Abstract
We herein report a case of a child with tuberculous meningitis and COVID-19 coinfection complicated by hydrocephalus, arterial ischaemic stroke and extensive cerebral sinus venous thrombosis. Both conditions induce a proinflammatory cytokine drive resulting, among others, in a prothrombotic state. The disruption of the coagulation system in this case was supported by elevated D-dimers, fibrinogen and ferritin levels, consistent with thrombotic complications reported in some adult patients infected with COVID-19. The child also exhibited prolonged viral shedding that suggests severe disease.
Keywords: radiology (diagnostics), infections, paediatrics (drugs and medicines), neurology (drugs and medicines), global health
Background
COVID-19 is caused by SARS-CoV-2 infection. Currently, South Africa is one of the worst affected COVID-19 pandemic countries with almost 450 000 cases1 and also one the highest tuberculosis (TB) burden countries with an incidence of 520/100 000 population in 2018.2 In the South African setting, tuberculous meningitis (TBM) was the presenting diagnosis in more than 10% of children presenting to a tertiary hospital with TB.3 Mortality in COVID-19 has been linked to the presence of the so-called ‘cytokine storm’ induced by the virus. Excessive production of proinflammatory cytokines leads to widespread tissue damage resulting in multiorgan failure and death. Similarly, most of the neurological complications of TBM are considered to be due to an overexuberant host inflammatory cytokine response. Adults with COVID-19 infection are at an increased risk of thrombotic complications such as venous thromboembolism and ischaemic stroke. The incidence of cerebral sinus venous thrombosis (CSVT) in COVID-19 remains unknown with only a single adult case series reporting a prevalence of 0.5%. Proposed mechanisms of CSVT include a cytokine-induced prothrombotic state as well as direct injury to the cerebral endothelial vessels.
Case presentation
A 2 year 7-month-old girl presented with acute onset left-sided weakness and lethargy. The preceding history revealed progressively enlarging cervical lymphadenopathy and decreased appetite. There was no history of either household contact with an adult TB source case or COVID-19. Immunisations, including BCG, was up to date.
Clinical examination revealed normal vital functions as well bilateral cervical lymphadenopathy measuring more than 2 cm. Neurological examination revealed a depressed level of consciousness, with a Glasgow Coma Scale of 11 (E-4, V-2 and M-5), right pupillary dilatation with right ptosis, left hemiplegia (arm affected more than the leg), globally brisk deep tendon reflexes and bilateral extensor plantar responses. The initial respiratory examination was normal with respiratory distress gradually developing within 72 hours after admission. Examination of the other systems proved unremarkable.
Investigations
Initial laboratory investigations showed a markedly elevated white cell count of 95×109/L (neutrophils 93%, lymphocyte 2.5%), haemoglobin of 12 g/dL and platelets of 253×109/L. The C reactive protein was raised (131 mg/L). Coagulation studies revealed an elevated international normalized ratio (INR) of 1.63, elevated prothrombin time 18.3 s (9.9–12.3), normal activated partial thromboplastin time, elevated fibrinogen (5.2 g/L), elevated D-Dimer (14.8 mg/L) as well as elevated ferritin (711 µg/L). The kidney function, serum electrolytes and liver function test all proved normal. Gastric washings were GeneXpert MTB/RIF positive and rifampicin sensitive. Culture was also positive for drug-sensitive Mycobacterium tuberculosis. The chest radiograph demonstrated a reticulonodular pattern in keeping with miliary TB (figure 1). Ventricular cerebrospinal fluid was clear, colourless and acellular. HIV PCR was non-reactive. The SARS-CoV-2 PCR nasal swab was positive on admission and subsequently remained positive on two further occasions until discharge after 1 month.
Figure 1.
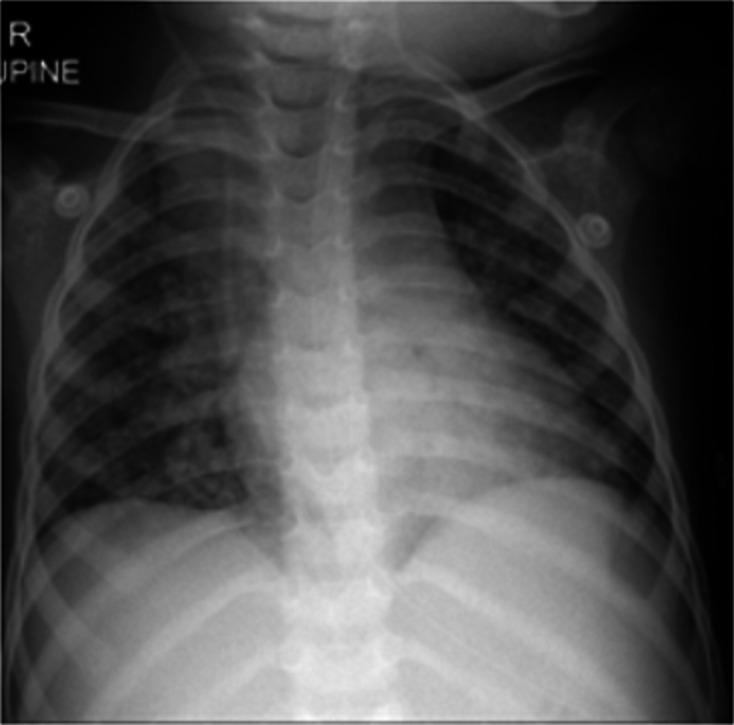
Chest radiograph demonstrated a reticulonodular pattern in keeping with miliary tuberculosis. A right-sided ventriculo-peritoneal shunt is visible.
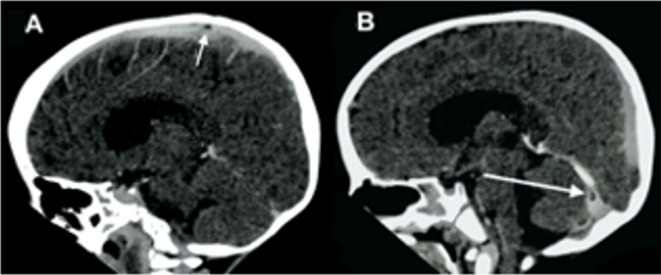
CT brain scan showed pan-hydrocephalus, basal meningeal enhancement and infarction involving the anterior limb of the right internal capsule, lentiform nucleus and thalamus (figure 2). The sagittal postcontrast images demonstrated multiple filling defects in the venous system, mainly superior sagittal sinus and the transverse sinuses (see arrows in figure 3A, B).
Figure 2.
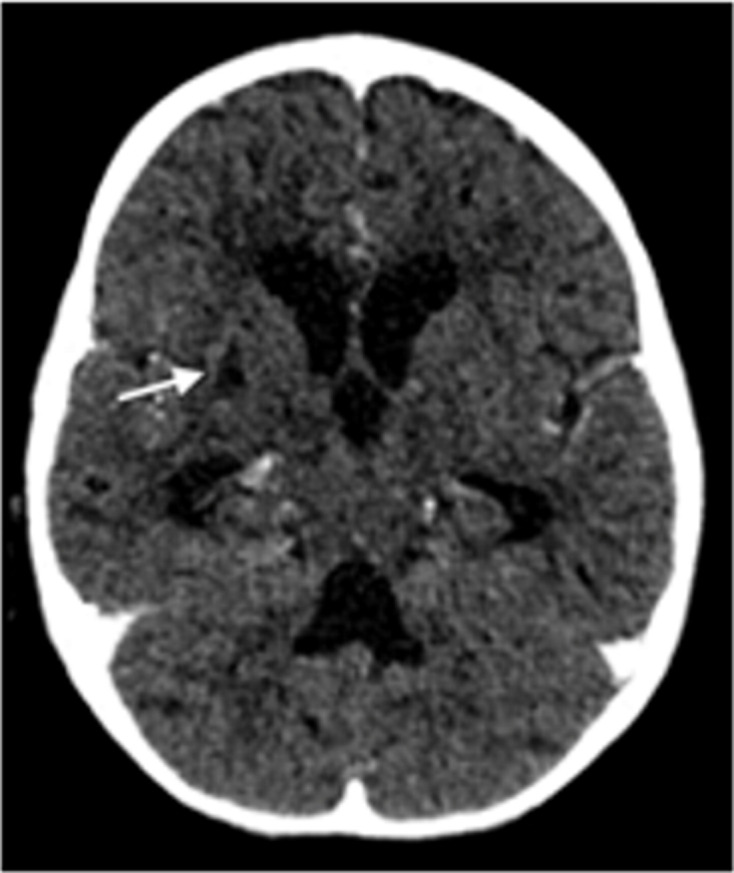
Axial contrasted CT showing infarction involving the right internal capsule extending to the lentiform nucleus (see arrow).
Figure 3.
(A and B) Sagittal contrasted CT showing a filling defect in the superior sagittal sinus (see arrow). (B) Sagittal contrasted CT showing a filling defect in the right transverse sinus (see arrow).
Treatment
The child was commenced on antituberculous treatment: isoniazid 20 mg/kg, rifampicin 20 mg/kg, pyrazinamide 40 mg/kg and ethionamide 20 mg/kg, as well as prednisone (2 mg/kg). Aspirin 3 mg/kg/day was prescribed in lieu of the extensive CSVT. Shortly after admission, raised intracranial pressure necessitated insertion of ventriculoperitoneal shunt. Within 72 hours following admission, the child’s respiratory status worsened that necessitated high dose dexamethasone (oral prednisone stopped), intravenous antibiotics and high flow nasal oxygen. During the recovery phase, she received speech, occupational and physiotherapy.
Outcome and follow-up
The patient required intensive neurorehabilitation for a month due to poor feeding and prolonged respiratory symptoms. On discharge, the child was stable with a GCS of 15/15, feeding orally and had a residual left haemiparesis. Currently, the child is recovering well at home with ongoing outpatient physiotherapy and occupational therapy. Monthly clinical review is planned.
Discussion
CVST in COVID-19 has only been reported in isolated adult cases, with no associated risk factors for CVST, with the youngest being a 23-year-old man.4–7 It is known that SARS-CoV-2 binds to ACE2 receptors, which are also present in the brain. It is therefore plausible that the virus can cause central nervous system injury by binding to the vascular endothelial cells leading to vessel injury and platelet aggregation, increasing the risk for thrombosis.5 Furthermore, numerous reports present an overwhelming evidence of a cytokine storm in COVID-19, causing coagulation system injury by a cascade of inflammatory reactions leading to platelet abnormalities, D-dimers and excess fibrinogen.6 7 CVST is an uncommon (4%) but recognised complication of TBM.8 A combination of the procoagulant, antithrombotic, platelet and vascular endothelial functions that occur during inflammation could contribute to the hypercoagulable state in TBM.9–11 Our patient had TBM and COVID-19 coinfection thereby having compounded risk for hypercoagulability, demonstrated by the markedly elevated fibrinogen, ferritin and D-dimers. The pathophysiology of a hypercoagulable state is similar for both the disease processes thereby increasing the risk for thrombosis.
The nasopharyngeal aspirate remained positive for COVID-19 for 30 days. Studies show 90% of patients with SARS-CoV-2 have repeat negative viral RNA test by 10 days.12 Longer positivity is associated with severity of disease, which was the case in our patient as she presented with stage III TBM and prolonged respiratory symptoms requiring high flow nasal oxygen for more than a week. Acute supportive management of CSVT include rehydration and treating any underlying infections, iron deficiency and seizures. None of the mentioned CSVT risk factors was present in our patient. Management consensus guidelines for CSVT in COVID-19 19 positive adult patients recommend low molecular weight heparin during admission and 7–14 days after discharge.7 We opted to use aspirin in our patient due to its antiplatelet, anti-ischaemic and anti-inflammatory properties. The hypercoagulable state in TBM lasts for approximately 1 month.9
This is the first reported paediatric case of TBM and COVID-19 coinfection and also the first reported case of CSVT. The case highlights the growing evidence of SARS-CoV-2 as a contributor to a hypercoagulable state. It also alerts clinicians to have a high index of suspicion of CSVT in patients with TBM and COVID-19 coinfection as both are risk factors for a hypercoagulable state, which is potentially life-threatening but treatable.
Learning points.
Cerebral sinus venous thrombosis (CSVT) is a known but uncommon complication of tuberculous meningitis (TBM).
COVID-19 may worsen the prothrombotic state in TBM and predispose to CSVT.
Aspirin may have a role in TBM and COVID-19 related CSVT due to its anti-inflammatory, anti-ischaemic and antiplatelet properties.
Footnotes
Contributors: FE wrote the case presentation and prepared images to be included with the manuscript. RS performed a literature review and wrote the discussion section. RVT assisted with literature review and discussion and also reviewed the paper. PG reviewed the paper and made several modifications to the discussion section.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.COVID-19 in the who African region. Available: https://who.maps.arcgis.com/apps/opsdashboard/index.html#/0c9b3a8b68d0437a8cf28581e9c063a9
- 2.World Health Organization Global tuberculosis report. Geneva, Switzerland: WHO Report, 2018. [Google Scholar]
- 3.du Preez K, Schaaf HS, Dunbar R, et al. Complementary surveillance strategies are needed to better characterise the epidemiology, care pathways and treatment outcomes of tuberculosis in children. BMC Public Health 2018;18:397. 10.1186/s12889-018-5252-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavalcanti DD, Raz E, Shapiro M, et al. Cerebral venous thrombosis associated with COVID-19. AJNR Am J Neuroradiol 2020;41:1370–6. 10.3174/ajnr.A6644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemasian H, Ansari B. First case of Covid-19 presented with cerebral venous thrombosis: a rare and dreaded case. Rev Neurol 2020;176:521–3. 10.1016/j.neurol.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poillon G, Obadia M, Perrin M, et al. Cerebral venous thrombosis associated with COVID-19 infection: causality or coincidence? J Neuroradiol 2020. 10.1016/j.neurad.2020.05.003. [Epub ahead of print: 11 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes C, Nichols T, Pike M, et al. Cerebral venous sinus thrombosis as a presentation of COVID-19. Eur J Case Rep Intern Med 2020;7:001691. 10.12890/2020_001691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhawan S, et al. Cerebral venous thrombosis in childhood. Arch Dis Child 2018;103:A1–212. [Google Scholar]
- 9.Schoeman J, Mansvelt E, Springer P, et al. Coagulant and fibrinolytic status in tuberculous meningitis. Pediatr Infect Dis J 2007;26:428–31. 10.1097/01.inf.0000261126.60283.cf [DOI] [PubMed] [Google Scholar]
- 10.Sharawat IK, Bhattacharya D, Saini L, et al. Multiple cerebral sinus venous thrombosis and venous infarct: rare complication of tuberculous meningitis in a child. BMJ Case Rep 2019;12:e231419. 10.1136/bcr-2019-231419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Natarajan V, George S, Eapen G, et al. An unusual association – tuberculous meningitis causing cerebral venous thrombosis. Journal of Case Reports 2015:343–6. 10.17659/01.2015.0088 [DOI] [Google Scholar]
- 12.Liu Y, Yan L-M, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis 2020;20:656–7. 10.1016/S1473-3099(20)30232-2 [DOI] [PMC free article] [PubMed] [Google Scholar]