Abstract
Background & Aims
Physical inactivity and sedentary lifestyle have contributed to the epidemic of obesity and non-alcoholic fatty liver disease (NAFLD). We assessed the association between physical activity, NAFLD, and sarcopenia, and their contributions to mortality.
Methods
Data from the National Health and Nutrition Examination Survey (NHANES) 1999–2004 with Linked Mortality file (through 2015) was utilised. NAFLD was determined by the US Fatty Liver Index in the absence of secondary causes of liver disease. Sarcopenia was defined using appendicular lean mass divided by body mass index by the Foundation for the National Institutes of Health criteria. Activity level was determined using standard self-reports. Publicly available imputed dual-energy X-ray absorptiometry data sets were used.
Results
Of 4,611 NHANES participants (48.2% males; 72.5% White; mean age 45.9 years), NAFLD was present in 1,351 (29.3%), of whom 17.7% had sarcopenia. Of the NAFLD group, 46.3% was inactive, whilst intermediate and ideal physical activity rates were observed in 14.2% and 39.5%, respectively. Sarcopenia was significantly and inversely related to higher physical activity level, both amongst NAFLD (odds ratio [OR] = 0.45 [95% CI 0.30–0.69]) and non-NAFLD (OR = 0.51 [0.35–0.75]) groups. During a median follow-up of 13.5 years, a total of 586 subjects died, of whom 251 had NAFLD. Amongst those who died with NAFLD, 33.0% had sarcopenia and 54.3% were inactive. Compared with NAFLD without sarcopenia, NAFLD with sarcopenia was associated with a higher risk of all-cause (hazard ratio [HR] = 1.78 [1.16–2.73]), cardiac-specific (HR = 3.19 [1.17–8.74]), and cancer-specific mortality (HR = 2.12 [1.08–4.15]).
Conclusions
Inactivity is associated with presence of sarcopenia, whilst sarcopenia is associated with increased mortality amongst NAFLD patients. Sarcopenia should be a part of clinical assessment of patients with NAFLD. Treatment of NAFLD should include optimal management of sarcopenia.
Lay summary
Nonalcoholic fatty liver disease (NAFLD) and sarcopenia have similar pathophysiological profiles. Our data show that sarcopenia is associated with inactivity in subjects with NAFLD. The presence of sarcopenia in patients with NAFLD poses increased risk for all-cause and cardiac-specific mortality.
Keywords: Sarcopenia, Non-alcoholic fatty liver disease, Physical activity
Abbreviations: ALM, appendicular lean mass; BMI, body mass index; CV, cardiovascular; CVD, cardiovascular disease; DXA, dual-energy X-ray absorptiometry; EWGSOP2, Revised European Working Group on Sarcopenia in Older People; FNIH, Foundation for the National Institutes of Health; GGT, gamma glutamyltransferase; HL, hyperlipidaemia; HR, hazard ratio; HTN, hypertension; MS, metabolic syndrome; NAFLD, non-alcoholic fatty liver disease; NFS, NAFLD fibrosis score; NHANES, National Health and Nutrition Examination Survey; T2DM, type 2 diabetes mellitus; US FLI, Fatty Liver Index for the multi-ethnic US population
Graphical abstract
Highlights
-
•
Non-alcoholic fatty liver disease (NAFLD) and sarcopenia have similar pathophysiological profiles.
-
•
In our study, amongst NAFLD patients, sarcopenia was inversely related to increased physical activity level.
-
•
The presence of sarcopenia in patients with NAFLD poses increased risk for all-cause and cardiac-specific mortality.
Introduction
Sarcopenia is a recently described condition that includes loss of muscle mass, muscle strength, and human function (i.e. gait speed).1 The condition implies both organ system change and its functional consequences.2 The European Working Group on Sarcopenia in Older People defined it as ‘a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength with the risk of adverse outcomes such as physical disability, poor quality of life and death’.3
The prevalence of sarcopenia varies widely, in part, depending on sex, age, method of diagnosis, and its classification (i.e. mild or severe). Best estimates for a US cohort is between 5% and 13% for those 60–70 years and between 11% and 50% of those >80 years of age.4 Worldwide prevalence, based on a systematic review, is 10% in both men and women.5
Patients with non-alcoholic fatty liver disease (NAFLD) have been reported to have an increased prevalence of sarcopenia.[6], [7], [8] They frequently meet the criteria for metabolic syndrome (MS), with at least 3 of the following abnormalities: obesity, type 2 diabetes, hypertension (HTN), increased waist circumference, elevation of alanine/aspartate aminotransferase, and dyslipidaemia. The role of these abnormalities and sarcopenia has been the subject of great interest recently, with investigators reporting results from large public data sets and retrospective reviews demonstrating that sarcopenia is associated with NAFLD and components of the MS.8,9 Furthermore, previous studies have shown that sarcopenia was independently associated with increased risk of NAFLD and NAFLD-associated advanced fibrosis independent of well-defined risk factors.10 Others have noted that the skeletal mass index calculations when based on height, as opposed to weight adjustments, provide different types of relationships.11
These publications, as well as the increasing prevalence of NAFLD and the associated risk factors for cardiovascular (CV) mortality, raise an important issue that is yet to be resolved. Is sarcopenia a risk for CV mortality? Is this risk independent of NAFLD? Further, patients with NAFLD or sarcopenia are often sedentary. As lack of exercise is believed to be a risk factor for sarcopenia and CV mortality, an important question would be to assess if increasing activity level will mitigate the adverse effects of sarcopenia in patients with NAFLD. All of these issues point to the potential impact of sarcopenia on mortality amongst NAFLD. Therefore, our aim was to assess the associations between sarcopenia, NAFLD, and mortality using population-based data.
Methods
Data source and population
We used the public data files for the 1999–2000, 2001–2002, and 2003–2004 cycles of the National Health and Nutrition Examination Survey (NHANES). NHANES is a population-based programme of studies conducted by the National Center for Health Statistics. To monitor the health and nutritional status of civilian, non-institutionalised individuals in the US population, cross-sectional socio-demographic, dietary, and medical data were collected through interviews, standardised physical examination, and laboratory testing with oversampling of certain subgroups of the US population (people aged more than 60 years, Hispanic, and African American). Full details of each survey have been described elsewhere.12
Mortality status of NHANES participants was ascertained through the end of 2015 via linkage of the NHANES data to the National Death Index.13 Using the 113 categories of underlying causes of death on the public use files, CV deaths were defined as death attributable to major CV disease and cerebrovascular diseases (International Classification of Diseases, Tenth Revision codes: I00–I90, I11, I13, I20–I51, and I60–I69).14 Participants who were not matched to any death records were presumed alive through the follow-up period. Time to death was counted from baseline (defined as the time when a subject participated in the NHANES survey) to date of death or December 31, 2015, whichever came first.
Definition of NAFLD and advanced fibrosis
NAFLD was defined using the improved Fatty Liver Index for the multi-ethnic US population (US FLI), a surrogate for the clinical diagnosis of NAFLD. The US FLI is a biochemical model that predicts the presence of fatty liver based on age, race/ethnicity, waist circumference, gamma glutamyltransferase (GGT) activity, fasting insulin, and fasting glucose, defined as follows:
US FLI = (e−0.8073 ∗ non-Hispanic black + 0.3458 ∗ Mexican American + 0.0093 ∗ age + 0.6151 ∗ loge(GGT) + 0.0249 ∗ waist circumference + 1.1792 ∗ loge(insulin) + 0.8242 ∗ loge(glucose) − 14.7812)/(1 + e−0.8073 ∗ non-Hispanic black + 0.3458 ∗ Mexican American + 0.0093 ∗ age + 0.6151 ∗ loge(GGT) + 0.0249 ∗ waist circumference + 1.1792 ∗ loge(insulin) + 0.8242 ∗ loge(glucose) − 14.7812) ∗ 100
This model has been previously validated with an area under the receiver operating characteristics curve of 0.80 (95% CI 0.77–0.83) for the detection of NAFLD in subjects with values ≥30.15 In this study, subjects were presumed to have NAFLD if they have a US FLI score of ≥30 in the absence of any other possible causes of chronic liver disease and excessive alcohol consumption. As a sensitivity analysis, NAFLD was also defined using a fatty liver index of ≥60. NAFLD fibrosis score (NFS) and Fibrosis-4 (FIB-4) score for liver fibrosis were used to categorise NAFLD patients into 2 groups, including low fibrosis risk (NFS ≤0.676; FIB-4 ≤2.67) and high fibrosis risk (NFS >0.676; FIB-4 >2.67).16
Dual-energy X-ray absorptiometry measurement and sarcopenia definitions
Dual-energy X-ray absorptiometry (DXA) has been considered the primary method for measuring body composition. In NHANES, the whole DXA scans used a Hologic QDR-4500A fan-beam densitometer (Hologic, Inc., Bedford, MA, USA) to assess the total body, for both arms and both legs, the trunk, and the head. Pregnant females were not scanned. Participants whose weights are over 300 lb (136 kg) or height over 6 ft, 5 in. (198 cm) were excluded because of DXA table limitations. Because of these exclusions for large body sizes, participants with missing data cannot be treated as a random subset of the original sample, leading CDC to perform multiple imputations to resolve the problem of potential biases as a result of missing DXA data. Details of the multiple-imputation protocol are described elsewhere.17 Our analysis used the DXA data sets released by NHANES from 1990 to 2004 with the publicly available 5 completed (imputed) DXA data files.18 Appropriate methods for the analysis of imputed data sets were described later in the statistical analysis section. From the DXA measures, appendicular lean mass (ALM) was the sum of lean mass for all 4 extremities (arms and legs). Sarcopenia was defined using the Foundation for the National Institutes of Health (FNIH) sarcopenia definition: ALM divided by body mass index (BMI) (men <0.789; women <0.512).19
Other definitions
General demographic characteristics were collected from self-reported information, including age (years), sex, race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, or other race), income level (poverty-to-income ratio [PIR] <1.3 as low, PIR 1.3–3.5 as middle, and PIR >3.5 as high),20 college degree, and history of medical conditions (cardiovascular disease [CVD], any cancer, and kidney).
The 10-year lifetime risk for developing atherosclerotic CVD was calculated from the atherosclerotic cardiovascular disease (ASCVD) risk score (American College of Cardiology/American Heart Association), which includes each participant's age, race, sex, smoking status, the presence of diabetes, systolic blood pressure, antihypertensive medication, serum cholesterol, and high-density lipoprotein levels. In this study, individuals with a 10-year ASCVD risk score of ≥7.5% were referred to as high risk for CVD.21 For physical activity, the total physical activity measures (moderate leisure-time physical activity + 2×vigorous leisure-time physical activity + transportation + work) were calculated by using a physical activity questionnaire. Along with the 2008 Adult Physical Activity Guidelines for Americans,22 total physical activity was categorised into inactive (<150 min/week), moderate (≥150 to <300 min/week), and ideal (≥300 min/week). Obesity pattern was categorised into lean (BMI 18.5–25 kg/m2), overweight (25–29.9 kg/m2), and obese (≥30 kg/m2). Type 2 diabetes mellitus (T2DM) was defined by a fasting glucose level ≥126 mg/dl, self-reported medical history of diabetes, oral hypoglycaemic agents, insulin use, or HbA1c of ≥6.5%. HTN was defined by systolic blood pressure measurements ≥130 mmHg or diastolic blood pressure measurements ≥80 mmHg from an average 3 measurements, or history of high blood measurements.23 Hyperlipidaemia (HL) was defined by either a serum cholesterol level ≥200 mg/dl, LDL level ≥130 mg/dl, HDL cholesterol level ≤40 mg/dl for men and 50 for women, or history of HL. Insulin resistance was defined as a homeostasis model assessment of insulin resistance of >3.24 Finally, MS was defined by the National Cholesterol Education Program Adult Treatment Panel III definition.25
Statistical analysis
Examination sample weights, accounting for non-response, non-coverage, and unequal selection probabilities for certain categories of the population, were incorporated to produce national estimates for all analyses. Sampling errors were estimated by the Taylor series linearisation method.26 For combining 3 NHANES study cycles, appropriate selection of sampling weights and adjustment coefficients was implemented in compliance with the NHANES Analytic and Reporting Guidelines.27
Age-standardised percentages were calculated by using the direct method to the 2000 projected census population using age groups 20–39, 40–59, and more than 60 years. Differences across groups were tested by the use of orthogonal contrasts. Multivariable logistic regression models were used to identify predictors of sarcopenia. Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% CIs for risk of all-cause mortality as well as cause-specific mortality associated with the presence of sarcopenia and NAFLD. Multivariable models were constructed in several stages. Model 1 was adjusted for age, sex, and race. Model 2 was also adjusted for socio-demographic characteristics (income, education, and height). Model 3 was further adjusted for health behaviour (smoking and physical activity). Model 4 was adjusted for all variables in model 3 with metabolic components (HTN, HL, and T2DM). Model 5 was adjusted for all variables in model 3 with a history of cancer, CVD, and kidney disease. Interactions between NAFLD and sarcopenia on mortality were tested, and no evidence interaction was found (p >0.05). The proportional hazards assumption of the Cox models was examined by testing time-dependent covariates,26,28 which showed no significant departure from proportionality over time.
All our analyses were based on the 5 imputed data sets. We independently analysed each of the 5 versions of the completed data in univariable and multivariable analyses. The 5 sets of results were combined to produce a single mean estimate and adjusted SEs according to Rubin's rules.29 The number of individuals in each group displayed in this study, except the numbers in the data flow chart, was determined by multiplying the estimated percentage by the total number of individuals in the full sample. All analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, NC, USA) using ‘SURVEY’ procedure, which incorporates the sample design. Statistical tests were considered significant at p <0.05 (2 tails).
Results
Of 12,976 non-pregnant adult (≥20 years) participants of 3 cycles of NHANES, 8,365 were excluded based on the study criteria (Fig. S1), and the final cohort included 4,611 participants. Clinico-demographic features of the study population are presented in Table S1.
Sarcopenia prevalence amongst NAFLD population
Individuals with NAFLD had a higher age-standardised prevalence of sarcopenia compared with individuals without NAFLD (16.0% vs. 6.4%), as well as across age group, sex, all race/ethnicities, and physical activity group (Fig. 1). Amongst individuals with NAFLD, sarcopenia was more common in women than in men (18.9% vs. 14.3%), and the prevalence of sarcopenia was highest amongst Hispanics (25.1%) compared with non-Hispanic Whites (15.1%), and lowest in non-Hispanic Blacks (5.1%).
Fig. 1.
Age-standardized prevalence of sarcopenia among participants with and without NAFLD, stratified by age, sex, race and physical activity: NHANES 1999-2004.
NAFLD, non-alcoholic fatty liver disease; NHANES, National Health and Nutrition Examination Survey.
In the age- and sex-adjusted model, individuals with NAFLD had 2.9 times higher odds of sarcopenia compared with individuals without NAFLD (Table S2). NAFLD was associated with sarcopenia even in the fully adjusted model (Table S3). Independent predictors of having sarcopenia amongst individuals with NAFLD included older age, male gender, non-Hispanic White ethnicity, low income, college degree, and physical activity.
Characteristics of individuals according to NAFLD and sarcopenia status
Of the entire cohort, 5.2% had both NAFLD and sarcopenia, 4.0% had sarcopenia without NAFLD, 24.1% had NAFLD without sarcopenia, and 66.7% had neither condition (Fig. 2). The demographic and clinical characteristics of individuals according to the presence of NAFLD and sarcopenia status are presented in Table 1. Compared with NAFLD patients without sarcopenia, individuals with both conditions were older, more likely to be female, and had a worse metabolic picture.
Fig. 2.
Distribution of NAFLD, sarcopenia and obesity: NHANES 1999-2004.
NAFLD, non-alcoholic fatty liver disease; NHANES, National Health and Nutrition Examination Survey.
Table 1.
Demographic and clinical characteristics of participants according to the presence of NAFLD and sarcopenia (multiple-imputation analysis).
| Covariate | Individuals with NAFLD |
Individuals without NAFLD |
||||
|---|---|---|---|---|---|---|
| Sarcopenia (n = 239a) | Non-sarcopenia (n = 1,112a) | p value | Sarcopenia (n = 183a) | Non-sarcopenia (n = 3,077a) | p value | |
| Ageb | 58.75 (1.32) | 49.04 (0.61) | <0.0001 | 58.75 (1.31) | 43.02 (0.50) | <0.0001 |
| Age (years) | ||||||
| 20–39 | 16.47 (3.25)% | 30.46 (1.87)% | <0.0001 | 20.78 (3.43)% | 47.70 (1.44)% | <0.0001 |
| 40–59 | 28.80 (3.63)% | 43.69 (1.65)% | 0.0009 | 26.25 (3.75)% | 36.69 (1.14)% | 0.0076 |
| >60 | 54.72 (3.73)% | 25.86 (1.69)% | <0.0001 | 52.97 (3.86)% | 15.61 (0.94)% | <0.0001 |
| Male | 51.44 (3.03)% | 61.87 (1.97)% | 0.0044 | 39.24 (3.88)% | 43.60 (0.92)% | 0.2650 |
| Race | ||||||
| Non-Hispanic White | 71.60 (3.96)% | 72.97 (2.70)% | 0.7491 | 68.89 (4.53)% | 72.63 (1.92)% | 0.3256 |
| Non-Hispanic Black | 1.84 (0.68)% | 6.60 (0.80)% | <0.0001 | 3.22 (1.18)% | 12.69 (1.37)% | <0.0001 |
| Hispanic | 22.19 (3.80)% | 16.33 (2.42)% | 0.0919 | 21.70 (3.82)% | 10.34 (1.44)% | 0.0012 |
| Other race | 4.37 (1.86)% | 4.11 (0.77)% | 0.9078 | 6.19 (2.54)% | 4.34 (0.60)% | 0.4308 |
| Income | ||||||
| Low | 27.99 (4.54)% | 18.20 (1.74)% | 0.0242 | 28.69 (3.81)% | 18.28 (1.29)% | 0.0041 |
| Medium | 40.13 (4.39)% | 34.10 (1.83)% | 0.1431 | 44.71 (3.90)% | 35.39 (1.68)% | 0.0335 |
| High | 31.88 (3.79)% | 47.70 (2.51)% | 0.0001 | 26.59 (3.81)% | 46.33 (2.01)% | <0.0001 |
| College degree | 17.19 (2.46)% | 20.74 (1.82)% | 0.2953 | 13.00 (2.33)% | 27.01 (1.62)% | <0.0001 |
| Married | 71.76 (3.46)% | 64.67 (2.22)% | 0.0800 | 61.50 (3.68)% | 59.26 (1.41)% | 0.5564 |
| Smoking status | ||||||
| Active | 15.27 (2.81)% | 17.94 (1.63)% | 0.3757 | 13.09 (2.73)% | 24.11 (1.25)% | <0.0001 |
| Former | 33.71 (3.20)% | 31.77 (1.56)% | 0.5516 | 29.40 (3.82)% | 22.36 (1.02)% | 0.0573 |
| Non-smoker | 51.02 (3.60)% | 50.29 (2.31)% | 0.8562 | 57.51 (4.10)% | 53.52 (1.38)% | 0.3063 |
| Heightb (cm) | 161.94 (0.55) | 172.37 (0.35) | <0.0001 | 157.33 (0.48) | 169.38 (0.23) | <0.0001 |
| Waistb (cm) | 112.16 (1.20) | 108.21 (0.60) | 0.0010 | 95.14 (1.05) | 89.08 (0.29) | <0.0001 |
| BMIb (kg/m2) | 34.62 (0.64) | 32.05 (0.22) | <0.0001 | 28.24 (0.43) | 25.63 (0.11) | <0.0001 |
| ALMb (g) | 21,018.52 (457.34) | 25,884.26 (244.72) | <0.0001 | 16,245.00 (290.62) | 21,059.24 (122.64) | <0.0001 |
| ALM/BMIb | 0.61 (0.01) | 0.82 (0.01) | <0.0001 | 0.58 (0.01) | 0.83 (0.00) | <0.0001 |
| Obesity | ||||||
| Lean | 3.57 (0.87)% BMI | 7.27 (0.99)% BMI | 0.0056 | 28.72 (3.78)% BMI | 50.20 (1.20)% BMI | <0.0001 |
| Overweight | 23.60 (3.03)% BMI | 34.71 (1.50)% BMI | 0.0005 | 39.91 (3.59)% BMI | 35.99 (1.16)% BMI | 0.2759 |
| Obese | 72.83 (3.24)% BMI | 58.02 (1.69)% BMI | <0.0001 | 31.37 (3.67)% BMI | 13.81 (0.84)% BMI | <0.0001 |
| Physical activity | ||||||
| Inactive | 63.47 (3.38)% | 42.56 (1.90)% | <0.0001 | 60.59 (3.86)% | 36.96 (1.34)% | <0.0001 |
| Intermediate | 12.79 (2.20)% | 14.52 (1.33)% | 0.4362 | 15.59 (2.84)% | 17.05 (0.70)% | 0.6241 |
| Ideal | 23.73 (3.34)% | 42.92 (1.88)% | <0.0001 | 23.82 (2.88)% | 45.99 (1.43)% | <0.0001 |
| Hypertension | 77.06 (3.60)% | 66.69 (2.00)% | 0.0093 | 64.97 (4.01)% | 39.57 (1.20)% | <0.0001 |
| Hyperlipidaemia | 88.14 (2.39)% | 87.79 (1.20)% | 0.9022 | 79.92 (3.19)% | 63.90 (1.20)% | <0.0001 |
| Insulin resistance | 82.90 (2.59)% | 82.41 (1.48)% | 0.8671 | 8.80 (2.42)% | 8.54 (0.73)% | 0.9094 |
| Diabetes | 29.87 (3.26)% | 18.76 (1.32)% | 0.0011 | 7.19 (1.96)% | 3.09 (0.33)% | 0.0381 |
| Metabolic syndrome | 71.05 (3.44)% | 62.21 (1.78)% | 0.0244 | 33.76 (4.79)% | 15.20 (0.96)% | <0.0001 |
| History of CVD | 17.64 (2.31)% | 10.48 (1.03)% | 0.0032 | 19.97 (2.98)% | 4.16 (0.37)% | <0.0001 |
| History of cancer | 12.52 (2.60)% | 8.65 (0.93)% | 0.1836 | 14.19 (3.54)% | 6.86 (0.50)% | 0.0451 |
| History of kidney disease | 2.47 (0.83)% | 2.43 (0.78)% | 0.9750 | 4.40 (1.31)% | 1.21 (0.19)% | 0.0152 |
| High risk for CVD | 61.74 (3.46)% | 35.75 (1.70)% | <0.0001 | 53.07 (3.41)% | 16.88 (0.92)% | <0.0001 |
| Advanced fibrosis (NFS) | 14.34 (2.81)% | 6.23 (0.68)% | 0.0082 | 7.09 (1.94)% | 1.24 (0.25)% | 0.0039 |
| Advanced fibrosis (FIB-4) | 3.08 (1.01)% | 1.18 (0.37)% | 0.0883 | 2.26 (0.87)% | 1.03 (0.18)% | 0.1482 |
| Cumulative mortalityc | ||||||
| All cause | 34.55 (3.71)% | 15.12 (1.22)% | <0.0001 | 36.94 (3.74)% | 8.73 (0.47)% | <0.0001 |
| Cardiac specific | 7.00 (1.78)% | 2.92 (0.53)% | 0.0397 | 9.47 (1.76)% | 1.41 (0.21)% | <0.0001 |
| Cancer specific | 8.85 (2.22)% | 3.74 (0.47)% | 0.0312 | 5.63 (1.53)% | 1.96 (0.24)% | 0.0164 |
All values are displayed weighted percentages (SE) except where otherwise noted. ALM, appendicular lean mass; BMI, body mass index; CVD, cardiovascular disease; FIB-4, Fibrosis-4; NAFLD, non-alcoholic fatty liver disease; NFS, NAFLD fibrosis score.
The number of individuals was reported by multiplying the estimated percentage by the total number of individuals in the full sample.
Mean (SE).
Median follow-up of 13.5 years.
Of the study cohort, 40.6% reported physical inactivity, 16.2% had intermediate physical activity, and 43.2% had ideal physical activity (Table S4). Accounting for socio-demographic and clinical risk factors, sarcopenia was significantly and inversely related to a higher physical activity level amongst individuals with NAFLD (odds ratio 0.45 [95% CI 0.30–0.69]) (Table S5).
All-cause mortality
Of the entire cohort, NAFLD and sarcopenia coexisted in 239 (5.2%) patients. After a median follow-up of 13.5 years, 587 individuals (12.7%) died. Cumulative all-cause mortality was higher for those with either sarcopenia or NAFLD compared with neither sarcopenia nor NAFLD (Table 1).
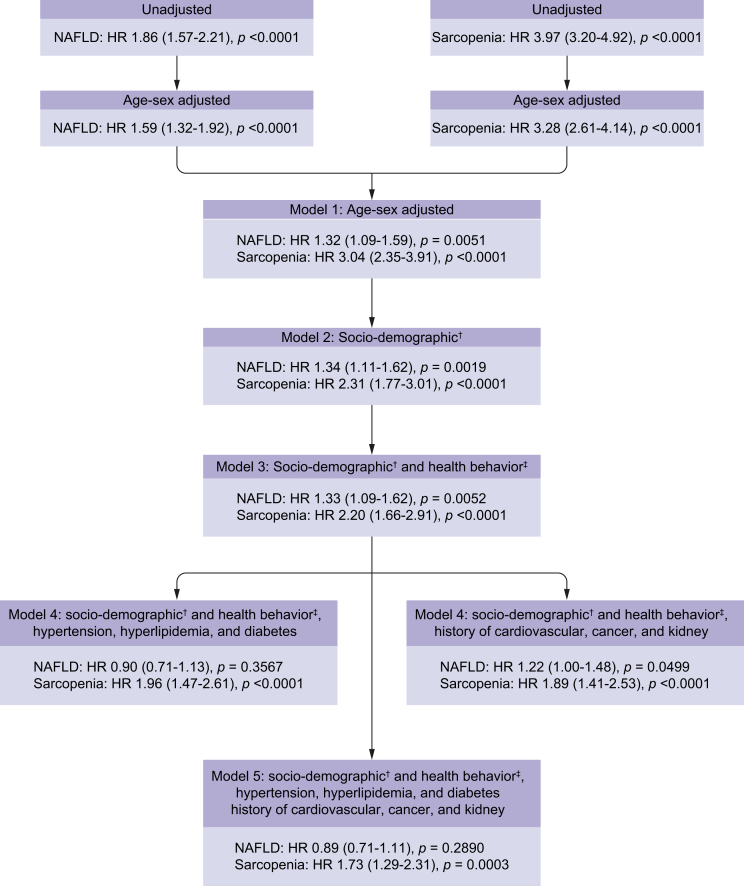
Changes in the HRs of NAFLD and sarcopenia for all-cause mortality were determined by successive adjustments for age, sex, race, socio-demographic, health behaviours and co-morbidities, and are displayed in Fig. 3 and Table S6. Interestingly, presence of sarcopenia was associated with mortality after all adjustments (models 1–6) (Fig. 3; Table S6). This was true in the fully adjusted model for subjects with or without NAFLD (Table 2). In stratified analyses, association of risk factors with all-cause mortality in the age- and sex-adjusted models was similar across presence of NAFLD and sarcopenia (Table 3).
Fig. 3.
Change in the hazard ratios (HRs) of NAFLD and sarcopenia for all-cause mortality by successive adjustments for age, sex, race, sociodemographic, health behaviors and comorbidities: NHANES 1999-2004.
HR, hazard ratio; NAFLD, non-alcoholic fatty liver disease; NHANES, National Health and Nutrition Examination Survey. †Age, male, race, height, income, education, ‡Physical activity and smoking status.
Table 2.
Fully adjusted HR of risk factors for all-cause mortality according to the presence of NAFLD (multiple-imputation analysis).
| Covariate | Individuals with NAFLD |
Individuals without NAFLD |
||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Sarcopenia | 1.78 (1.16–2.73) | 0.0089 | 1.69 (1.19–2.40) | 0.0032 |
| Age (years) | ||||
| 20–39 | Reference | Reference | ||
| 40–59 | 0.40 (0.23–0.72) | 0.0021 | 0.36 (0.22–0.58) | <0.0001 |
| >60 | 1.50 (1.07–2.09) | 0.0174 | 1.35 (0.93–1.97) | 0.1112 |
| Male | 2.11 (1.43–3.10) | 0.0001 | 2.04 (1.56–2.67) | <0.0001 |
| Race | ||||
| Non-Hispanic White | Reference | Reference | ||
| Non-Hispanic Black | 1.14 (0.72–1.81) | 0.569 | 1.02 (0.76–1.37) | 0.9115 |
| Hispanic | 0.73 (0.48–1.09) | 0.1215 | 0.72 (0.49–1.07) | 0.1040 |
| Other race | 0.60 (0.18–2.03) | 0.4163 | 0.61 (0.30–1.22) | 0.1590 |
| Low income | 1.08 (0.74–1.58) | 0.689 | 1.38 (1.15–1.66) | 0.0006 |
| College | 0.63 (0.41–0.99) | 0.0436 | 0.67 (0.47–0.95) | 0.0255 |
| Height (cm) | 0.98 (0.96–1.00) | 0.1044 | 0.97 (0.95–0.98) | 0.0005 |
| Physical inactivity | 1.19 (0.83–1.71) | 0.3459 | 1.13 (0.88–1.45) | 0.3272 |
| Active smoker | 1.48 (0.93–2.34) | 0.0983 | 1.07 (0.81–1.42) | 0.6344 |
| Hypertension | 2.10 (1.37–3.23) | 0.0007 | 2.59 (1.84–3.67) | <0.0001 |
| Hyperlipidaemia | 0.75 (0.51–1.10) | 0.1379 | 1.26 (0.96–1.65) | 0.1019 |
| Diabetes | 2.23 (1.57–3.15) | <0.0001 | 1.68 (1.14–2.46) | 0.0082 |
| History of cancer | 2.91 (2.25–3.76) | <0.0001 | 3.02 (2.29–3.98) | <0.0001 |
| History of CVD | 2.31 (1.49–3.60) | 0.0002 | 2.80 (1.93–4.05) | <0.0001 |
| History of kidney disease | 1.59 (0.82–3.08) | 0.1692 | 2.31 (1.54–3.48) | <0.0001 |
CVD, cardiovascular disease; HR, hazard ratio; NAFLD, non-alcoholic fatty liver disease.
Table 3.
Age- and sex-adjusted HR of risk factors for all-cause mortality by NAFLD and sarcopenia status (multiple-imputation analysis).
| Covariate | Individuals with NAFLD |
Individuals without NAFLD |
Individuals with both NAFLD and sarcopenia |
Individuals with NAFLD but non-sarcopenia |
Individuals with sarcopenia but non-NAFLD |
Individuals with neither sarcopenia nor NAFLD |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Sarcopenia (unadjusted) | 2.58 (1.84–3.63) | <0.0001 | 5.12 (3.90–6.72) | <0.0001 | ||||||||
| Sarcopenia | 1.54 (1.07–2.21) | 0.0192 | 1.95 (1.38–2.75) | 0.0001 | ||||||||
| Age | 1.08 (1.06–1.10) | 0.001 | 1.10 (1.09–1.11) | <0.0001 | 1.06 (1.03–1.09) | 0.001 | 1.08 (1.06–1.11) | <0.0001 | 1.07 (1.05–1.09) | <0.0001 | 1.10 (1.09–1.11) | <0.0001 |
| Male | 1.67 (1.24–2.23) | <0.0001 | 1.65 (1.36–2.00) | <0.0001 | 2.04 (1.34–3.11) | 0.001 | 1.55 (1.11–2.18) | 0.0109 | 1.59 (1.03–2.45) | 0.0371 | 1.67 (1.37–2.03) | <0.0001 |
| Non-Hispanic White | 0.78 (0.55–1.10) | 0.1498 | 0.75 (0.55–1.01) | 0.0589 | 0.92 (0.54–1.56) | 0.7501 | 0.74 (0.52–1.06) | 0.103 | 1.11 (0.67–1.84) | 0.6647 | 0.71 (0.52–0.97) | 0.0318 |
| Non-Hispanic Black | 1.52 (1.05–2.22) | 0.0289 | 1.37 (1.00–1.86) | 0.0467 | 2.00 (0.99–4.06) | 0.0543 | 1.64 (1.13–2.37) | 0.0104 | 0.74 (0.44–1.25) | 0.248 | 1.58 (1.16–2.16) | 0.0052 |
| Hispanic | 1.14 (0.76–1.73) | 0.5139 | 1.28 (0.82–2.00) | 0.2702 | 1.12 (0.69–1.82) | 0.6252 | 1.02 (0.63–1.67) | 0.9282 | 0.79 (0.48–1.32) | 0.362 | 1.30 (0.73–2.33) | 0.367 |
| Other race | 1.12 (0.38–3.28) | 0.8298 | 1.00 (0.39–2.53) | 0.9926 | 0.68 (0.14–3.25) | 0.6227 | 1.40 (0.42–4.65) | 0.5757 | 1.37 (0.38–4.97) | 0.6219 | 0.81 (0.27–2.36) | 0.6869 |
| Low income | 1.51 (1.08–2.11) | 0.0172 | 2.29 (1.86–2.82) | <0.0001 | 1.27 (0.83–1.93) | 0.2624 | 1.50 (0.99–2.27) | 0.054 | 1.34 (0.79–2.29) | 0.2713 | 2.47 (1.91–3.19) | <0.0001 |
| College degree | 0.44 (0.28–0.68) | 0.0004 | 0.58 (0.41–0.83) | 0.0033 | 0.50 (0.22–1.14) | 0.0967 | 0.43 (0.28–0.65) | 0.0002 | 0.86 (0.51–1.46) | 0.5684 | 0.56 (0.38–0.83) | 0.0046 |
| Married | 0.64 (0.46–0.89) | 0.0085 | 0.52 (0.40–0.68) | <0.0001 | 0.62 (0.38–1.01) | 0.0545 | 0.61 (0.42–0.89) | 0.0111 | 0.45 (0.32–0.64) | <0.0001 | 0.54 (0.38–0.76) | 0.0007 |
| Active smoker | 2.10 (1.35–3.26) | 0.0014 | 1.62 (1.23–2.14) | 0.001 | 1.24 (0.57–2.71) | 0.5766 | 2.55 (1.54–4.25) | 0.0006 | 0.83 (0.45–1.54) | 0.5426 | 1.88 (1.43–2.47) | <0.0001 |
| Lean | 1.66 (1.31–2.10) | <0.0001 | 1.35 (1.09–1.67) | 0.0074 | 1.52 (0.77–2.99) | 0.2235 | 1.82 (1.37–2.43) | 0.0001 | 1.22 (0.77–1.94) | 0.3822 | 1.49 (1.18–1.88) | 0.0012 |
| Physical inactivity | 1.45 (1.09–1.93) | 0.0128 | 1.60 (1.25–2.05) | 0.0004 | 1.20 (0.77–1.87) | 0.4217 | 1.45 (1.01–2.08) | 0.0425 | 1.25 (0.80–1.95) | 0.3258 | 1.55 (1.19–2.01) | 0.0014 |
| Hypertension | 1.30 (0.92–1.84) | 0.1393 | 1.40 (0.97–2.02) | 0.0703 | 1.02 (0.45–2.31) | 0.9668 | 1.37 (0.86–2.20) | 0.1835 | 1.08 (0.66–1.75) | 0.7583 | 1.42 (0.96–2.10) | 0.0755 |
| Hyperlipidaemia | 0.73 (0.52–1.02) | 0.062 | 0.94 (0.65–1.34) | 0.7104 | 0.73 (0.44–1.22) | 0.2247 | 0.74 (0.48–1.13) | 0.1553 | 0.61 (0.38–1.00) | 0.0483 | 0.98 (0.63–1.53) | 0.9284 |
| Insulin resistance | 0.92 (0.70–1.20) | 0.5142 | 0.83 (0.52–1.33) | 0.4229 | 0.88 (0.49–1.57) | 0.6582 | 0.88 (0.65–1.19) | 0.4119 | 0.59 (0.22–1.60) | 0.2901 | 0.86 (0.53–1.38) | 0.5159 |
| Diabetes | 2.05 (1.53–2.74) | <0.0001 | 2.51 (1.91–3.28) | <0.0001 | 2.42 (1.55–3.79) | 0.0003 | 1.79 (1.25–2.56) | 0.0022 | 0.90 (0.40–2.06) | 0.8035 | 3.21 (2.35–4.39) | <0.0001 |
| Metabolic syndrome | 1.23 (0.94–1.61) | 0.1236 | 1.13 (0.78–1.63) | 0.5122 | 0.94 (0.60–1.48) | 0.789 | 1.31 (0.99–1.73) | 0.0595 | 0.77 (0.50–1.18) | 0.2189 | 1.18 (0.78–1.79) | 0.4265 |
| History of CVD | 2.08 (1.54–2.80) | <0.0001 | 23.16 (13.85–38.73) | <0.0001 | 2.07 (1.33–3.23) | 0.0018 | 2.13 (1.51–3.01) | <0.0001 | 1.58 (1.02–2.45) | 0.0411 | 19.60 (12.44–30.87) | <0.0001 |
| History of cancer | 1.56 (1.07–2.28) | 0.0211 | 3.42 (2.71–4.31) | <0.0001 | 1.73 (1.00–2.98) | 0.0495 | 1.55 (0.98–2.45) | 0.0613 | 1.35 (0.81–2.25) | 0.2445 | 4.16 (3.15–5.50) | <0.0001 |
| History of kidney disease | 2.47 (1.05–5.83) | 0.0394 | 16.82 (10.99–25.72) | <0.0001 | 1.60 (0.68–3.78) | 0.2715 | 3.03 (0.99–9.25) | 0.0519 | 1.80 (0.84–3.85) | 0.1236 | 17.17 (9.05–32.57) | <0.0001 |
| High risk for CVD | 3.83 (2.20–6.69) | <0.0001 | 9.40 (3.40–25.94) | <0.0001 | 4.91 (2.28–10.56) | 0.0002 | 3.22 (1.55–6.68) | 0.0023 | 1.96 (0.94–4.08) | 0.0725 | 11.30 (3.19–40.02) | 0.0004 |
| Advanced fibrosis (NFS) | 1.67 (1.28–2.18) | 0.0003 | 2.44 (1.73–3.44) | <0.0001 | 1.99 (1.18–3.34) | 0.0108 | 1.44 (1.03–2.00) | 0.0339 | 1.92 (1.11–3.32) | 0.0205 | 25.13 (15.79–39.99) | <0.0001 |
| Advanced fibrosis (FIB-4) | 2.89 (1.73–4.84) | 0.0001 | 33.96 (21.91–52.62) | <0.0001 | 2.48 (1.00–6.18) | 0.051 | 3.20 (1.70–6.02) | 0.0006 | 2.34 (1.22–4.48) | 0.0116 | 35.43 (22.24–56.44) | <0.0001 |
CVD, cardiovascular disease; FIB-4, Fibrosis-4; HR, hazard ratio; NAFLD, non-alcoholic fatty liver disease; NFS, NAFLD fibrosis score.
In contrast, NAFLD was associated with mortality after adjustments when T2DM (type 2 diabetes mellitus) was not included in the model (models 1–4; Fig. 3; Table S6). On the other hand, after adjustment for T2DM, NAFLD was no longer associated with mortality (Fig. 3; Table S6).
Cause-specific mortality
Amongst 587 individuals who died of all causes, 110 deaths (18.7%) were cardiac specific, 133 (22.7%) were cancer specific, and 344 (58.6%) were neither. The age- and sex-adjusted HR for cardiac-specific mortality was 1.71 [1.10–2.67] for NAFLD and 3.95 [2.08–7.49] for sarcopenia (Table S7). After including sarcopenia and NAFLD together (model 1), the HRs of NAFLD for cardiac-specific mortality decreased by 18.7% from those in the age- and sex-adjusted model and moved to non-significance (HR = 1.39 [0.89–2.16]). In the fully adjusted model, sarcopenia was associated with increased risk for cardiac-specific mortality (HR = 2.52 [1.07–5.93]) in the entire study cohort and in subjects with NAFLD (HR = 3.19 [1.17–8.74]) (Table 4). Table S8 demonstrates stratified analysis of different variables by age- and sex-adjusted Cox models.
Table 4.
Fully adjusted HR of risk factors for cardiac-specific mortality according to the presence of NAFLD (multiple-imputation analysis).
| Covariate | Individuals with NAFLD |
Individuals without NAFLD |
||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Sarcopenia | 3.19 (1.17–8.74) | 0.0239 | 2.37 (0.94–5.99) | 0.068 |
| Age (years) | ||||
| 20–39 | Reference | Reference | ||
| 40–59 | 0.62 (0.14–2.78) | 0.5289 | 0.39 (0.06–2.52) | 0.325 |
| >60 | 2.86 (1.22–6.70) | 0.0153 | 2.49 (1.20–5.17) | 0.0142 |
| Male | 3.32 (1.11–9.91) | 0.0312 | 3.30 (1.61–6.76) | 0.0011 |
| Race | ||||
| Non-Hispanic White | Reference | Reference | ||
| Non-Hispanic Black | 2.04 (0.86–4.86) | 0.1060 | 0.53 (0.23–1.18) | 0.1205 |
| Hispanic | 1.57 (0.53–4.63) | 0.4125 | 0.44 (0.13–1.52) | 0.1955 |
| Other race | N/A | 0.17 (0.02–1.54) | 0.1156 | |
| Low income | 0.69 (0.26–1.81) | 0.45 | 2.31 (1.33–4.01) | 0.0031 |
| College | 0.88 (0.36–2.14) | 0.7833 | 0.39 (0.14–1.12) | 0.0812 |
| Height (cm) | 1.00 (0.96–1.05) | 0.8556 | 0.98 (0.94–1.02) | 0.3903 |
| Physical inactivity | 1.15 (0.50–2.69) | 0.7401 | 1.29 (0.64–2.58) | 0.4784 |
| Active smoker | 2.04 (0.94–4.45) | 0.0726 | 0.62 (0.25–1.52) | 0.2931 |
| Hypertension | 1.44 (0.52–3.96) | 0.4821 | 2.99 (1.29–6.93) | 0.0107 |
| Hyperlipidaemia | 2.00 (0.59–6.82) | 0.2691 | 1.15 (0.47–2.84) | 0.7562 |
| Diabetes | 3.06 (1.26–7.42) | 0.0133 | 3.93 (1.83–8.47) | 0.0005 |
| History of cancer | 4.42 (2.20–8.89) | <0.0001 | 3.18 (1.48–6.84) | 0.0031 |
| History of CVD | 1.81 (0.77–4.27) | 0.1765 | 1.30 (0.59–2.85) | 0.5115 |
| History of kidney disease | 3.21 (0.78–13.24) | 0.1058 | 3.65 (1.18–11.34) | 0.0249 |
CVD, cardiovascular disease; HR, hazard ratio; NAFLD, non-alcoholic fatty liver disease.
Patterns for cancer-specific mortality were similar as all-cause mortality. The age- and sex-adjusted HR for cancer-specific mortality was 1.71 [1.24–2.37] for NAFLD and 2.74 [1.75–4.00] for sarcopenia (Table S9). A multivariable model, including metabolic components (model 4), demonstrated that the HR of NAFLD for cancer-specific mortality shifted towards non-significance (HR = 1.40 [0.93–2.11]). Table 5 demonstrates the risk of cancer-specific mortality in the fully adjusted model (Table 5). The association of variables with cancer-specific mortality in stratified analyses is shown in Table S10.
Table 5.
Fully adjusted HR of risk factors for cancer-specific mortality according to the presence of NAFLD (multiple-imputation analysis).
| Covariate | Individuals with NAFLD |
Individuals without NAFLD |
||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Sarcopenia | 2.12 (1.08–4.15) | 0.0293 | 1.68 (0.59–4.80) | 0.3299 |
| Age (years) | ||||
| 20–39 | Reference | Reference | ||
| 40–59 | 0.36 (0.08–1.68) | 0.1948 | 0.57 (0.27–1.23) | 0.1534 |
| >60 | 3.43 (1.78–6.60) | 0.0002 | 1.70 (0.82–3.53) | 0.153 |
| Male | 2.20 (0.92–5.24) | 0.0747 | 1.96 (0.94–4.06) | 0.071 |
| Race | ||||
| Non-Hispanic White | Reference | Reference | ||
| Non-Hispanic Black | 1.17 (0.34–4.06) | 0.8008 | 3.08 (1.70–5.56) | 0.0002 |
| Hispanic | 0.45 (0.24–0.87) | 0.0164 | 1.51 (0.60–3.80) | 0.3864 |
| Other race | 0.18 (0.02–1.61) | 0.1254 | 1.76 (0.49–6.33) | 0.3882 |
| Low income | 1.35 (0.64–2.86) | 0.4376 | 1.14 (0.61–2.13) | 0.6716 |
| College | 0.55 (0.20–1.52) | 0.2508 | 0.80 (0.37–1.74) | 0.5751 |
| Height (cm) | 0.99 (0.96–1.02) | 0.4819 | 1.00 (0.96–1.03) | 0.8443 |
| Physical inactivity | 0.93 (0.49–1.78) | 0.8301 | 0.75 (0.42–1.34) | 0.3397 |
| Active smoker | 1.87 (0.94–3.73) | 0.0738 | 1.83 (1.18–2.84) | 0.0067 |
| Hypertension | 1.49 (0.77–2.87) | 0.2367 | 1.89 (1.11–3.20) | 0.0181 |
| Hyperlipidaemia | 2.04 (0.82–5.06) | 0.1261 | 1.17 (0.68–1.99) | 0.5724 |
| Diabetes | 1.00 (0.56–1.81) | 0.9913 | 0.83 (0.32–2.18) | 0.7044 |
| History of cancer | 1.91 (1.05–3.46) | 0.0341 | 2.49 (1.18–5.26) | 0.0171 |
| History of CVD | 2.13 (0.99–4.59) | 0.0532 | 6.12 (3.72–10.06) | <0.0001 |
| History of kidney disease | 0.91 (0.18–4.49) | 0.9073 | 1.52 (0.52–4.45) | 0.4475 |
CVD, cardiovascular disease; HR, hazard ratio; NAFLD, non-alcoholic fatty liver disease.
Discussion
In the general population, prevalence of NAFLD is estimated to be around 24%,30 whilst prevalence of sarcopenia is about 10%.5 Historically, sarcopenia has been more frequently reported in the frail elderly individuals and in people with long-standing chronic illness and disability.31 Furthermore, sarcopenia is associated with increased mortality.32 In contrast, sarcopaenic obesity may occur at a younger age, often associated with insulin resistance and in associated with frailty.33,34 Given the association of NAFLD with body composition and MS, these patients are at risk for sarcopaenic obesity.35 Given the high prevalence of sarcopenia amongst patients with NAFLD, the impact on long-term outcomes will be important.
Consistent with previous reports, our data show that the prevalence of NAFLD and sarcopenia amongst the general population is 29.3% and 9.2%, respectively.36,37 Our analysis shows that patients with NAFLD have significantly higher rates of sarcopenia than patients without NAFLD, and this finding was persistent across all age, sex, race, and physical activity groups. In fact, our age- and sex-adjusted analysis showed that patients with NAFLD were 2.9 times more likely to have sarcopenia than patients without NAFLD. Furthermore, older age, female gender, non-Hispanic White ethnicity, and lower physical activity were the independent predictors of sarcopenia amongst patients with NAFLD. These data are consistent with previous studies, which have documented a close relationship between NAFLD and sarcopenia.[38], [39], [40], [41], [42], [43] In a study by Bhanji et al., the main potential pathophysiological mechanisms leading to sarcopenia in patients with non-alcoholic steatohepatitis (NASH) were reported to be insulin resistance and increased inflammation.44 In this context, these abnormalities could lead to triglyceride accumulation in myocytes and hepatocytes, causing proteolysis and muscle depletion.44 Although in our study, NAFLD patients with or without sarcopenia had similar rates of insulin resistance, they were significantly more likely to have T2DM.
Another important link between NAFLD and sarcopenia is related to the level of physical activity. In our study, amongst NAFLD, sarcopenia was inversely related to increased physical activity level. Although sarcopenia and physical deconditioning have been reported in patients with end-stage liver disease, the association with NAFLD with relatively early liver disease has been fully reported.45,46 In this context, our data make an important contribution linking NAFLD, sarcopenia, and level of inactivity.
The association of sarcopenia with adverse outcomes of patients with NAFLD is of great interest. In fact, a recent meta-analysis amongst more than 3,000 NAFLD patients demonstrated a significant direct association between sarcopenia, NASH, and advanced fibrosis.47 Additionally, other studies have confirmed these associations.7,[48], [49], [50] Given the lack of liver biopsy, we were unable to provide additional data supporting the association of sarcopenia with histological severity as documented by NASH activity or stage of advanced hepatic fibrosis.
Nevertheless, it is important to know that stage of fibrosis and histological NASH are surrogates of long-term mortality. In this context, establishing the association of sarcopenia with long-term outcomes amongst patients with NAFLD will be of utmost importance. In fact, our analysis shows that amongst patients with NAFLD, the presence of sarcopenia was associated with a 78% increase in all-cause mortality. More strikingly, in the NAFLD population, sarcopenia was associated with a 320% increase in cardiac-specific mortality. In this context, sarcopenia should be regarded as a key factor playing a significant role in worsening of both overall and cardiac-specific mortality amongst patients with NAFLD. Our data suggest that the presence of sarcopenia is independently associated with all-cause mortality, CV mortality, and cancer-related mortality in patients with NAFLD.
There are a few limitations to this study. First, we have utilised US FLI as a non-invasive diagnostic method for NAFLD in the absence of secondary causes of liver disease. Although a radiological- or histological-based diagnosis of NAFLD may be more accurate, ultrasound data are only available at the outdated NHANES III database (1988–1994) amongst NHANES survey cycles. However, the US FLI was established as a reliable method for non-invasive diagnosis of NAFLD in the US population and is associated with a higher risk of liver-specific mortality and all-cause mortality. Second, with respect to sarcopenia, there is evidence that its definition requires strength measures, a functional measure (usually of ambulation), and a measure of percent body fat/lean appendicular muscle mass. NHANES does not provide data on grip strength, the most commonly used measure of strength in many studies. Third, we used the FNIH guideline for sarcopenia instead of the Revised European Working Group on Sarcopenia in Older People (EWGSOP2) guideline, based on height adjustment. In these data, the crude prevalence of sarcopenia, defined by the EWGSOP2 guideline, was lower amongst individuals with NAFLD than amongst individuals without NAFLD (4.3% vs. 16.8%). We believed that the definition of FNIH would be a better choice for this study. Although the FNIH definition would lead to misclassification and overestimate the true prevalence of sarcopenia, we hope that the likelihood of misclassification may be alleviated by adjusting the height on multivariable analyses. Fourth, whilst we have reported mortality data, they come from a separate national database and have to be matched with the NHANES data. The association of sarcopenia and NAFLD with liver-specific mortality was not evaluated because of the unavailability of a specific cause of death in the public-use mortality files. Finally, the study we report here is cross sectional and does not provide data on the progression or regression of liver status. Another limitation is the lack of data on liver-specific mortality. Given that publicly available causes of mortality are only available for top 10 causes of death, liver-specific mortality was not available. Despite these study limitations, NHANES provides a nationally representative sample of the US population and the evaluation of various features of NAFLD with sarcopenia using multivariate analyses. To our knowledge, this approach has not been previously reported and we believe reduces bias. Finally, we believe this is the first study to link the prevalence data of NAFLD and sarcopenia and demographic and clinical findings to all-cause and CVD mortality.
In summary, our data show that sarcopenia is associated with inactivity in subjects with NAFLD. Furthermore, the presence of sarcopenia in patients with NAFLD poses increased risk for all-cause and cardiac-specific mortality. Given that exercise is an effective treatment for NAFLD and sarcopenia, these data make it imperative that clinicians should aim to diagnose and optimally mange sarcopenia in patients with NAFLD.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
Conceptualisation, investigation, and article writing: P.G. Conceptualisation, supervision, visualisation, writing, review, and editing: L.G. Data curation, formal analysis, and methodology: J.M.P. Article writing: R.D., L.deA. Resources, supervision, visualisation, writing, review, and editing: Z.M.Y. Z.M.Y. is the guarantor of this work, had full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final version of the article. The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Conflicts of interest
Z.M.Y. is a consultant to Bristol Myers Squibb, Gilead, Intercept, Novo Nordisk, Novartis, Terns, Merck, Viking, and Shinogi. All other authors have no conflicts of interest to disclose.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
The data were presented during the American Association for the Study of Liver Diseases 2019 meeting in Boston, MA.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100171.
Supplementary data
References
- 1.Rosenberg I.H. Sarcopenia: origins and clinical relevance. Clin Geriatr Med. 2011;27:337–339. doi: 10.1016/j.cger.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Dhillon R.J.S., Hasni S. Pathogenesis and management of sarcopenia. Clin Geriatr Med. 2017;33:17–26. doi: 10.1016/j.cger.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Haehling S., Morley J.E., Anker S.D. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1:129–133. doi: 10.1007/s13539-010-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shafiee G., Keshtkar A., Soltani A., Ahadi Z., Larijani B., Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta-analysis of general population studies. J Diabetes Metab Disord. 2017;16:21. doi: 10.1186/s40200-017-0302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Fré C.H., De Fré M.A., Kwanten W.J., Op de Beeck B.J., Van Gaal L.F., Francque S.M. Sarcopenia in patients with non-alcoholic fatty liver disease: is it a clinically significant entity? Obes Rev. 2019;20:353–363. doi: 10.1111/obr.12776. [DOI] [PubMed] [Google Scholar]
- 7.Wijarnpreecha K., Kim D., Raymond P., Scribani M., Ahmed A. Associations between sarcopenia and nonalcoholic fatty liver disease and advanced fibrosis in the USA. Eur J Gastroenterol Hepatol. 2019;31:1121–1128. doi: 10.1097/MEG.0000000000001397. [DOI] [PubMed] [Google Scholar]
- 8.Chung G.E., Kim M.J., Yim J.Y., Kim J.S., Yoon J.W. Sarcopenia is significantly associated with presence and severity of nonalcoholic fatty liver disease. J Obes Metab Syndr. 2019;28:129–138. doi: 10.7570/jomes.2019.28.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee Y.-H., Jung K.S., Kim S.U., Yoon H.-J., Yun Y.J., Lee B.-W. Sarcopenia is associated with NAFLD independently of obesity and insulin resistance: nationwide surveys (KNHANES 2008–2011) J Hepatol. 2015;63:486–493. doi: 10.1016/j.jhep.2015.02.051. [DOI] [PubMed] [Google Scholar]
- 10.Hong H.C., Hwang S.Y., Choi H.Y., Yoo H.J., Seo J.A., Kim S.G. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology. 2014;59:1772–1778. doi: 10.1002/hep.26716. [DOI] [PubMed] [Google Scholar]
- 11.Peng T.-C., Wu L.-W., Chen W.-L., Liaw F.-Y., Chang Y.-W., Kao T.-W. Nonalcoholic fatty liver disease and sarcopenia in a Western population (NHANES III): the importance of sarcopenia definition. Clin Nutr. 2019;38:422–428. doi: 10.1016/j.clnu.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 12.Johnson C.L., Dohrmann S.M., Burt V.L., Mohadjer L.K. National health and nutrition examination survey: sample design, 2011-2014. Vital Health Stat 2. 2014, (162):1–33. [PubMed] [Google Scholar]
- 13.National Center for Health Statistics Office of Analysis and Epidemiology, the linkage of National Center for Health Statistics survey data to the National Death Index—2015 linked mortality file (LMF): methodology overview and analytic considerations. http://www.cdc.gov/nchs/data_access/data_linkage/mortality.htm Available at.
- 14.Centers for Disease Control and Prevention, National Center for Health Statistics . Maryland; Hyattsville: 2015. NCHS 2011 Linked Public Use Data Dictionary.https://www.cdc.gov/nchs/data/datalinkage/Public_use_Data_Dictionary_23_2015.pdf Available at. [Google Scholar]
- 15.Ruhl C.E., Everhart J.E. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment Pharmacol Ther. 2015;41:65–76. doi: 10.1111/apt.13012. [DOI] [PubMed] [Google Scholar]
- 16.Angulo P., Hui J.M., Marchesini G., Bugianesi E., George J., Farrell G.C. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention National Health and Nutrition Examination Survey: technical documentation for the 1999-2004 dual energy X-ray absorptiometry (DXA) multiple imputation data files, 2008. https://wwwn.cdc.gov/nchs/data/nhanes/dxa/dxa_techdoc.pdf Available at.
- 18.Centers for Disease Control and Prevention NHANES 1999-2006 DXA multiple imputation data files. https://wwwn.cdc.gov/nchs/nhanes/dxa/dxa.aspx Available at.
- 19.Studenski S.A., Peters K.W., Alley D.E., Cawthon P.M., McLean R.R., Harris T.B. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention National Center for Health Statistics (NCHS). Analytic and reporting guidelines: the Third National Health and Nutrition Examination Survey, NHANES III (1988-94) Available at: https://wwwn.cdc.gov/nchs/data/nhanes/analyticguidelines/88-94-analytic-reporting-guidelines.pdf.
- 21.Goff D.C., Lloyd-Jones D.M., Bennett G., Coady S., D'Agostino R.B., Gibbons R. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. J Am Coll Cardiol. 2014;63:2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Araújo J., Cai J., Stevens J. Prevalence of optimal metabolic health in American adults: national health and nutrition examination survey 2009-2016. Metab Syndr Relat Disord. 2019;17:46–52. doi: 10.1089/met.2018.0105. [DOI] [PubMed] [Google Scholar]
- 23.Whelton P.K., Carey R.M., Aronow W.S., Casey D.E., Collins K.J., Himmelfarb C.D. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. J Am Coll Cardiol. 2018;71:e127–248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Wallace T.M., Levy J.C., Matthews D.R. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 25.Rezaianzadeh A., Namayandeh S.-M., Sadr S.-M. National Cholesterol Education Program Adult Treatment Panel III versus International Diabetic Federation definition of metabolic syndrome, which one is associated with diabetes mellitus and coronary artery disease? Int J Prev Med. 2012;3:552–558. [PMC free article] [PubMed] [Google Scholar]
- 26.Wolter K.M. 2nd edn. Springer; New York: 2007. Taylor Series Methods. Introduction to Variance Estimation. [Google Scholar]
- 27.Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, et al. National Health and Nutrition Examination Survey: analytic guidelines, 1999-2010. Vital Health Stat 2 2013;(161):1–24. [PubMed]
- 28.Allison P.D. 1995. Survival Analysis Using the SAS System: A Practical Guide. Cary, NC, SAS Institute. [Google Scholar]
- 29.Rubin D.B. John Wiley & Sons, Inc., Hoboken, NJ; 2004. Multiple Imputation for Nonresponse in Surveys. [Google Scholar]
- 30.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 31.Cesari M., Landi F., Vellas B., Bernabei R., Marzetti E. Sarcopenia and physical frailty: two sides of the same coin. Front Aging Neurosci. 2014;6:192. doi: 10.3389/fnagi.2014.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown J.C., Harhay M.O., Harhay M.N. Sarcopenia and mortality among a population-based sample of community-dwelling older adults. J Cachexia Sarcopenia Muscle. 2016;7:290–298. doi: 10.1002/jcsm.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srikanthan P., Hevener A.L., Karlamangla A.S. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS One. 2010;5:e10805. doi: 10.1371/journal.pone.0010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stenholm S., Harris T.B., Rantanen T., Visser M., Kritchevsky S.B., Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim T.N., Yang S.J., Yoo H.J., Lim K.I., Kang H.J., Song W. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: the Korean sarcopenic obesity study. Int J Obes (Lond) 2009;33:885–892. doi: 10.1038/ijo.2009.130. [DOI] [PubMed] [Google Scholar]
- 36.Wijarnpreecha K., Panjawatanan P., Thongprayoon C., Jaruvongvanich V., Ungprasert P. Sarcopenia and risk of nonalcoholic fatty liver disease: a meta-analysis. Saudi J Gastroenterol. 2018;24:12–17. doi: 10.4103/sjg.SJG_237_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu Y.-B., Chen Z., Fu R.-Q. The association between sarcopenia and non-alcoholic fatty liver disease. J Hepatol. 2017;66:243–244. doi: 10.1016/j.jhep.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 38.Abdulla H., Smith K., Atherton P.J., Idris I. Role of insulin in the regulation of human skeletal muscle protein synthesis and breakdown: a systematic review and meta-analysis. Diabetologia. 2016;59:44–55. doi: 10.1007/s00125-015-3751-0. [DOI] [PubMed] [Google Scholar]
- 39.Zhai Y., Xiao Q. The common mechanisms of sarcopenia and NAFLD. Biomed Res Int. 2017;2017:6297651. doi: 10.1155/2017/6297651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abbatecola A.M., Paolisso G., Fattoretti P., Evans W.J., Fiore V., Dicioccio L. Discovering pathways of sarcopenia in older adults: a role for insulin resistance on mitochondria dysfunction. J Nutr Health Aging. 2011;15:890–895. doi: 10.1007/s12603-011-0366-0. [DOI] [PubMed] [Google Scholar]
- 41.Kim C.W., Kim H.Y. The association between sarcopenia and non-alcoholic fatty liver disease: potential pitfalls in non-invasive prediction models. J Hepatol. 2016;64:519–520. doi: 10.1016/j.jhep.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 42.Zhai Y., Xiao Q., Miao J. The relationship between NAFLD and sarcopenia in elderly patients. Can J Gastroenterol Hepatol. 2018;2018:5016091. doi: 10.1155/2018/5016091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hashimoto Y., Osaka T., Fukuda T., Tanaka M., Yamazaki M., Fukui M. The relationship between hepatic steatosis and skeletal muscle mass index in men with type 2 diabetes. Endocr J. 2016;63:877–884. doi: 10.1507/endocrj.EJ16-0124. [DOI] [PubMed] [Google Scholar]
- 44.Bhanji R.A., Narayanan P., Allen A.M., Malhi H., Watt K.D. Sarcopenia in hiding: the risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology. 2017;66:2055–2065. doi: 10.1002/hep.29420. [DOI] [PubMed] [Google Scholar]
- 45.Tandon P., Raman M., Mourtzakis M., Merli M. A practical approach to nutritional screening and assessment in cirrhosis. Hepatology. 2017;65:1044–1057. doi: 10.1002/hep.29003. [DOI] [PubMed] [Google Scholar]
- 46.Duarte-Rojo A., Ruiz-Margáin A., Montaño-Loza A.J., Macías-Rodríguez R.U., Ferrando A., Kim W.R. Exercise and physical activity for patients with end-stage liver disease: improving functional status and sarcopenia while on the transplant waiting list. Liver Transpl. 2018;24:122–139. doi: 10.1002/lt.24958. [DOI] [PubMed] [Google Scholar]
- 47.Yu R., Shi Q., Liu L., Chen L. Relationship of sarcopenia with steatohepatitis and advanced liver fibrosis in non-alcoholic fatty liver disease: a meta-analysis. BMC Gastroenterol. 2018;18:51. doi: 10.1186/s12876-018-0776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koo B.K., Kim D., Joo S.K., Kim J.H., Chang M.S., Kim B.G. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol. 2017;66:123–131. doi: 10.1016/j.jhep.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 49.Lee Y.-H., Kim S.U., Song K., Park J.Y., Kim D.Y., Ahn S.H. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: nationwide surveys (KNHANES 2008-2011) Hepatology. 2016;63:776–786. doi: 10.1002/hep.28376. [DOI] [PubMed] [Google Scholar]
- 50.Petta S., Ciminnisi S., Di Marco V., Cabibi D., Cammà C., Licata A. Sarcopenia is associated with severe liver fibrosis in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2017;45:510–518. doi: 10.1111/apt.13889. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.