Abstract
Acute liver failure (ALF) is a rare initial presentation of metastatic liver disease and is associated with high fatality. Our case report describes acute hepatic decompensation from an occult pancreatic malignancy. A 64-year-old man presented with abdominal distension for 2 weeks associated with decreased appetite and a weight loss of 13.6 kg, over the past 8 months. Significant admission labs were serum creatinine: 6.15 mg/dL, serum bilirubin: 27 mg/dL, aspartate aminotransferase (AST): 316 u/L, alanine aminotransferase (ALT): 198 u/L and serum alkaline phosphatase: 2121 u/L. He was admitted to the medical intensive care unit and was started on dialysis for acute renal failure. MRI of the abdomen showed multiple masses in the liver concerning for metastatic disease, cystic lesions in the pancreatic body and ascites. He underwent paracentesis and ascitic fluid analysis was positive for adenocarcinoma. CA 19-9 was 17 828 u/mL. The patient’s condition gradually deteriorated, and he died of cardiac arrest.
Keywords: cancer - see oncology, pancreatic cancer
Background
Acute liver failure (ALF), also known as fulminant hepatic failure, is defined as rapid loss of liver function in a patient with either no pre-existing liver disease or an undiagnosed condition.1 ALF is a true medical emergency with a high mortality rate if left untreated that necessitates aggressive medical management and often liver transplantation.2 3 ALF therapy is mainly etiology-directed.2 Common causes, include viral hepatitis, drug-induced hepatitis, commonly related to acetaminophen toxicity, hypoxia-induced liver injury, autoimmune disease, Budd-Chiari syndrome, Wilson’s disease, pregnancy-related causes include acute fatty liver of pregnancy and haemolysis elevated liver enzymes low platelets syndrome.2
Although the liver is the most common site of metastasis in solid tumours,4 there are limited reports of a rapid decline of liver function due to metastatic infiltrates.5–9 ALF due to malignant metastasis commonly results due to infiltration of the liver with leukaemia and lymphoma cells.10 11 However, such a decompensation due to metastasis of solid tumours, however, is extremely rare.6–8 12–14 Here, we present a rare case of pancreatic cancer presenting as ALF.
Case presentation
A 64-year-old man with a history of hypertension presented with a chief problem of jaundice and abdominal distension for 2 weeks associated with diarrhoea, decreased appetite and a weight loss of 30 lbs, over the past 8 months. On presentation, the patient was found hypothermic, hypotensive and bradycardic.
Investigations
Admission lab work revealed: white cell count: 11.4×109/L, haemoglobin: 155 g/L, platelet count: 337 000×109/L, serum albumin: 2.8 g/dL, sodium was 131 mEq/L, K: 5.6 mEq/L, Cl: 100 mEq/L, HCO3: 16 mEq/L and anion gap of 14, serum creatinine: 6.15 mg/dL, AST: 316 u/L, ALT: 198 u/L, serum bilirubin: 27 mg/dL, direct bilirubin: 20 mg/dL, serum alkaline phosphatase: 2121 u/L, prothromin time (PT): 21 s and international normalised ratio (INR) 1.76.
Treatment
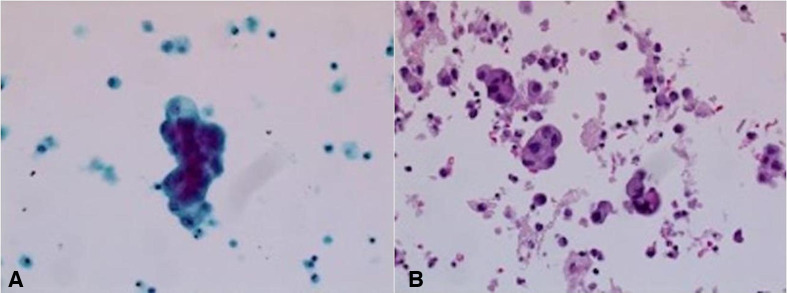
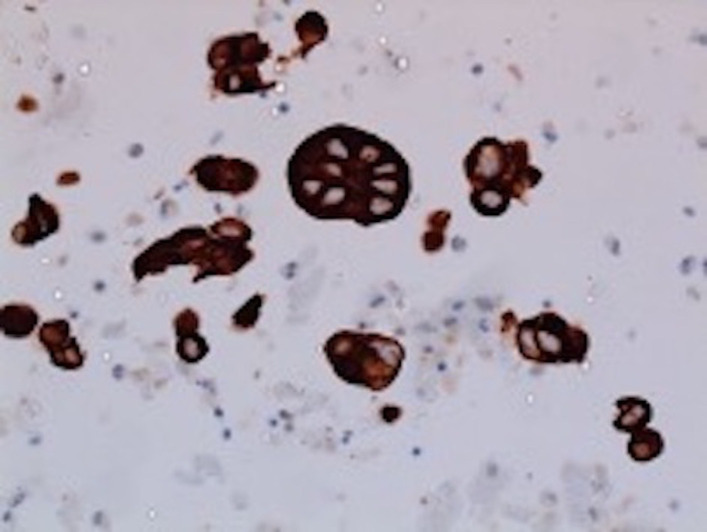
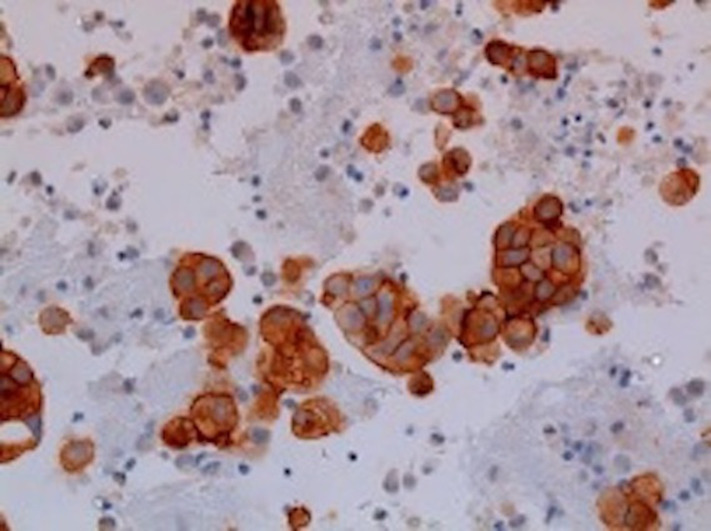
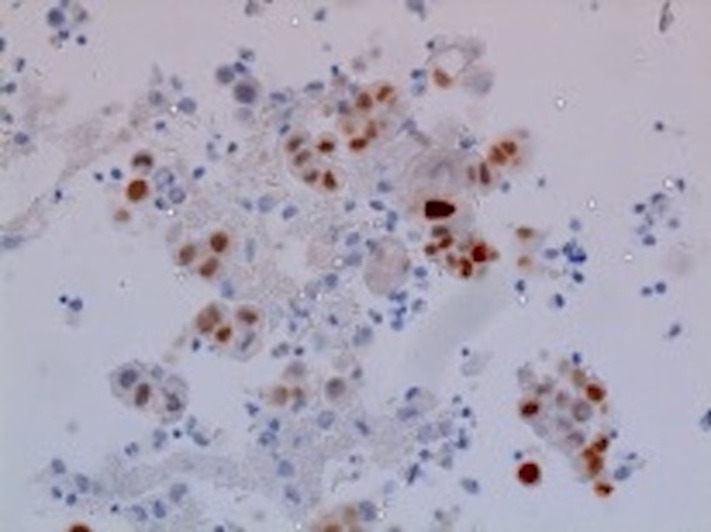
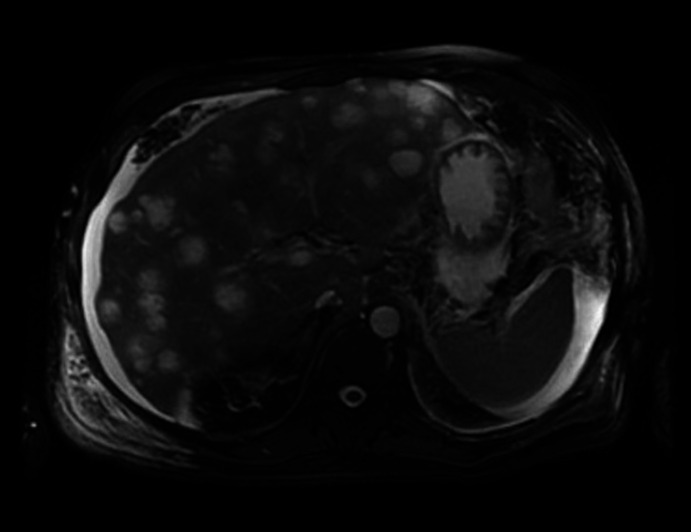
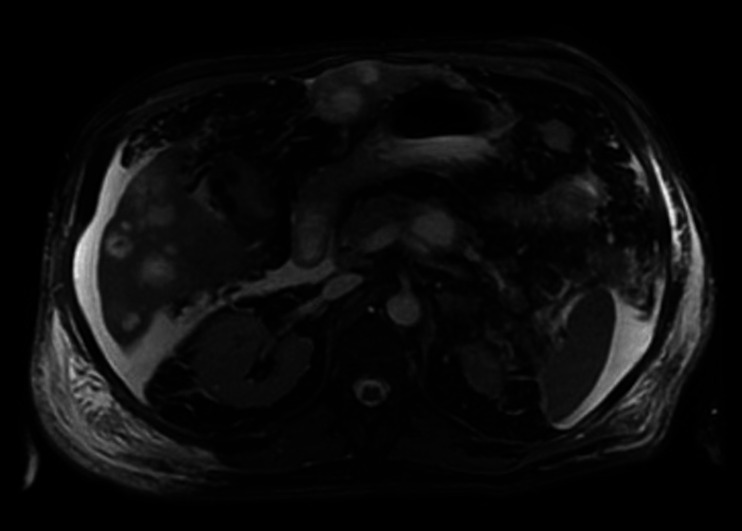
The patient was admitted to the medical intensive care unit with ALF. The patient had a Model For End-stage disease (MELD) score of 39 points (estimated 90-day mortality of 65%–66%). Hepatitis panel, acetaminophen levels, CPK, C3 and C4 were within normal limits. Urinalysis was unremarkable for urine eosinophils. Urine toxicology was negative. He received intravenous albumin, octreotide and was started on continuous venovenous haemofiltration for acute renal failure due to hepatorenal syndrome. The ascitic fluid cytology was positive for adenocarcinoma, as shown in figure 1. Immunohistochemical finding was positive for cytokeratin 7 (CK7), MOC-31 and CDX-2, as shown in figures 2–4. MRI of the abdomen showed numerous masses throughout the liver, suspicious for metastatic disease, cystic lesions in the pancreatic body associated with pancreatic tail atrophy and pancreatic duct dilatation as shown in figures 5 and 6.
Figure 1.
(A) Thin prep, papanicolaou stain, ×400. (B) Cell block, H&E stain, ×200: the ascitic fluid in this patient shows three-dimensional clusters of epithelial cells with smooth community borders, raising the suspicion for adenocarcinoma. No windows are present. Background shows inflammatory cells.
Figure 2.
Ascitic fluid specimen: positive immunohistochemical stain for CK7 (shows epithelial origin of malignant cells).
Figure 3.
Ascitic fluid specimen: positive immunohistochemical stain for MOC31 (this favours adenocarcinoma over malignant mesothelioma).
Figure 4.
Ascitic fluid specimen: focal positivity for CDX2 immunohistochemical stain.
Figure 5.
MRI of the abdomen: numerous T1 hypointense, T2 intermediate to hyperintense lesions are present throughout the liver involving the left and right lobes. These are highly suspicious for metastatic disease.
Figure 6.
MRI of the abdomen demonstrating multiple cystic masses within the pancreas body, the largest measuring 29×19×18 mm. In the distal body in the pancreatic tail, proximal to the cystic lesions, there is pancreatic duct dilatation measuring up to 6 mm and parenchymal atrophy indicating ductal obstruction.
Outcome and follow-up
During the course of hospitalisation, he started developing slurred speech, mild confusion and behavioural changes. CT scan of head did not reveal acute or chronic infarct or bleed. The ammonia levels were 104 µmol/L. He was diagnosed with grade 1 hepatic encephalopathy. The patient remained unstable and a core biopsy could not be performed. Tumour markers revealed: Carcinoembryonic antigen (CEA): 8.8 u/mL, Alpha Fetoprotein(AFP): 6.3 u/mL and Cancer Antigen 19-9 (CA 19–9): 17 828 u/mL, supporting the diagnosis of pancreatic cancer. He continued to deteriorate and gradually developed grade 4 encephalopathy and died of sudden cardiac arrest within 2 weeks of admission.
Discussion
ALF is a rare but critical illness that occurs either in patients who do not have preexisting liver disease or have an undiagnosed underlying condition. The clinical picture includes hepatic dysfunction, abnormal liver biochemical values, impaired synthetic function and or encephalopathy with multiorgan failure. ALF can be classified as hyperacute (<7 days), acute (7–21 days) or subacute (>21 days and <26 weeks).15 Our patient presented with a chief problem of jaundice over the last 14 days with an underlying weight loss over the last 8 months suggested acuity of liver failure in likely a pre-existing underlying condition.
A thorough history and physical examination is imperative to determine the aetiology of ALF. Our patient had constitutional signs and symptoms for more than 6 months determining the chronicity and systemic nature of the disease. However, symptoms of ALF do not generally help distinguish between the aetiology as seen in our case where the symptoms were rather non-specific. Previous studies have also shown that patients with metastasis induced ALF may report non-specific prodromal symptoms such as malaise, weight loss, right upper quadrant abdominal pain and fever. Jaundice usually develops later in the disease course and often indicates the onset of ALF.5 6
Laboratory evaluations include prothrombin time/INR, serum chemistries, liver blood tests, complete blood count, acetaminophen level, toxicology screen, viral hepatitis serologies, HIV, autoimmune markers, ammonia levels and abdominal ultrasonography. Other investigations are specific to the diagnosis and include CT scan or MRIof the abdomen to rule out metastases. Liver biopsy may be considered for suspected liver metastases, autoimmune hepatitis and Wilson’s disease.16 Especially in some cases, radiological evidence maybe absent altogether and histological/cytopathological evidence of malignancy is imperative.12 17 Often, radiological and clinical picture may mimic cirrhosis. This ‘pseudocirrhosis’ is a result of a desmoplastic response of the liver. The infiltrating tumour and the associated inflammation causes the activation of stellate cells which produce collagen, causing extensive fibrosis. This extensive fibrosis results in the atrophy of hepatocytes.12 18 19 In such cases, the histopathology or cytology is diagnostic. Tumour markers are also helpful in diagnosing and differentiating the primary malignancy.
Even in the setting of known or suspected malignancy, other etiologies of ALF should be considered. In our case, we first excluded all other causes of ALF prior to concluding malignancy as a cause. Our patient had a cholestatic picture of ALF as evident by direct bilirubinemia and elevated alkaline phosphatase. Malignancy induced ALF is a rare initial presentation of a solid tumour and portends a poor prognosis. On review of literature, there are very few case reports of solid malignancy presenting with sudden hepatic decompensation and no case reports in database on pancreatic cancer with such a dramatic presentation.5 20
The mechanism of ALF in malignancy is explained by infiltration of small intrahepatic bile ducts by metastatic cells which may result in extensive cholangitis, duct necrosis and subsequent ALF. Also, infiltration of liver parenchyma by tumour cells results in hepatocyte destruction and can result in subsequent ALF. It has been hypothesised that loss of adhesion molecules such as E-cadherin and CD4+ as well as ischaemia-induced by infiltration of hepatic vessels by tumour cells could be a possible mechanism.5 7
In almost all cases of malignancy causing ALF, rapid deterioration of liver failure is inevitable and the prognosis is dismal. As in our patient, patients with ALF should be treated with supportive care and intensive medical management. Although liver transplantation might be the only salvageable therapy, the presence of active malignancy is an absolute contraindication for liver transplantation, limiting the options for further management in patients with malignancy-induced ALF. Cases of liver transplantation in patients with malignancy induced ALF were previously reported, however, data are limited and this practice is controvertial.15 Chemotherapeutic agents, although have a role, its utility is extremely limited as the patients with impaired hepatic function are often not suitable candidates for chemotherapy given concomitant immunosuppressed states, infection and multiorgan failure.15 21
Learning points.
Although rare, physicians must have a high index of suspicion to consider cancer as an aetiology when approaching a case of acute liver failure (ALF) after excluding other common causes.
A thorough history, physical examination and appropriate laboratory and radiological imaging are imperative in differentiating and diagnosing the aetiology of the ALF.
Even though the prognosis remains poor, early recognition of the aetiology of ALF could impact at least short-term survival in this patient population.
Patients often require prompt workup and medical management in an intensive care unit preferably by a multidisciplinary team of physicians.
Treatment options are limited as metastatic malignancy with poor survival is a contraindication to liver transplantation, and the role of chemotherapy is limited, given hepatic dysfunction.
Footnotes
Contributors: PG, MP and TG involved in conception, design and managing the patient. PG, SD, MP and TG involved in drafting the work and revising critically, and finally approved the manuscript to be published. All authors agreed to be accountable for all aspects of work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Next of kin consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.O'Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet 1993;342:273–5. 10.1016/0140-6736(93)91818-7 [DOI] [PubMed] [Google Scholar]
- 2.Patton H, Misel M, Gish RG. Acute liver failure in adults: an evidence-based management protocol for clinicians. Gastroenterol Hepatol 2012;8:161–212. [PMC free article] [PubMed] [Google Scholar]
- 3.Tavabie OD, Bernal W. How to manage: acute liver failure. Frontline Gastroenterol 2020;11:70–4. 10.1136/flgastro-2018-101105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obenauf AC, Massagué J. Surviving at a distance: organ-specific metastasis. Trends Cancer 2015;1:76–91. 10.1016/j.trecan.2015.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rowbotham D, Wendon J, Williams R. Acute liver failure secondary to hepatic infiltration: a single centre experience of 18 cases. Gut 1998;42:576–80. 10.1136/gut.42.4.576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanamornroongruang S, Sangchay N. Acute liver failure associated with diffuse liver infiltration by metastatic breast carcinoma: a case report. Oncol Lett 2013;5:1250–2. 10.3892/ol.2013.1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nazario HE, Lepe R, Trotter JF. Metastatic breast cancer presenting as acute liver failure. Gastroenterol Hepatol 2011;7:65–6. [PMC free article] [PubMed] [Google Scholar]
- 8.Hussain SZ, Jaiswal A, Bader AA, et al. Fatal acute liver failure in a child with metastatic gastric adenocarcinoma. J Pediatr Gastroenterol Nutr 2006;43:116–8. 10.1097/01.mpg.0000189365.91792.bf [DOI] [PubMed] [Google Scholar]
- 9.van Marcke C, Coulier B, Gielen I, et al. Acute liver failure secondary to metastatic liver infiltration: case report and review of the literature. Acta Gastroenterol Belg 2013;76:436–8. [PubMed] [Google Scholar]
- 10.Bhat YM, Krasinskas A, Craig FE, et al. Acute liver failure as an initial manifestation of an infiltrative hematolymphoid malignancy. Dig Dis Sci 2006;51:63–7. 10.1007/s10620-006-3085-3 [DOI] [PubMed] [Google Scholar]
- 11.Kheyri Z, Ali Asgari A, Zare Mehrjerdi A, et al. Fulminant hepatic failure due to primary hepatic lymphoma: a case report. Middle East J Dig Dis 2013;5:168–70. [PMC free article] [PubMed] [Google Scholar]
- 12.Bernardo S, Carvalhana S, Antunes T, et al. A rare cause of acute liver failure- a case report. BMC Gastroenterol 2017;17:166. 10.1186/s12876-017-0730-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogata T, Kikawa Y, Ogata M, et al. Acute liver failure with diffuse liver metastasis from breast cancer, not detected by computed tomography: 2 case reports. Case Rep Oncol 2018;11:699–704. 10.1159/000493848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varghese J, Jayanthi V, Patra S, et al. Massive infiltration of liver by metastatic adenocarcinoma: a rare cause of acute hepatic failure. J Clin Exp Hepatol 2012;2:286–8. 10.1016/j.jceh.2012.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernal W, Wendon J. Acute liver failure. N Engl J Med 2013;369:2525–34. 10.1056/NEJMra1208937 [DOI] [PubMed] [Google Scholar]
- 16.Lee WM, Stravitz TR, Larson AM. Introduction to the revised American association for the study of liver diseases position paper on acute liver failure. Available: http://www.aasld.org/practiceguidelines/Documents/AcuteLiverFailureUpdate2011.pdf [DOI] [PMC free article] [PubMed]
- 17.Millard T, Gupta A, Brenin C, et al. Radiographically occult carcinomatous spread of breast cancer to the liver: a challenging case. Case Rep Oncol Med 2019;2019:1–5. 10.1155/2019/4935615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sass DA, Clark K, Grzybicki D, et al. Diffuse desmoplastic metastatic breast cancer simulating cirrhosis with severe portal hypertension: a case of "pseudocirrhosis". Dig Dis Sci 2007;52:749–52. 10.1007/s10620-006-9332-9 [DOI] [PubMed] [Google Scholar]
- 19.Mogrovejo E, Manickam P, Amin M, et al. Characterization of the syndrome of acute liver failure caused by metastases from breast carcinoma. Dig Dis Sci 2014;59:724–36. 10.1007/s10620-013-2943-z [DOI] [PubMed] [Google Scholar]
- 20.Rich NE, Sanders C, Hughes RS, et al. Malignant infiltration of the liver presenting as acute liver failure. Clin Gastroenterol Hepatol 2015;13:1025–8. 10.1016/j.cgh.2014.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giuliani J, Bonetti A. Acute liver failure caused by metastatic breast cancer: can we expect some results from chemotherapy? Dig Dis Sci 2015;60:2541–3. 10.1007/s10620-015-3741-6 [DOI] [PubMed] [Google Scholar]