Abstract
Objective.
The current opioid crisis was fueled by escalation of opioid dosing among patients with chronic pain. Yet, there are few evidence-based psychological interventions for opioid dose reduction among chronic pain patients receiving long-term opioid analgesics. Mindfulness-Oriented Recovery Enhancement (MORE), which was designed to target mechanisms underpinning chronic pain and opioid misuse, has shown promising results in two randomized clinical trials (RCTs), and could facilitate opioid sparing and tapering by bolstering self-regulation. Here we tested this hypothesis with secondary analyses of data from a Stage 2 RCT.
Method.
Chronic pain patients (N = 95) on long-term opioid therapy were randomized to 8 weeks of MORE or a support group (SG) control delivered in primary care. Opioid dose was assessed with the Timeline Followback through 3-month follow-up. Heart rate variability (HRV) during mindfulness meditation was quantified as an indicator of self-regulatory capacity.
Results.
Participants in MORE evidenced a greater decrease in opioid dosing (a 32% decrease) by follow-up than the SG [F(2,129.77) = 5.35, p = .006, d = 1.07]. MORE was associated with a significantly greater increase in HRV during meditation than the SG. Meditation-induced change in HRV partially mediated the effect of MORE on opioid dose reduction [p = .034].
Conclusions.
MORE may boost self-regulatory strength via mindfulness and thereby facilitate self-control over opioid use, leading to opioid dose reduction in people with chronic pain.
Keywords: addiction, heart rate variability, mindfulness, opioid tapering, self-regulation
According to the 2015 National Survey on Drug Use and Health, over a third of U.S. adults used prescription opioids in the past year, and 15.3 million Americans met criteria for an opioid use disorder (OUD) (Han et al., 2017). The over-prescription of opioid medications to treat chronic pain contributed to the current OUD epidemic. Over-prescribing has proven pernicious, as chronic, high dose, opioid analgesic use increases the risk of developing opioid misuse and OUD (Chou et al., 2015). One of the common pathways to OUD begins with a valid opioid prescription for pain management and leads, via opioid dose escalation, to opioid misuse and eventually to the loss of control over opioid use that is characteristic of addiction.
The downward spiral linking chronic pain to opioid dose escalation, opioid misuse, and OUD is thought to be driven by the neuropsychopharmacologic effects of chronic opioid exposure on dysregulation of brain stress and reward systems (Garland et al., 2013). During this process, known as allostasis, chronic opioid use triggers a cascade of neurobiological processes in corticostriatal circuits that disrupt hedonic homeostasis, increasing sensitization to drug-related cues that entrenches opioid use as a habitual behavior while reducing the reward derived from natural reinforcers in the socioenvironment (Koob & Volkow, 2016). Simultaneously, allostatic effects of chronic, high dose opioid use on corticolimbic circuitry results in increased reactivity to stressors, including pain (Shurman et al., 2010). Furthermore, chronic opioid exposure can weaken cognitive control (Baldacchino et al., 2012), undermining the capacity for self-regulation of the opioid use habit, particularly under conditions of heightened stress – which has been shown to prompt habitual behaviors (Schwabe & Wolf, 2009). Weakened self-regulatory capacity coupled with increasing opioid tolerance and overwhelming distress and pain drives opioid dose escalation as a means of obtaining hedonic equilibrium, further propelling the downward spiral of allostasis and increasing dependence on opioids (Garland et al., 2013). Thus, chronic opioid users with pain become caught in a vicious cycle where the perceived solution (i.e., opioid use) to their problem (i.e., pain and distress) only magnifies the problem. Alternative treatments are urgently needed to halt opioid dose escalation among people with chronic pain.
Mindfulness-Oriented Recovery Enhancement (MORE) is a mindfulness-based intervention that was developed to reduce opioid use and misuse among people with chronic pain via a treatment sequence involving three distinct, yet interrelated, therapeutic components: mindfulness, reappraisal, and savoring (Garland, 2013). MORE begins with a foundation of mindfulness training to increase attentional control and metacognitive awareness. Next, MORE draws from the cognitive-behavioral tradition, providing direct instruction in cognitive reappraisal to improve negative emotion regulation. Finally, MORE introduces savoring, a technique designed to boost natural reward processing and positive emotion regulation. Results from two, Stage 2 randomized clinical trials (N = 115 and N = 95) of opioid-treated chronic pain patients indicate significant effects of MORE on pain severity, stress arousal, desire for opioids, and opioid misuse risk as well as on positive psychological functioning (e.g., positive affectivity, meaning in life) relative to a therapist-led support group control condition (Garland et al., 2014a; Garland, Hanley, Riquino, et al., 2019). Although a broad spectrum of intervention effects have been observed, MORE’s effect on opioid dose escalation has yet to be established.
Preventing opioid dose escalation is likely dependent on improving self-regulation. Among pain management patients in primary care, opioid dosing is self-administered, and given that patients are often prescribed ≥ 30-day supplies (Mundkur et al., 2018), patients may take higher doses than prescribed. In light of the powerfully reinforcing effects of opioids, opioid dose escalation has been shown to occur over time among some individuals with chronic pain (Henry et al., 2015; Mystakidou et al., 2003; Paice et al., 1996; Zenz et al., 1992). Prospective cohort studies indicate that 19% of chronic pain patients double their dose over two years (Morasco et al., 2020), with national epidemiologic data indicating that 34% double their dose over five years (Fredheim et al., 2013). Plausibly, such opioid dose escalation is likely to occur in the absence of effective self-regulation. In their neurovisceral integration model, Thayer & Lane (2000) propose self-regulation is comprised of three core components: control of attention, emotion, and autonomic function. Within this model, attention and emotion regulation are subserved by the same functional and structural brain networks as autonomic control (Jennings et al., 2015); by virtue of this common neural architecture, regulating attention and emotion should also regulate autonomic processes. Mindfulness training, which has been shown to improve self-regulatory capacities (Ostafin et al., 2015), is intended to strengthen both attention and emotion regulation (Tang, Hölzel, & Posner, 2015; Vago & Silbersweig, 2012), and MORE, which rests on a foundation of mindfulness training, has been shown to enhance both of these capacities. Specifically, MORE improves the capacity to regulate attention to emotional information, as revealed by dot probe (Garland, Baker, et al., 2017; Garland & Howard, 2013) and emotional go/no-go tasks (Garland, Bryan, Priddy, et al., 2019). In addition, MORE improves top-down regulation of emotional responses, as revealed by neurophysiology (Froeliger et al., 2017; Garland, Atchley, Hanley, et al., 2019). Following the neurovisceral integration model, to the extent that MORE strengthens self-regulatory capacity, such improvements should be reflected in increased autonomic control.
According to the neurovisceral integration model, self-regulation in the face of homeostatic perturbations (such as those evoked by chronic pain and/or the urge to consume opioids) is thought to elicit downstream activation of a central-autonomic network that modulates attention and emotion (e.g., medial prefrontal cortex → anterior cingulate cortex and anterior insula → central nucleus of the amygdala → hypothalamus → nucleus of the solitary tract → ventrolateral medullary) with downstream effects on visceral physiological responses, including the beat-to-beat modulation of heart rate by the vagus nerve, known as heart rate variability (HRV) (Thayer & Lane, 2009; Thayer & Lane, 2000). Prefrontal cortical activation is significantly positively associated with HRV (Thayer, Åhs, Fredrikson, Sollers III, & Wager, 2012). Thus, HRV may index top-down, prefrontally-mediated self-regulation of brainstem activity and autonomic responses. In that regard, a meta-analysis including 123 studies found a statistically significant, albeit small, relation between HRV and various measures of self-regulation (Holzman & Bridgett, 2017), including the capacity to regulate appetitive urges. For example, in a classic experiment, Segerstrom and Nes (2007) found that exerting self-regulation strength to resist the urge to eat an appetizing food increased HRV relative to indulging in the urge. In contrast, decreased HRV is associated with reduced self-regulation of craving for other substances, including alcohol (Garland et al., 2012; Quintana et al., 2013), methamphetamine (Wang et al., 2019), nicotine (Ashare et al., 2012), and opioids (Baker & Garland, 2019; Garland, Bryan, et al., 2017).
As a self-regulatory strategy, mindfulness meditation should impact HRV. In that regard, Krygier et al. (2013) identified increases in HRV during mindfulness Vipassana meditation among novice practitioners during a 10-day mindfulness retreat. Practitioners of an integrative body-mind training involving mindfulness meditation showed similarly elevated levels of HRV during meditation that were correlated with increases in activation in the anterior cingulate cortex (Tang et al., 2009) – a key region implicated in the frontoparietal control system, which may suggest a cortical (attentional) control mechanism over autonomic function. Furthermore, Takahashi et al., (2005) found that Zen-meditation also increased HRV and frontal slow-alpha/theta EEG rhythms, again suggesting attention regulation over autonomic function during meditation. With respect to MORE, significant HRV effects have been observed during attention to drug-related stimuli and other emotionally salient cues in the context of cognitive tasks (Garland et al., 2014; Garland et al., 2010; Garland, Howard, et al., 2017). These findings demonstrate that MORE modulates HRV during attention to exteroceptive stimuli. However, to date, no study has directly examined effects of MORE on autonomic control during mindfulness meditation with an internal locus of attention on cognitive, affective, and interoceptive experience, nor has any study tied self-regulation of autonomic responses via meditation to opioid use.
The present study examined effects of MORE on opioid dosing and HRV during meditation among a sample of patients with opioid-treated chronic pain participating in a recently completed Stage 2 RCT (NCT03298269). Self-reported outcomes from this trial demonstrated that MORE significantly decreased chronic pain severity and opioid misuse risk by 3-month follow-up (Garland, Hanley, Riquino, et al., 2019). In this secondary analysis of proximal outcome data from this trial, we hypothesized that patients with opioid-treated chronic pain allocated to MORE would report significantly greater increases in HRV during meditation than patients allocated to an active support group (SG) control condition, and that this increase in HRV would mediate the effect of MORE on reducing opioid dosing by 3-month follow-up.
METHODS
Participants
Participants met study inclusion criteria if they reported chronic non-cancer pain on more days than not and had been prescribed and taking opioid analgesics nearly every day for at least 90 days. Participants were recruited from primary care clinics in Salt Lake City, UT through a variety of means, including electronic health record, opt out letters, flyers, and radio advertisements. Participants were excluded if they were actively suicidal or psychotic (determined by the Mini-International Neuropsychiatric Interview 6.0, Sheehan et al., 1998) or had previously completed an 8-week mindfulness-based intervention (e.g., Mindfulness-Based Cognitive Therapy, MBCT). Over two-years, 304 patients were screened, 95 of whom met study criteria and were randomly assigned to MORE (n=50) or SG (n=45) conditions (see CONSORT chart in Garland, Hanley, Riquino, et al., 2019b). Of these participants, 76 (80% of the randomly allocated sample; 74% of MORE participants and 86% of SG participants) completed treatment - defined as attending ≥4 treatment sessions, a threshold established in the original MBCT efficacy trial (Teasdale et al., 2000). Of participants allocated to MORE, 72% completed post-treatment assessments, and 68% completed the 3-month follow-up. Of participants allocated to SG, 84% completed post-treatment assessments, and 69% completed the 3-month follow-up.
Procedures
Following screening, individuals who met eligibility criteria and gave informed consent completed a series of validated questionnaires (reported in Garland, Hanley, Riquino, et al., 2019b). Next, participants were interviewed by a clinically trained research assistant using the Timeline Followback procedure (Sobell & Sobell, 1992) to assess their daily opioid use. After the pre-treatment assessment, participants were randomly allocated by a project coordinator, who was uninvolved with assessment or treatment, to one of two undisclosed treatment groups. Participants were informed that they would be randomized to a behavioral treatment group that would help them to cope with pain, stress, and opioid-related problems by providing either mindfulness training or group support. To prevent bias and maintain allocation concealment, the identity of treatment allocation was not revealed to the participant until he or she arrived to the first treatment session. The allocation sequence (which was inaccessible to research staff involved in assessment or treatment) was generated via computerized random number table by a researcher who was uninvolved in assessment, treatment, or enrollment using simple randomization in blocks of varying sizes (2 - 4) to preserve allocation unpredictability. Assessments were conducted by research staff blinded to group assignment (which remained concealed throughout the study), and participants were reminded to not disclose their group assignment to research staff before or during each assessment. After participants had completed the 8-week MORE or SG treatment, they returned to the lab to complete a post-treatment assessment consisting of: 1) the same questionnaires administered at pre-treatment, 2) the Timeline Followback to assess for opioid use, and 3) a laboratory-based, mindfulness practice session during which HRV was assessed. Participants returned for a 3-month follow-up where they again completed the Timeline Followback. All study procedures were IRB approved.
Study Interventions
MORE intervention.
The manualized MORE intervention (Garland, 2013) was delivered by a Master’s level social worker in 8 weekly, 2-hour group therapy sessions conducted in a community-based primary care clinic. MORE sessions involved: 1) mindfulness training to facilitate self-regulation of cognitive, affective, and physiological responses; 2) reappraisal training to reframe adversity as an opportunity for psychological growth; and 3) training in savoring pleasant events to boost positive affective responses and promote natural reward processing. MORE treatment sessions addressed the following topics on successive weeks: distinguishing nociception from pain and suffering; cultivating mindful awareness of automaticity in the context of chronic pain and opioid use; elucidating the role of cognitive reappraisal in fueling negative emotions and pain catastrophizing; shifting attention from pain and stress to savor pleasant experiences; self-regulating opioid use and misuse through mindful attention and awareness; reducing stress as a means of preventing opioid misuse; finding meaning in life through self-transcendence; and developing a mindful recovery plan. No explicit motivation or instruction for opioid dose reduction was provided in the intervention groups. Mindfulness training involved mindful breathing and body scan techniques, with specific instructions to help patients shift from affective to sensory processing of pain sensations and savor positive emotions and pleasant body sensations (Hanley & Garland, 2019), as well as develop meta-awareness. Participants were asked to engage in daily 15-minute mindfulness practice sessions at home guided by an audio recording. In addition, participants were asked to pause before taking their next opioid dose to practice three minutes of mindful breathing. This mindfulness practice aimed to disrupt habitual (automatic) use of opioids, and was intended to clarify whether opioid use was driven by craving or a legitimate need for pain relief. Ultimately, this set of techniques was intended to strengthen self-regulatory capacity and provide a means of reducing unneeded opioid dosing.
Support group intervention.
To control for non-specific factors including attention by a caring professional, therapeutic expectancy, and social support, we employed a therapist-led support group (SG) as the active control condition in this study. The SG consisted of 8 weekly, 2-hour group sessions, in which a Master’s level social worker led discussion on topics pertinent to chronic pain and long-term opioid use that were selected to roughly match corresponding themes in the MORE intervention: the lived experience of chronic pain; ways of coping with chronic pain; ways of coping with negative emotions; how stressful life events impact pain; the stigma of opioid use and dependence; adverse effects of opioids; acceptance versus denial; and plans for the future. To match the MORE homework requirement, SG participants were asked to engage in 15 minutes of journaling a day on the weekly session topics. This Rogerian SG format, which did not involve learning of any experiential skills, was validated as a control condition in several prior trials of MORE (Garland, Manusov, et al., 2014; Garland, Gaylord, Boettiger, & Howard, 2010) and was largely based on the active intervention condition outlined in the Matrix Model intensive outpatient treatment manual (Rawson & McCann, 2006). During the SG sessions, participants were guided via client-centered reflective listening techniques to disclose feelings and thoughts about group topics, as well as to provide advice and emotional support for their peers. Therapists engaged in empathic responding and promoted mutual support among group members, but no specific recommendations for change were provided. This intervention, which typifies a widely-available form of conventional, process-oriented group therapy, was found in three prior RCTs to have equivalent perceived credibility to mindfulness-based interventions (Garland et al., 2010, 2014; Gaylord et al., 2011). MORE and SG sessions were held in a primary care clinic in groups of 8 to 12 people, were 2 hours in length, and were administered by Masters-level therapists supervised by the first author.
Treatment fidelity.
MORE therapist competence was rated on a Likert scale (1 = very poor, 4 = adequate, 7 = excellent) while therapist adherence was rated on a similar Likert scale (1 = not at all, 4 = somewhat, 7 = extensively) across 16 items developed by the first author (validated in R34DA037005). Therapist competence and adherence to the SG treatment manual were rated with similar Likert scales across 14 items (also validated in R34DA037005). As reported in Garland, Hanley, Riquino, et al. (2019b), MORE and SG therapists achieved a high level of fidelity (MORE competence = 5.9, SD = 0.7, MORE adherence = 5.9, SD = 0.6; SG competence = 6.4, SD = 0.4, SG adherence = 5.6, SD = 0.6). The first author reviewed all MORE and SG treatment session recordings weekly to monitor fidelity and provide clinical supervision until a level of adequate or greater therapist competence and adherence had been achieved (mean scores > 4). At this time, clinical supervision was provided every few weeks, and clinically trained research staff (e.g., graduate-level social workers and psychologists) continued to conduct fidelity monitoring on a random sample of ≥ two sessions per cohort.
Measures
Opioid dose.
Average daily opioid dose throughout the study was assessed using the validated Timeline Followback procedure (Sobell & Sobell, 1992). Self-reports of opioid dosing data were triangulated through electronic health record review of opioid prescription data. Opioid dose was converted in morphine milligram (mg) equivalents.
Heart rate variability during mindfulness practice.
At the beginning of the experimental laboratory session, disposable Ag-AgCl adhesive electrodes were attached to participants’ right and left pectoral muscles and electrocardiogram (ECG) data were continuously sampled at 1,000 Hz on a Biopac MP 150 system (Biopac Systems, Goleta, CA). Baseline HRV was collected during a 5-minute period in which participants were asked to remain silent and motionless, after which they completed the 10-minute mindfulness practice. For this practice all participants received the same instruction: “Now practice mindfulness, which means focusing on your thoughts, feelings and body sensations in the present moment in a nonjudgmental way, without reacting to them.” In keeping with methods used in previous mindfulness studies (E. L. Garland, Atchley, Hanley, et al., 2019), to control for demand characteristics, these task instructions were kept constant across both treatment conditions (MORE and SG), allowing us to isolate the effects of mindfulness training through the MORE intervention from any potential instruction effects. We assumed that meditation-naive participants randomized to the SG control would be unable to successfully practice mindfulness meditation with such nondescript instructions, whereas participants randomized to MORE would be able to use these simple instructions to successfully practice mindfulness following 8 weeks of training in the MORE intervention.
During the resting baseline and mindfulness practice session, R-R intervals in the ECG waveforms were initially detected automatically in Acqknowledge 4.1 (BIOPAC, Inc.), and then visually inspected to correct missed or incorrectly identified R-waves. Kubios 2.0 (Biosignal Analysis and Medical Imaging Group, University of Finland) was used for time domain analysis of R-waves. The root mean square of successive differences (RMSSD) in R-R intervals was used to compute HRV. This measure has been well-established to be sensitive to short-term fluctuations in HRV (Task Force of the European Society of Cardiology the North American Society of Pacing and Electrophysiology, 1996). Although some researchers suggest RMSSD is not a pure measure of vagal control of the heart (Berntson et al., 2007), we selected RMSSD as our primary measure of HRV in this study given that it less affected by respiratory influences than spectral frequency-domain HRV measures (Hill et al., 2009). RMSSD is recommended as a psychophysiological measure of phasic, short-term changes in HRV in multiple best practice guidelines (Laborde et al., 2017; Shaffer & Ginsberg, 2017; Smith et al., 2020). It has been demonstrated that RMSSD is preferable to spectral frequency-domain HRV (e.g.., high frequency HRV, HF-HRV) measures in experiments where respiratory confounds are likely to be present (Hill et al., 2009; Penttilä et al., 2001). Indeed, HF-HRV is well-known to be confounded by respiration effects (Shaffer & Ginsberg, 2017). In light of well-documented effects of opioids on respiratory depression (Boom et al., 2012; Jarzyna et al., 2011), as well as the effects of mindfulness training on respiration rate (Adler-Neal et al., 2019; Wielgosz et al., 2016), we selected RMSSD as our primary HRV measure in an effort to remove the potentially confounding influence of respiration inherent to spectral frequency-domain measures of HRV. As an ancillary HRV measure, we also report HF-HRV. HRV values were averaged across the 5-min baseline and the 10-minute mindfulness induction.
Therapeutic skill practice.
Each day during the 8-week MORE intervention, participants completed ecological momentary assessments (EMAs) via smartphones to report the number of minutes they spent engaged in each type of therapeutic skill (i.e., mindful breathing, reappraisal, and savoring) that day. We asked participants to report skill practice daily to prevent retrospective memory biases.
Statistical Analysis
Intendon-to-treat (ITT) analyses were conducted on the entire randomized sample. To analyze patterns of missing data, we performed Little’s MCAR test (Little, 1988). The nonsignificant MCAR test (χ2 = 75.32, df = 75, p = .47) and pattern of missing data were consistent with data being missing completely at random; thus, maximum likelihood estimation (MLE) was employed to handle missing data. To reduce bias resulting from listwise deletion, MLE estimates the variance-covariance matrix for all available data, including data from cases assessed at only one time point. Given that all available data were used for ITT analysis, raw means from the available data for the entire sample (without listwise deletion, in mean ± 1 standard error) were reported for interpretation of study findings (e.g., changes in opioid dose).
We undertook a stepwise analytic approach. We first employed linear mixed modeling in SPSS 24.0 to assess effects of MORE on opioid dose trajectories from pre-treatment to followup. This model was composed of variables representing treatment group (Group) and assessment time point (Time), as well as their interaction, and was specified with a random intercept. In the SG, two participants had very high opioid dose values (i.e., greater than 1000 mg of morphine equivalents at 3-month follow-up). As such, the opioid dosing variable was found to be skewed (skewness = 6.33, SE = .16). To account for these outliers and adjust for the non-normality of the data, opioid dose at each study timepoint was natural log transformed before analysis, resulting in distributions that did not significantly differ from normal (one-sample Kolmogorov-Smirnov test ps= .20). After establishing differences in opioid dose trajectory by treatment condition, we then conducted a path analysis (Baron & Kenny, 1986) to test mindfulness-induced change in HRV at post-treatment as a mediator of the effects of opioid dose reduction by 3-month followup. This path model was composed of a single independent variable representing treatment group (Group), a mediator variable representing change in HRV (RMSSD during mindfulness meditation – resting baseline RMSSD at post-treatment), and dependent variable representing change in opioid dose by follow-up. The R Lavaan package obtained direct and indirect effects with bootstrapping (Preacher & Hayes, 2008) to test the significance of the indirect effect.
Finally, to better determine which therapeutic skill (i.e., mindfulness, reappraisal, and savoring) was most strongly related to the effect of MORE on increased HRV during mindfulness meditation and lowered opioid dose at 3-month follow-up, we examined Pearson correlations between the total number of self-reported minutes of each type of therapeutic skill practice over the 8-week intervention and opioid dose and HRV outcomes.
RESULTS
Sample Characteristics
Participants were predominately Caucasian (89.5%) and female (66.3%), with a mean age of 56.6 ± 1.21 years. Approximately half of participants (53.6%) had no more than a high school education, and the majority (69.5%) had an annual household income < $49,999. Back pain was the most commonly reported pain location (38.9%), followed by pain in the joints (13.7%), lower extremities (11.6%), and neck/shoulders (8.4%), with the remaining participants reporting other types of pain as their primary pain condition. The mean pain severity was 5.29 ± 0.15 out of 10. Participants had used opioids for an average of 10.2 ± 0.8 years. Hydrocodone was the most commonly reported primary opioid type (40.0%), followed by oxycodone (20.0%), tramadol (15.7%), and morphine (10.5%), with fewer participants reporting use of other opioids.
Effect of MORE On Opioid Dose
In ITT analysis via linear mixed modeling, although neither the main effect of Group (F(1,92.68) = 1.41, p = .24 nor Time (F(2,129.77) = .99, p = .38), were significant, a significant Group X Time interaction was observed on the log-transformed opioid dose variable (Figure 1), F(2,129.77) = 5.35, p = .006, d = 1.07, such that participants in MORE evidenced a significantly greater decrease in opioid dosing from pre- to post-treatment and 3-month follow-up than the SG. Inspection of the available opioid dose data (non-transformed for ease of interpretation) revealed that by 3-month follow-up, participants in MORE reported a 31.8% reduction in opioid dose (from 66.31 ± 10.75 mg at pre-treatment to 45.24 ± 10.08 mg at follow-up), compared to a 124.0% increase in opioid dose among participants in the SG (from 69.56 ± 15.05 mg at pretreatment to 155.84 ± 63.82 mg at follow-up). In a sensitivity analysis dropping the two outliers from the SG, the Group X Time interaction remained significant, F(2,59.92) = 4.41, p = .016, d = .77, with the SG reporting a 16.1% increase in opioid dose (from 61.04 ± 14.05 mg at pretreatment to 70.89 ± 19.54 mg at follow-up).
Figure 1.
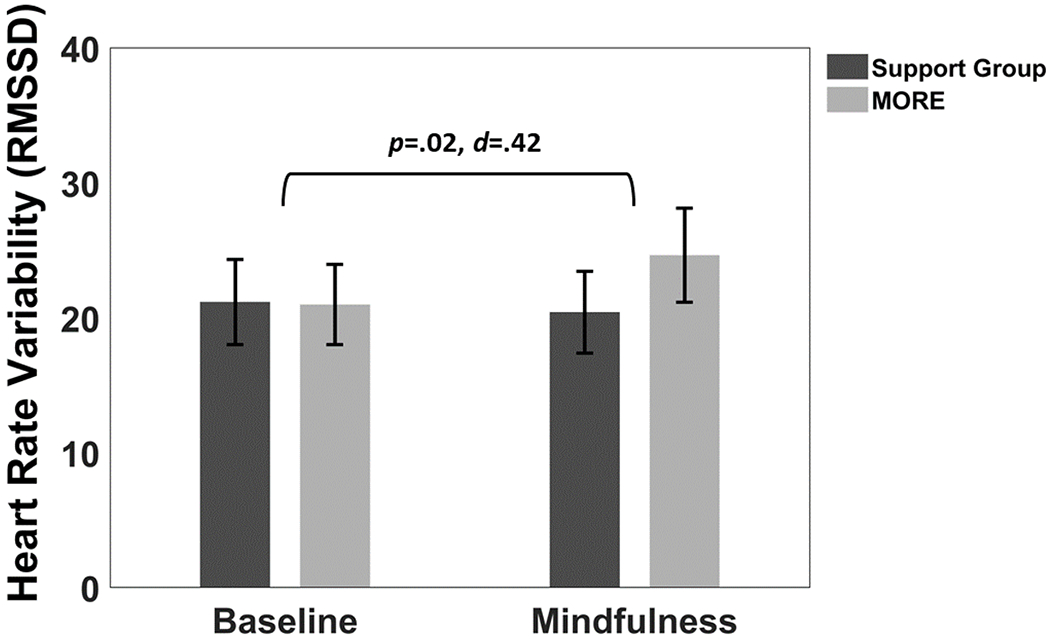
Effects of Mindfulness-Oriented Recovery Enhancement (MORE) versus a Support Group control condition on heart rate variability (mean RMSSD ± 1 S.E.; RMSSD = root mean square of successive differences) during a laboratory-based mindfulness meditation practice session and resting baseline at post-treatment.
HRV During Meditation as a Mediator of Treatment Effect on Opioid Dose
Participation in MORE was associated with significantly greater increases in RMSSD from resting baseline through mindfulness meditation (baseline: 32.81 ± 4.55, meditation: 38.51 ± 4.62) than the support group (baseline: 30.36 ± 4.62, meditation: 29.65 ± 4.69); this difference was statistically significant (B = 6.41, SE = 2.78, p = .02, see Figure 1). MORE was also associated with greater increases in HF-HRV from resting baseline through meditation (baseline: 251.04 ± 144.38, meditation: 689.18 ± 271.92) than the support group (baseline: 383.35 ± 146.69, meditation: 353.28 ± 276.27), but this effect was not statistically significant (p = .16).
In our path model (Figure 2), the direct effect of Group on change in RMSSD from resting baseline through mindfulness meditation in the post-treatment laboratory session was significant (‘a’ path: B = 6.48, SE = 2.36, p = .006). Mindfulness-induced change in RMSSD significantly predicted changes in opioid dose by 3-month follow-up (‘b’ path: B = −.03, SE = .008, p = .001), such that individuals who evidenced the largest increases in RMSSD during mindfulness meditation at the post-treatment laboratory assessment evidenced the greatest decreases in opioid dose over time. The direct effect of Group on opioid dose remained significant (‘c’ path: B = −.58, SE = .20, p = .004). The indirect effect of Group on opioid dose reduction via change in RMSSD was significant (B = −.17, SE = .07, p = .034).
Figure 2.
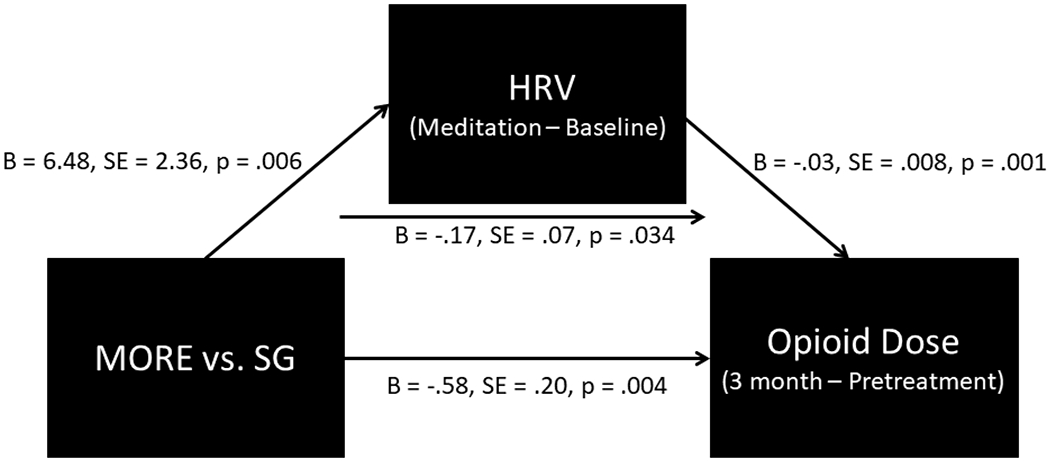
Path model indicating that the effect of Mindfulness-Oriented Recovery Enhancement (MORE) versus a Support Group (SG) control condition on reducing opioid dose was statistically mediated by increasing heart rate variability (HRV) during mindfulness meditation.
Therapeutic Skill Practice and its Relation to Study Outcomes
With regard to home therapeutic skill practice, participants in MORE reported a daily mean practice of mindful breathing for 17.4 (SD = 8.9), reappraisal for 10.5 (SD = 7.6), and savoring for 17.0 (SD = 17.1) minutes. Further analysis indicated that the total number of minutes of mindful breathing practice over the 8 weeks of the intervention correlated significantly with both RMSSD increases during meditation (r = .42, p = .048) and opioid dose at the 3-month follow-up (r = −.40, p = .046). Total number of minutes practicing savoring also correlated significantly with opioid dose at the 3-month follow-up (r = −.39, p = .049), but not with RMSSD during meditation (r = .25, p = .25). Total number of minutes of reappraisal practice showed no significant correlations with either RMSSD during meditation (r = .25, p = .26) or opioid dose at the 3-month follow-up (r = −.38, p = .08).
DISCUSSION
Mindfulness-based interventions are theorized to improve self-regulation (Tang et al., 2015; Vago & Silbersweig, 2012), and self-regulation may be critical for managing opioid medications over time. Previous evidence suggests MORE improves attentional and emotional markers of self-regulation (Garland, Baker, et al., 2017; Garland, Atchley, Hanley, et al., 2019; Garland, Bryan, Priddy, et al., 2019; Garland & Howard, 2013) and decreases opioid misuse (Garland et al., 2014a; Garland, Hanley, Riquino, et al., 2019). Here we tested whether MORE could strengthen self-regulation of autonomic function (i.e., HRV) during an acute meditative state and whether meditation-related increases in HRV during meditation predict less opioid use 3-months after treatment ends. Results from the present study indicate that MORE significantly reduced opioid dosing by three-month follow-up in a sample of primary care patients prescribed opioid pain management. Comparatively, opioid use increased in the SG control condition over the same time period. Additionally, MORE increased HRV during meditation, and these HRV increases predicted less opioid use at three-month follow-up. Taken together, study results suggest that MORE may reduce opioid dose by strengthening self-regulation of autonomic function during mindfulness practice.
Evidence that behavioral interventions can reduce prescribed opioid dose in chronic pain patients is limited (Eccleston et al., 2017; Frank et al., 2017). However, a recent systematic review and meta-analysis concluded that mindfulness-based interventions showed particular promise for opioid-treated chronic pain patients (Garland, Brintz, et al., 2020). To date, only six RCTs of behavioral interventions are known to have reported positive opioid-related outcomes in samples of chronic pain patients (Garland et al., 2014b; Garland, Hanley, Kline, et al., 2019; Garland, Hanley, Riquino, et al., 2019; Jamison et al., 2010; Naylor et al., 2010; Wilson et al., 2015). Cognitive-behavioral therapy was tested in three of the six studies, whereas MORE was tested in the remaining three. Jamison et al. (2010) found that an in-person cognitive-behavioral intervention decreased opioid misuse among chronic pain patients. Naylor et al. (2010) found an automated cognitive behavioral therapy maintenance enhancement program delivered via telephone reduced chronic pain patients’ opioid dose at 4-month and 8-month follow-ups. Wilson et al. (2015) found a cognitive behaviorally based pain self-management program delivered via the internet decreased chronic pain patients’ opioid misuse at post-treatment. In two previous, stage 2 RCTs Garland and colleagues found that MORE decreased chronic pain patients’ opioid misuse at post-treatment (Garland et al., 2014b), and at 3-month follow-up (Garland, Hanley, Riquino, et al., 2019). Additionally, a stage 1 RCT found MORE decreased moment-to-moment opioid craving (as indexed by ecological momentary assessment), and increased self-control over craving among individuals with OUD and chronic pain (Garland, Hanley, Kline, et al., 2019). Findings from the present study compliment and extend these previous results, providing the first evidence in the scientific literature that a mindfulness-based intervention can reduce opioid dosing among people prescribed opioid analgesics.
MORE’s ability to improve opioid-related outcomes is likely dependent on improving self-regulation through mindfulness meditation – a practice of cultivating meta-awareness to self-regulate thoughts, emotions, and body sensations (Tang et al., 2015). Self-regulation is held to be integral to restraint of addictive behavior, and evidenced by the exertion of self-control over the impulse to consume the appetitive substance (Baumeister & Vonasch, 2015; Heatherton & Wagner, 2011). Yet, self-regulation failure occurs when resources for self-control are depleted over time (Baumeister et al., 2018). In the present study, we assessed HRV during mindfulness as an indicator of self-regulatory strength. Meta-analyses suggest that HRV is positively associated with self-regulation (Holzman & Bridgett, 2017) and self-control (Zahn et al., 2016). With respect to addictive behavior, HRV has been shown to index self-control strength over appetitive responses to food (Geisler et al., 2016; Segerstrom & Nes, 2007) and alcohol (Garland et al., 2012; Ingjaldsson et al., 2003), and in meta-analyses individuals with substance use disorders evidence reduced resting HRV (Cheng et al., 2019) and attenuated task-elicited HRV (Beauchaine et al., 2019). Specific to problematic opioid use, blunted HRV among opioid misusers is associated with deficits in self-regulation of attention (Garland et al., 2015) and emotion (Garland, Bryan, et al., 2017). Here we show that mindfulness meditation, which has been shown to counteract self-control depletion (Friese et al., 2012), may increase physiological resources for self-regulation. In support of this contention, we observed that participants receiving 8 weeks of mindfulness training through the MORE intervention exhibited increases in HRV during mindfulness meditation from resting baseline levels that mediated the effect of MORE on reducing opioid dose. Plausibly, these findings may reflect MORE participants’ increased capacity to use mindfulness to regulate autonomic reactions to homeostatic perturbations incurred by internal (e.g., pain) and external stimuli (e.g., opioid cues). Of note, because participants in MORE received 8 weeks of mindfulness training whereas those in the SG did not, HRV responses in the SG likely reflected a resting state whereas HRV responses in the MORE group likely indexed the autonomic effects of mindfulness practice. Thus, the current design was able to assess the state effects of mindfulness practice on HRV and their associations with opioid dose escalation, but could not evaluate trait-like changes in HRV produced by the intervention. Plausibly, if chronic pain patients learn to use mindfulness as an endogenous means of effectively self-regulating their internal milieu back to homeostasis, this may reduce the demand for opioids as an exogenous means of self-regulating the body’s internal state.
Mindfulness is a skill, and like other skills, requires practice. This notion suggests that the greater duration of mindfulness practice, the greater self-regulatory strength one may cultivate and therefore be able to exert over opioid use in the face of chronic pain. In the present study, we found that number of minutes of mindful breathing practice were associated with increases in HRV during meditation and opioid dose. In data from another RCT, we observed parallel findings: greater duration of mindfulness practiced during the intervention period predicted a greater extent of emotional response inhibition observed in a laboratory task, that was in turn correlated with reduced pain interference (Garland, Bryan, Priddy, et al., 2019). That said, MORE is a multimodal intervention comprised of mindfulness, reappraisal, and savoring techniques. It is possible that techniques other than mindfulness meditation might account for the observed results. Although neither savoring nor reappraisal practice minutes were associated with HRV during the laboratory-based mindfulness meditation session, the number of minutes of savoring practice was indeed associated with opioid dose by 3-month follow-up. This result is perhaps unsurprising given that MORE uses savoring techniques to increase responsivity to natural rewards, which in prior psychophysiological studies has been associated with reduced opioid craving (Garland et al., 2014) and misuse (Garland, Howard, et al., 2017). Future studies should employ tasks from cognitive and affective neuroscience to parse the independent and interactive contributions of mindfulness, reappraisal, and savoring on HRV and opioid dosing.
The current study has several other limitations. First, the Timeline Followback measure of opioid dose is based on participant recall, and could be prone to errors due to participants forgetting or intentionally misrepresenting their opioid dose. Although there is no gold standard means of quantifying medication adherence (Weiss, 2004), the Timeline Followback has been shown through meta-analysis to validity detect substance use and to evidence agreement rates with biological measures of opioid use as high as 94% (Hjorthoj et al., 2012). A more accurate quantification of opioid use and misuse would require triangulation of self-reports, clinician evaluation, electronic monitoring systems (e.g., MEMS caps), statewide monitoring of prescription data, and urine toxicology screens. Second, due to the demographic characteristics of the study location, the sample recruited for this study was rather homogenous with respect to ethnicity and race. Future studies should increase participant racial and ethnic heterogeneity to obtain greater generalizability of study results. Third, as a Stage 2 RCT, the study sample size was relatively small, thus reducing statistical power. A future Stage 3 RCT with three to four times the sample size would improve our ability to test effects of MORE on HRV and opioid dosing outcomes. Lastly, we followed a reasonable conjecture that the mindfulness component of MORE was causally responsible for generating the observed effect of increased HRV during meditation at post-treatment, but it still remains to be seen exactly which components of MORE may have causally contributed to the observed effects on HRV and opioid dose, as MORE is an integrated bio-behavioral intervention program that unites three therapeutic components: mindfulness, reappraisal, and savoring. We were unable to examine the differential effects of each of these practices because high multicollinearity between practice duration measures precluded multiple regression analysis. Also, when the present study was conducted, we did not have a decisive means to decipher presumably complex chains of causes and effects that synergistically emerge in the actual delivery of MORE to pain patients who can greatly benefit from it in many ways. A future study employing ecological momentary assessment (EMA) may address this type of question relevant to intervention-induced dynamics of change.
In summary, we show that MORE, a mindfulness-based intervention for chronic pain patients on long-term opioid therapy, can reduce opioid dosing by 3-month follow-up. Increases in HRV during mindfulness meditation were found to mediate the effect of treatment on dose reduction, with larger HRV increases associated with greater dose reductions. Plausibly, the mindfulness meditation component of MORE provides a means of cultivating self-regulatory strength, as reflected by autonomic function, which might in turn be useful for exerting self-control over opioid dosing. In light of a recent systematic review of the efficacy of mind-body therapies for opioid-treated pain (Garland, Brintz, et al., 2019), this study represents the first RCT to demonstrate the effects of a mindfulness-based intervention on quantitative reductions in opioid dose. Effects of MORE on opioid dose, coupled with other clinical outcomes such as reduced pain, craving, and misuse demonstrated in previous studies (Garland et al., 2014b; Garland, Hanley, Kline, et al., 2019; Garland, Hanley, Riquino, et al., 2019), indicate robust empirical support for MORE as an intervention to combat the current opioid epidemic. Lastly, the present study provides intriguing implications for the question of how to best optimize the approach to opioid dose reduction as the goal of the intervention. Although no motivational enhancement therapy or explicit instructions for dose reduction were integrated into the intervention, participants in MORE, relative to those in SG, acquired skills to boost self-regulatory capacity and thereby self-initiated opioid dose reduction. Thus, MORE might be useful as part of a comprehensive opioid sparing or tapering strategy for patient-centered reduction of long-term opioid analgesic therapy (Dowell et al., 2016). Future treatment development research should address what optimized intervention factors and therapeutic requirements (e.g., motivational interviewing, see Rollnick & Miller, 1995) will be ultimately responsible for facilitating opioid dose reduction for patients undergoing an integrated treatment program such as MORE. This is an empirically tractable problem that will be pursued in our future research program.
Public significance:
Mindfulness-Oriented Recovery Enhancement reduced opioid dose among people with chronic pain by increasing heart rate variability – a marker of self-control strength.
Acknowledgements:
This work was supported by a grant from the Fahs Beck Fund for Research and Experimentation (PI: Garland) and a gift from Stephanie Loker Harpst. E.L.G. was also supported by R01DA042033 (PI: Garland) from the National Institute on Drug Abuse, and R61AT009296 (PI: Garland) from the National Center for Complementary and Integrative Health during the preparation of this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Biographies




Footnotes
Disclosures: Dr. Garland has received honoraria and payment for teaching engagements (related to training clinicians in MORE and mindfulness) sponsored by institutions of higher education, government agencies, and medical center.
References
- Adler-Neal AL, Waugh CE, Garland EL, Shaltout HA, Diz DI, & Zeidan F (2019). The role of heart rate variability in mindfulness-based pain relief. The Journal of Pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Sinha R, Lampert R, Weinberger AH, Anderson GM, Lavery ME, Yanagisawa K, & McKee SA (2012). Blunted vagal reactivity predicts stress-precipitated tobacco smoking. Psychopharmacology, 220(2), 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AK, & Garland EL (2019). Autonomic and affective mediators of the relationship between mindfulness and opioid craving among chronic pain patients. Experimental and Clinical Psychopharmacology, 27(1), 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldacchino A, Balfour DJK, Passetti F, Humphris G, & Matthews K (2012). Neuropsychological consequences of chronic opioid use: A quantitative review and meta-analysis. Neuroscience & Biobehavioral Reviews, 36(9), 2056–2068. [DOI] [PubMed] [Google Scholar]
- Baron RM, & Kenny DA (1986). The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology, 51, 1173–1182. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Tice DM, & Vohs KD (2018). The strength model of self-regulation: Conclusions from the second decade of willpower research. Perspectives on Psychological Science, 13(2), 141–145. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, & Vonasch AJ (2015). Uses of self-regulation to facilitate and restrain addictive behavior. Addictive Behaviors, 44, 3–8. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Bell Z, Knapton E, McDonough-Caplan H, Shader T, & Zisner A (2019). Respiratory sinus arrhythmia reactivity across empirically based structural dimensions of psychopathology: A meta-analysis. Psychophysiology, 56(5), e13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Quigley KS, & Lozano D (2007). Cardiovascular psychophysiology In Handbook of psychophysiology (3rd ed., Vol. 3, pp. 182–210). Cambridge University Press. [Google Scholar]
- Boom M, Niesters M, Sarton E, Aarts L, W Smith T, & Dahan A (2012). Non-analgesic effects of opioids: Opioid-induced respiratory depression. Current Pharmaceutical Design, 18(37), 5994–6004. [DOI] [PubMed] [Google Scholar]
- Cheng Y-C, Huang Y-C, & Huang W-L (2019). Heart rate variability as a potential biomarker for alcohol use disorders: A systematic review and meta-analysis. Drug and Alcohol Dependence, 204, 107502. [DOI] [PubMed] [Google Scholar]
- Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, Dana T, Bougatsos C, & Deyo RA (2015). The Effectiveness and Risks of Long-Term Opioid Therapy for Chronic Pain: A Systematic Review for a National Institutes of Health Pathways to Prevention Workshop. Annals of Internal Medicine, 162(4), 276. [DOI] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, & Chou R (2016). CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA, 315(15), 1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston C, Fisher E, Thomas KH, Hearn L, Derry S, Stannard C, Knaggs R, & Moore RA (2017). Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. Cochrane Database of Systematic Reviews, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank JW, Lovejoy TI, Becker WC, Morasco BJ, Koenig CJ, Hoffecker L, Dischinger HR, Dobscha SK, & Krebs EE (2017). Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: A systematic review. Annals of Internal Medicine, 167(3), 181–191. [DOI] [PubMed] [Google Scholar]
- Fredheim OMS, Borchgrevink PC, Mahic M, & Skurtveit S (2013). A pharmacoepidemiological cohort study of subjects starting strong opioids for nonmalignant pain: A study from the Norwegian Prescription Database. PAIN®, 154(11), 2487–2493. [DOI] [PubMed] [Google Scholar]
- Friese M, Messner C, & Schaffner Y (2012). Mindfulness meditation counteracts self-control depletion. Consciousness and Cognition, 21(2), 1016–1022. [DOI] [PubMed] [Google Scholar]
- Froeliger B, Mathew AR, McConnell PA, Eichberg C, Saladin ME, Carpenter MJ, & Garland EL (2017). Restructuring reward mechanisms in nicotine addiction: A pilot fMRI study of Mindfulness-Oriented Recovery Enhancement for cigarette smokers. Evidence-Based Complementary and Alternative Medicine, 2017, e7018014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL (2013). Mindfulness-oriented recovery enhancement for addiction, stress, and pain. NASW Press, National Association of Social Workers. [Google Scholar]
- Garland EL, Atchley RM, Hanley AW, Zubieta J-K, & Froeliger B (2019). Mindfulness-Oriented Recovery Enhancement remediates hedonic dysregulation in opioid users: Neural and affective evidence of target engagement. Science Advances, 5(10), eaax1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Baker AK, & Howard MO (2017). Mindfulness-oriented recovery enhancement reduces opioid attentional bias among prescription opioid-treated chronic pain patients. Journal of the Society for Social Work and Research, 8(4), 493–509. [Google Scholar]
- Garland EL, Brintz CE, Hanley AW, Roseen EJ, Atchley RM, Gaylord SA, Faurot KR, Yaffe J, Fiander M, & Keefe FJ (2020). Mind-body therapies for opioid-treated pain: A systematic review and meta-analysis. JAMA Internal Medicine, 180(1), 91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Bryan CJ, Nakamura Y, Froeliger B, & Howard MO (2017). Deficits in autonomic indices of emotion regulation and reward processing associated with prescription opioid use and misuse. Psychopharmacology, 234(4), 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Bryan MA, Priddy SE, Riquino MR, Froeliger B, & Howard MO (2019). Effects of Mindfulness-Oriented Recovery Enhancement versus social support on negative affective interference during inhibitory control among opioid-treated chronic pain patients: A pilot mechanistic study. Annals of Behavioral Medicine, 53(10), 865–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Carter K, Ropes K, & Howard MO (2012). Thought suppression, impaired regulation of urges, and Addiction-Stroop predict affect-modulated cue-reactivity among alcohol dependent adults. Biological Psychology, 89(1), 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, & Howard MO (2014). Effects of Mindfulness-Oriented Recovery Enhancement on reward responsiveness and opioid cue-reactivity. Psychopharmacology, 231(16), 3229–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, & Howard MO (2015). Allostatic dysregulation of natural reward processing in prescription opioid misuse: Autonomic and attentional evidence. Biological Psychology, 105, 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, Zeidan F, Partin K, & Howard MO (2013). The downward spiral of chronic pain, prescription opioid misuse, and addiction: Cognitive, affective, and neuropsychopharmacologic pathways. Neuroscience & Biobehavioral Reviews, 37(10), 2597–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Gaylord SA, Boettiger CA, & Howard MO (2010). Mindfulness training modifies cognitive, affective, and physiological mechanisms implicated in alcohol dependence: Results of a randomized controlled pilot trial. Journal of Psychoactive Drugs, 42(2), 177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Hanley AW, Kline A, & Cooperman NA (2019). Mindfulness-Oriented Recovery Enhancement reduces opioid craving among individuals with opioid use disorder and chronic pain in medication assisted treatment: Ecological momentary assessments from a stage 1 randomized controlled trial. Drug and Alcohol Dependence, 203, 61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Hanley AW, Riquino MR, Reese SE, Baker AK, Salas K, Yack BP, Bedford CE, Bryan MA, Atchley R, Nakamura Y, Froeliger B, & Howard MO (2019). Mindfulness-oriented recovery enhancement reduces opioid misuse risk via analgesic and positive psychological mechanisms: A randomized controlled trial. Journal of Consulting and Clinical Psychology, 87(10), 927–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, & Howard MO (2013). Mindfulness-oriented recovery enhancement reduces pain attentional bias in chronic pain patients. Psychotherapy and Psychosomatics, 82(5), 311–318. [DOI] [PubMed] [Google Scholar]
- Garland EL, Howard MO, Zubieta J-K, & Froeliger B (2017). Restructuring hedonic dysregulation in chronic pain and prescription opioid misuse: Effects of mindfulness-oriented recovery enhancement on responsiveness to drug cues and natural rewards. Psychotherapy and Psychosomatics, 86(2), 111–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Manusov EG, Froeliger B, Kelly A, Williams JM, & Howard MO (2014). Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: Results from an early-stage randomized controlled trial. Journal of Consulting and Clinical Psychology, 82(3), 448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaylord SA, Palsson OS, Garland EL, Faurot KR, Coble RS, Mann JD, Frey W, Leniek K, & Whitehead WE (2011). Mindfulness training reduces the severity of irritable bowel syndrome in women: Results of a randomized controlled trial. The American Journal of Gastroenterology, 106(9), 1678–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler FCM, Kleinfeldt A, & Kubiak T (2016). Restrained eating predicts effortful self-control as indicated by heart rate variability during food exposure. Appetite, 96, 502–508. [DOI] [PubMed] [Google Scholar]
- Han B, Compton WM, Blanco C, Crane E, Lee J, & Jones CM (2017). Prescription Opioid Use, Misuse, and Use Disorders in U.S. Adults: 2015 National Survey on Drug Use and Health. Annals of Internal Medicine, 167(5), 293. [DOI] [PubMed] [Google Scholar]
- Hanley AW, & Garland EL (2019). Mapping the affective dimension of embodiment with the Sensation Manikin: Validation among chronic pain patients and modification by Mindfulness-Oriented Recovery Enhancement. Psychosomatic Medicine, 81(7), 612. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, & Wagner DD (2011). Cognitive neuroscience of self-regulation failure. Trends in Cognitive Sciences, 15(3), 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry SG, Wilsey BL, Melnikow J, & Iosif A-M (2015). Dose escalation during the first year of long-term opioid therapy for chronic pain. Pain Medicine, 16(4), 733–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill LK, Siebenbrock A, Sollers JJ, & Thayer JF (2009). Are all measures created equal? Heart rate variability and respiration. Biomedical Sciences Instrumentation, 45, 71–76. [PubMed] [Google Scholar]
- Hjorthøj CR, Hjorthøj AR, & Nordentoft M (2012). Validity of Timeline Follow-Back for self-reported use of cannabis and other illicit substances—Systematic review and meta-analysis. Addictive Behaviors, 37(3), 225–233. [DOI] [PubMed] [Google Scholar]
- Holzman JB, & Bridgett DJ (2017). Heart rate variability indices as bio-markers of top-down self-regulatory mechanisms: A meta-analytic review. Neuroscience & Biobehavioral Reviews, 74, 233–255. [DOI] [PubMed] [Google Scholar]
- Ingjaldsson JT, Laberg JC, & Thayer JF (2003). Reduced heart rate variability in chronic alcohol abuse: Relationship with negative mood, chronic thought suppression, and compulsive drinking. Biological Psychiatry, 54, 1427–1436. [DOI] [PubMed] [Google Scholar]
- Jamison RN, Ross EL, Michna E, Chen LQ, Holcomb C, & Wasan AD (2010). Substance misuse treatment for high-risk chronic pain patients on opioid therapy: A randomized trial. PAIN, 150(3), 390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarzyna D, Jungquist CR, Pasero C, Willens JS, Nisbet A, Oakes L, Dempsey SJ, Santangelo D, & Polomano RC (2011). American Society for Pain Management Nursing guidelines on monitoring for opioid-induced sedation and respiratory depression. Pain Management Nursing, 12(3), 118–145. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Allen B, Gianaros PJ, Thayer JF, & Manuck SB (2015). Focusing neurovisceral integration: Cognition, heart rate variability, and cerebral blood flow. Psychophysiology, 52(2), 214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, & Volkow ND (2016). Neurobiology of addiction: A neurocircuitry analysis. The Lancet Psychiatry, 3(8), 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krygier JR, Heathers JA, Shahrestani S, Abbott M, Gross JJ, & Kemp AH (2013). Mindfulness meditation, well-being, and heart rate variability: A preliminary investigation into the impact of intensive Vipassana meditation. International Journal of Psychophysiology, 89(3), 305–313. [DOI] [PubMed] [Google Scholar]
- Laborde S, Mosley E, & Thayer JF (2017). Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research — Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Frontiers in Psychology, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RJA (1988). A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association, 1198–1202. [Google Scholar]
- Morasco BJ, Smith N, Dobscha SK, Deyo RA, Hyde S, & Yarborough BJH (2020). Outcomes of prescription opioid dose escalation for chronic pain: Results from a prospective cohort study. Pain. [DOI] [PubMed] [Google Scholar]
- Mundkur ML, Rough K, Huybrechts KF, Levin R, Gagne JJ, Desai RJ, Patorno E, Choudhry NK, & Bateman BT (2018). Patterns of opioid initiation at first visits for pain in United States primary care settings. Pharmacoepidemiology and Drug Safety, 27(5), 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mystakidou K, Parpa E, Tsilika E, Mavromati A, Smyrniotis V, Georgaki S, & Vlahos L (2003). Long-term management of noncancer pain with transdermal therapeutic system-fentanyl. The Journal of Pain, 4(6), 298–306. [DOI] [PubMed] [Google Scholar]
- Naylor MR, Naud S, Keefe FJ, & Helzer JE (2010). Therapeutic Interactive Voice Response (TIVR) to reduce analgesic medication use for chronic pain management. The Journal of Pain, 11(12), 1410–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostafin BD, Robinson MD, & Meier BP (2015). Handbook of mindfulness and self-regulation. Springer. [Google Scholar]
- Paice JA, Penn RD, & Shott S (1996). Intraspinal morphine for chronic pain: A retrospective, multicenter study. Journal of Pain & Symptom Management, 11(2), 71–80. [DOI] [PubMed] [Google Scholar]
- Penttilä J, Helminen A, Jartti T, Kuusela T, Huikuri HV, Tulppo MP, Coffeng R, & Scheinin H (2001). Time domain, geometrical and frequency domain analysis of cardiac vagal outflow: Effects of various respiratory patterns. Clinical Physiology, 21(3), 365–376. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40(3), 879–891. [DOI] [PubMed] [Google Scholar]
- Quintana DS, Guastella AJ, McGregor IS, Hickie IB, & Kemp AH (2013). Heart rate variability predicts alcohol craving in alcohol dependent outpatients: Further evidence for HRV as a psychophysiological marker of self-regulation. Drug and Alcohol Dependence, 132(1), 395–398. [DOI] [PubMed] [Google Scholar]
- Rawson R, & McCann MJ (2006). Counselor’s Treatment Manual: Matrix Intensive Outpatient Treatment for People With Stimulant Use Disorders. DHHS. [Google Scholar]
- Rollnick S, & Miller WR (1995). What is motivational interviewing? Behavioural and Cognitive Psychotherapy, 23(4), 325–334. [DOI] [PubMed] [Google Scholar]
- Schwabe L, & Wolf OT (2009). Stress Prompts Habit Behavior in Humans. The Journal of Neuroscience, 29(22), 7191–7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC, & Nes LS (2007). Heart rate variability reflects self-regulatory strength, effort, and fatigue. Psychological Science, 18, 275–281. [DOI] [PubMed] [Google Scholar]
- Shaffer F, & Ginsberg JP (2017). An Overview of Heart Rate Variability Metrics and Norms. Frontiers in Public Health, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, & Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry, 59, 22–33. [PubMed] [Google Scholar]
- Shurman J, Koob GF, & Gutstein HB (2010). Opioids, pain, the brain, and hyperkatifeia: A framework for the rational use of opioids for pain. Pain Medicine, 11(7), 1092–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TW, Deits-Lebehn C, Williams PG, Baucom BR, & Uchino BN (2020). Toward a social psychophysiology of vagally mediated heart rate variability: Concepts and methods in self-regulation, emotion, and interpersonal processes. Social and Personality Psychology Compass, e12516. [Google Scholar]
- Sobell LC, & Sobell MB (1992). Timeline follow-back In Measuring alcohol consumption (pp. 41–72). Springer. [Google Scholar]
- Takahashi T, Murata T, Hamada T, Omori M, Kosaka H, Kikuchi M, Yoshida H, & Wada Y (2005). Changes in EEG and autonomic nervous activity during meditation and their association with personality traits. International Journal of Psychophysiology, 55(2), 199–207. [DOI] [PubMed] [Google Scholar]
- Tang Yi-Yuan, Hölzel BK, & Posner MI (2015). The neuroscience of mindfulness meditation. Nature Reviews Neuroscience, 16(4), 213–225. [DOI] [PubMed] [Google Scholar]
- Tang Y-Y, Ma Y, Fan Y, Feng H, Wang J, Feng S, Lu Q, Hu B, Lin Y, Li J, Zhang Y, Wang Y, Zhou L, & Fan M (2009). Central and autonomic nervous system interaction is altered by short-term meditation. Proceedings of the National Academy of Sciences, 106(22), 8865–8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology the North American Society of Pacing and Electrophysiology. (1996). Heart rate variability: Standards of measurement, physiological Interpretation, and clinical use. Circulation, 93, 1043–1065. [PubMed] [Google Scholar]
- Teasdale JD, Segal ZV, Williams JMG, Ridgeway VA, Soulsby JM, & Lau MA (2000). Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. Journal of Consulting and Clinical Psychology, 68(4, 615. [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2009). Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience and Biobehavioral Reviews, 33, 81–88. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers JJ III, & Wager TD (2012). A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews, 36(2), 747–756. [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61, 201–216. [DOI] [PubMed] [Google Scholar]
- Vago DR, & Silbersweig DA (2012). Self-awareness, self-regulation, and self-transcendence (S-ART): A framework for understanding the neurobiological mechanisms of mindfulness. Frontiers in Human Neuroscience, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu M, & Shen Z (2019). A virtual reality counterconditioning procedure to reduce methamphetamine cue-induced craving. Journal of Psychiatric Research, 116, 88–94. [DOI] [PubMed] [Google Scholar]
- Weiss RD (2004). Adherence to pharmacotherapy in patients with alcohol and opioid dependence. Addiction, 99(11), 1382–1392. [DOI] [PubMed] [Google Scholar]
- Wielgosz J, Schuyler BS, Lutz A, & Davidson RJ (2016). Long-term mindfulness training is associated with reliable differences in resting respiration rate. Scientific Reports, 6(1), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M, Roll JM, Corbett C, & Barbosa-Leiker C (2015). Empowering patients with persistent pain using an internet-based self-management program. Pain Management Nursing, 16(4), 503–514. [DOI] [PubMed] [Google Scholar]
- Zahn D, Adams J, Krohn J, Wenzel M, Mann CG, Gomille LK, Jacobi-Scherbening V, & Kubiak T (2016). Heart rate variability and self-control—A meta-analysis. Biological Psychology, 115, 9–26. [DOI] [PubMed] [Google Scholar]
- Zenz M, Strumpf M, & Tryba M (1992). Long-term oral opioid therapy in patients with chronic nonmalignant pain. Journal of Pain and Symptom Management, 7(2), 69–77. [DOI] [PubMed] [Google Scholar]


