Abstract
Background
Pulmonary arterial hypertension (PAH) is a severe chronic condition associated with poor quality of life and high risks of mortality and hospitalisation. The utilisation of novel diagnostic technologies has improved survival rates although the effectiveness of Electronic Health (eHealth) interventions in patients with a chronic cardiopulmonary disease remains controversial. As the effectiveness of eHealth can be established by specific evaluation for different chronic health conditions, the aim of this study was to explore and summarise the utilisation of eHealth in PAH.
Method
We searched PubMed, CINAHL and Embase for all studies reporting clinical trials on eHealth solutions for the management of PAH. No limitations in terms of study design or date of publication were imposed.
Results
18 studies (6 peer-reviewed journal papers and 12 conference papers) were identified. Seven studies addressed the accuracy, safety or reliability of eHealth technologies such as intra-arterial haemodynamic monitoring of the pulmonary artery pressure, self-administered 6-Minute walk test App, computerised step-pulse oximeter and ambulatory impedance cardiography. Two studies evaluated eHealth as part of the medical management and showed a reduction in hospitalisation rate.
Conclusions
The evidence of eHealth supporting the management of people with PAH is limited and only embraced through a few studies of small sample size and short-term duration. Given the proposed clinical benefits in heart failure, we postulate that the evaluation of eHealth for the clinical management of PAH is highly warranted.
Keywords: patient care, health care
Introduction
Pulmonary arterial hypertension (PAH) is an incurable and progressive disease caused by the narrowing of the blood vessels of the lungs. This disease is characterised by high blood pressure in the pulmonary artery that, if maintained over time, may lead to right heart failure and premature death.1 PAH is a complex and multifactorial chronic disease2 that pervades the holistic functioning of the individuals and their quality of life (QoL).3 Over the past few years, the efficacy of combination drug therapy and the utilisation of diagnostic technologies (including imaging techniques and right heart catheterisation) have contributed to increasing the efficacy of therapeutic regimes, reducing morbidity and mortality in people with PAH.4 5
The management of PAH is complex and requires intense and regular communication, in the first place between the patient and caregivers, but also among all members of the highly specialised centre-based multidisciplinary care team6 and the external caregivers, including primary care centres, regional hospitals, supporting family and carers. In PAH care, all contributions to enhance communication can be of particular interest, for example, during the almost inevitable periods of drug modification or up-titration, or when drug side effects may potentially appear.7
Electronic Health (eHealth), which is referred to as a broad range of informatics applications for facilitating the management and delivery of healthcare,8 has been proposed to support the management of patients with chronic disease and as a possible tool to improve clinical outcomes in patients with heart failure (HF) and chronic obstructive pulmonary disease (COPD).9 10 The adoption of eHealth enables remote monitoring of patients and helps to identify early symptoms and respond promptly to exacerbations.11 In addition, eHealth innovations enable self-management and contribute to shifting the balance of power and responsibility from healthcare professionals to patients,12 which in turn is closely related to improvements in health-related QoL.13 In addition, mobile health (mHealth), which is a subset of eHealth and defined as the medical and public health practice supported by mobile devices, such as mobile phones, patient monitoring devices, personal digital assistants and other wireless devices,14 has been envisioned to play an important role for the management of chronic conditions.15 As the impact of eHealth needs to be established specifically for different targeted populations,16 the objective of this study was reviewing the existing literature in an attempt to better understand what types of eHealth solutions have been tested, and the possible benefits that eHealth has brought about for the management of people with PAH.
Methods
Search strategy
We searched PubMed, CINAHL and Embase for studies on eHealth interventions for people with PAH. A combination of MeSH terms and free-text keywords pertaining to the two main concepts of interest (ie, eHealth and pulmonary arterial hypertension) was used to develop a sensitive search query following the recent guides on PubMed searches.17–19 The detailed search strategy for PubMed is available (online supplementary appendix 1). The search query was modified for CINAHL and Embase according to their user guide. The results of electronic searches were exported to an EndNote library, and duplicate records were removed.
bmjhci-2020-100176supp001.pdf (105.5KB, pdf)
Study selection
Two independent reviewers screened the records at the title/abstract level to identify potentially relevant studies. The inclusion criteria were journal papers as well as conference proceedings in English language in which eHealth had been used for improving the care of people with PAH, with no limitations on date of publication. The exclusion criteria were non-clinical studies (either pharmacological or technology-focused publications related to the development of technology devices), clinical trials published in a language other than English, expert opinions and letters to the Editor. Research articles on imaging and echocardiography, as well as reported surgical procedures, were excluded as considered out of the scope of this review.
Data extraction
The full text of potentially relevant papers was obtained for a further check against the study selection criteria and determining the final set of included papers for review. We extracted the following data: author and country, characteristics of the study (retrospective or prospective cohort study or case-series), trial quality (level of evidence, according to the Centre for Evidence-Based Medicine [CEBM], Oxford University),20 the year and the type of publication (conference paper or journal paper), the study population, brief descriptions of the eHealth solution and the results of the study. Some of the authors had conducted more than one study on the same eHealth solution. For simplicity reasons, all studies presented by the same group of researchers are presented in a single row of the table, indicating their correspondent author’s name, type and year of publication.
Results
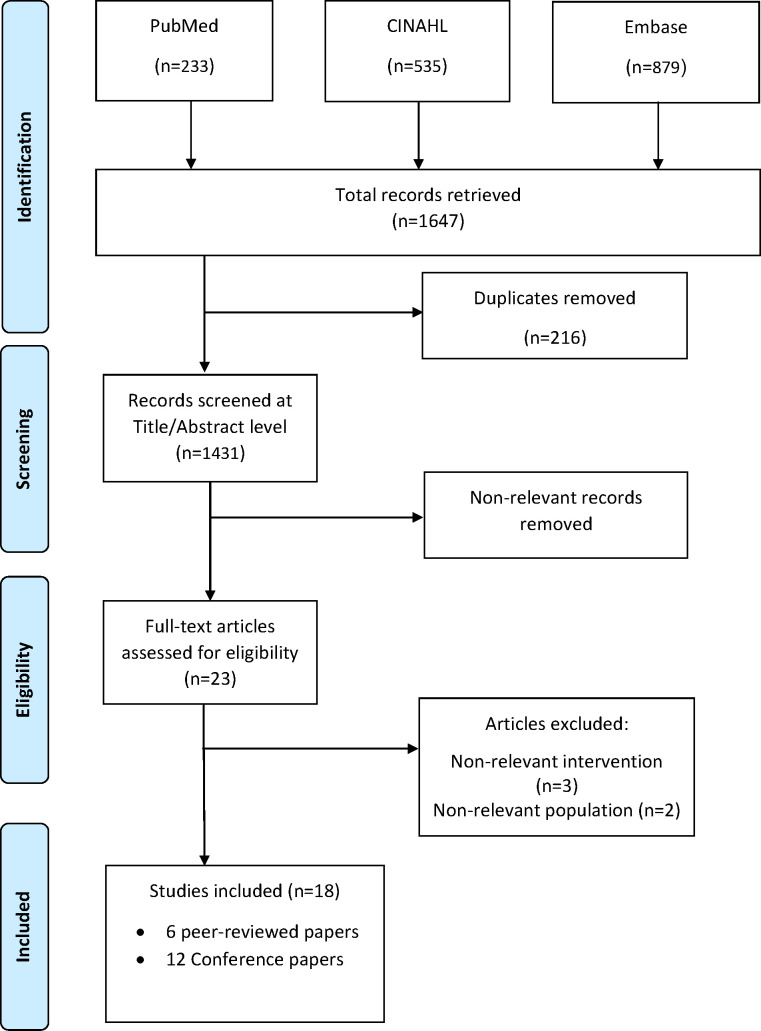
The electronic search resulted in 1431 unique records. Of these, 1406 non-relevant records were filtered out at title/abstract screening, based on the exclusion criteria. The full text of 23 studies were inspected for eligibility. Of these, five papers were excluded either due to non-relevant study setting (eg, the purpose of the trial was not assessing eHealth) or non-relevant patient population (eg, patients with HF without PAH). Eighteen studies were considered eligible for inclusion in this review: 6 peer-reviewed journal papers and 12 conference papers published in scientific journals. The PRISMA flow diagram of study selection is shown in figure 1. To facilitate the analysis and the discussion of the findings of this review, we split the studies into two groups: one group includes the studies that aimed to test the validity and/or the reliability of a specific eHealth solution (table 1), and the other group includes the studies that evaluated eHealth as part of the medical management of PAH, with the possibility of reporting clinical endpoints such as hospitalisation and QoL (table 2).
Figure 1.
PRISMA flow diagram of the screening and selection of the studies.
Table 1.
Summary of studies evaluating the validity/reliability of eHealth for PAH
| Author Country (year of publication) |
Type of study/level of evidence (CEBM)/type of publication | Study population | Intervention | Summary of results | Significance level |
| Arelli et al
USA (2012)37 |
Case-series/level 4/CP | 18 patients with right heart catheterisation–confirmed PH | A portable IC device with real-time wireless monitoring via a Bluetooth adapter to determine HR, CO, CI and SV during the 6MWT | The IC increased the value of 6MWT and provided insight into the haemodynamic changes during exercise in PH | All the comparisons at rest vs activity were statistically significant (p<0.001) |
| Biederman et al
USA (2015)28 |
Case-series study/level 4/CP | 10 patients with PAH | Longitudinal CardioMEMS measuring of PAP, HR and CO and CMR at baseline and during pharmacological stress were performed | Cardiomems may contribute to providing an accurate calculation of non-invasive CO in PAH | NR |
| Brooks et al
USA (2014, 2015)31 32 |
|
CP: 52 participants half of whom had CHF and PH JP: 103 participants with PH and CHF |
SA-6MWT app for independent use at home |
|
|
| Fox et al
Israel (2011, 2013)35 36 |
|
|
Step oximetry system linked to a computer | 1. The step-oximetry test was an informative test of functional capacity among patients with PH. 2. Patients with PH showed significant limitation in step climbing ability that correlated with functional class and 6MWT |
|
| Fruhwald et al
Austria (2003)29 |
Case-series study/level 4/JP | 5 patients with PAH who had received long-term treatment with aerosolized Iloprost | IHM in patients who had received long-term treatment with Iloprost | The IHM enabled reproducible measuring of drug-induced variations in PAP | Mean PAP from 68±13 to 49±11 mm Hg (mean time of 49±8 min). Thereafter, PAP returned to pre-inhalation levels |
| Gregorietti et al
Argentina (2015 and 2016)33 34 |
Case-series study/level 4/CP | 162 and 118 patients, respectively, with PH | Utilisation of a DP during the 6MWT, and its possible association with other parameters of clinical and prognostic relevance in PH | The addition of a DP to the 6MWT provided valuable data for evaluation and follow-up of patients with PH The number of footsteps was inversely correlated to clinical and laboratory parameters of prognostic relevance in PAH |
In both studies, there were significant associations between pro-BNP levels and the number of footsteps (p=0.001) and WHO functional class (p=0.003 and p=0.009, respectively) |
| Tonelli et al
USA (2013, 2014)38 39 |
Prospective cohort study with good follow-up (>80%)/level 1b/
|
|
A portable impedance cardiography with wireless monitoring via a Bluetooth to determine HR and CO during a 6MWT and HR acceleration and decay slopes during the 6MWT |
|
All the comparisons in HR curves and acceleration rates were statistically significant (p<0.001) |
CEBM, Centre for Evidence-Based Medicine; CHF, congestive heart failure; CI, cardiac index; CMR, cardiovascular magnetic resonance; CO, cardiac output; CP, conference paper; CV, coefficient of variation; DLCO, diffusing capacity of the lungs for carbon monoxide; DP, digital pedometer; HR, heart rate; IC, impedance cardiography; IHM, implantable haemodynamic monitor; JP, journal paper; 6MWT, 6-minute walk test; 6MWTApp, 6-minute walk test app; NR, not reported; PAH, pulmonary arterial hypertension; PAP, positive airway pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; SV, stroke volume.
Table 2.
Summary of studies evaluating eHealth as part of the medical management in PAH
| Author/country | Type of study/level of evidence (CEBM)/type (and year) of publication | Study population | Intervention | Results | Significance level |
| Benza et al
USA21–26 |
|
11 patients with advanced PAH, 6 of them with a recent hospitalisation for RHF CHAMPION study population |
Haemodynamically guided management of patients through Cardiomems |
|
1. 36% reduction in HF-related hospitalisation rates (0.60 vs 0.94, HR 0.64, 95% CI 0.51 to 0.81, p=0.0002) 2. Composite endpoint: HR 0.74, 95% CI 0.55 to 0.99, p=0.04 Survival: HR 0.78, 95% CI 0.50 to 1.22, p=0.28 |
| Raina et al
USA30 |
Retrospective cohort study/level 2b/CP (2014) | 314 WHO Group 2 patients with PH from the CHAMPION study | Retrospective analysis of Cardiomems patients | Cardiomems associated with hospitalisation reductions in Group 2 patients with PAH | HR 0.64, 95% CI 0.51 to 0.81 |
CEBM: Centre for Evidence-Based Medicine, Oxford University(https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/)). CHAMPION study population: 550 patients with heart failure with Cardiomems; 314 of them with WHO Group 2 PAH.
CEBM, Centre for Evidence-Based Medicine; CP, conference paper; JP, journal paper; PAH, pulmonary arterial hypertension; PAP, pulmonary arterial pressure; PH, pulmonary hypertension; RHF, right heart failure.
In the reviewed studies, four different eHealth solutions have been tested: (1) wireless implantable haemodynamic monitor (IHM) to measure pulmonary artery pressure, (2) wireless pedometers and smartphone apps for self-administered 6-minute walk test (6MWT), (3) step-oximetry devices to capture wireless information on exercise capacity and (4) ambulatory impedance cardiography to evaluate haemodynamic variables during all daytime activity. The findings are summarised as follows:
Benza et al explored the feasibility and safety of CardioMEMS® (Abbott Laboratories; Atlanta, USA) demonstrating that its use was safe at rest, during exercise21–23 and in combination with the use of cardiac MRI,24 and was associated with a greater pulmonary artery pressure reduction through medication changes in the ambulatory setting.25 The use of CardioMEMS was associated with a 36% reduction in heart failure–related hospitalisation rates (0.60 vs 0.94, HR 0.64, 95% CI 0.51 to 0.81, p=0.00020).26 In a retrospective analysis of the CHAMPION trial, 314 patients with HF who met the definition of pulmonary hypertension (PH) (151 with CardioMEMS vs 163 control) were followed for an average of 15 months. The CardioMEMS group experienced a reduction in a composite endpoint of death and HF hospitalisation (HR 0.74, 95% CI 0.55 to 0.99, p=0.04).27 Biederman et al showed that the use of CardioMEMS allowed reliable measurements of right ventricle cardiac output.28 Fruhwald et al used an early model of IHM (Chronicle, model 9520; Medtronic, USA) to demonstrate the need for improvement of treatment models with long-term aerosolised Iloprost.29 Raina et al conducted a retrospective analysis of the CHAMPION trial concluding that in patients with HF with marked limitation of their physical activity, those with pulmonary hypertension (Group 2 of the WHO classification) had higher hospitalisation rates than those without pulmonary hypertension (0.77/year vs 0.37/year, HR 0.49, 95% CI 0.39 to 0.61), and that in patients with and without PH, ongoing knowledge of CardioMEMS data resulted in a significant reduction in HF hospitalisation (HR 0.64, 95% CI 0.51 to 0.81 for patients with PH and HR 0.60, 95% CI 0.41 to 0.89 for non-PH). The relative risk reduction (RRR) was lowest in the patients with transpulmonary pressure gradient >15 mm Hg (RRR 30%) and pulmonary vascular resistance >3 Woods Units (RRR 33%). In addition, there was a non-significant trend towards improved survival with knowledge of CardioMEMS parameters (HR 0.78, 95% CI 0.50 to 1.22).30
Brooks et al and Gregorietti et al showed the feasibility and accuracy of a smartphone-based self-administered 6-MWT (6MWTApp) and a digital pedometer in measuring physical activity level and exercise capacity. The number of footsteps during the 6MWT correlated with clinical and laboratory parameters of prognostic relevance in patients with PH such as pro-BNP levels and WHO functional class and provided valuable data for the evaluation and the follow-up of people with PH.31–34
Fox et al demonstrated that a step oximetry system equipped with pressure sensors and pulse oximeter linked to a computer provided useful information on the functional capacity of patients with WHO group 1 PAH (idiopathic form of PAH) and group 4 PAH (chronic thromboembolic PH), strongly correlating with functional class and 6MWT walk distance.35 36
Arelli et al showed a real-time wireless impedance cardiography (Physioflow Enduro; Manatec Biomedical, Paris, France) for non-invasive determination of haemodynamic parameters in pulmonary hypertension patients during their 6MWT. Stroke volume, cardiac output and cardiac index increased with activity in direct relation to the distance walked, providing valuable insights into the haemodynamic changes during exercise.37 Other authors (Tonelli et al) used this portable device to enable continuous non-invasive evaluation of haemodynamic variables on a beat-to-beat basis during all daytime activity. In another study, the same researchers demonstrated that the acceleration and decay of the heart rate slopes during a 6MWT exercise in patients with PAH were different compared with lung airway diseases and healthy controls.38 39
Discussion
To our knowledge, this is the first literature review investigating the use of eHealth in PAH. In general, the reported eHealth solutions have demonstrated to be safe, accurate and reliable, and could contribute to the clinical management of people with pulmonary hypertension. In particular, the reported association between CardioMEMS and a reduction in hospitalisations rates in PAH Group 2 (patients with left-sided HF and concomitant PH) appears promising. In another study (based on the same HF population), the use of this device was associated with a reduction in a composite endpoint of death and HF hospitalisation, although without any difference in survival. CardioMEMS combined with CMR could also contribute to providing an accurate calculation of non-invasive cardiac output, necessary to estimate non-invasive pulmonary vascular resistance, a key feature in the diagnosis of patients with PAH.
The smartphone-based self-administered 6MWT app (SA-6MWT App) demonstrated to be accurate and reliable at clinics and at home. A digital footstep counting and a computerised step-pulse oximeter during the 6MWT added valuable data for evaluation and follow-up of these patients, showing a strong correlation to clinical and laboratory parameters of prognostic relevance in PAH. The ambulatory impedance cardiography increased the value of 6MWT providing valuable insights into the haemodynamic changes that occur during exercise in patients with PAH, under treatment and in comparison, with other lung diseases and healthy controls.
In general, this review highlights a lack of studies assessing the impact of eHealth on hard clinical endpoints (such as mortality and hospitalisations) and on QoL. This makes it difficult to draw further conclusions on the effects that the introduction of eHealth may have for the people living with PAH, their health professionals and their families. However, based on promising reports in the HF domain,40 41 possible benefits of using eHealth in the management of PAH can be postulated.
In particular, some additional observations may be considered. First, looking at the characteristics of the study participants, most participants pertained to Group 2 of the WHO classification, haemodynamically referred to as post-capillary PAH. This is a crucial observation as the patients with left-sided HF have a completely different management (and treatment) compared with the WHO Group 1 of patients with PAH (haemodynamically referred to as pre-capillary PAH). This type of PAH includes, along with the idiopathic form of the disease, the connective tissue disease–associated PAH. Both conditions are considered rare diseases42 (or perhaps underdiagnosed).43 These entities present a management challenge for PAH services and health organisations derived from a high dependency of frequent visits at tertiary centres and highly specialised care, with close supervision of health measures, as well as symptoms and treatment monitoring. We propose that any future attempt to trial the impact of eHealth in PAH should only recruit patients with precapillary PAH, excluding participants with PH associated with HF or severe chronic obstructive lung disease.
Second, one of the most important factors determining the success versus failure of an eHealth intervention may be dependent on delivery of an effective medical intervention and the presence or absence of required medical action. Thus, it is important to examine the whole workflow that the eHealth intervention will support and the impact on the clinical management.44 In the present review, only two studies25 30 considered the use of eHealth as part of the medical management (table 2). In both cases, most of the patients pertained to the post-capillary form of PAH, which represents a serious limitation in terms of validating the usefulness of these eHealth interventions in patients living with precapillary forms of PAH.
Third, in the two studies referred to in table 2, the use of eHealth showed reductions in hospitalisation and, in one of them, a non-significant trend towards improved survival.26 30 Again, it was not possible to extrapolate this positive outcome to the patients with the precapillary form of the disease, as most of the participants included in these studies were patients with post-capillary PH.
Lastly, none of these studies assessed the impact that eHealth has on the well-being of the patients, usually represented by the assessment of QoL. Hence, the assessment of QoL is of great interest in PAH,44 as in any other incurable disease where new treatments with small survival benefits may be offset by QoL deterioration.45 Thus, specific tools have been developed to measure QoL in people with PAH.46
Opportunities and future directions
Since most of the patients with PAH live geographically away from highly specialised PAH centres (especially in sparsely populated areas), these patients generally need to travel long distances for their regular clinical appointments. The remote monitoring of health data and vital signs (such as blood pressure, heart rate and oxygen saturation [at rest or during exercise]) could bring some benefits.41 47 This possibility becomes more relevant in such cases where urgent consultations and first-line visits can only be attended by local non-PAH specialised health services.48 The monitoring of clinical symptoms such as weight gain, light-headedness, hypotension, fainting episodes (syncope), chest pain and palpitations or increasing shortness of breath, either at rest, during daily exercise or during home-based pulmonary rehabilitation sessions,49 could contribute to the prompt detection of clinical deterioration and early intervention.
Considering the effects of eHealth in empowering people with chronic disease by improving their self-management skills50 and their QoL,51 a similar positive impact in patients with PAH could be proposed. Unfortunately, based on the current evidence, this hypothesis cannot be corroborated. For this reason, QoL should be considered as the main endpoint in future studies. Some learnings can be taken from eHealth trials in patients with HF. For example, the indistinct inclusion in HF trials of patients with different aetiology (preserved ejection fraction vs reduced ejection fraction) has led to uncertainties in the study outcomes.52 In a similar way, PAH studies should include only patients with pre-capillary forms of PAH, differentiated from left-sided–associated disease, as they are different entities with a dissimilar management and treatment.
Finally, as the success (or failure) of eHealth may rely on delivery of an effective medical intervention, the impact of eHealth on patients with PAH should be evaluated with the technology integrated into their medical management. In addition, the use and grade of satisfaction from patients and caregivers could be a relevant domain to evaluate.
Conclusion
The evidence on the use of eHealth in patients with PAH is scant and mainly envisioned to validate the accuracy and reliability of specific technology tools. However, the potentials of using eHealth to improve the management of patients with PAH needs to be considered. A comprehensive analysis of the impact that the utilisation of eHealth may have on hard clinical outcomes and QoL needs to be addressed. We see this study as a general call for researchers to investigate the impact that eHealth may have on the holistic management of people with PAH.
Footnotes
Twitter: @farhadfatehi
Contributors: MCG-G and FF conceived the study and conducted the search. MCG-G and HD extracted data and synthesised the results. MCG-G wrote the first draft of the manuscript. FF and HD contributed to the writing of the manuscript. MV, MK, IY, RC and JF contributed to the interpretation of data and provided insights to the synthesis of the results. All authors read and approved the final draft of the manuscript.
Funding: FF was financially supported by the Queensland Government through an Advance Queensland Fellowship.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study. No additional data are available for this study.
Ethics statements
Patient consent for publication
Not required.
References
- 1. McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association.. J Am Coll Cardiol 2009;53:1573–619. 10.1016/j.jacc.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Fact sheets: noncommunicable diseases, 2015. Available: http://www.who.int/mediacentre/factsheets/noncommunicable-diseases/en/
- 3. Delcroix M, Howard L. Pulmonary arterial hypertension: the burden of disease and impact on quality of life. Eur Respir Rev 2015;24:621–9. 10.1183/16000617.0063-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sitbon O, Gaine S. Beyond a single pathway: combination therapy in pulmonary arterial hypertension. Eur Respir Rev 2016;25:408–17. 10.1183/16000617.0085-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Badlam JB, Bull TM. Steps forward in the treatment of pulmonary arterial hypertension: latest developments and clinical opportunities. Ther Adv Chronic Dis 2017;8:47–64. 10.1177/2040622317693218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barberà JA, Escribano P, Morales P, et al. [Standards of care in pulmonary hypertension. Consensus Statement of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) and the Spanish Society of Cardiology (SEC)]. Arch Bronconeumol 2008;44:87–99. [PubMed] [Google Scholar]
- 7. Wu RC, Lo V, Rossos P, et al. Improving hospital care and collaborative communications for the 21st century: key recommendations for general internal medicine. Interact J Med Res 2012;1:e9. 10.2196/ijmr.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pagliari C, Sloan D, Gregor P, et al. What is eHealth (4): a scoping exercise to map the field. J Med Internet Res 2005;7:e9. 10.2196/jmir.7.1.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Comín-Colet J, Enjuanes C, Verdú-Rotellar JM, et al. Impact on clinical events and healthcare costs of adding telemedicine to multidisciplinary disease management programmes for heart failure: results of a randomized controlled trial. J Telemed Telecare 2016;22:282–95. 10.1177/1357633X15600583 [DOI] [PubMed] [Google Scholar]
- 10. Tupper OD, Gregersen TL, Ringbaek T, et al. Effect of tele-health care on quality of life in patients with severe COPD: a randomized clinical trial. Int J Chron Obstruct Pulmon Dis 2018;13:2657–62. 10.2147/COPD.S164121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bashi N, Karunanithi M, Fatehi F, et al. Remote monitoring of patients with heart failure: an overview of systematic reviews. J Med Internet Res 2017;19:e18. 10.2196/jmir.6571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alpay L, van der Boog P, Dumaij A. An empowerment-based approach to developing innovative e-health tools for self-management. Health Informatics J 2011;17:247–55. 10.1177/1460458211420089 [DOI] [PubMed] [Google Scholar]
- 13. Cramm JM, Nieboer AP. Disease management: the need for a focus on broader self-management abilities and quality of life. Popul Health Manag 2015;18:246–55. 10.1089/pop.2014.0120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kay M, Santos J, Takane M. mHealth: new horizons for health through mobile technologies, 2011: 66–71. [Google Scholar]
- 15. Varnfield M, Karunanithi M, Lee C-K, et al. Smartphone-based home care model improved use of cardiac rehabilitation in postmyocardial infarction patients: results from a randomised controlled trial. Heart 2014;100:1770–9. 10.1136/heartjnl-2014-305783 [DOI] [PubMed] [Google Scholar]
- 16. Flodgren G, Rachas A, Farmer AJ, et al. Interactive telemedicine: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2015:CD002098. 10.1002/14651858.CD002098.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fatehi F, Gray LC, Wootton R. How to improve your PubMed/MEDLINE searches: 1. background and basic searching. J Telemed Telecare 2013;19:479–86. 10.1177/1357633X13512061 [DOI] [PubMed] [Google Scholar]
- 18. Fatehi F, Gray LC, Wootton R. How to improve your PubMed/MEDLINE searches: 2. display settings, complex search queries and topic searching. J Telemed Telecare 2014;20:44–55. 10.1177/1357633X13517067 [DOI] [PubMed] [Google Scholar]
- 19. Fatehi F, Gray LC, Wootton R. How to improve your PubMed/MEDLINE searches: 3. advanced searching, MeSH and My NCBI. J Telemed Telecare 2014;20:102–12. 10.1177/1357633X13519036 [DOI] [PubMed] [Google Scholar]
- 20. Howick J. Levels of evidence. Oxford university 2009, 2018. Available: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/
- 21. Benza RL, Correa-Jaque P, Thompson DV, et al. Exercise hemodynamics in PAH: lessons utilizing an indwelling hemodynamic monitor. J Heart Lung Transplant 2016;35:S118–9. 10.1016/j.healun.2016.01.326 [DOI] [Google Scholar]
- 22. Benza RL, Doyle M, Cham M, et al. A study to explore the feasibility and safety of using an implantable hemodynamc monitor in PAH patients. J Heart Lung Transplant 2015;34:S142. 10.1016/j.healun.2015.01.383 [DOI] [Google Scholar]
- 23. Benza RL, Doyle M, Jaque PC, et al. A study to explore the feasibility and safety of using Cardiomems HF system in PAH patients. Am J Resp Crit Care 2015;191. [Google Scholar]
- 24. Benza RL, Doyle M, Correa-Jaque P, et al. Exploring the feasibility and safety of the combined use of cardiac MRI and the CardioMEMS™ HF system in PAH: the utility of coincident pressure and volume in RV failure. Am J Resp Crit Care 2015;191. [Google Scholar]
- 25. Benza RL, Raina A, Abraham WT, et al. Pulmonary hypertension related to left heart disease: insight from a wireless implantable hemodynamic monitor. J Heart Lung Transplant 2015;34:329–37. 10.1016/j.healun.2014.04.014 [DOI] [PubMed] [Google Scholar]
- 26. Benza R, Bourge R, Adamson P, et al. Heart failure hospitalizations are reduced in heart failure patients with comorbid pulmonary hypertension using a wireless implanted pulmonary artery pressure monitoring system. J Card Fail 2012;18:S99. 10.1016/j.cardfail.2012.06.378 [DOI] [Google Scholar]
- 27. Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377:658–66. 10.1016/S0140-6736(11)60101-3 [DOI] [PubMed] [Google Scholar]
- 28. Biederman RW, Doyle M, Rayarao G, et al. RV cardiac output calculated from an implantable hemodynamic device: a correlative approach using CMR in PAH patients. Circulation 2015;132. [Google Scholar]
- 29. Fruhwald FM, Kjellström B, Perthold W, et al. Continuous hemodynamic monitoring in pulmonary hypertensive patients treated with inhaled iloprost. Chest 2003;124:351–9. 10.1378/chest.124.1.351 [DOI] [PubMed] [Google Scholar]
- 30. Raina A, Bourge RC, Abraham W, et al. Use of a wireless implantable hemodynamic monitor leads to reductions in heart failure hospitalizations among who group II pulmonary hypertension patients. J Heart Lung Transplant 2014;33:S92. 10.1016/j.healun.2014.01.282 [DOI] [Google Scholar]
- 31. Brooks GC, Vittinghoff E, Iyer S, et al. Diagnostic accuracy of a smartphone based six-minute walk test. Circulation 2014;130. [Google Scholar]
- 32. Brooks GC, Vittinghoff E, Iyer S, et al. Accuracy and usability of a self-administered 6-minute walk test smartphone application. Circ Heart Fail 2015;8:905–13. 10.1161/CIRCHEARTFAILURE.115.002062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gregorietti VE, Bortman GR, Ferreyra RE, et al. Integrating pedometer to the 6MWT in patients with PH. J Heart Lung Transplant 2015;34:S314. 10.1016/j.healun.2015.01.887 [DOI] [Google Scholar]
- 34. Gregorietti V, Perrone S, Machain A, et al. PS162 integrating pro-BNP and pedometer to the 6MWT in patients with PH in public hospital center of Argentina. Glob Heart 2016;11:e40. 10.1016/j.gheart.2016.03.138 [DOI] [Google Scholar]
- 35. Fox B, Hirsch A, Boutet K, et al. Use of a novel step-oximetry test for evaluating functional capacity in patients with pulmonary arterial hypertension. Am J Resp Crit Care 2011;183. [Google Scholar]
- 36. Fox BD, Langleben D, Hirsch A, et al. Step climbing capacity in patients with pulmonary hypertension. Clin Res Cardiol 2013;102:51–61. 10.1007/s00392-012-0495-4 [DOI] [PubMed] [Google Scholar]
- 37. Arelli V, Ramos J, McCarthy K, et al. Determination of hemodynamic parameters during 6-minute walk test in pulmonary hypertension. Am J Resp Crit Care 2012;185. [Google Scholar]
- 38. Tonelli AR, Alkukhun LHR, Arelli V, et al. Measurement of hemodynamic parameters during 6-minute walk test in patients with pulmonary hypertension and healthy controls. Am J Resp Crit Care 2013;187. [Google Scholar]
- 39. Tonelli AR, Wang X-F, Alkukhun L, et al. Heart rate slopes during 6-min walk test in pulmonary arterial hypertension, other lung diseases, and healthy controls. Physiol Rep 2014;2. 10.14814/phy2.12038. [Epub ahead of print: 11 Jun 2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Inglis SC, Clark RA, Dierckx R, et al. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Cochrane Database Syst Rev 2015:CD007228. 10.1002/14651858.CD007228.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet 2018;392:1047–57. 10.1016/S0140-6736(18)31880-4 [DOI] [PubMed] [Google Scholar]
- 42. Lai Y-C, Potoka KC, Champion HC, et al. Pulmonary arterial hypertension: the clinical syndrome. Circ Res 2014;115:115–30. 10.1161/CIRCRESAHA.115.301146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. The Lancet Respiratory Medicine . Pulmonary hypertension: still a rare disease? Lancet Respir Med 2016;4:241. 10.1016/S2213-2600(16)00103-X [DOI] [PubMed] [Google Scholar]
- 44. Granja C, Janssen W, Johansen MA. Factors determining the success and failure of eHealth interventions: systematic review of the literature. J Med Internet Res 2018;20:e10235. 10.2196/10235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gotay CC, Korn EL, McCabe MS, et al. Quality-of-life assessment in cancer treatment protocols: research issues in protocol development. J Natl Cancer Inst 1992;84:575–9. 10.1093/jnci/84.8.575 [DOI] [PubMed] [Google Scholar]
- 46. Ganderton L, Jenkins S, McKenna SP, et al. Validation of the Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR) for the Australian and New Zealand population. Respirology 2011;16:1235–40. 10.1111/j.1440-1843.2011.02030.x [DOI] [PubMed] [Google Scholar]
- 47. Ding H, Moodley Y, Kanagasingam Y, et al. A mobile-health system to manage chronic obstructive pulmonary disease patients at home. Conf Proc IEEE Eng Med Biol Soc 2012;2012:2178–81. 10.1109/EMBC.2012.6346393 [DOI] [PubMed] [Google Scholar]
- 48. Delcroix M, Naeije R. Optimising the management of pulmonary arterial hypertension patients: emergency treatments. Eur Respir Rev 2010;19:204–11. 10.1183/09059180.00004910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chan L, Chin LMK, Kennedy M, et al. Benefits of intensive treadmill exercise training on cardiorespiratory function and quality of life in patients with pulmonary hypertension. Chest 2013;143:333–43. 10.1378/chest.12-0993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Williams V, Price J, Hardinge M, et al. Using a mobile health application to support self-management in COPD: a qualitative study. Br J Gen Pract 2014;64:e392–400. 10.3399/bjgp14X680473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Koch S. Improving quality of life through eHealth - the patient perspective. Stud Health Technol Inform 2012;180:25–9. [PubMed] [Google Scholar]
- 52. Kelly JP, Mentz RJ, Mebazaa A, et al. Patient selection in heart failure with preserved ejection fraction clinical trials. J Am Coll Cardiol 2015;65:1668–82. 10.1016/j.jacc.2015.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjhci-2020-100176supp001.pdf (105.5KB, pdf)
Data Availability Statement
Data sharing not applicable as no datasets generated and/or analysed for this study. No additional data are available for this study.