Abstract
Recent studies have shown that BRAF inhibitors, such as vemurafenib, are effective in inducing long periods of remission in relapsed hairy cell leukaemia. Acute pancreatitis is one of the rare complications that is reported with vemurafenib use. As severe pancreatitis can be life threatening, physicians should be vigilant of this side effect and promptly treat patients that develop clinical signs and symptoms while receiving vemurafenib. We present an interesting case of vemurafenib-induced pancreatitis that not only resolved but also did not recur after reintroduction of the drug at a reduced dose.
Keywords: haematology (drugs and medicines), malignant and benign haematology, pancreatitis, safety, unwanted effects / adverse reactions
Background
Hairy cell leukaemia (HCL) is a rare lymphoproliferative disorder of mature B cells with an incidence of about 1000 new cases in the USA per year.1 Although HCL responds well when treated with purine nucleoside analogues such as cladribine, relapse occurs in about 50% of cases and the disease becomes less sensitive to repeat treatment. More recent studies have shown that BRAF inhibitors such as vemurafenib, through the upregulation of proapoptotic genes resulting in the loss of hairy morphology, are effective in inducing long periods of remission in the relapsed setting.2 Vemurafenib is generally well tolerated and the most common side effects are rash and arthritis. Pancreatitis has been reported as a rare, but serious adverse event.3 We present an interesting case of vemurafenib-induced pancreatitis that not only resolved but also did not recur after reintroduction of the drug at a reduced dose.
Case presentation
An 84-year-old man initially presented to the hospital with pancytopenia and was discovered to have HCL after undergoing a diagnostic bone marrow biopsy. He received initial treatment with cladribine and maintained a complete haematological response for 6 years before relapsing. He repeated treatment with cladribine and rituximab, to which he had a short response lasting 18 months. Given his age and multiple comorbidities (hypertension, hypercholesterolaemia, pulmonary fibrosis, atrial fibrillation, benign prostatic hyperplasia, cardiomegaly with global left ventricular dysfunction and pulmonary hypertension), salvage therapy was started outpatient, with oral vemurafenib dosed 960 mg two times per day. Baseline laboratory values prior to starting vemurafenib included normal comprehensive metabolic panel (CMP), normal baseline amylase and lipase, and a normal electrocardiogram (EKG). He continued to take his usual medications in addition to vemurafenib, namely, diltiazem, tamsulosin, losartan, lovastatin and warfarin—all of which had been unchanged.
One week after starting vemurafenib, he presented to the clinic for scheduled monitoring of bloodwork, and on questioning, he reported mild back pain.
Investigations
Blood tests showed elevated lipase and amylase levels of 1893 U/L (reference range 7–60 U/L) and 411 U/L (25–125 U/L), respectively (figure 1). He was sent to the emergency room and abdominal imaging revealed infiltration of the peripancreatic fat adjacent to the pancreatic body and tail consistent with acute pancreatitis (figure 2) without the presence of gallstones. The patient had no history of alcohol use and had a normal triglyceride level of 61 mg/dL (10–149 mg/dL) on admission. Other relevant laboratory values include white cell count 1.2×109/L, glucose 97 mg/dL, Aspartate Aminotransferase (AST) 59 U/L (10–40 U/L) and serum Blood Urea Nitrogen (BUN) 20 mg/dL (7–23 mg/dL). He remained afebrile and did not meet criteria for systemic inflammatory response syndrome. Pancreatitis risk stratification was performed using Bedside Index for Severity in Acute Pancreatitis (BISAP) and Ranson’s scoring criteria. For each, the patient only scored one point, indicating that he belonged to a low mortality group (0%–3%).
Figure 1.
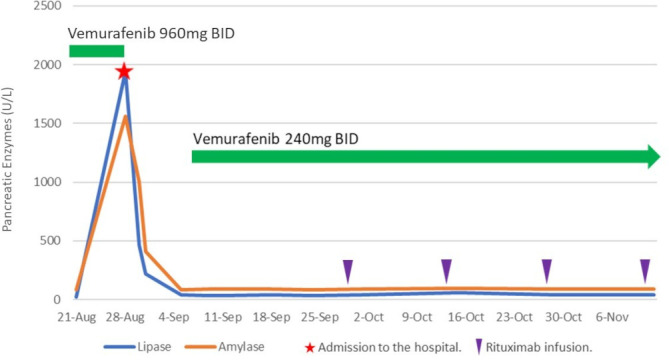
Pancreatic enzymes and vemurafenib use. Back pain resolved around 31 August, and the patient became asymptomatic.
Figure 2.
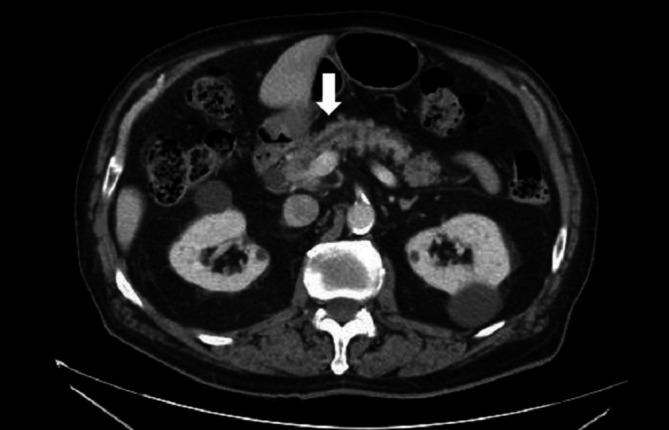
CT image showing peripancreatic fat and pancreatic body inflammation with mild pancreatic duct dilatation (arrow).
Treatment
Vemurafenib was discontinued immediately. His symptoms ultimately resolved with intravenous hydration without the need for analgesics. He was discharged on the third day of hospitalisation. His amylase and lipase levels normalised over the 5 days post-discharge.
Outcome and follow-up
The patient was restarted on vemurafenib 2 days after his bloodwork normalised. The total dose was reduced to 25% of the original dose (240 mg two times per day) and was given in combination with four doses of biweekly intravenous rituximab infusions (375 mg/m²). He was able to tolerate the medication well without any further recurrence of pancreatitis while undergoing weekly bloodwork consisting of Complete Blood Count (CBC), CMP, amylase and lipase. His pancytopenia completely resolved on the fifth week of the dose-reduced regimen. A follow-up bone marrow biopsy was performed on the 21st week, and it confirmed complete remission (CR) of disease. Of note, the patient was on the 22nd week of dose-reduced vemurafenib when this case report was written.
Discussion
To our knowledge, this is the first case reporting tolerance of dose-reduced vemurafenib with good haematological response in a patient with HCL following an episode of drug-induced pancreatitis. Another report described a case of pancreatitis that recurred in a patient who had metastatic melanoma and was administered vemurafenib at a 50% dose reduction–480 mg two times per day.3 However, our patient notably had no recurrence of pancreatitis and successfully achieved CR even at a lower drug dose.
Of note, the patient did not undergo abdominal ultrasound or MRI studies, which are more sensitive and specific tests to detect gallstone pancreatitis. The clinical presentation and the normal patient’s liver function testing (including normal bilirubin and alkaline phosphatase) make this a less likely aetiology of his pancreatitis. However, this is considered a limitation of our case report.
BRAF-V600E is a somatic mutation that is identified in almost all cases of HCL and has become a new molecular target in treating HCL.4 Vemurafenib, an oral BRAF serine-threonine kinase inhibitor, has been used in the relapsed and refractory setting for HCL,1 2 5 with overall response rates of 96% in an Italian study (n=26, median of 8 weeks) and 100% in an American study (n=24, median of 12 weeks), respectively. Of note, the overall survival rate was 91% at 1 year in the American study.5 Currently, there is no established standard dose of vemurafenib for the treatment of HCL, although lower doses of vemurafenib (240 mg or 480 mg two times per day) have shown to achieve a 95% haematological response in one study with a small group of patients.2 6
Common adverse effects of vemurafenib include various degrees of dermatologic events, arthralgias and fatigue. Elevation of liver enzymes, in particular, is observed in up to 19% of patients.5 Vemurafenib is not considered myelotoxic, which makes it a favourable drug to use in the relapsed setting wherein patients may have pancytopenia from prior bone marrow suppressive therapies. In a 2015 Italian trial (n=28) of patients being treated for relapsed or refractory HCL, pancreatitis was reported in three patients (11%) who received a dose of 960 mg two times per day5; however, it is unclear if this was a dose-related toxicity.
Other new targeted agents, such as immune checkpoint inhibitors, tyrosine kinase inhibitors and proteasome inhibitors,7 have also been implicated in possibly inciting pancreatitis. Although immune-mediated processes are the most likely cause, the exact mechanism of pancreatitis in these newer agents is still not well understood. Further guidelines regarding how to monitor and prevent this rare side effect are needed. More studies with a larger group of patients are also needed to define the optimal dose of vemurafenib in treating relapsed/refractory HCL.
BRAF gene mutations have recently been found to be targetable in several malignancies. Therefore, the use of BRAF inhibitors, such as vemurafenib, will likely continue to rise. Performing a thorough history and physical, laboratory test monitoring and appropriate radiologic studies can promote early detection of vemurafenib-induced pancreatitis. As severe pancreatitis can be life threatening, physicians should be vigilant of this side effect and promptly treat patients that develop clinical signs and symptoms while receiving the medication. Additional cases reported by individual physicians will also help increase our knowledge base in order to develop a standard safe dose for treating HCL.
Learning points.
As severe pancreatitis can be life threatening, physicians should be vigilant of this side effect in patients who are treated with vemurafenib.
Clinicians should promptly treat patients that develop clinical signs and symptoms of pancreatitis while receiving targeted agents, as many agents have been reported for possibly causing pancreatitis recently.
Treatment for vemurafemib-induced pancreatitis includes discontinuation of the agent, intravenous hydration and analgesics as needed. Physicians may consider restarting vemurafenib carefully, at lower dose, when laboratory values and patient’s clinical symptoms resolve.
More studies with a larger group of patients are needed to define the optimal dose of vemurafenib in treating relapsed/refractory HCL.
Footnotes
Contributors: CMG provided the main conceptual ideas and outline of this case report. SYC and JGS worked out almost all of the technical details. SYC designed and worked on the figures. All authors equally contributed in writing this manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Troussard X, Cornet E. Hairy cell leukemia 2018: update on diagnosis, risk-stratification, and treatment. Am J Hematol 2017;92)::1382–90. Dec 1;. 10.1002/ajh.24936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falini B, Tiacci E. New treatment options in hairy cell leukemia with focus on BRAF inhibitors. Hematol Oncol 2019;37 Suppl 1:30–7. 10.1002/hon.2594 [DOI] [PubMed] [Google Scholar]
- 3.Muluneh B, Buie LW, Collichio F. Vemurafenib-associated pancreatitis: case report. Pharmacotherapy 2013;33:e43–4. 10.1002/phar.1208 [DOI] [PubMed] [Google Scholar]
- 4.Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med 2011;364:2305–15. 10.1056/NEJMoa1014209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tiacci E, Park JH, De Carolis L, et al. Targeting mutant BRAF in relapsed or refractory hairy-cell leukemia. N Engl J Med 2015;373:1733–47. 10.1056/NEJMoa1506583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietrich S, Pircher A, Endris V, et al. BRAF inhibition in hairy cell leukemia with low-dose vemurafenib. Blood 2016;127:2847–55. 10.1182/blood-2015-11-680074 [DOI] [PubMed] [Google Scholar]
- 7.Clamon G, Patel R, Mott S. Pancreatitis associated with newer classes of antineoplastic therapies. J Community Support Oncol 2017;15:e135–41. 10.12788/jcso.0347 [DOI] [Google Scholar]


