Abstract
A 33-year-old man with paranoid schizophrenia and a ventriculoperitoneal (VP) shunt was sent to our institution from an inpatient psychiatric facility due to concerns for the 2019 novel coronavirus (COVID-19). Per the facility, the patient had a fever and non-productive cough. On admission, the patient was afebrile and lacked subjective symptoms. A RNA reverse transcriptase PCR (RNA RT-PCR) test for COVID-19 was positive. A chest X-ray contained a small patchy opacity in the right middle lobe and another in the retrocardiac region concerning for pneumonia. Inflammatory markers were mildly elevated. He remained COVID-19 positive and asymptomatic for 36 days. This case details one asymptomatic carrier’s course with persistently positive COVID-19 nasopharyngeal swabs. It demonstrates that a VP shunt could be a possible predisposition for prolonged viral shedding.
Keywords: infectious diseases, respiratory medicine
Background
In December 2019, a novel virus, COVID-19, was identified in Wuhan, China. Its origin has since been linked to a large seafood market involved in the illegal selling of live animals.1 2 The virus has since rapidly spread throughout the world, and was declared a global pandemic by the WHO on 11 March 2020.3 As of 21 June 2020 there are 8 708 008 total confirmed cases and 461 715 confirmed deaths globally.4 However, the actual number of cases is believed to significantly exceed reported cases due to undiagnosed asymptomatic carriers. These asymptomatic carriers as well as pre-symptomatic individuals are essential to the virus’ rapid spread with estimated infection rates of 34% and 40% according to the Centre of Disease Control.5 However, much is still unknown about the intricate interplay between transmission, viral RNA shedding patterns, viral loads, infectivity and individual predispositions to prolong viral shedding.
Case presentation
A 33-year-old man with paranoid schizophrenia and a ventriculoperitoneal (VP) shunt was sent to our institution from an inpatient psychiatric facility due to concerns for the COVID-19. Per the facility, the patient had a fever and non-productive cough; however, no records accompanied the patient on arrival to our emergency department. On admission, the patient was afebrile, non-tachycardic, respiratory rate was within normal limits and his oxygen saturation was 100% on room air. The remaining physical examination was unremarkable except for his psychiatric examination, which showed intermittent response to internal stimuli.
Per hospital policy the following was obtained: nasopharyngeal swab for RNA RT-PCR for COVID-19, creatine kinase (CPK), C reactive protein (CRP), lactate dehydrogenase (LDH), ferritin and a chest radiograph. CRP and CPK were mildly elevated at 27.2 mg/L and 286 units/L, respectively. The LDH and ferritin were within normal limits at 221 units/L and 112.5 ng/mL, respectively. The chest radiograph demonstrated a small patchy opacity in the right middle lobe and another in the retrocardiac region concerning for pneumonia (figure 1). The following day, the patient’s RNA RT-PCR returned positive for COVID-19.
Figure 1.
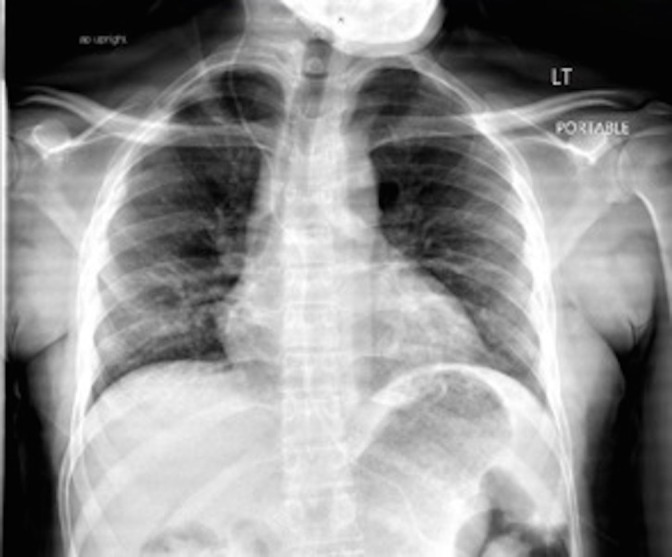
Chest radiograph showing small patchy opacities in the right middle lobe and in the retrocardiac region concerning for pneumonia.
The patient was court-mandated to live in a long-term inpatient psychiatric facility due to poor insight into his paranoid schizophrenia. Consequently, social work was involved in the patient’s case. At this point in the COVID-19 global crisis, no inpatient psychiatric facility in our system could accommodate a COVID-19 positive patient. As a result, the patient remained under the medicine service for a total of 36 days until two consecutive negative nasopharyngeal RNA RT-PCR tests were obtained. He was then discharged according to psychiatric recommendations.
Treatment
The patient was admitted under the medicine service to a COVID-positive unit requiring both airborne and contact precautions. At the time, there were two major treatment options for COVID-19: (1) hydroxychloroquine and (2) high-dose oral corticosteroids. Per hospital policy, if an individual had a positive nasopharyngeal swab and a positive chest radiograph, as our patient did, then they qualified for hydroxychloroquine therapy. Dosing was 400 mg two times the first day followed by 400 mg daily for 4 days. As he was asymptomatic, he did not qualify for corticosteroid therapy; his oxygen saturation remained at 100% on room air; therefore, no supplemental oxygen was needed during hospitalisation.
Outcome and follow-up
The remainder of the patient’s clinical course was complicated by placement issues with the community outreach for psychiatric emergencies. Once appropriate placement was found, the patient was discharged with follow-up appointments scheduled with his mental health professionals and primary care physician.
Discussion
The novel coronavirus is an enveloped, single-stranded RNA virus that belongs to the Coronavirus family within the Betacoronavirus genus (β-CoV). It is the seventh virus of its family that has demonstrated the ability to infect humans.1–3 6 Rapid human-to-human transmission occurs via respiratory droplets such as coughing and sneezing. Non-respiratory viral detection in the stool, blood and semen has also been observed, but transmission capability from these sites has not been established. Infectivity begins before the onset of symptoms with an estimated mean incubation period of 5 days and basic reproduction number (R0) of 2.5.5 7 This explains the well-known transmission of pre-symptomatic and asymptomatic individuals and the exponential spread of infection.
The novel virus exhibits marked genetic structural similarity to members within its genus, notably Severe Acute Respiratory Syndrome-associated coronavirus (SARS-CoV). Despite variability among amino acid sequencing, the s-protein of the viruses maintain almost identical electrostatic properties allowing COVID-19 to use the same ACE2 receptor for viral entry as SARS-CoV.2 The ACE2 receptors are ubiquitous throughout the human body, but are concentrated in the respiratory system, gastrointestinal tract and systemic small vessels.8 9 These locations correlate with the well-established clinical symptomology of respiratory distress, diarrhoea, vasculitis and anosmia and dysgeusia. ACE2 receptors have also been found in the brain. β-CoVs, including SARS-CoV, have a well-documented propensity for neuroinvasion; thus, it is reasonable to believe COVID-19 shares this tendency.10 Ren et al also showed that the most common coronaviruses may well survive or persist on surfaces in vitro for up to 1 month.11 These surfaces include plastic, which is the material VP shunts are made out of. Therefore, one can postulate that COVID-19 may have neuroinvasive potential based on its affinity to the ACE2 receptor, and potentially could have prolonged viral survival on the plastic of a VP shunt in vivo. This pathophysiology may explain why our patient with a VP shunt had prolonged viral shedding thus illustrating a VP shunt as a possible predisposition for prolonged viral shedding and a potential viral niche for COVID-19.
The precise duration and relationships between transmission, viral shedding and infectivity are uncertain. The length of viral shedding is variable and may depend on disease severity. In a retrospective cohort analysis of 191 patients, viral shedding ranged from 8 to 37 days with a median duration of 20 days.12 Another study of 96 patients revealed a median of 21 days in patients with severe disease compared with 14 days in those with mild disease.13 Additional factors associated with extended viral shedding include the male sex, delayed hospital treatment, mechanical ventilation and treatment with glucocorticoids.14 The longest reported viral shedding is 60 days in a 47-year-old woman including her symptomatic course and ongoing recovery.15 To our knowledge, our case of viral shedding for 36 days is the longest reported in a relatively young, asymptomatic patient or in a patient with a VP shunt demonstrating a VP shunt as a possible predisposition for prolonged viral shedding and possible asymptomatic infectivity. However, further studies and case reports are needed to draw this conclusion.
Learning points.
A VP shunt may predispose individuals to prolonged viral shedding of the novel coronavirus.
Further insight into prolonged viral shedding is warranted to know whether this shedding leads to infectivity.
For persistently positive patients, consider all risk factors for prolonged infectivity.
Footnotes
Contributors: FE-B, DG and AF were all involved in this project. All provided substantial contributions to the conception of case report, drafted the original work and revised the copy, all approved the final project and agreed to be accountable for all aspects of the work ensuring that questions related to the accuracy or integrity of any part of the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Hui DS, I Azhar E, Madani TA, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis 2020;91:264–6. 10.1016/j.ijid.2020.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 2020;63:457–60. 10.1007/s11427-020-1637-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270–3. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Coronavirus disease (COVID-19) situation report-153, 2020
- 5.Center of Disease Control Coronavirus disease 2019: pandemic planning scenarios. Available: https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html [Accessed 19 Jun 2020].
- 6.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–33. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med 2020;172:577–82. 10.7326/M20-0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamming I, Timens W, Bulthuis MLC, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631–7. 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia H, Lazartigues E. Angiotensin-Converting enzyme 2 in the brain: properties and future directions. J Neurochem 2008;107:1482–94. 10.1111/j.1471-4159.2008.05723.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y-C, Bai W-Z, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol 2020;92:552–5. 10.1002/jmv.25728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren S-Y, Wang W-B, Hao Y-G, et al. Stability and infectivity of coronaviruses in inanimate environments. World J Clin Cases 2020;8:1391–9. 10.12998/wjcc.v8.i8.1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng S, Fan J, Yu F, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang Province, China, January-March 2020: retrospective cohort study. BMJ 2020;369:m1443. 10.1136/bmj.m1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu K, Chen Y, Yuan J, et al. Factors associated with prolonged viral RNA shedding in patients with coronavirus disease 2019 (COVID-19). Clin Infect Dis 2020;71:799–806. 10.1093/cid/ciaa351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Li C, Zhou Y, et al. Persistent viral shedding lasting over 60 days in a mild COVID-19 patient with ongoing positive SARS-CoV-2. Quant Imaging Med Surg 2020;10:1141–4. 10.21037/qims.2020.04.08 [DOI] [PMC free article] [PubMed] [Google Scholar]


