | 5,343 (17.9%) | 309 (12.9%) | 1,306 (15.6%) | 2,545 (17.6%) | 25,815 (17.6%) | 2,313 (18.2%) | 11,520 (18.3%) | |
| 10-12y | 14,028 (46.9%) | 1,180 (49.3%) | 4,131 (49.3%) | 6,548 (45.2%) | 69,579 (47.4%) | 5,923 (46.7%) | 29,610 (46.9%) | |
| >12y | 10,361 (34.7%) | 887 (37.1%) | 2,911 (34.7%) | 5,327 (36.8%) | 51,171 (34.8%) | 4,318 (34.0%) | 21,552 (34.2%) | |
| Missing | 147 (0.5%) | 17 (0.7%) | 39 (0.5%) | 64 (0.4%) | 369 (0.3%) | 133 (1.0%) | 415 (0.7%) | |
| Comorbidities¶, n (%) | ||||||||
| Type 2 diabetes | 2,153 (7.2%) | 109 (4.6%) | 494 (5.9%) | 1,064 (7.3%) | 8,763 (6.0%) | 748 (5.9%) | 3,181 (5.0%) | |
| Cardiovascular disease | 3,158 (10.6%) | 150 (6.3%) | 667 (8.0%) | 1,455 (10.0%) | 11,059 (7.5%) | 1,298 (10.2%) | 4,173 (6.6%) | |
| Hypertension | 8,561 (28.7%) | 441 (18.4%) | 1,959 (23.4%) | 4,058 (28.0%) | 37,086 (25.2%) | 3,156 (24.9%) | 13,129 (20.8%) | |
| Chronic kidney disease | 876 (2.9%) | 56 (2.3%) | 201 (2.4%) | 247 (1.7%) | 1,882 (1.3%) | 310 (2.4%) | 706 (1.1%) | |
| Stroke | 411 (1.4%) | 20 (0.8%) | 92 (1.1%) | 199 (1.4%) | 1,616 (1.1%) | 186 (1.5%) | 669 (1.1%) | |
| Rheumatic diseases* | 1,618 (5.4%) | 301 (12.6%) | 575 (6.9%) | 389 (2.7%) | 3,280 (2.2%) | 549 (4.3%) | 1,229 (1.9%) | |
| Medication use, n (%) | ||||||||
| Anti-TNF agents with approved indication in CD or UC | 2,739 (9.2%) | 2,369 (99.0%) | 224 (2.7%) | 70 (0.5%) | 188 (0.1%) | 119 (0.9%) | 68 (0.1%) | |
| Other biologics | 43 (0.1%) | 37 (1.5%) | 2 (0.0%) | 0 | 29 (0.0%) | 6 (0.0%) | 1 (0.0%) | |
| Immunomodulators | 9,533 (31.9%) | 1,146 (47.9%) | 8,387 (100.0%) | 0 | 1,253 (0.9%) | 997 (7.9%) | 478 (0.8%) | |
| 5-aminosalicylic acid | 18,738 (62.7%) | 749 (31.3%) | 3,505 (41.8%) | 14,484 (100.0%) | 44 (0.0%) | 8,254 (65.1%) | 18 (0.0%) | |
| Corticosteroids* | 12,154 (40.7%) | 963 (40.2%) | 3,035 (36.2%) | 3,541 (24.4%) | 7,347 (5.0%) | 7,720 (60.8%) | 2,817 (4.5%) | |
| Other drugs | 27,408 (91.7%) | 2,229 (93.1%) | 7,699 (91.8%) | 12,950 (89.4%) | 103,328 (70.3%) | 12,276 (96.8%) | 43,632 (69.2%) | |
Medication use was defined at the time of eligibility for prevalent cases and within the first month of diagnosis for incident cases.
Patients with >1 of anti-TNF, Immunomodulators, or 5-ASA treatment during the study period are included in more than one group, resulting in the n patients overall are not equal to the sum of drug groups.
Represents any diagnostic codes within the last 5 years.
Includes rheumatoid arthritis, systemic lupus, psoriasis, and spondyloarthropathies. Includes budesonide use.
Cost of Patients with prevalent CD and UC
Healthcare costs:
Among working age patients (age 18-64 years), the mean healthcare cost in 2014 was $10,096 for CD and $5,926 for UC (Table 2). In CD, compared to working age patients, the cost of hospital care was higher in older patients ($6,438 vs $4,265). Nevertheless, the overall healthcare cost in older patients was similar to younger patients with CD ($9,726 vs $10,096). This was related to lower medication cost in older CD patients ($3,288 vs $5,83), due to lower utilization of anti-TNF therapy in this population (6.8% vs 21.9%). In UC, despite similar cost related to medication use, compared to working age patients, healthcare cost was significantly higher in older patients ($8,072 vs. $5,926). This was largely due to higher cost of hospital care ($5,711 vs $3,067). In both CD and UC, the increased hospital care in older patients compared to working adults was related to longer average duration of hospitalization (mean = 1.9 vs 4.0 days for CD and 1.2 vs. 3.5 days for UC).
Table 2:
Accumulated resource use and mean costs during 1 year by cost components for prevalent CD and UC patients
| Cost Component | Prevalent treated CD (2014) | Prevalent treated UC (2014) | ||||||
|---|---|---|---|---|---|---|---|---|
| 18-64 years (n=7,663) | ≥ 65 years (n=2,454) | 18-64 years (n=14,631) | ≥ 65 years (n=5,131) | |||||
| Resource use | Mean cost (SD) | Resource use | Mean cost (SD) | Resource use | Mean cost (SD) | Resource use | Mean cost (SD) | |
| Variable | ||||||||
| Drug use, % of patients | ||||||||
| Biologic drug use | ||||||||
| Anti-TNF with approved indication in IBD | 21.9% | 4,444 (10,001) | 6.8% | 1,386 (5,762) | 7.4% | 1,456 (6,074) | 2.4% | 435 (3,173) |
| Other biologics | 0.4% | 50 (924) | 0.2% | 20 (593) | 0.1% | 15 (510) | 0.0% | 1 (84) |
| Total biologic drug use | 22.2% | 4,495 (10,029) | 6.9% | 1,407 (5,807) | 7.4% | 1,471 (6,095) | 2.4% | 436 (3,175) |
| Other non-biologic drug use | ||||||||
| Immunomodulators | 50.8% | 181 (413) | 30.3% | 101 (316) | 25.2% | 96 (325) | 14.9% | 58 (275) |
| 5-aminosalicylic acid | 26.9% | 200 (406) | 29.5% | 225 (422) | 70.1% | 598 (645) | 63.1% | 501 (573) |
| Corticosteroids | 33.4% | 114 (350) | 48.1% | 209 (438) | 25.0% | 27 (141) | 34.3% | 44 (172) |
| Other drugs | 90.3% | 889 (5,076) | 98.6% | 1,365 (3,127) | 85.9% | 679 (2,459) | 97.2% | 1,321 (5,971) |
| Total drug use | 97.9% | 5,830 (11,131) | 99.1% | 3,288 (6,524) | 97.2% | 2,858 (6,622) | 99.2% | 2,361 (6,806) |
| Hospital care, mean (std) | ||||||||
| Non-primary outpatient care visits | 3.9 (5.6) | 1,926 (3,133) | 4.4 (4.9) | 2,092 (2,515) | 2.9 (4.4) | 1,447 (2,341) | 3.5 (5.0) | 1,706 (2,640) |
| Gastroenterologist/internist | 1.8 (3.2) | 913 (1,912) | 1.4 (2.1) | 720 (1,210) | 1.3 (2.7) | 654 (1,523) | 1.0 (1.7) | 538 (1,006) |
| Other specialists | 2.1 (4.3) | 1,013 (2,352) | 2.9 (4.2) | 1,371 (2,084) | 1.6 (3.3) | 792 (1,674) | 2.5 (4.5) | 1,168 (2,378) |
| Inpatient days | 1.9 (9.4) | 2,338 (8,233) | 4.0 (11.9) | 4,346 (11,112 | 1.2 (7.2) | 1,619 (7,610 | 3.5 (10.7) | 4,004 (11,378 |
| Bowel surgery | 0.3 (2.7) | 479 (2,980) | 0.3 (2.8) | 381 (3,517) | 0.1 (1.5) | 220 (2,163) | 0.2 (2.2) | 288 (3,301) |
| Total hospital care | 4,265 (9,796) | 6,438 (12,025) | 3,067 (8,614) | 5,711 (12,230) | ||||
| Total healthcare cost | 10,096 (15,442) | 9,726 (14,161) | 5,926 (11,480) | 8,072 (14,338) | ||||
| Work loss | ||||||||
| Sick leave, n days | 19.3 (62.4) | 3,900 (12,571) | 15.5 (54.6) | 3,118 (11,015) | ||||
| Disability pension, n days | 43.7 (110.3) | 8,816 (22,235) | 25.3 (86.4) | 5,091 (17,416) | ||||
| Total work loss, n days | 63.1 (122.0) | 12,717 (24,602) | 40.7 (100.2) | 8,209 (20,196) | ||||
| Societal cost | 22,813 (31,454) | 9,726 (14,161) | 14,136 (25,684) | 8,072 (14,338) | ||||
Productivity loss:
Rates of workforce participation among working-age CD patients and their population comparators were 80% and 85%, respectively. Unemployment rate was at 5% in both working-age CD patents and their comparators. Working-age CD patients had on average 63 work loss days from sick leave (among 20% of patients with an average = 19 days) and disability pension (among 15% of patients with an average = 44 days) per year. The mean annual productivity loss in working age CD patients was $12,717. Similarly, working-age UC patients had an average of 41 work loss days from sick leave (among 18% of patients with an average = 16 days) and disability pension (among 9% of patients with an average = 25 days). The mean annual productivity loss in working age UC patients was $8,209. Working-age UC patients and their comparators had similar rates of work force participation (86%) and unemployment (5%).
Total Societal Costs:
We calculated the annual total societal cost in prevalent CD and UC patients from healthcare and productivity loss. The mean annual societal cost for the working age and older patients were $22,813 and $9,726, respectively for CD and $14,136 and $8,072, respectively for UC. The higher societal cost in CD compared to UC was related to increased healthcare cost from greater utilization of anti-TNF therapy (22.2% vs. 7.4%) and increased disability pension (44 days vs. 25 days).
Relation to costs in the general population:
We also compared the total annual societal cost of prevalent CD and UC patients to their respective general population comparators (Figure 2). In comparison to the population comparator with an annual societal cost of $7,532, the incremental increase in societal cost among working age patients with CD was $15,281 (95% CI, $14,491-$15,943), a nearly three-fold increase in cost. Work loss accounted for majority of this increase in cost ($12,717 vs $5,946, mean difference = $6,771) followed by medication cost ($5,830 vs $375, mean difference = $5,455). The mean annual societal costs of working age CD patients were $39,238 in the anti-TNF group, $16,779 for immunomodulator group, and $15,583 for 5-ASA group compared to $7,532 in the population comparators (Figure 2a). Similarly, in UC compared to population comparators with annual societal cost of $7,351, there was an increase among the working age UC patients of $6,786 (95% CI $6,342 - $7,199). Work loss ($8,209 vs $5,718, mean difference = $2,491) followed by medication cost ($2,858 vs $374, mean difference = $2,484) accounted for the majority of increase in cost. The mean annual societal costs of working age UC patients were $37,357 in the anti-TNF group, $12,260 for immunomodulator group, and $11,236 for 5-ASA group compared to $7,351 in the population comparators (Figure 2b).
Figure 2:
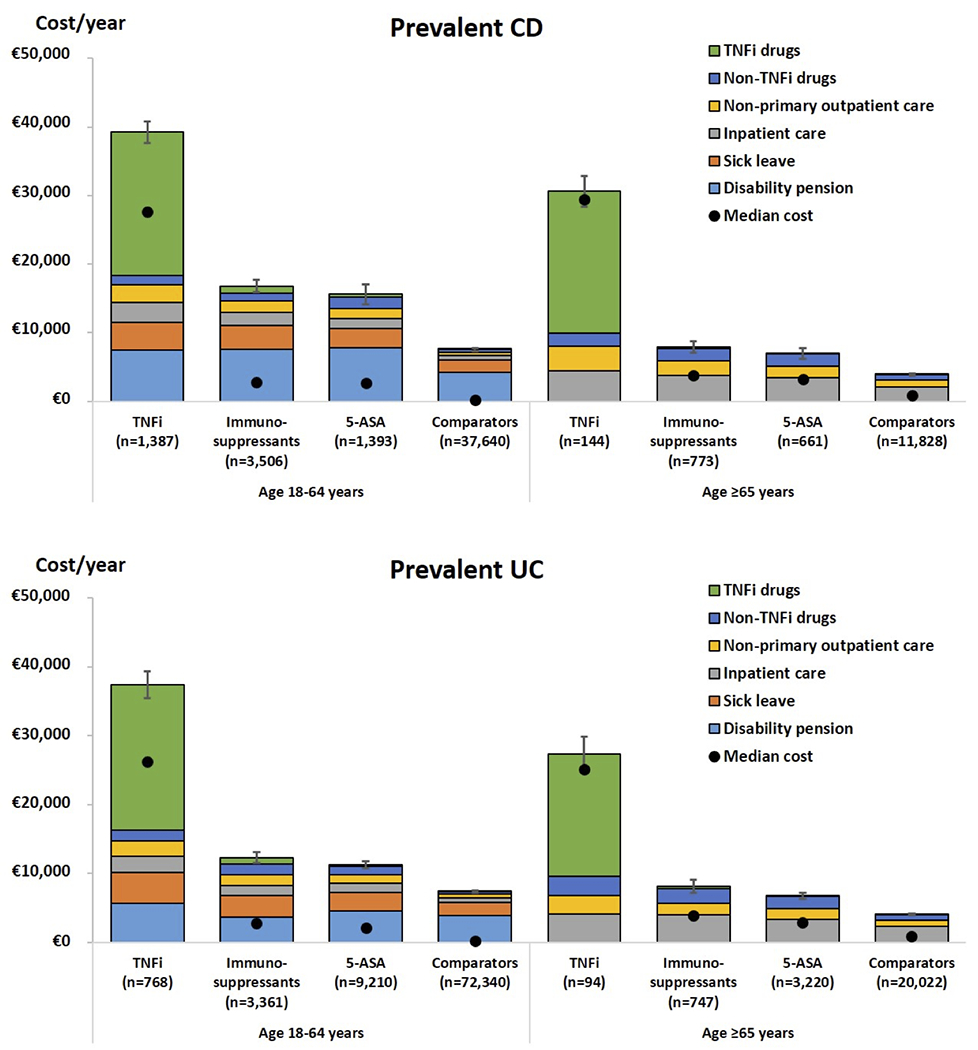
Annual mean cost of prevalent patients with CD and UC compared to general population comparators in working age and older adults
In older CD patients, compared to population comparators with total cost of $3,875, the cost increased by $5,852 (95% CI $5,268 - $6,440), a greater than two-fold increase in cost. This increase in cost was related to in average longer hospitalization ($4,346 vs $2,096) and higher medication cost ($3,288 vs. $822). The mean annual costs of older CD patients were $30,629 in the anti-TNF group, $7,919 in the oral immunomodulator group, and $6,964 in the 5-ASA group compared to $3,875 in the population comparator group (Figure 2a).
Similarly, in UC patients, compared to population comparators, we estimated a mean increase in total cost of $4,057 (95% CI, $3,674 - $4,508), which was also related to in average longer hospitalization ($4,004 vs $2,289) and higher medication cost ($2,361 vs $786). The mean annual societal costs of older UC patients were $27,314 in the anti-TNF group, $8,100 for oral immunomodulator group, and $6,729 for 5-ASA group compared to $4,016 in the population comparators (Figure 2b).
Cost of Patients with Incident CD and UC:
Healthcare costs:
Similar to prevalent cases, the use of immunomodulators and anti-TNF therapy was higher in working age compared to older patients during the first year of diagnosis (CD: 12.8% vs 4.2% for anti-TNF and 45.7% vs 26.1% for immunomodulators; UC: 5.0% vs. 1.4% for anti-TNF and 18.8% vs 10.7% for immunomodulators). Nevertheless, due to significantly higher cost of non-IBD medications in older patients, these differences resulted in only small annual increase in medication cost in working-age patients as compared to older patients within the first year of diagnosis (CD: $3,837 vs $3,010 and UC: $2,509 vs $2,324). The number of outpatient gastroenterology visits in the first year of CD or UC diagnosis was similar in both age groups and ranged between approximately 3 visits for CD and 2 visits for UC. However, the cost related to hospitalization for older CD and UC patients increased by over 2-fold when compared to working age patients yielding higher overall healthcare cost in the first year of diagnosis (CD: $16,282 vs $11,784 and UC: $12,191 vs $7,926).
Productivity loss:
Among working age adults with incident CD and UC, the number of days of sick leave (29 days for CD and 20 days for UC) and disability pension (31 days for CD and 24 days for UC) were similar in the first year of diagnosis. The mean productivity losses in the first year of diagnosis among working age CD and UC patients were $12,102 and $8,852, respectively.
Total societal cost:
The mean annual societal costs in the first year of CD and UC diagnosis were $23,886 and $16,778 for working age patients and $16,282 and $12,191 for older patients, respectively.
Relation of cost to diagnosis and population comparators:
We examined the dynamic changes in cost from one year before to one year after the diagnosis of CD and UC and compared that to the matched population comparators (Figure 3). The monthly cost increased from a level twice that of the comparators 1 year before register identification of CD diagnosis (18-64 years: $1,043 vs $505; mean difference $538 95% CI $466 - $611; ≥ 65 years $622 vs $283; mean difference $340 95% CI $211 - $468), peaked the month following the diagnosis and decreased to around three times the cost of the comparators thereafter (18-64 years: $1,686 vs $570; mean difference $1,116, 95% CI $1,036-$1,196; ≥ 65 years $1,083 vs $354; mean difference $729, 95% CI $577-$882) (Figure 3a). Similarly in UC, the monthly cost increased from a level close to the comparators 1 year before register identification of UC diagnosis (18-64 years: mean difference $228 95% CI $178 - $279; ≥ 65 years: mean difference $175 95% CI $101 - $249), peaked the month following the diagnosis and decreased to around twice the cost of the comparators thereafter (18-64 years: mean difference $585, 95% CI $534-$636; ≥ 65 years: mean difference $459, 95% CI $372-$547) (Figure 3a).
Fgure 3:
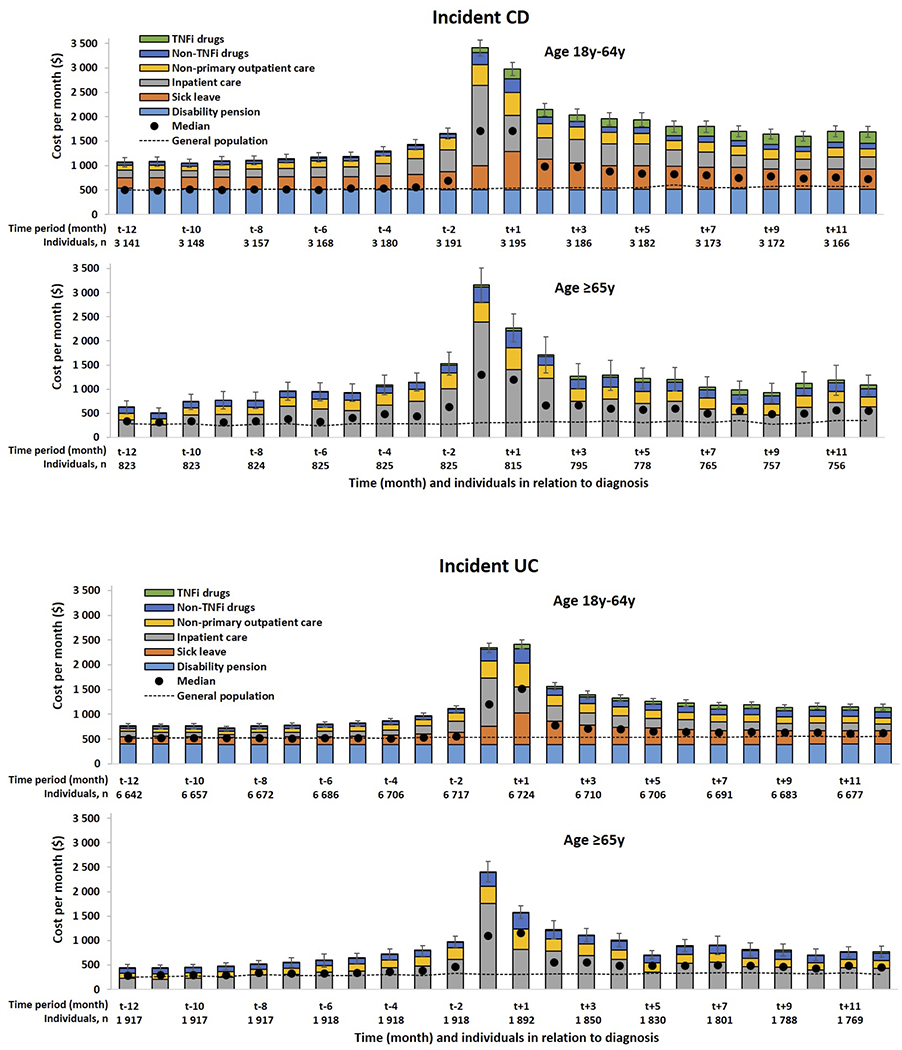
Monthly mean cost in relation to time of diagnosis in incident patients and in general population comparator
Impact of calendar year on healthcare cost in patients with incident CD and UC:
Because of the increase in utilization of anti-TNF therapy over time, we explored whether healthcare costs for incident patients significantly changed during the 2010-2013 period. Healthcare cost was similar across these calendar years. Specifically, in CD, the estimates ranged between $22,199 and $24,669 for working age adults and $13,121 and $18,879 for older patients. In UC, these estimates ranged between $15,401 and $18,077 for working age adults and $11,733 and $13,318 with older patients.
Cost distribution in Incident and Prevalent CD and UC:
The cost distribution in both incident and prevalent CD and UC cohorts were significantly right-skewed albeit to a lesser degree than population comparators. (Figure 4). Approximately 15% of patients with CD and 9% of patients with UC accounted for 50% of the annual total cost in both incident and prevalent cohorts. In the population comparators, the distribution was more skewed with approximately 5% of the population accounting for 50% of the annual cost. The characteristics of CD and UC patients and population comparators in the highest decile of cost distribution are reported in Supplementary Table 3. The primary difference between these two groups were younger age and higher proportion of men in the CD and UC group. Education level and prevalence of cardiometabolic comorbidities were similar across the two groups.
Figure 4:
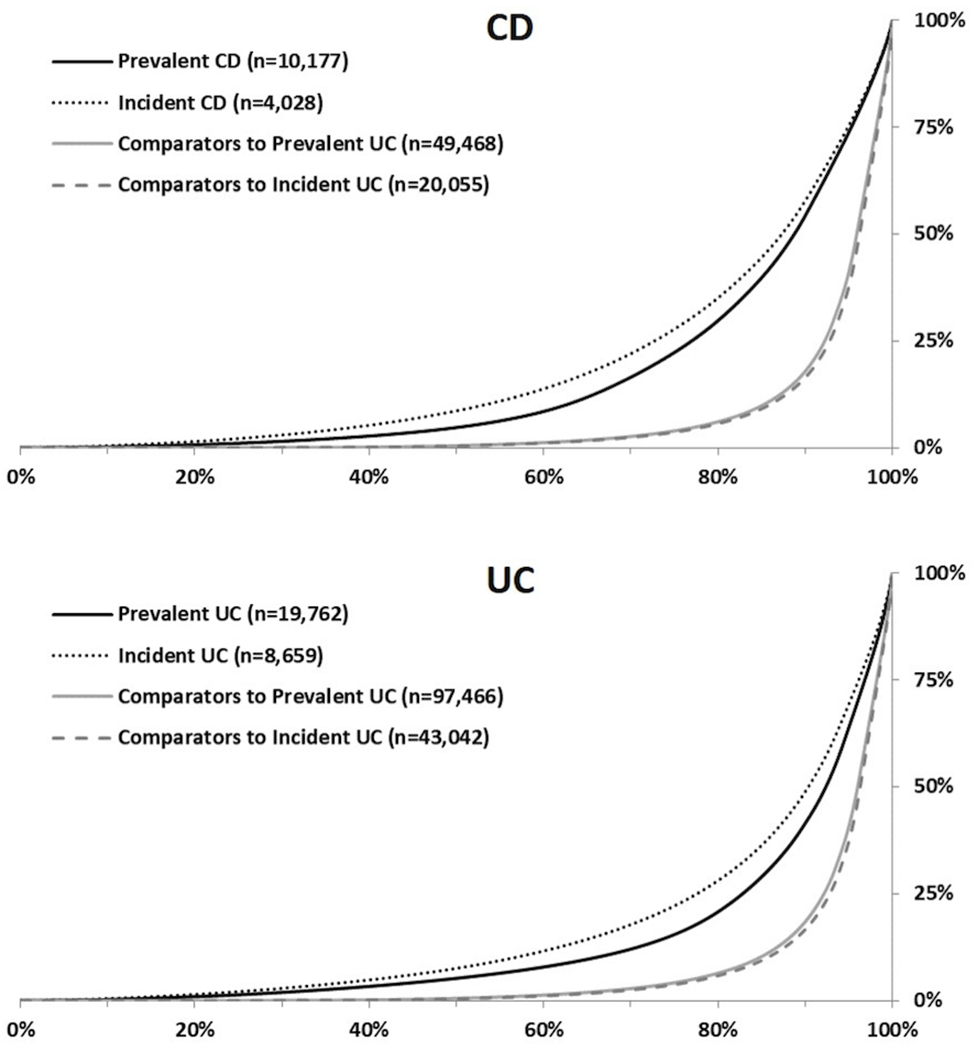
Cost distribution in prevalent and incident patients and their comparators
DISCUSSION:
In a nationwide cohort study in Sweden, we estimated the total annual societal cost of CD and UC derived from inpatient and hospital-based outpatient care, medications, surgery, and productivity loss in prevalent working age patients to be $22,813 and $14,136, respectively. Strikingly, majority of the cost (56% for CD and 59% for UC) originated from productivity losses in the form of sick leave and disability pension25. These costs were 2-3 times higher than the general population comparators corresponding to an annual cost of $15,281 and $6,786 attributable to CD and UC, respectively. Higher economic burden of working age adults with CD as compared to UC was related to greater utilization of anti-TNF therapies and increased disability pension. In patients treated with anti-TNF therapy, the societal cost was 5-7 times that of the general population comparators in both working age and older patients with CD and UC.
Among incident cases, beginning a year before the first register identification of CD and UC, the societal cost increased from a level just above that of the general population for UC and twice of that for CD and peaked the month after the diagnosis. Although the societal cost decreased following the diagnosis, it continued to be 2-3 times that of the general population one year after the diagnosis, mainly due to increase in productivity losses26. Lastly, we demonstrated that the societal cost of CD and UC followed a skewed distribution, with a small proportion of patients accounting for most of the cost. This finding provides opportunities for cost-reducing interventions in the high-cost group. These interventions may include implementation of integrated approach that addresses patients’ need beyond just treatment of their underlying disease such as nutrition, social, and mental health support. Several examples of these include multidisciplinary clinics and patient-center medical homes that have been shown to reduce cost in the US27.
A recent systematic review and meta-analysis highlighted the challenges in comparing cost estimation across studies28, particularly with regards to indirect cost where diverse methodological approaches are used. A few prior studies in Australia and Europe have estimated the cost of CD and UC using inception cohorts but these studies have primarily focused on healthcare related cost without assessment of indirect cost, which based on our study accounts for a substantial proportion of the total societal cost12, 13. In Australia, the estimated mean total health care cost in the first year of diagnosis was $10,477 for CD and $6,292 for UC while in the Netherlands, the mean annual healthcare cost was 7,836 euros (~$8,924) for CD and 3,600 euros (~$4,100) for UC. Since these studies included mostly academic or university-based centers and had limited number of patients, the estimates are likely not an accurate reflection of the real-world cost of the disease. Few other studies have estimated cost based on a single center or region also limiting the generalizability of these studies8–11. Therefore, our analyses that include comprehensive assessment of indirect healthcare cost on nearly 20,000 prevalent and over 4,000 incident cases significantly extend prior findings and provide a more precise and comprehensive picture of the societal cost of the disease.
In the US, one prior analysis of a private insurance carrier among 4,314 working adult (18-64 years) prevalent UC patients found a total direct cost of $15,548 and indirect cost of $4,12529. The large difference in the direct cost between this study and our current study is primarily driven by the significantly higher medication cost in the US. Nevertheless, consistent with our study, Cohen and colleagues found a 2-3-fold higher total societal cost among UC patients compared to controls (total cost = $19,619 vs $6,787).
Our findings were also in line with prior studies of cost in other immune-mediated diseases in Sweden23. Specifically, we have previously demonstrated that the total societal cost of rheumatoid arthritis (RA) among working age adult is 23,000 euros ($29,900), 2-3 fold greater than the general population comparators, corresponding to an annual cost of 15,000 euros ($19,500) attributable to RA. The overall increased in economic burden of RA compared to IBD was related to significantly higher number of disability pension days in RA (118 days) as compared to CD (63 days) and UC (47 days).
Our study has several strengths. All cost data were derived from prospectively collected nationwide data on healthcare and work loss. These data are updated monthly which allowed for finer ascertainment of dynamic changes in cost over the period of the study. Additionally, we used general population comparators to estimate the increase in societal cost related to CD and UC. The main limitation of our study is lack of data on other components of cost including rehabilitation stay, primary care visits, presenteeism, sick leave less than 14 days, and family and personal cost related to travel to various appointments and loss of promotional or educational opportunities. This could suggest that our observed total and indirect costs are underestimated. We also acknowledge that although not all diagnoses of CD and UC were confirmed by medical record review, our prior validation studies have demonstrated high validity18. Another limitation of our study is that findings with regards to cost in Sweden may not be applicable to other countries with different healthcare or social systems. However, our estimates of cost were similar to those reported from Europe and Australia and relative increase in cost is comparable to countries with higher cost of prescription medication such as the US. Additionally, data in RA, which has similar patterns of healthcare and indirect costs to IBD suggest that cost estimates related to anti-TNF use from Sweden are similar to those observed in the rest of Europe and the US30, 31. We also note that although our study did not show a significant change in healthcare cost during the study period, with the advent of biosimilars and their increased use, the direct healthcare cost may decrease over time in subgroups of patients requiring biologic therapy. Nevertheless, the lower medication cost may also result in expanded use, increasing the treatment cost in the patient segment currently not receiving biologic drugs. Lastly, because of the time period of our study, we could not take into consideration cost related to use of other biologic therapy such as ustekinumab, vedolizumab and oral JAK inhibitors as well as the increase in cost related to greater utilization of anti-TNF therapy in CD and UC. However, we note that the true economic impact of these medications particularly with ustekinumab and oral JAK inhibitors on indirect cost may not be fully appreciated until they have been in the market for several years.
In summary, we demonstrate that the annual societal cost of prevalent CD and UC patients and monthly cost in incident CD and UC patients are substantial, and 2-3 times higher than the general population. The skewed distribution of the cost, with a small proportion of patients accounting for most of the cost, provides opportunities in the future for identification of segments of the patient population amendable to cost-reducing interventions.
Supplementary Material
Table 3:
Accumulated resource use and mean costs during 1 year by cost components for incident CD and UC patients
| Cost Component | Incident treated CD (2010-2013) | Incident treated UC (2010-2013) | ||||||
|---|---|---|---|---|---|---|---|---|
| 18-64 years (n=3,201) | ≥ 65 years (n=827) | 18-64 years (n=6,736) | ≥ 65 years (n=1,923) | |||||
| Resource use | Mean cost (SD) | Resource use | Mean cost (SD) | Resource use | Mean cost (SD) | Resource use | Mean cost (SD) | |
| Variable | ||||||||
| Drug use, % of patients | ||||||||
| Biologic drug use | ||||||||
| Anti-TNF with approved indication in IBD | 12.8% | 2,289 (7,024) | 4.2% | 746 (3,959) | 5.0% | 830 (4,491) | 1.4% | 215 (2,266) |
| Other biologics | 0.1% | 11 (441) | 0.2% | 12 (262) | 0.0% | 1 (54) | 0.1% | 10 (473) |
| Total biologic drug use | 12.9% | 2,301 (7,045) | 4.2% | 759 (4,029) | 5.0% | 831 (4,493) | 1.5% | 226 (2,314) |
| Other non-biologic drug use | ||||||||
| Immunomodulators | 45.7% | 99 (261) | 26.1% | 49 (181) | 18.8% | 37 (152) | 10.7% | 19 (116) |
| 5-aminosalicylic acid | 40.0% | 325 (563) | 30.4% | 241 (485) | 90.3% | 834 (766) | 73.4% | 618 (700) |
| Corticosteroids | 83.6% | 324 (507) | 87.4% | 361 (553) | 54.9% | 74 (199) | 66.0% | 119 (296) |
| Other drugs | 94.6% | 797 (2,176) | 99.6% | 1,611 (3,106) | 90.2% | 733 (2,423) | 98.5% | 1,261 (2,258) |
| Total drug use | 100.0% | 3,837 (7,342) | 100.0% | 3,010 (5,059) | 100.0% | 2,509 (5,252) | 100.0% | 2,234 (3,361) |
| Hospital care, mean (std) | ||||||||
| Non-primary outpatient care visits | 5.7 (5.8) | 2,794 (3,006) | 6.1 (8.4) | 2,905 (4,347) | 4.3 (4.4) | 2,212 (2,310) | 4.8 (5.9) | 2,400 (3,216) |
| Gastroenterologist/internist | 3.2 (3.3) | 1,548 (1,651) | 2.6 (3.3) | 1,229 (1,637) | 2.4 (2.8) | 1,244 (1,551) | 2.0 (2.4) | 1,021 (1,352) |
| Other specialists | 2.5 (4.7) | 1,246 (2,472) | 3.5 (7.7) | 1,676 (4,031) | 1.9 (3.2) | 967 (1,694) | 2.8 (5.3) | 1,378 (2,912) |
| Inpatient days | 3.4 (9.9) | 5,152 (10,597 | 8.8 (18.4) | 10,367 (21,437 | 2.4 (9.2) | 3,204 (9,324 | 7.0 (16.6) | 7,556 (14,654 |
| Bowel surgery | 0.6 (3.2) | 1,093 (4,530) | 0.8 (3.9) | 1,225 (5,110) | 0.2 (2.1) | 392 (2,885) | 0.6 (4.1) | 901 (5,348) |
| Total hospital care | 7,946 (11,931) | 13,272 (22,689) | 5,416 (10,429) | 9,956 (15,654) | ||||
| Healthcare cost | 11,784 (14,678) | 16,282 (23,645) | 7,926 (12,691) | 12,191 (16,485) | ||||
| Work loss | ||||||||
| Sick leave, n days | 29.1 (73.8) | 5,858 (14,882) | 20.2 (58.3) | 4,073 (11,756) | ||||
| Disability pension, n days | 31.0 (96.5) | 6,243 (19,448) | 23.7 (84.8) | 4,778 (17,087) | ||||
| Total work loss, n days | 60.0 (115.5) | 12,102 (23,277) | 43.9 (99.9) | 8,852 (20,137) | ||||
| Societal cost | 23,886 (30,797) | 16,282 (23,645) | 16,778 (27,043) | 12,191 (16,485) | ||||
Acknowledgments
Financial Support:
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R03 DK113337 to HK. HK is also supported by American Gastroenterological Association (AGA) Pfizer Young IBD Investigator Grant. The project was also supported by Pfizer
Potential Competing Interests:
Hamed Khalili receives grant support from Takeda and Pfizer. Karolinska Institutet has received study grants and fees for lectures and consulting performed by Ola Olén for Janssen, Pfizer, Ferring, and Takeda. Ola Olén was supported by grants from the Swedish medical society (Project grants, the fund for research in gastroenterology and the Ihre foundation), Mag-tarmfonden, the Jane and Dan Olsson foundation, and Karolinska Institutet foundations while working on this project. Financial support was also provided through the regional agreement on medical training and clinical research between Stockholm County Council and Karolinska Institutet (ALF). Jonas Halvarson served as speaker and/or advisory board member: AbbVie, Celgene, Celltrion, Dr. Falk Pharma and the Falk Foundation, Ferring, Hospira, Janssen, MEDA, Medivir, MSD, Olink Proteomics, Pfizer, Prometheus Laboratories, Sandoz/Novartis, Shire, Takeda, Tillotts Pharma, Vifor Pharma, UCB and received grant support from Janssen, MSD, and Takeda. Par Myrelid has been involved in projects partly financed by investigator-initiated grants from Janssen, Ferring, Takeda, and Pfizer (whereof none of those studies have any relation to the present study) and has received fees for lectures and participation on advisory boards from Abbvie, Janssen, Ferring, Takeda, and Tillotts Pharma not related to the present study. Jonas Ludvigsson coordinates a study on behalf of the Swedish IBD quality register (SWIBREG). This study has received funding from Janssen corporation. Åsa H Everhov has previously worked on projects partly financed by grants from Jansen and Ferring, and was in this project supported by grants from Karolinska Institutet and the Bengt Ihre Foundation. The remaining authors have no conflicts to disclose.
Footnotes
Copyright: The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, a worldwide license to the Publishers and its licensees in perpetuity, in all forms, formats and media (whether known now or created in the future), to i) publish, reproduce, distribute, display and store the Contribution, ii) translate the Contribution into other languages, create adaptations, reprints, include within collections and create summaries, extracts and/or, abstracts of the Contribution, iii) create any other derivative work(s) based on the Contribution, iv) to exploit all subsidiary rights in the Contribution, v) the inclusion of electronic links from the Contribution to third party material where-ever it may be located; and, vi) license any third party to do any or all of the above. The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive license (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in BMJ editions and any other BMJPGL products and sublicenses such use and exploit all subsidiary rights, as set out in our license.
Details of ethics approval: This study was approved by the Regional Ethics Committee, Stockholm, Sweden.
Transparency declaration: The lead author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Data sharing statement: No additional data available due to Swedish regulation.
REFERENCES
- 1.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2018;390:2769–2778. [DOI] [PubMed] [Google Scholar]
- 2.Yarlas A, Rubin DT, Panes J, et al. Burden of Ulcerative Colitis on Functioning and Well-being: A Systematic Literature Review of the SF-36(R) Health Survey. J Crohns Colitis 2018;12:600–609. [DOI] [PubMed] [Google Scholar]
- 3.Kappelman MD, Rifas-Shiman SL, Porter CQ, et al. Direct health care costs of Crohn's disease and ulcerative colitis in US children and adults. Gastroenterology 2008;135:1907–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Longobardi T, Jacobs P, Bernstein CN. Work losses related to inflammatory bowel disease in the United States: results from the National Health Interview Survey. The American journal of gastroenterology 2003;98:1064–72. [DOI] [PubMed] [Google Scholar]
- 5.Panaccione R, Ghosh S, Middleton S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology 2014;146:392–400 e3. [DOI] [PubMed] [Google Scholar]
- 6.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med 2010;362:1383–95. [DOI] [PubMed] [Google Scholar]
- 7.Paschos P, Katsoula A, Salanti G, et al. Systematic review with network meta-analysis: the impact of medical interventions for moderate-to-severe ulcerative colitis on health-related quality of life. Aliment Pharmacol Ther 2018;48:1174–1185. [DOI] [PubMed] [Google Scholar]
- 8.Aldeguer X, Sicras-Mainar A. Costs of ulcerative colitis from a societal perspective in a regional health care area in Spain: A database study. Gastroenterol Hepatol 2016;39:9–19. [DOI] [PubMed] [Google Scholar]
- 9.Bassi A, Dodd S, Williamson P, et al. Cost of illness of inflammatory bowel disease in the UK: a single centre retrospective study. Gut 2004;53:1471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dan A, Boutros M, Nedjar H, et al. Cost of Ulcerative Colitis in Quebec, Canada: A Retrospective Cohort Study. Inflamm Bowel Dis 2017;23:1262–1271. [DOI] [PubMed] [Google Scholar]
- 11.Kamat N, Ganesh Pai C, Surulivel Rajan M, et al. Cost of Illness in Inflammatory Bowel Disease. Dig Dis Sci 2017;62:2318–2326. [DOI] [PubMed] [Google Scholar]
- 12.Niewiadomski O, Studd C, Hair C, et al. Health Care Cost Analysis in a Population-based Inception Cohort of Inflammatory Bowel Disease Patients in the First Year of Diagnosis. J Crohns Colitis 2015;9:988–96. [DOI] [PubMed] [Google Scholar]
- 13.van der Valk ME, Mangen MJ, Severs M, et al. Evolution of Costs of Inflammatory Bowel Disease over Two Years of Follow-Up. PLoS One 2016;11:e0142481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawalec P Indirect costs of inflammatory bowel diseases: Crohn's disease and ulcerative colitis. A systematic review. Arch Med Sci 2016;12:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, et al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol 2009;24:659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Socialstyrelsen. In English - the National Patient Register. 2011.
- 18.Jakobsson GL, Sternegard E, Olen O, et al. Validating inflammatory bowel disease (IBD) in the Swedish National Patient Register and the Swedish Quality Register for IBD (SWIBREG). Scand J Gastroenterol 2017;52:216–221. [DOI] [PubMed] [Google Scholar]
- 19.Wettermark B, Hammar N, Fored CM, et al. The new Swedish Prescribed Drug Register--opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 2007;16:726–35. [DOI] [PubMed] [Google Scholar]
- 20.Ludvigsson JF, Almqvist C, Bonamy AK, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 2016;31:125–36. [DOI] [PubMed] [Google Scholar]
- 21.Ludvigsson JF, Svedberg P, Olen O, et al. The longitudinal integrated database for health insurance and labour market studies (LISA) and its use in medical research. Eur J Epidemiol 2019;34:423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johannesson M The willingness to pay for health changes, the human-capital approach and the external costs. Health Policy 1996;36:231–44. [DOI] [PubMed] [Google Scholar]
- 23.Eriksson JK, Johansson K, Askling J, et al. Costs for hospital care, drugs and lost work days in incident and prevalent rheumatoid arthritis: how large, and how are they distributed? Ann Rheum Dis 2015;74:648–54. [DOI] [PubMed] [Google Scholar]
- 24.Eriksson JK, Neovius M, Jacobson SH, et al. Healthcare costs in chronic kidney disease and renal replacement therapy: a population-based cohort study in Sweden. BMJ Open 2016;6:e012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Everhov AH, Khalili H, Askling J, et al. Sick Leave and Disability Pension in Prevalent Patients With Crohn's Disease. J Crohns Colitis 2018;12:1418–1428. [DOI] [PubMed] [Google Scholar]
- 26.Everhov AH, Khalili H, Askling J, et al. Work Loss Before and After Diagnosis of Crohn's Disease. Inflamm Bowel Dis 2019;25:1237–1247. [DOI] [PubMed] [Google Scholar]
- 27.Click B, Regueiro M. The Inflammatory Bowel Disease Medical Home: From Patients to Populations. Inflamm Bowel Dis 2019;25:1881–1885. [DOI] [PubMed] [Google Scholar]
- 28.Kawalec P, Malinowski KP. Indirect health costs in ulcerative colitis and Crohn's disease: a systematic review and meta-analysis. Expert Rev Pharmacoecon Outcomes Res 2015;15:253–66. [DOI] [PubMed] [Google Scholar]
- 29.Cohen R, Skup M, Ozbay AB, et al. Direct and indirect healthcare resource utilization and costs associated with ulcerative colitis in a privately-insured employed population in the US. J Med Econ 2015;18:447–56. [DOI] [PubMed] [Google Scholar]
- 30.Eriksson JK, Karlsson JA, Bratt J, et al. Cost-effectiveness of infliximab versus conventional combination treatment in methotrexate-refractory early rheumatoid arthritis: 2-year results of the register-enriched randomised controlled SWEFOT trial. Ann Rheum Dis 2015;74:1094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Hout WB, Goekoop-Ruiterman YP, Allaart CF, et al. Cost-utility analysis of treatment strategies in patients with recent-onset rheumatoid arthritis. Arthritis Rheum 2009;61:291–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


