Abstract
The SARS-CoV-2 can lead to severe illness with COVID-19. Outcomes of patients requiring mechanical ventilation are poor. Awake proning in COVID-19 improves oxygenation, but on data clinical outcomes is limited. This single-centre retrospective study aimed to assess whether successful awake proning of patients with COVID-19, requiring respiratory support (continuous positive airways pressure (CPAP) or high-flow nasal oxygen (HFNO)) on a respiratory high-dependency unit (HDU), is associated with improved outcomes. HDU care included awake proning by respiratory physiotherapists. Of 565 patients admitted with COVID-19, 71 (12.6%) were managed on the respiratory HDU, with 48 of these (67.6%) requiring respiratory support. Patients managed with CPAP alone 22/48 (45.8%) were significantly less likely to die than patients who required transfer onto HFNO 26/48 (54.2%): CPAP mortality 36.4%; HFNO mortality 69.2%, (p=0.023); however, multivariate analysis demonstrated that increasing age and the inability to awake prone were the only independent predictors of COVID-19 mortality. The mortality of patients with COVID-19 requiring respiratory support is considerable. Data from our cohort managed on HDU show that CPAP and awake proning are possible in a selected population of COVID-19, and may be useful. Further prospective studies are required.
Keywords: respiratory infection, non invasive ventilation, viral infection
Background
COVID-19 is an acute respiratory syndrome caused by the SARS-CoV-2, which was first described in the Wuhan region of China and has since become the most serious global health crisis in a century.1
Previous studies across the world have reported characteristics of patients presenting with COVID-19 with high rates of mortality and admission to intensive care units (ICU).2 3 Worldwide, the mortality rate of patients treated with invasive ventilation is high.4 5 Use of non-invasive respiratory support, such as continuous positive airways pressure (CPAP), was recommended to reduce the need for invasive ventilation and improve outcomes in lung injury associated with COVID-19. In addition, in view of the benefits of proning in the setting of invasive ventilation, ‘awake proning’ was suggested as an adjunct to respiratory support in patients with COVID-19. Both CPAP and proning may be beneficial for patients in two main ways: first by recruiting poorly ventilated lung units, and second by distributing pulmonary blood flow more evenly, thereby improving ventilation-perfusion matching.6–8 A number of case series studies have suggested improved short-term oxygenation with awake proning in patients with COVID-19,9–13 including in the emergency department.14 15 Small retrospective case series with 2 and 10 patients, respectively, have suggested that awake proning may reduce intubation and improve survival,16 17 although these data have significant limitations.
In order to deliver enhanced respiratory care outside of the intensive care setting during the COVID-19 pandemic, a dedicated respiratory high-dependency unit (HDU), led by respiratory physicians, and supported by specialist respiratory nursing and physiotherapy staff, was established in the John Radcliffe Hospital, Oxford University Hospitals NHS Foundation Trust (OUHNFT) to manage patients requiring or expected to require respiratory support beyond that deliverable on general medical wards (ie, more than supplemental oxygen), but not requiring or not suitable for admission to ICU for invasive ventilation. Respiratory support was defined as CPAP, high-flow nasal oxygen (HFNO), or bilevel non-invasive ventilation (NIV).
Here, we describe our experience and outcomes for the first 48 patients admitted to this respiratory HDU requiring additional respiratory support as described above.
Methods
Criteria for admission to the respiratory HDU
Patients were transferred to HDU if there was an increasing oxygen requirement, or an absolute oxygen requirement of: either FiO2≥40% or ≥8 L/min via mask face. Referrals were made from medical wards or directly from the emergency department and all transfers were approved by a respiratory consultant. Patients were not transferred to HDU if they were rapidly deteriorating and required immediate ICU admission, or if they were deemed to be for ward-based care. Decisions about ceilings of care and escalation to the HDU were made within an agreed ethical framework, and on the basis of clinical need and suitability for escalation. There was no resource limitation.
Respiratory support
Respiratory support was given in a protocolised fashion (see online supplemental figure S1), and CPAP was initiated (initial positive pressure support of 6–8 cmH20) with supplemental oxygen entrained, aiming for target oxygen saturations (SaO2) of 92%–96% (or 88%–92% in those with evidence of chronic type 2 respiratory failure). Specialist respiratory physiotherapists provided input to medical ward rounds and, separately, reviewed patients twice daily and attempted awake proning, in which patients were encouraged to lie in a prone or semiprone position as tolerated for periods of ≥2 hours at least twice daily (in line with recent guidance18 19). ‘Successful’ proning was defined as at least 2 hours in the prone position, twice a day for two consecutive days. Awake proning in the HDU was set up and began on the 6 April 2020, 2 weeks after the first cases in our institution, and in response to anecdotal reports of success. Oxygen requirement prior to commencement of respiratory support was defined as moderate (FiO2 ≤60%) or high (FiO2 >60%).
bmjresp-2020-000678supp001.pdf (1.3MB, pdf)
Data analysis
Summary statistics were used to define the population. Logistic regression analysis was used to compare risk of death between types of respiratory support adjusting for age and clinical frailty score. χ2 tests were used to make proportion comparisons between groups (with Yates correction used where one cell had an expected count <5). Multivariate logistic regression was used to estimate the OR for death with CPAP therapy, proning and starting respiratory therapy when on moderate oxygen therapy with the potential confounders of age and clinical frailty score (dichotomised into scores of 1–4 and 5–9).20 Due to the low numbers of observations in some cell counts, Firth bias-reduced logistic regression was used via R software (R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/) logistic package.21
Patient and public involvement
Given the severe nature of the disease, the rapidity of the set-up of the respiratory HDU, and the high mortality rate, patient and public involvement was not deemed suitable for the design, conduct and reporting of this retrospective study.
Results
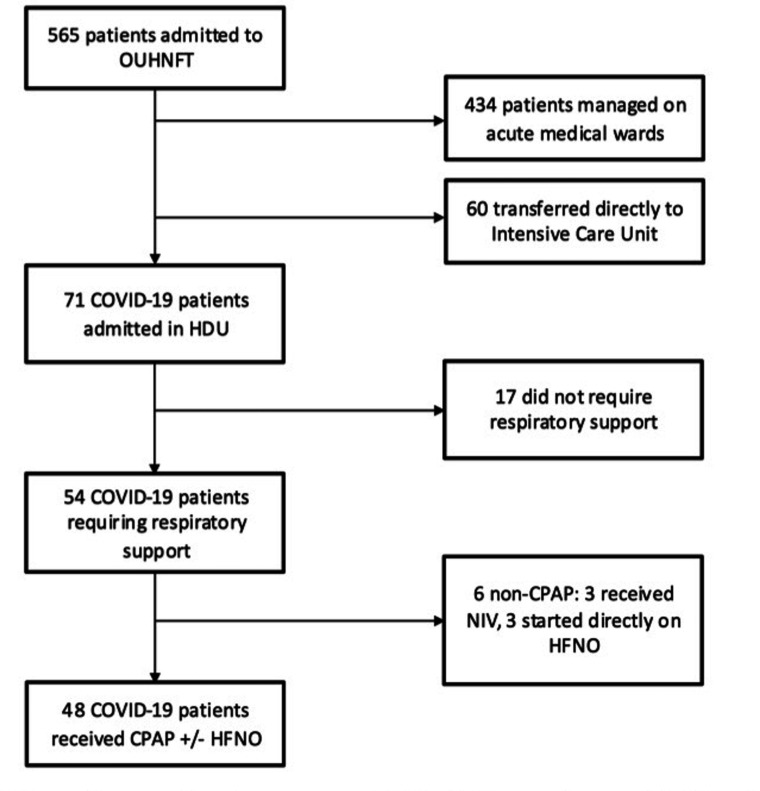
Between 21 March and 2 May 2020, 565 patients were admitted (ie, stayed at least one night in hospital) to the OUHNFT with confirmed (SARS-CoV-2 PCR positive) or a high clinical/radiological suspicion of COVID-19. Of all 565 patients, 131 (23.2%) were admitted to either ICU or HDU, with 71 (12.6%) managed on the respiratory HDU and 60 (10.6%) transferred directly to ICU. Fifty-four patients (9.6% of total COVID-19 admissions and 76.1% of HDU COVID-19 admissions) required non-invasive respiratory support on HDU (CPAP and/or HNFO; figure 1).
Figure 1.
Flow chart of COVID-19 admitted to Oxford University Hospitals NHS Foundation Trust (OUHNFT. CPAP, continuous positive airways pressure; HDU, high-dependency unit; HFNO, high-flow nasal oxygen; ICU, transferred to intensive care unit for intubation and ventilation; NIV, (bilevel) non-invasive ventilation.
Forty-eight (88.9%) of the 54 patients on HDU were managed with CPAP initially as per protocol (. The other six included three (5.6%) managed directly with HFNO for severe hypoxia (SaO2 <84% on 15 L/min non-rebreathe mask) and three (5.6%) patients initially managed with NIV for hypercapnic respiratory failure (pCO2 mean=7.3 kPa). These six patients were excluded from subsequent analysis.
Baseline characteristics
Baseline demographics and baseline characteristics for the 48 patients are presented in table 1. Median age was 69 years (IQR, 54–80) and 36 (66.7%) were men. The majority (83.6%) of the patients were white, with the remainder being of Asian (9.1%) or Black (7.3%) ethnicity. The most common comorbidities were hypertension (47.9%), diabetes (35.4%), chronic lung disease (31.3%), chronic kidney disease (18.8%) and cardiovascular disease (16.7%). The median (IQR) number of comorbidities per patient was 2 (1-3) and the median (IQR) Clinical Frailty Score22 was 3 (3–4).
Table 1.
Demographics of 48 patients requiring respiratory support on respiratory high-dependency unit managed by continuous positive airways pressure (CPAP) initially
| CPAP only | CPAP to HFNO | Overall | |
| Number of patients | 22 (45.8%) | 26 (54.2%) | 48 (100%) |
| Age (years; median, IQR) | 63 (54–77) | 73 (57–83) | 69 (54–80) |
| Male | 17 (77.3%) | 15 (57.7%) | 32 (66.7%) |
| Female | 5 (22.7%) | 11 (42.3%) | 16 (33.3%) |
| BMI (kg/m2) (median, IQR) |
29.9 (25.5–33.3) | 28.3 (26.2–31.8) | 29.5 (25.6–33.4) |
| Comorbidities | |||
| Hypertension | 11 (50%) | 12 (46.2%) | 23 (47.9%) |
| Diabetes | 10 (45.5%) | 7 (26.9%) | 17 (35.4%) |
| Chronic lung disease: | 10 (45.5%) | 5 (19.2%) | 15 (31.3%) |
| - Asthma | 3 (13.6%) | 3 (11.5%) | 6 (12.5%) |
| - COPD | 5 (22.7%) | 1 (3.8%) | 6 (12.5%) |
| - ILD | 2 (9.1%) | 1 (3.8%) | 3 (6.3%) |
| CKD | 3 (13.6%) | 6 (23.1%) | 9 (18.8%) |
| Cardiovascular | 4 (18.2%) | 4 (15.4%) | 8 (16.7%) |
| Anticoagulated | 5 (22.7%) | 2 (7.7%) | 7 (14.6%) |
| Immunosuppressed | 0 (0%) | 6 (23.1%) | 6 (12.5%) |
| Stroke | 2 (9.1%) | 2 (7.7%) | 4 (8.3%) |
| Autoimmune | 0 (0%) | 2 (7.7%) | 2 (4.2%) |
| Other | 4 (18.2%) | 1 (3.8%) | 5 (10.4%) |
| Number of comorbidities median (IQR) | 2.5 (1.25–4) | 2 (1–3) | 2 (1–3) |
| Clinical Frailty Score median (IQR) | 3 (2–3) | 4 (3–5) | 3 (3–4) |
| Laboratory findings Median (IQR) |
|||
| Lymphocytes (109/L)* | 0.7 (0.5–1.1) | 0.7 (0.5–0.9) | 0.7 (0.5–0.9) |
| CRP (mg/L) | 163.0 (101.3–195.5) |
182.5 (142.3–206.5) |
172.5 (127.8–199.0) |
| D dimer (µg/L) | 1272.0 (818.3–2132.8) | 1377.5 (805.3–2808.0) | 1291.5 (793.3–2220.3) |
Data presented as number (%) unless otherwise stated.
*Excluding one patient with chronic lymphocytic leukaemia.
BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; HFNO, high-flow nasal oxygen; ILD, interstitial lung disease.
The median (IQR) duration of symptoms prior to hospitalisation was 6 (3–9) days and patients were transferred to HDU a median of 1 (0–3) day after admission to hospital. Immediately prior to commencement of respiratory support on HDU, 22/48 (45.8%) had moderate and 26/48 (54.2%) had high oxygen requirements, as defined above.
Management
Of the 48 patients initially managed with CPAP, 26 (54.2%) required transfer of respiratory support to HFNO. The most common indication was poor tolerance of CPAP (21/26, 80.8%), followed by persistent hypoxia (5/26, 19.2%).
Awake proning was attempted in 30/48 (62.5%) patients. Proning was not attempted in 18 patients: 7 (38.9%) were deemed too unstable or were being managed as end-of-life care, 5 (27.7%) were mobilising independently and able to sit out of bed and therefore deemed too well to benefit, 3 (16.7%) were admitted prior to commencement of routine proning, and 2 (11.1%) were unable to tolerate prone position. Successful (full) proning was achieved in 11/30 (36.7%), and semiproning in 17 (56.7%) patients. Two (6.7%) patients declined proning after initial attempt. Patients managed on CPAP alone were more likely to successfully prone (9/17, 52.9%), than those needing HFNO (2/13, 15.4%) although this did not reach statistical significance (χ2 1 df=3.0 (Yates), p=0.083).
Outcomes
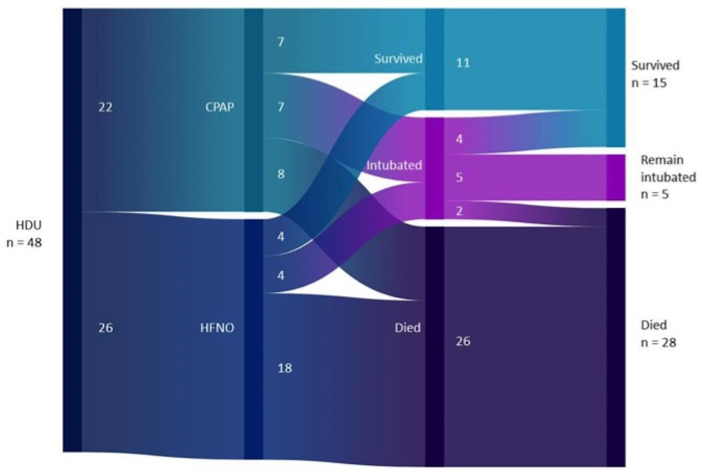
Outcomes for all patients with COVID-19 on HDU are presented in table 2. Of the 48 patients managed on CPAP initially, 11 (22.9%) were successfully managed with respiratory support in HDU alone and were discharged home, 26 (54.2%) patients died with HDU as their planned ceiling of care and 11 (22.9%) required ICU admission for intubation and invasive ventilation (figure 2).
Table 2.
Outcomes of 48 patients requiring respiratory support on respiratory high-dependency unit managed by continuous positive airways pressure (CPAP) initially
| CPAP only (n=22) | CPAP to HFNO (n=26) | Overall (n=48) | |
| Outcome | |||
| Discharged | 7 (31.8%) | 4 (15.4%) | 11 (22.9%) |
| ICU | 7 (31.8%) | 4 (15.4%) | 11 (22.9%) |
| Died | 8 (36.4%) | 18 (69.2%) | 26 (54.2%) |
HFNO, high flow nasal oxygen; ICU, transferred to intensive care unit for intubation and ventilation.
Figure 2.
Sankey plot of 48 patients requiring continuous positive airways pressure (CPAP): patient management and outcomes. HDU, high-dependency unit; HFNO, high-flow nasal oxygen.
Patients managed on CPAP alone were more likely to be discharged home (7/22, 31.8%) than those who required transfer onto HFNO (4/26, 15.4%), although this did not reach statistical significance (χ2 1 df=1.8, p=0.177). Patients managed with CPAP alone were significantly less likely to die than patients who required transfer onto HFNO (CPAP mortality 8/22, 36.4%; ‘CPAP to HFNO’ mortality 18/26, 69.2%; χ2 1 df=5.2, p=0.023).
Achievement of full proning was associated with lower mortality than failed or semiproning in the HDU setting (full proning mortality 0/11, 0.0%; non-full proning mortality 12/19, 63.2%; χ2 1 df=9.1 (Yates), p=0.003). Successful proning was significantly associated with reduced odds of death (OR 0.06) but with a wide CI (95% CI 0.01 to 0.55).
Given the statistically significant associations between CPAP, successful proning and mortality, we used multivariate logistic regression to evaluate independent predictors of COVID-19 mortality adjusting for age, clinical frailty score and FiO2 requirement at the start of respiratory therapy. The ORs (table 3) for death by age (OR 1.08 95% CI 1.02 to 1.15, p=0.007) and ability to fully prone (OR 0.06, 95% CI 0.00 to 0.80, p=0.031) were independently significant.
Table 3.
OR for mortality
| OR | 95% CI | χ2 | P value | |
| Age | 1.08 | 1.02 to 1.15 | 7.22 | 0.007* |
| Clinical frailty score | ||||
| CFS 1–4 | Ref | 0.617 | ||
| CFS 5–9 | 1.56 | 0.27 to 11.09 | 0.25 | |
| Oxygen therapy prior to respiratory support | ||||
| Moderate | Ref | 0.088 | ||
| High | 4.18 | 0.81 to 29.70 | 2.91 | |
| Full proning | ||||
| Unsuccessful | Ref | 0.031* | ||
| Successful | 0.06 | 0.00 to 0.80 | 4.68 | |
| CPAP | ||||
| Required transfer onto HFNO | Ref | 0.761 | ||
| Required CPAP only | 0.76 | 0.13 to 4.88 | 0.09 |
Multivariate logistic regression (Firth bias reduced).
*p<0.05
CFS, clinical frailty score; CPAP, continuous positive airway pressure; HFNO, high flow nasal oxygen.
Of the 11 patients admitted to ICU for intubation and ventilation: 6 (54.5%) were successfully extubated and eventually discharged home, 3 (27.3%) died in ICU and 2 (18.2%) remained intubated (as of 19 May 2020).
Discussion
These data represent the first description of patients treated for COVID-19 in the UK with non-invasive respiratory support in an HDU, and the first dataset demonstrating that there appear to be favourable clinical outcomes in patients who are able to awake prone. To date, there is only one study in the UK,23 describing a cohort of 95 patients admitted with COVID-19, of whom 10 (10.5%) required non-invasive ventilation and 6 required (6.3%) invasive ventilation.
Compared with large non-UK cohorts, the age of our patients was similar (median 65 years)5 24, although lower than the UK cohort (median 74 years).23 Most of our patients were white but Black and Asian patients were over-represented compared with local ethnicity data, which is in keeping with other UK data.4 Other patient demographics were comparable with existing cohorts, showing a male predominance (66.7%), with high proportions of obesity (median body mass index 29.5 kg/m2), hypertension (46.3%) and diabetes (31.5%). The rate of chronic respiratory disease (29.6%) was comparable with the first UK cohort (33.0%) but higher than a large New York cohort of hospitalised patients (16.1%)5 and a large ICU cohort from Lombardy (4.0%)3.
The mortality rate in our patients requiring non-invasive respiratory support is similar (54.2%, 26/48) to those requiring non-invasive ventilation in the first UK cohort (60%, 6/10),23 and one Wuhan cohort of patients admitted to ICU (62.9%, 39/62),2 but lower than a second Wuhan cohort of patients who required HFNO or non-invasive respiratory support (85.1%, 57/67).3 However, direct comparisons should be treated with caution given the lack of specific characteristic data on this subset of patients in other cohorts, and high rates of missing outcome data in other cohorts. A large cohort of patients in an Italian ICU reported an overall mortality of 21%.24 However, only 12% of patients received mechanical ventilation. The reported mortality of patients who are invasively ventilated on ICU is high: between 48% and 97%.2 4 5 The precise reasons for the high mortality in ventilated patients are unclear, and while this may relate to more physiologically compromised patients requiring mechanical ventilation, it may be that mechanical ventilation itself is associated with worse outcomes. Optimal timing for mechanical ventilation is difficult to determine. While the early acute lung injury seen in COVID-19 pneumonia appears to be associated with compliant lungs and significant hypoxia, some post-intubation patients display a more classic acute respiratory distress syndrome (ARDS) phenotype with stiff lungs, potentially arguing for barotrauma or other deleterious effect of mechanical ventilation. It may therefore be argued that early mechanical ventilation should only be employed in patients with rapid deterioration and impending failure to end-organ oxygenate.25 Conversely, delayed intubation could mean withholding treatment from some patients who may benefit from mechanical ventilation.26
Early in the COVID-19 pandemic, we adopted a strategy of avoiding early intubation and ventilation where possible, and therefore attempted to prioritise other interventions such as non-invasive respiratory support and awake proning in a dedicated unit. Our data demonstrate that 8.5% of the total admitted COVID-19 cases required HDU support, and 31.8% patients with high oxygen requirements can be managed with CPAP alone and survive to discharge. Tolerance of CPAP therapy was associated with improved survival, whereas ‘failing’ CPAP therapy and requiring transition to either intensive care or HFNO was associated with a poor outcome. Interestingly, the majority of those who failed CPAP treatment did so for reasons of tolerance (80.8%) rather than oxygen requirement. This might argue for a more aggressive sedation strategy alongside CPAP to enhance tolerability and prolong treatment, but further data are needed on whether such a strategy will improve outcomes.
To our knowledge, this study is the first to report survival and outcome data from awake proning in COVID-19 or any other cause of respiratory failure. While randomised data have established the efficacy of early and prolonged proning in ventilated patients with severe ARDS,27 it is unclear whether the mortality benefits relate to improvements in oxygenation, or to other mechanisms such as reduced ventilator-induced lung injury. It is therefore unknown whether these benefits will apply to awake patients with severe hypoxaemia secondary to COVID-19 pneumonia. In this setting, previous case series suggest an improvement in oxygen saturations with awake proning to date.10 11 14 We adopted a pragmatic approach to awake proning in all patients, aiming for 2 hours prone at least twice daily. These data suggest that the majority of patients with high oxygen requirements are able to fully prone or semiprone in 97% of cases, although full proning was only achieved in 41%. Our data demonstrate a significant association between full proning and reduced mortality (8% vs 67%). This is a potentially important signal, but there are limitations to this study. This was a pragmatic study in which all patients admitted to our HDU were given a trial of CPAP or HFNO and considered for awake proning. These included 28 patients in the cohort were not deemed suitable for escalation beyond HDU level care (ie, not for ICU and intubation). As an observational cohort study, the dataset suffers with potential selection bias and lack of a control group and, as such, it is not possible to infer whether it is the ability to prone fully (which might imply lack of other markers of severe disease or comorbidities, or a simple marker of functional status) or proning itself which is associated with reduced mortality. Nevertheless, given the tolerability of awake proning using an expert physiotherapy team demonstrated in our study, awake proning is clearly achievable in some sick patients with respiratory failure. Randomised studies are now urgently needed to assess its risks and benefits.
Conclusions
The mortality of patients with COVID-19 requiring respiratory support is considerable. Data from our cohort of patients managed on a respiratory HDU providing CPAP and respiratory physiotherapy support to enable awake proning show an association with successful awake proning and improved outcomes in patients receiving non-invasive respiratory support.
Acknowledgments
The authors thank and acknowledge the contribution of all the patients, and members of the clinical team (Alphabetically: Andrew Achaiah, Dinesh Addala, Radhika Banka, Eihab Bedawi, Kat Bowyer, Mat Bulpett, Simon Couillard De L'Espinay, Becky Dalton, Hannah Dewey, Alexandra Dudina, Jayne Faulkner, Gilly Fry, Ali Gates, Vineeth George, Jo Hobbs, Natasha Hough, Karen Humphries, Cat Kemp, Namrata Kewalramani, Kallirroi Lamprou, Becky Masterman, Phoebe Montgomery, Shefaly Patel, Clare Scott-Dempster, Davide Strappelli, Anand Sundaralingam, Chia Ling Tey, and Alexa Thomas).
Footnotes
Contributors: The study was conceived by RJH, BMLP, PJDE, CDT, MB, NP and NMR. Clinical care of the patients was conducted by all authors. Data collection was conducted by BMLP, PJDE, SBE, RL and KA. Data and statistical analysis was conducted by RJH and CDT. RJH, BMLP and CDT performed the literature search, and initial manuscript preparation. All authors reviewed and approved the final manuscript.
Funding: This paper was not directly funded by any grant or funding body. RJ Hallifax and CD Turnbull are Academic Clinical Lecturers in Respiratory Medicine funded by the National Institute for Health Research (NIHR). NM Rahman, ID Pavord and N Petousi are funded by the NIHR Oxford Biomedical Research Centre.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The study was discussed and approved by the local lead for ethics, and considered to be an audit of clinical practice.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data can be made available upon request at the discretion of the corresponding and senior authors.
References
- 1.WHO COVID-19 situation report 119, 2020. Available: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200518-covid-19-sitrep-119.pdf?sfvrsn=4bd9de25_4
- 2.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475–81. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ICNARC ICNARC report on COVID-19 in critical care, 2020. Available: https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports
- 5.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the new York City area. JAMA 2020. 10.1001/jama.2020.6775. [Epub ahead of print: 22 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nyrén S, Mure M, Jacobsson H, et al. Pulmonary perfusion is more uniform in the prone than in the supine position: scintigraphy in healthy humans. J Appl Physiol 1999;86:1135–41. 10.1152/jappl.1999.86.4.1135 [DOI] [PubMed] [Google Scholar]
- 7.Johnson NJ, Luks AM, Glenny RW. Gas exchange in the prone posture. Respir Care 2017;62:1097–110. 10.4187/respcare.05512 [DOI] [PubMed] [Google Scholar]
- 8.Gattinoni L, Taccone P, Carlesso E, et al. Prone position in acute respiratory distress syndrome. rationale, indications, and limits. Am J Respir Crit Care Med 2013;188:1286–93. 10.1164/rccm.201308-1532CI [DOI] [PubMed] [Google Scholar]
- 9.Sun Q, Qiu H, Huang M, et al. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care 2020;10:33. 10.1186/s13613-020-00650-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elharrar X, Trigui Y, Dols A-M, et al. Use of prone positioning in Nonintubated patients with COVID-19 and hypoxemic acute respiratory failure. JAMA 2020. 10.1001/jama.2020.8255. [Epub ahead of print: 15 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sartini C, Tresoldi M, Scarpellini P, et al. Respiratory parameters in patients with COVID-19 after using noninvasive ventilation in the prone position outside the intensive care unit. JAMA 2020. 10.1001/jama.2020.7861. [Epub ahead of print: 15 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul V, Patel S, Royse M, et al. Proning in Non-Intubated (pini) in times of COVID-19: case series and a review. J Intensive Care Med 2020;35:818–24. 10.1177/0885066620934801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coppo A, Bellani G, Winterton D, et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med 2020;8:765–74. 10.1016/S2213-2600(20)30268-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caputo ND, Strayer RJ, Levitan R. Early Self-Proning in awake, Non-intubated patients in the emergency department: a single ED's experience during the COVID-19 pandemic. Acad Emerg Med 2020;27:375–8. 10.1111/acem.13994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang LG, LeBaron J, Bodnar D, et al. Conscious Proning: an introduction of a Proning protocol for Nonintubated, awake, hypoxic emergency department COVID-19 patients. Acad Emerg Med 2020;27:566–9. 10.1111/acem.14035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sztajnbok J, Maselli-Schoueri JH, Cunha de Resende Brasil LM, et al. Prone positioning to improve oxygenation and relieve respiratory symptoms in awake, spontaneously breathing non-intubated patients with COVID-19 pneumonia. Respir Med Case Rep 2020;30:101096. 10.1016/j.rmcr.2020.101096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Q, Wang T, Qin X, et al. Early awake prone position combined with high-flow nasal oxygen therapy in severe COVID-19: a case series. Crit Care 2020;24:250. 10.1186/s13054-020-02991-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bentley SK, Iavicoli L, Cherkas D, et al. Guidance and patient Instructions for Proning and repositioning of awake, Nonintubated COVID-19 patients. Acad Emerg Med 2020. 10.1111/acem.14067. [Epub ahead of print: 29 Jun 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding L, Wang L, Ma W, et al. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care 2020;24:28. 10.1186/s13054-020-2738-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NICE COVID-19 rapid guideline: critical care in adults, 2020. Available: https://www.nice.org.uk/guidance/ng159 [PubMed]
- 21.Heinze G, Ploner M. Technical report 2/2004: a SAS-macro, S-PLUS library and R package to perform logistic regression without convergence problems. Vienna, Austria: Section of Clinical Biometrics, Department of Medical Computer Sciences, Medical University of Vienna; 2004. [Google Scholar]
- 22.Chong E, Chan M, Tan HN, et al. COVID-19: use of the clinical frailty scale for critical care decisions. J Am Geriatr Soc 2020;68:E30–2. 10.1111/jgs.16528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomlins J, Hamilton F, Gunning S, et al. Clinical features of 95 sequential hospitalised patients with novel coronavirus 2019 disease (COVID-19), the first UK cohort. J Infect 2020;81:e59–61. 10.1016/j.jinf.2020.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020;323:1574. 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leisman DE, Deutschman CS, Legrand M. Facing COVID-19 in the ICU: vascular dysfunction, thrombosis, and dysregulated inflammation. Intensive Care Med 2020;46:1105–8. 10.1007/s00134-020-06059-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Telias I, Katira BH, Brochard L. Is the prone position helpful during spontaneous breathing in patients with COVID-19? JAMA 2020. 10.1001/jama.2020.8539. [Epub ahead of print: 15 May 2020]. [DOI] [PubMed] [Google Scholar]
- 27.Bloomfield R, Noble DW, Sudlow A. Prone position for acute respiratory failure in adults. Cochrane Database Syst Rev 2015:CD008095. 10.1002/14651858.CD008095.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2020-000678supp001.pdf (1.3MB, pdf)