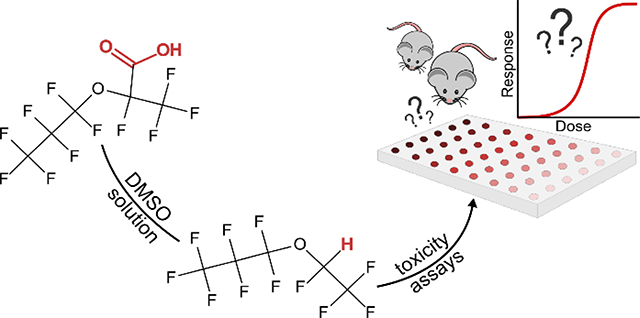
Abstract
Per- and polyfluorinated alkyl substances (PFAS) are of significant interest because of their prevalence and environmental persistence. Further, for many PFAS, including fluorinated ethers, such as hexafluoropropylene oxide dimer acid (HFPO-DA, or the parent acid of “GenX”), toxicological data are sparse. In general, in vitro testing frequently uses dimethyl sulfoxide (DMSO) as a carrier solvent due to its low toxicity, solubility across vast chemical space, and permeation across biological barriers. For PFAS, laboratory practice has assumed that the materials are stable across a wide range of solvents, pHs, and temperatures. In this study, HFPO-DA stability was evaluated with DMSO and other commonly used solvents to determine each solvent’s suitability for use in toxicity assays. The formation of HFPO-DA’s degradation product, heptafluoropropyl 1,2,2,2-tetrafluoroethyl ether (Fluoroether E-1), was monitored by headspace gas chromatography-mass spectrometry (GC-MS) over time. These experiments revealed degradation of HFPO-DA to Fluoroether E-1 in DMSO and other aprotic, polar solvents, with half-lives on the order of hours (1 h, 1.25 h, and 5.2 h for DMSO, acetone, and acetonitrile, respectively). This rapid degradation suggests the need for caution when performing or using data from toxicity assessments on HFPO-DA and closely related PFAS compounds.
Graphical Abstract
INTRODUCTION
Per- and polyfluorinated alkyl substances (PFAS) are a diverse chemical class containing aliphatic chains of fluorine-bonded carbons1 and have long been a focus of significant public and ecological health interest due to their environmental persistence.2 This class of compounds is broadly used in commercial products, including applications in flame-retardants/fire-suppressants, water-resistant coatings, and as surfactant additives.3, 4 Concerns over the health impacts of the legacy PFAS, perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS)5, led to their voluntary phase-out in the U.S. in the early 2000s.6, 7 Despite the phase-out of these specific chemicals, the number of extant PFAS species is believed to be in the thousands1 with a more limited number, on the order of hundreds, in active commercial usage.8
To specifically address concerns related to PFOS/PFOA toxicity, replacement chemistries have been developed with shorter fluoroalkyl chains, such as perfluorobutanesulfonic acid (PFBS), and/or the inclusion of fluoroether moieties, such as perfluoro-2-propoxypropanoic acid, also referred to as hexafluoropropylene oxide dimer acid (HFPO-DA) or its trade name “GenX”. PFAS with these newer chemistries have been increasingly detected in drinking water and soil, including many chemicals which are not explicitly in commercial usage.9–13 The continued discovery of new PFAS with limited health effects information has, unsurprisingly, led to a heightened demand for toxicity data.14–16 The prospect of generating mammalian toxicity data for all emerging PFAS species is daunting, and has resulted in substantial investment in new approach methodologies, including alternate model organisms and in vitro toxicity assays,8, 10, 11, 17–19 which can yield high-throughput data at a rate consistent with the breadth of chemical substances being identified.
The chemical, HFPO-DA (“GenX” parent acid), has offered an exemplary scenario demonstrating the need for rapid integration of environmental monitoring information with relevant toxicity testing. HFPO-DA contamination was first reported in the Cape Fear River of North Carolina in 2015, downstream of a chemical manufacturing plant in Fayetteville, NC.10, 11, 18 Intense scrutiny of the compound ensued due to its relatively high abundance in drinking water and the scarcity of information regarding long-term exposure effects in biological organisms. Focus on the compound in NC also triggered interest in its prevalence in areas near other fluorochemical facilities in the U.S.20, 21 and worldwide.22–24
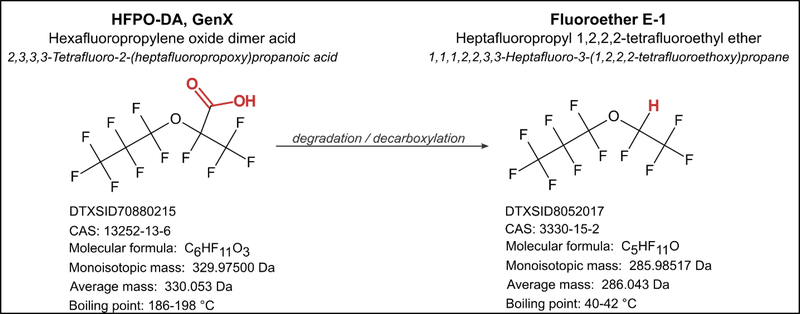
As toxicologists have begun to assess HFPO-DA, we have had reason to doubt the stability of HFPO-DA in dosing solutions relative to the long lifetimes of legacy PFAS species. A study by Sheng et al. noted the apparent loss of HFPO-DA in the solvents they used for testing purposes,25 hypothesizing “high volatility” of the compound, and we had previously noted loss of HFPO-DA in DMSO in some of our own studies.19, 26, 27 Based on our observations of solvent-specific stability issues with HFPO-DA, we hypothesize that degradation is occuring to produce the H-substituted ether derivative, Fluoroether E-1 (heptafluoropropyl 1,2,2,2-tetrafluoroethyl ether [Figure 1]), mediated by reaction with solvent. If not assessed and accounted for, degradation in stock and dosing solutions is likely to result in underestimation of HFPO-DA’s toxicological effects in vitro and in vivo, perhaps instead quantifying the combined effects of parent and product compound or the product compound alone. We therefore set out to assess the stability of HFPO-DA in a variety of toxicologically relevant solvents by monitoring the formation of its degradation product, Fluoroether E-1, by headspace gas chromatography-mass spectrometry (GC-MS) analysis.
Figure 1.
HFPO-DA degradation via decarboxylation to Fluoroether E-1.
MATERIALS AND METHODS
Standards and Solvents
HFPO-DA (EPA DSSTox28 substance identifier [DTXSID] 70880215) and Fluoroether E-1 (DTXSID8052017) standards were purchased from SynQuest Laboratories, Inc. (Alachua, FL) at the highest purity available (97%). All solvents evaluated for HFPO-DA degradation were HPLC-grade or of equivalent purity (≥99.5%). Methanol (MeOH), dimethyl sulfoxide (DMSO), acetone (ACE), dichloromethane (DCM) and acetonitrile (ACN) were Burdick & Jackson (Muskegon, MI) brand solvents; methyl tert-butyl ether (MTBE) and ethanol (EtOH) were obtained from Sigma-Aldrich (St. Louis, MO). Laboratory-grade refined sesame seed oil was obtained from Jedwards International, Inc. (Quincy, MA) and deionized water was obtained from a laboratory deionized water system (EASYPure II, Thermo Scientific) and measured resistivity ≥18MΩ-cm.
Headspace GC-MS Method for Fluoroether E-1
A headspace gas chromatography-mass spectrometry (GC-MS) method was developed for the quantification of Fluoroether E-1 using a Thermo Scientific (Waltham, MA) Trace 1310 gas chromatograph coupled to a Q Exactive GC Orbitrap mass spectrometer. All samples and standards were prepared in 20-mL headspace vials containing 10 mL solvent and analyzed according to the instrument method outlined in Table S1. Samples were maintained at room temperature (~22 °C) between injections, with a 2-min incubation/agitation period at 30 °C before 0.5 mL was drawn from the headspace and injected onto the GC. It should be noted that a short incubation period above ambient temperature was required as fluctuations in laboratory air temperature would cause variation in the measurements. The incubation temperature of 30 °C was chosen so there would be consistency between measurements, but the results for rate of formation of E-1 would not be markedly different than what would be observed at typical room temperatures.
The GC inlet was maintained at 200 °C with a split flow of 22.5 mL/min and helium carrier gas maintained at a constant flow rate of 1.5 mL/min (split ratio 15:1). GC separations were conducted using a Thermo TraceGOLD TG-5SilMS capillary column (30 m × 0.25 mm × 0.25 μm, 5% diphenyl/95% dimethyl polysiloxane). The 17-min GC oven program began at an initial temperature of 35 °C, which was held for 2 min, then ramped at 15 °C/min to 150 °C and held for 0.25 min. An electron ionization (EI) MS source was used, with an electron energy of 70 eV and 50 μA emission current. The ion source and MS transfer line were both maintained at 280 °C. MS data were acquired for m/z range 40–600 with a resolving power of 60,000 (at m/z 200). Area counts obtained from extracted ion chromatograms (XICs) of two characteristic ions, m/z 168.9882 and 68.9946 (within 5 ppm mass accuracy), were used for calibration and concentration determination of E-1 (Figure S1).
Calibrations of Fluoroether E-1
A high-concentration (~10 parts-per-thousand [ppth; mg/mL]) standard of E-1 was prepared in ACN and utilized as the stock solution for all calibration standards prepared in this study. This stock solution was used within 20 days of preparation and stored at −20 °C between uses. Calibration standards of E-1 were prepared in each test solvent at 0.05, 0.1, 0.5, 1, 5, 10, 15, and 20 parts-per-million (ppm; mg/L; μg/mL) Because analyte volatilization and method sensitivity are solvent-dependent (Figure S2), care was taken to maintain consistent solvent composition across all calibration samples. A neat ACN-calibration set was first prepared at 100-fold higher concentrations (5 to 2,000 ppm), and 100 μL of each was diluted in 9.9 mL solvent, with each resulting headspace-analysis standard consisting of 1% ACN. Linear regressions (R2 ≥ 0.99) were used for E-1 quantification in each solvent, and calibrations were run within three days prior to each respective degradation study performed. Immediately before each timed experiment, a randomly chosen calibration standard was re-analyzed as a check standard to verify calculated concentration from constructed calibration curves were accurate within 15% error.
Degradation Studies of HFPO-DA
A high-concentration (~10 ppth) stock solution of HFPO-DA was prepared in MeOH and used within ten days for all degradation experiments. Degradation experiments were performed with 20 ppm HFPO-DA in a series of solvent vehicles (DMSO, ACE, ACN, MeOH, EtOH, water, DCM, MTBE, and sesame oil). Each solvent’s respective calibration curve was used to calculate E-1 concentration over time in solvent spiked with HFPO-DA. Percent conversion of HFPO-DA to E-1 was calculated based on the initial spike of 20 ppm HFPO-DA (equivalent to 60.6 μM, 17.3 ppm as E-1).
The degradation of HFPO-DA to Fluoroether E-1 was monitored over 25-h periods in all solvents included in this study, with time point intervals as often as every 17 min (minimum allowable due to GC run time of 17 min). For toxicologically relevant solvents (DMSO, EtOH, water, and sesame oil), degradation experiments were performed in triplicate. Half-lives of HFPO-DA in different solvent conditions were estimated using a single-first order decomposition equation implemented in R package, mkin (v. 0.9.49.8).29
RESULTS AND DISCUSSION
Stability/Degradation of HFPO-DA in Solvent
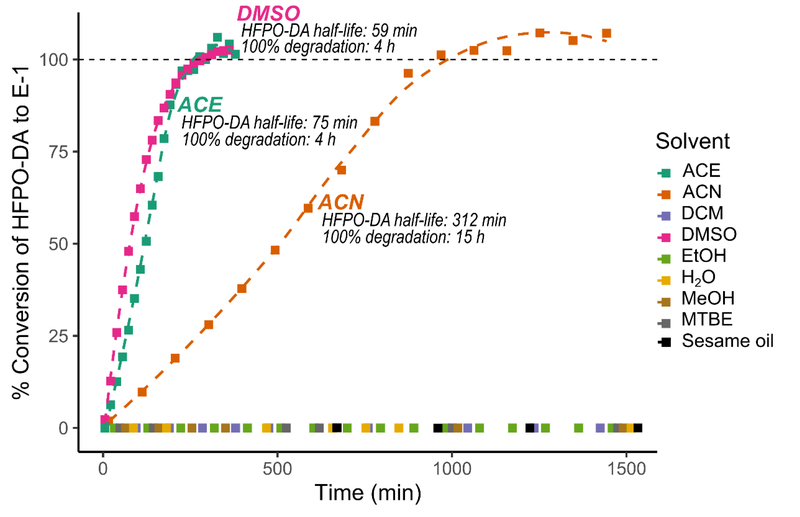
Degradation experiments (25-h) of HFPO-DA were performed for all solvents in this study, with E-1 headspace measurements taken in 96-min intervals for each. Conversion of HFPO-DA to E-1 in DMSO And ACE was rapid enough to reach 100% by the third time point; additional experimental replicates were conducted for DMSO and ACE with shorter, 17-min sampling intervals to more accurately model the degradation (Figure 2).
Figure 2.
Formation of E-1 from HFPO-DA spiked into each solvent monitored over time; HFPO-DA half-life calculated using fit of single first-order kinetics using R package mkin; 100% degradation time based on observed degradation curve.
As shown in Figure 2, degradation of HFPO-DA was observed in DMSO and ACE with estimated half-lives of 59 min (0.98 h) and 75 min (1.25 h), respectively. In ACN, degradation was slower, reaching 100% conversion to E-1 in 15 h (half-life of 312 min [5.2 h]). In water, MeOH, EtOH, MTBE, DCM, and sesame oil, no significant formation of E-1 was observed. Replicates for the degradation in toxicologically relevant solvents are shown in Figures S3 and S4.
Solvent-mediated degradation was observed in three of the nine solvents tested (DMSO, ACE, ACN) to produce the hypothesized Fluoroether E-1 product. No additional volatile products of degradation were observed in any of the tested solvents based on the GC-high-resolution-MS scans. The degradation pathway for this particular molecule is believed to be mediated by the solvent parameters, with degradation occurring only in polar, aprotic solvents with H-bond accepting character.30 Computational modeling of similar decarboxylation of a perchlorinated species found the process was likewise solvent dependent, being most favored in polar, aprotic solvents like DMSO.31
The carboxylic acid moiety of HFPO-DA has been noted to be highly thermally labile,11 and we conclude that observed formation of Fluoroether E-1 was not due to thermal degradation of HFPO-DA in the hot GC inlet, as: (1) sample incubation at 30 °C would not volatilize a significant portion of HFPO-DA (boiling point ~190 °C), and (2) even if HFPO-DA was present in the headspace and injected, E-1 would have been detected in every HFPO-DA standard analyzed, and formation over time would not have been observed under these conditions. We hypothesize aprotic solvents facilitate decarboxylation and protonation of the stabilized carbanion intermediate via solvent-mediated proton transfer (Figure S5), even at room temperature, whereas protic solvents stabilize the carboxylic acid via hydrogen bonding. Stability of the intermediate carbanion would be crucial to this pathway, which likely explains why we have only observed this effect in branched fluoroether PFAS and not in linear, legacy compounds such as PFOA. Efforts to fully understand the mechanism and effects of various solvent compositions and additives on the stability of different emerging PFAS is a vital focus for future work.
Implications for Toxicological Assessment of HFPO-DA
Traditional handling of PFAS has assumed that they are stable across a wide range of solvents and conditions.2 DMSO is one of the most commonly used solvents in toxicological assays, especially in vitro, due to its low acute toxicity, wide-ranging compound solubilization capability, and high degree of permeation through biological membranes.32, 33 However, results of this study indicate that it is unsuitable for use as the carrier solvent in toxicity testing of HFPO-DA. It is probable that other compounds with a similar branched perfluorinated carboxylic acid (e.g., perfluoro-2-methoxypropanoic acid [PMPA], perfluoro-2-ethoxypropanoic acid [PEPA], other HFPO-polymer acids) could undergo degradation by a similar mechanism. For example, HFPO-trimer acid (HFPO-TA; DTXSID00892442) and tetramer acid (HFPO-TeA; DTXSID40892441) likely degrade to Fluoroethers E-2 (DTXSID50880192) and E-3 (DTXSID10880193), respectively.
There is an ongoing large-scale effort at the U.S. Environmental Protection Agency to test PFAS materials and ensure the presence of testing materials, as well as determine solvent stability and appropriateness for assay usage. These efforts have also noted loss of HFPO-DA, -TA, and -TeA from DMSO stocks of these compounds.17 As toxicological efforts on PFAS expand, consideration of sample stability in any solvent should be carefully assessed. For example, while methanol provides stability for HFPO-DA, it is known that perfluorinated carboxylic acids, such as PFOA, can react in methanol to form fluorinated fatty acid methyl esters (FAMEs), and the reaction rates can be modified by pH.34
Our results indicate that toxicological assessment must take care in the preparation of both stock solutions and dosing solutions involving HFPO-DA. Stock solutions of HFPO-DA should not be prepared in DMSO, ACE, or ACN, given these solvents facilitated degradation of the analyte within hours at room temperature; alternatives such as MeOH should be used preferably, while still monitoring for stability. For assay dosing, we suggest that procedural adjustments are made to utilize either EtOH or water, including all appropriate negative and positive controls. In general, these results indicate the importance of monitoring effective concentrations of chemicals (i.e., in-serum, in-well, etc.) in addition to nominal dose concentrations. Failure to verify the nominal dose could result in toxicity results that underestimate the effect of PFAS due to loss of parent, or combined effects of parent and product compound.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Marci Smeltz and Adam Swank for sharing their initial observations on HFPO-DA, -TA, and -TeA stability in toxicological solutions, as well as Seth Newton, Alli Phillips, Bevin Blake, Theresa Guillette, Anna Robuck, Matthew Henderson, and David DeMarini for their input.
Footnotes
This article was reviewed by the U.S. Environmental Protection Agency and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. EPA.
SUPPORTING INFORMATION
Instrumental parameters for headspace GC-MS analysis (Table S1), additional figures for Fluoroether E-1 formation (Figures S1–S4), proposed E-1 formation mechanism in solvent (Figure S5)
REFERENCES
- 1.OECD Toward a New Comprehensive Global Database of Per- and Polyfluoroalkyl Substances (PFASs): Summary Report on Updating the OECD 2007 List of Per- and Polyfluoroalkyl Substances (PFASs); 2018. [Google Scholar]
- 2.Lindstrom AB; Strynar MJ; Libelo EL, Polyfluorinated Compounds: Past, Present, and Future. Environ Sci Technol 2011, 45, (19), 7954–7961. [DOI] [PubMed] [Google Scholar]
- 3.Kissa E, Fluorinated surfactants and repellants. 2nd ed.; Marcel Dekker: New York, 2001; Vol. 97. [Google Scholar]
- 4.Bowman JS, Fluorotechnology Is Critical to Modern Life: The FluoroCouncil Counterpoint to the Madrid Statement. Environ Health Perpect 2015, 123, (5), A112–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lifetime Health Advisories and Health Effects Support Documents for Perfluorooctanoic Acid and Perfluorooctane Sulfonate; U.S. Environmental Protection Agency; Federal Register: 2016; pp 33250–33251. [Google Scholar]
- 6.Meng CK; Zweigenbaum J; Furst P; Blanke E, Finding and confirming nontargeted pesticides using GC/MS, LC/quadrupole-time-of-flight MS, and databases. J AOAC Int 2010, 93, (2), 703–11. [PubMed] [Google Scholar]
- 7.Williams AJ; Grulke CM; Edwards J; McEachran AD; Mansouri K; Baker NC; Patlewicz G; Shah I; Wambaugh JF; Judson RS; Richard AM, The CompTox Chemistry Dashboard: a community data resource for environmental chemistry. J Cheminform 2017, 9, (1), 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.EPA’s Per- and Polyfluoroalkyl Substances (PFAS) Action Plan; EPA 823R18004; U.S. Environmental Protection Agency; 2019. [Google Scholar]
- 9.Pan Y; Zhang H; Cui Q; Sheng N; Yeung LW; Guo Y; Sun Y; Dai J, First Report on the Occurrence and Bioaccumulation of Hexafluoropropylene Oxide Trimer Acid: An Emerging Concern. Environ Sci Technol 2017, 51, (17), 9553–9560. [DOI] [PubMed] [Google Scholar]
- 10.Strynar M; Dagnino S; Lindstrom A; Andersen E; McMillan L; Thurman M; Ferrer I; Ball C In Identification of novel polyfluorinated compounds in natural waters using accurate mass TOFMS, 33rd SETAC North America Annual Meeting, Long Beach, USA, Nov 11–15, 2012, 2012; Long Beach, USA, 2012. [Google Scholar]
- 11.Strynar M; Dagnino S; McMahen R; Liang S; Lindstrom A; Andersen E; McMillan L; Thurman M; Ferrer I; Ball C, Identification of Novel Perfluoroalkyl Ether Carboxylic Acids (PFECAs) and Sulfonic Acids (PFESAs) in Natural Waters Using Accurate Mass Time-of-Flight Mass Spectrometry (TOFMS). Environ Sci Technol 2015, 49, (19), 11622–11630. [DOI] [PubMed] [Google Scholar]
- 12.Newton S; McMahen R; Stoeckel JA; Chislock M; Lindstrom A; Strynar M, Novel Polyfluorinated Compounds Identified Using High Resolution Mass Spectrometry Downstream of Manufacturing Facilities near Decatur, Alabama. Environ Sci Technol 2017, 51, (3), 1544–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCord J; Strynar M, Identification of Per- and Polyfluoroalkyl Substances in the Cape Fear River by High Resolution Mass Spectrometry and Nontargeted Screening. Environ Sci Technol 2019, 53, (9), 4717–4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blum A; Balan SA; Scheringer M; Trier X; Goldenman G; Cousins IT; Diamond M; Fletcher T; Higgins C; Lindeman AE; Peaslee G; de Voogt P; Wang Z; Weber R, The Madrid Statement on Poly- and Perfluoroalkyl Substances (PFASs). Environ Health Perspect 2015, 123, (5), A107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borg D; Ivarsson J; Andersson A; Moore G, Nordic Workshop on PFASs: Outcomes. In Nordic Council of Ministers: Copenhagen, 2017. [Google Scholar]
- 16.Scheringer M; Trier X; Cousins IT; de Voogt P; Fletcher T; Wang ZY; Webster TF, Helsingor Statement on poly- and perfluorinated alkyl substances (PFASs). Chemosphere 2014, 114, 337–339. [DOI] [PubMed] [Google Scholar]
- 17.Patlewicz G; Richard AM; Williams AJ; Grulke CM; Sams R; Lambert J; Noyes PD; DeVito MJ; Hines RN; Strynar M; Guiseppi-Elie A; Thomas RS, A Chemical Category-Based Prioritization Approach for Selecting 75 Per- and Polyfluoroalkyl Substances (PFAS) for Tiered Toxicity and Toxicokinetic Testing. Environ Health Perspect 2019, 127, (1), 14501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun M; Arevalo E; Strynar M; Lindstrom A; Richardson M; Kearns B; Pickett A; Smith C; Knappe DRU, Legacy and Emerging Perfluoroalkyl Substances Are Important Drinking Water Contaminants in the Cape Fear River Watershed of North Carolina. Environ Sci Technol Lett 2016, 3, (12), 415–419. [Google Scholar]
- 19.Gaballah S; Swank A; Sobus JR; Howey XM; Schmid J; Catron T; McCord J; Hines E; Strynar MJ; Tal T, Evaluation of developmental toxicity, developmental neurotoxicity, and tissue dose in zebrafish exposed to GenX and other PFAS. Environ Health Perpect 2020, 128, (4), 047005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Statewide PFAS Directive, Information Request, and Notice to Insurers; New Jersey Department of Environmental Protection; Trenton, New Jersey, 2019. [Google Scholar]
- 21.Information for Communities Impacted by Per- and Poly-fluorinated Alkyl Substances (PFAS). https://www.dec.ny.gov/chemical/108791.html
- 22.Gebbink WA; van Asseldonk L; van Leeuwen SPJ, Presence of Emerging Per- and Polyfluoroalkyl Substances (PFASs) in River and Drinking Water near a Fluorochemical Production Plant in the Netherlands. Environ Sci Technol 2017, 51, (19), 11057–11065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang P; Lu YL; Wang TY; Fu YN; Zhu ZY; Liu SJ; Xie SW; Xiao Y; Giesy JP, Occurrence and transport of 17 perfluoroalkyl acids in 12 coastal rivers in south Bohai coastal region of China with concentrated fluoropolymer facilities. Environ Pollut 2014, 190, 115–122. [DOI] [PubMed] [Google Scholar]
- 24.Pan Y; Zhang H; Cui Q; Sheng N; Yeung LWY; Sun Y; Guo Y; Dai J, Worldwide Distribution of Novel Perfluoroether Carboxylic and Sulfonic Acids in Surface Water. Environ Sci Technol 2018, 52, (14), 7621–7629. [DOI] [PubMed] [Google Scholar]
- 25.Sheng N; Cui R; Wang J; Guo Y; Wang J; Dai J, Cytotoxicity of novel fluorinated alternatives to long-chain perfluoroalkyl substances to human liver cell line and their binding capacity to human liver fatty acid binding protein. Arch Toxicol 2018, 92, (1), 359–369. [DOI] [PubMed] [Google Scholar]
- 26.Conley JM; Lambright CS; Evans N; Strynar MJ; McCord J; McIntyre BS; Travlos GS; Cardon MC; Medlock-Kakaley E; Hartig PC; Wilson VS; Gray LE Jr., Adverse Maternal, Fetal, and Postnatal Effects of Hexafluoropropylene Oxide Dimer Acid (GenX) from Oral Gestational Exposure in Sprague-Dawley Rats. Environ Health Perspect 2019, 127, (3), 37008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blake BE; Cope HA; Hall SM; Keys RD; Mahler BW; McCord J; Scott B; Stapleton HM; Strynar MJ; Elmore SA; Fenton SE, Evaluation of Maternal, Embryo, and Placental Effects in CD-1 Mice following Gestational Exposure to Perfluorooctanoic Acid (PFOA) or Hexafluoropropylene Oxide Dimer Acid (HFPO-DA or GenX). Environ Health Perspect 2020, 128, (2), 27006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.U.S. EPA CompTox Chemicals Dashboard. https://comptox.epa.gov/dashboard
- 29.Ranke J ‘mkin’: Kinetic Evaluation of Chemical Degradation Data, v. 0.9.49.8; 2019, https://CRAN.R-project.org/package=mkin.
- 30.Kamlet MJ; Abboud JLM; Abraham MH; Taft RW, Linear Solvation Energy Relationships .23. A Comprehensive Collection of the Solvatochromic Parameters, Pi-Star, Alpha and Beta, and Some Methods for Simplifying the Generalized Solvatochromic Equation. J Org Chem 1983, 48, (17), 2877–2887. [Google Scholar]
- 31.da Silva GCQ; Cardozo TM; Amarante GW; Abreu CRA; Horta BAC, Solvent effects on the decarboxylation of trichloroacetic acid: insights from ab initio molecular dynamics simulations. Phys Chem Chem Phys 2018, 20, (34), 21988. [DOI] [PubMed] [Google Scholar]
- 32.Wetmore BA; Wambaugh JF; Allen B; Ferguson SS; Sochaski MA; Setzer RW; Houck KA; Strope CL; Cantwell K; Judson RS; LeCluyse E; Clewell HJ; Thomas RS; Andersen ME, Incorporating High-Throughput Exposure Predictions With Dosimetry-Adjusted In Vitro Bioactivity to Inform Chemical Toxicity Testing. Toxicol Sci 2015, 148, (1), 121–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galvao J; Davis B; Tilley M; Normando E; Duchen MR; Cordeiro MF, Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J 2014, 28, (3), 1317–30. [DOI] [PubMed] [Google Scholar]
- 34.Hanari N; Itoh N; Ishikawa K; Yarita T; Numata M, Variation in concentration of perfluorooctanoic acid in methanol solutions during storage. Chemosphere 2014, 94, 116–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.