Introduction
Stroke continues to be one of the prominent causes of mortality and long-term disability among diseases in the USA [1]. Every 40 s, someone has a stroke in the USA. Stroke is primarily classified into two types: ischemic and hemorrhagic. Almost 86% of strokes result from cerebral ischemia, which occurs as a result of interrupted blood flow to the brain from a clot, which leads to oxygen and nutrient deficiency in the brain, ultimately resulting in primary brain injury [2]. After this, secondary injury cascades of the brain can initiate. Depletion of cellular energy leads to intracellular homeostasis impairment and increases intracellular calcium and neurotoxic levels of dopamine and glutamate. This can also lead to ion gradient dissipation because of the interruption of ATP-dependent ion channels, accumulation of reactive oxygen species (ROS), mitochondrial dysfunction, and excitotoxicity [3]. Brain edema, either cellular or vasogenic, are crucial factors that can worsen stroke outcome because of a cellular homeostasis imbalance and the blood–brain barrier (BBB) disruption. The latter increases the uptake of fluids into the brain tissue, raising the intracranial pressure [4]. Therefore, ischemic stroke is a combination of neuronal and vascular disorders. So far, r-tPA is the only US Food and Drug Administration (FDA)-approved drug for the treatment of acute ischemic stroke [5]. More recently, endovascular mechanical thrombectomy has also been used for ischemic stroke treatment in cases of large vessel occlusion. In addition to these reperfusion techniques, neuroprotective strategies have also been investigated in ischemic stroke to improve neuronal survival and stroke outcome [6]. Penumbra, the viable surrounding tissue of the lethally and irreversibly injured ischemic core, can be protected if appropriate treatments are administered quickly following an injury. This is possible because the cells within this area die slowly; therefore, progressively ongoing damage can be prevented with specific targeting. However, delivering neuroprotective therapeutics to the ischemic brain region has always been challenging (Box 1). Different physiological, chemical, and pharmacological approaches can be utilized to facilitate drug delivery in ischemic stroke. Some novel drug delivery strategies are also being investigated in stroke therapy, which could impact ischemic stroke outcome.
BOX 1. Challenges of CNS drug delivery.
Intricacy of brain diseases.
Inefficient technology for brain drug delivery.
Requirement for meticulous understanding of pathophysiological alterations in body following disease.
Existence and structural components of biological barriers: BBB and blood–CSF barrier, including paucity of fenestrations on cerebral microvessels, inefficient, nonspecific, energy, and temperature-dependent pinocytosis, and involvement of adherence, tight, and gap junctional proteins to create a seal between brain microvessel endothelial cells.
Existence of influx and efflux transporters, such as P-gp, Breast Cancer Resistance Protein (BCRP), and Multidrug Resistance Protein-1 (MRP-1).
Enzymatic role of BBB in form of endopeptidases, carboxypeptidases, and aminopeptidases.
Current stroke therapy and limitations
Ischemic stroke displays a complex pathobiology involving several pathways and factors that contribute to ischemic brain damage at different stages after the ischemic episode. Anoxic depolarization, BBB disruption, excitotoxic cell death, oxidative stress, reactive astrogliosis, edema formation, white matter injury, and inflammation are some of the most crucial mechanisms involved in ischemic brain injury and neuronal cell death [3].
Current approaches that have been developed for the treatment of ischemic stroke so far are based on either thrombolysis or neuroprotection. The main goal of treatment in ischemic stroke is to preserve the penumbra in a short period of time and prevent progression of the ischemic core, which worsens the neurological outcomes. The only FDA-approved medication for the treatment of ischemic stroke is r-tPA, which is a thrombolytic agent that lyses the fibrin clots. However, several limitations have been reported of its use, such as the need to administer it within a short period of time after stroke onset in eligible patients who meet the clinical criteria. It also has some possible serious adverse effects, such as hemorrhagic transformation and cellular damage after cerebral blood flow restoration [2]. tPA also upregulates brain matrix metalloproteinases in cerebral endothelial cells. Increased proteolytic activity of these enzymes further complicates stroke outcome by promoting edema, increasing inflammatory signals and degradation of BBB substrates [7]. Furthermore, ischemic stroke has neural components. The pathophysiology of ischemic stroke involves primary and secondary brain damage, which result in neuronal injury. Therefore, alongside improved canalization, there is an obvious demand for neuroprotective approaches that can salvage ischemic brain injury to improve both short and long-term outcomes of ischemic stroke. Recently, endovascular treatment has advanced to be a standard treatment for acute ischemic stroke, especially when large blood vessels are occluded and tPA will not show desired outcomes. Endovascular treatment can improve canalization, which can provide greater opportunities for testing the effectiveness of neuroprotective drugs using localized drug delivery to the site of action.
Neuroprotective therapy of ischemic stroke
Neuroprotective therapies tend to reduce brain injury after acute ischemic stroke by targeting brain parenchyma to reduce toxic molecular and cellular events caused by ischemia (Fig. 1). More than 1000 neuroprotective drugs have been evaluated in preclinical stroke research, several with promising results to offset stroke injury. With these preclinical studies, ~200 neuroprotective clinical trials have been completed to date, unfortunately with little success. Excitotoxic brain damage was identified as the first molecular mechanism to target to ameliorate ischemic brain damage. Interruption of cerebral blood flow causes ATP depletion, resulting in neuronal depolarization. This causes release of the neurotransmitter glutamate, which leads to excessive activation of the ionotropic (AMPA, kainate, and NMDA) glutamatergic receptors. These events further cause enhanced calcium entry and ultimately result in protein, lipid, and DNA damage [8]. Unfortunately, almost all promising therapeutics targeting excitotoxic brain damage were ineffective in clinical trials.
FIGURE 1.
Cellular and molecular target(s) of neuroprotection in acute ischemic stroke. (a) Excitotoxicity. Following oxygen and glucose deprivation, glutamate and calcium are released, which causes excitotoxic neuronal damage by overactivation of ionotropic glutamate receptors. (b) Inflammation. Membrane depolarization causes ATP depletion, resulting in failure of ionic pumps, which in turn causes cytotoxic brain edema. Inflammatory pathways, such as leukocyte infiltration, inflammatory mediators, and microglial activation, also contribute to neuronal cell death. (c) Oxidative stress. Mitochondrial dysfunction leads to reactive oxygen species and free radical generation, resulting in increased oxidative stress. (d) Apoptosis. Apoptosis is also increased in the ischemic brain because of DNA damage and mitochondrial dysfunction. Created with BioRender. Abbreviations: NO, nitric oxide; ROS, reactive oxygen species.
Another crucial step in the pathophysiology of ischemic stroke is the generation of free radicals, which include ROS and reactive nitrogen species (RNS) [9]. Immunological reaction to acute ischemic stroke is also a key part of the ischemic cascade. Thus, oxidative stress and inflammation became major targets for neuroprotection after acute ischemic stroke, which resulted in the concept of treating neurovascular units (NVUs) as a whole in stroke instead of only targeting neurons. Disodium 2,4-disulphophenyl-N-tert-butylnitrone (NXY-059) is a novel nitrone that affects all cells of the NVU by its free radical-trapping properties [10]. Although it showed promising results in preclinical studies [11], it failed to show any neuroprotective actions in clinical trials [12]. Edaravone is another novel free radical scavenger that has demonstrated potent neuroprotective activity by reducing neuronal damage and improving functional outcome in preclinical and clinical stroke studies [13].
The reasons suggested for the shortcomings of preclinical neuroprotective investigations include erroneous dosage, timing, and models used in the preclinical settings. Sufficient rigor of preclinical testing should be maintained to overcome these shortcomings, including multiple dosing, timing, and testing in both permanent occlusion and reperfusion models, and behavioral studies performed in addition to brain tissue damage evaluation. Also, the differences between rodent and human brain must be considered while designing a preclinical study and testing in nonhuman primates should be done after investigations in rodents. The Stroke Therapy Academic Industry Roundtable (STAIR) publication provides further recommendations for the preclinical development of therapeutics for acute ischemic stroke [14,15].
In recent years, research into the roles of the innate immune response to ischemic brain injury has gained traction. Targeting these immunological pathways has resulted in promising neuroprotection in preclinical animal models [16] and some clinical trials [17]. Drugs targeting multiple pathways, exerting pleiotropic effects, in ischemic stroke pathobiology have also shown improved neuroprotection in preclinical stroke studies. DL-3-n-butylphthalide (NBP) provides neuroprotection in ischemic stroke by improving mitochondria health and energy metabolism [11], reducing apoptotic cell death and oxidative injury [18], decreasing inflammatory responses and improving regional blood flow and angiogenesis [19]. Clinical trials have also shown that NBP can improve the outcomes of patients with acute ischemic stroke [20]. Other drugs with multiple mode of actions for neuroprotection in ischemic stroke include statins [21], citicoline [22], and stem cells [23]. Modulation of brain opioid receptors has been demonstrated to exert neuroprotective activities in ischemic stroke by targeting multiple pathways [24]. Given that BBB disruption during ischemic stroke impacts significantly on the long-term outcomes of stroke, novel strategies targeting BBB stabilization are also being considered as promising therapeutic options [5]. Novel drug delivery approaches might also provide effective neuroprotection in stroke because of their multifarious mechanisms of actions and independence from blood flow restoration. Examples include trans-acting activator of transcription (TAT) protein transduction and the nasal delivery of peptides [25]. Ongoing preclinical and clinical studies with investigational agents for stroke neuroprotection are summarized in Table 1. A major challenge to providing effective neuroprotection in stroke is to deliver therapeutics to the appropriate brain regions, where they can exert their maximum beneficial actions. This can be accomplished with novel strategies of brain drug delivery in acute ischemic stroke.
TABLE 1.
Neuroprotective therapeutics currently under investigation for acute ischemic strokea
| Target(s) | Investigational therapeutic(s) | Finding(s) | Refs |
|---|---|---|---|
| Excitotoxicity | Magnesium sulfate | Safe but not effective clinically | [121] |
| Verapamil | Efficacy not proven yet clinically | [122] | |
| NA-1 | Prevented iatrogenic stroke clinically | [123] | |
| Ketamine | Efficacy not proven yet clinically | [124] | |
| Oxidative stress and free radical | NXY-059 | Failed to replicate efficacy in larger clinical trial | [125] |
| Citicoline | Not effective clinically | [22] | |
| Edaravone | Showed promising efficacy clinically | [13] | |
| Uric acid | Safe but not effective clinically | [126] | |
| Immune system | Fingolimod | Can increase therapeutic window for thrombolysis in clinical settings | [127] |
| Natalizumab | Not effective clinically | [128] | |
| Growth factors | G-CSF | Safe and promise of efficacy clinically | [129,130] |
| EPO | Safe and promising efficacy clinically | [131] | |
| VEGF | Promising results in animal models | [132] | |
| BDNF | Promising preclinical results | [133] | |
| Cerebral vasculature | Glyceryl trinitrate | Not effective clinically | [134] |
| mTOR | Sodium hydrosulfide | Promising preclinical results | [135] |
| C21 | [136] | ||
| Melatonin | [137] | ||
| Resveratrol | [138] | ||
| Blood coagulation and inflammation | 3K3A-activated protein C | Promising preclinical results | [139] |
| Multiple targets/pathways | |||
| Metabolism, oxidative stress, collateral blood flow | Albumin | Not effective and exerted toxic adverse effects clinically | [140] |
| Excitotoxicity, free radical | Neu2000KWL | Effective but has safety concerns clinically | [141] |
| Energy metabolism, oxidative stress, inflammation, | NBP | Promising safety and efficacy in clinical settings | [20] |
| angiogenesis | |||
| Metabolism, oxidative stress, hemodilution | Minocycline | Not effective clinically | [142] |
| Free radical, free fatty acid | Citicoline | Not effective clinically | [143] |
| Oxidative stress, inflammation, proliferation | Polyphenols | Promising results shown in preclinical studies | [144] |
| Excitotoxicity, inflammation, free radical | Therapeutic hypothermia | Safe, but efficacy not proven clinically | [145] |
| Excitotoxicity, ischemic tolerance | Remote ischemic conditioning | Promising safety and efficacy in clinical settings | [146] |
Abbreviation: G-CSF, granulocyte-colony stimulating factor.
Drug delivery strategies for improved therapeutic outcomes in ischemic stroke
For the cells in the central nervous system (CNS) to function ideally, specific control of their surrounding microenvironment is vital, and a significant component in this regulation is the BBB. The BBB is a semipermeable physical and functional barrier between the CNS and blood. It preserves brain homeostasis by maintaining the components of extracellular fluid surrounding the neural cells and protecting the CNS from injury, neurotoxins, and pathogens. It also has a role in supplying oxygen and nutrients to brain cells and eliminating waste products and, thus, has both selective and restrictive properties [26]. Different immune, neural, and vascular endothelial cells, astrocytes, and pericytes all have major regulatory roles. They interact in paracrine manner to provide a selective barrier between the brain and CNS that functions as a dynamic structure referred to as the NVU [27]. Several stressors can alter BBB integrity and permeability to increase CNS drug delivery, including heat, inflammation, pain, reperfusion following hypoxia in stroke, and numerous other CNS diseases [28,29].
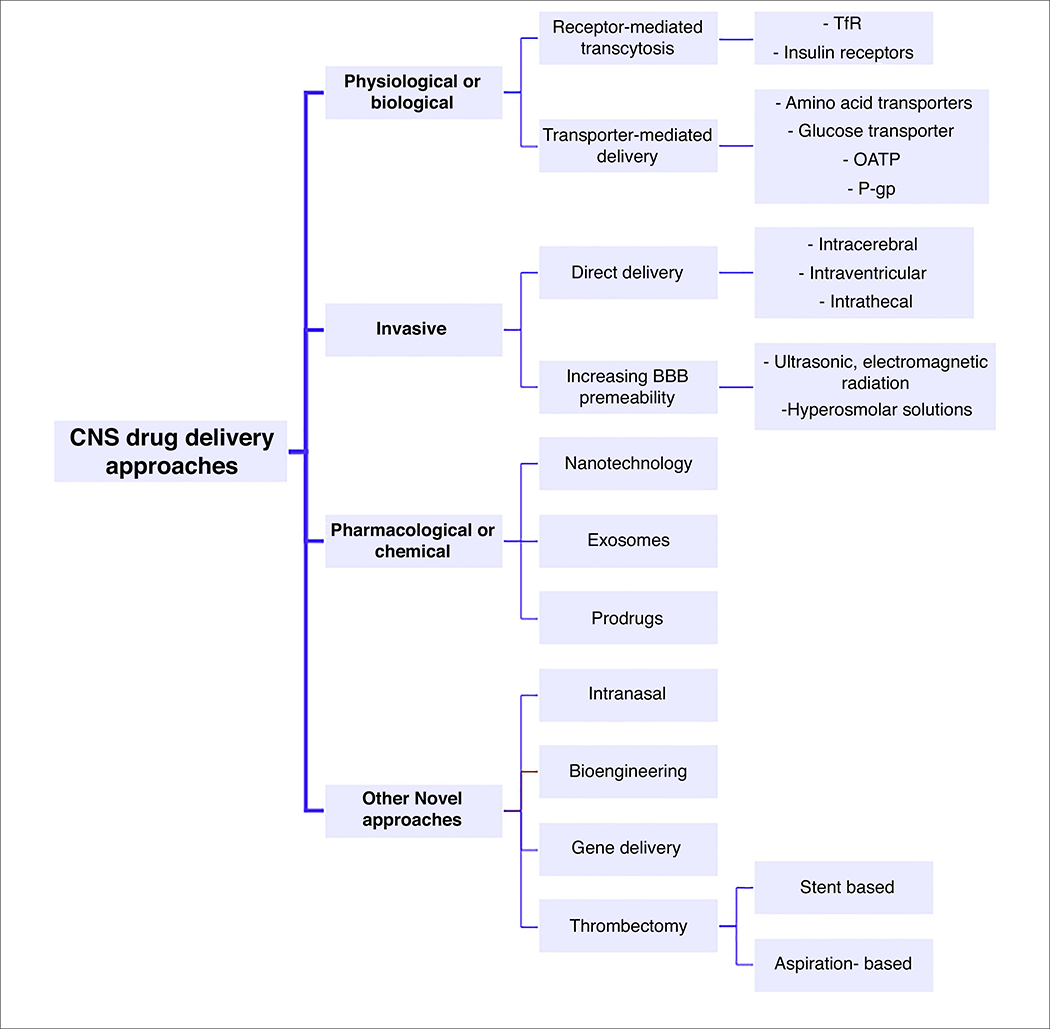
To deliver therapeutics to the brain, they should either bypass the BBB or pass through it without being degraded. This provides a challenge given both the physical and enzymatic barrier elements of the BBB. However, exploiting unique properties of the cerebrovasculature creates promising opportunities for brain drug delivery. Several approaches are being established to augment the concentration of therapeutic compounds in the brain and circumvent the restrictive properties of the BBB (Fig. 2). These strategies are currently categorized into three main procedures: physiological or biological approaches that involve exploiting different transporters and receptors, invasive physical-based approaches that mechanically increase the BBB permeability, and pharmacological or chemical approaches in which the physicochemical properties of the drug molecule are modified to increase their transport across the BBB [30,31].
FIGURE 2.
Overview of different approaches for central nervous system (CNS) drug delivery. Abbreviations: BBB, blood–brain barrier; OATP, organic anion-transporting polypeptide; TfR, transferrin receptor; P-gp, P-glycoprotein.
Physiological approaches
The micro- and/or macromolecules essential for brain metabolism and survival are recognized and taken up by specific transporters or receptors. Drug molecules can be modified or conjugated to substrates of these receptors or transporters to create a pseudonutrient structure that can be carried into the brain. This method is one of the most attractive potential strategies to supersede other approaches for brain drug delivery [32]. To use this approach, various factors should be taken into account, such as considering the suitability of the drug molecular structure required for binding to the receptor or, in the case of structural manipulation, the ability of the drug to maintain its activity toward the CNS target and achieve a high enough brain concentration for proper binding.
Different transporters at the BBB can be targeted for brain drug delivery of both therapeutic agonists and antagonists. Endogenous neutral amino acids, nucleosides, monocarboxylic acids, and some small molecules use transporter-mediated delivery systems. These transporters and/or carriers are members of solute-linked carrier (SLC) transporter families, are expressed on both sides of the cerebral microvascular endothelial cell membrane, and transfer molecules inside and outside of the brain without energy consumption [33]. L-type amino acid transporter 1 (LAT-1) has been used for the delivery of synthesized dopamine prodrugs and lysine-conjugated methotrexate. Brain uptake and brain tissue availability were increased because of the improved affinity of these therapeutics for the receptors [34]. Choline transporters have also been used for the active transport of choline and nicotine analogs into the CNS [35]. Likewise, sodium-independent glucose transporters (GLUT1) are highly expressed at the BBB and are responsible for the transport of hexoses, such as glucose. Peptides conjugated to D-glucose to create glycopeptide derivatives provided higher brain concentrations compared with nonglycosylated peptides. The expression of glucose transporters increases significantly in cerebral endothelial cells during hypoxic conditions [36]. However, these facilitative carriers are also expressed in other organs of the body, which makes them less intriguing for targeted therapy of the CNS [5]. The expression of sodium-dependent glucose cotransporter 1 (SGLT1) also increases in the penumbra after ischemia and reperfusion, and inhibition of this transporter has been reported to improve stroke outcomes and provide antiedematous effects in animal models of ischemic stroke [37]. Therefore, targeting or pharmacological modulation of these transporters with consideration of their characteristics during brain injuries, could provide a suitable therapeutic site or opportunity for CNS drug delivery in stroke.
An additional physiological approach to enhance ischemic brain drug delivery is the use of nucleosides, which have crucial roles in the CNS. Among different nucleosides, implementation of adenosine receptor signaling through administration of receptor agonists was shown to increase BBB permeability temporarily, facilitating the entry of neurotherapeutics into the CNS [38].
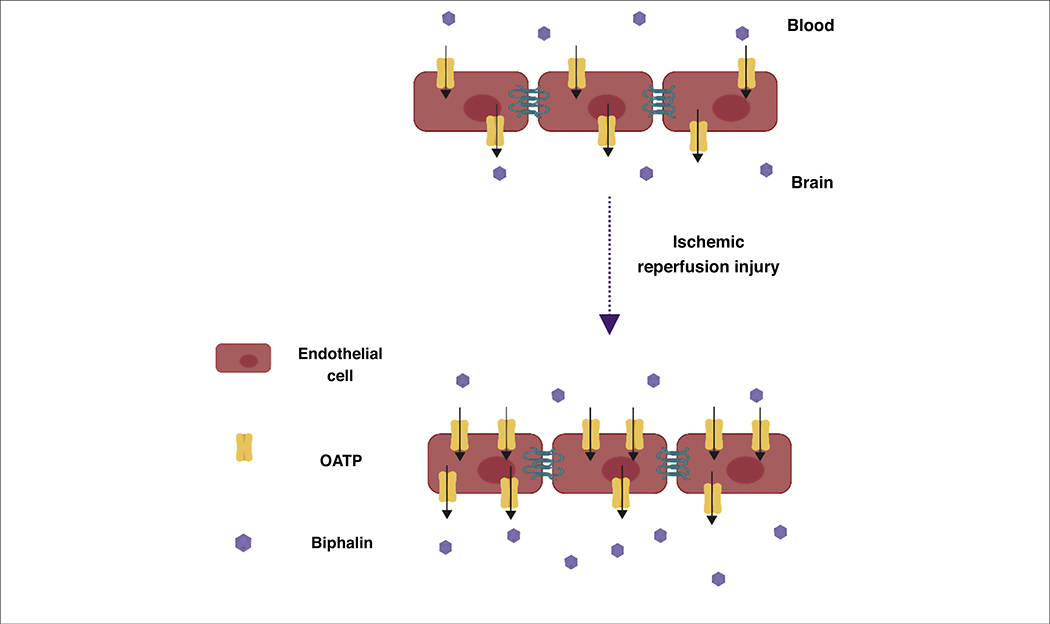
Moreover, brain influx and delivery of some molecules are mediated by groups of unidirectional transport systems, such as organic anion-transporting polypeptides (OATP), which are sodium-independent members of the SLC superfamily. Recently, the expression and activity of OATP1 in induced pluripotent stem cell-derived brain microvascular endothelial cells (iPSC-BMECs) was reported to be increased during oxygen–glucose deprivation (OGD) and reoxygenation. It was also found that OATP1 contributes to the transport of biphalin across the in vitro brain endothelial cell monolayer of a BBB model (Fig. 3). Thompson et al. also reported that the expression of oatp1a4 (an ortholog of human OATP1A2) in rats increased during hypoxia-reoxygenation conditions. It has also been observed that the brain uptake of the oatp1a4 substrates, taurocholate and atorvastatin, increased when administered at the time point of higher oatp1a2 expression [39]. These findings suggest that OATP could be used as a novel target to improve ischemic brain delivery of biphalin, a potent opioid receptor agonist, and also other neurotherapeutics that have affinity for OATP1 at the BBB [40].
FIGURE 3.
Role of organic anion-transporting polypeptide 1 (OATP1) in the increased transport of biphalin across the blood–brain barrier (BBB) during ischemic stroke. The expression of OATP1 in induced pluripotent stem cell-derived brain microvascular endothelial cells (iPSC-BMECs) increases after oxygen glucose deprivation (OGD) and reoxygenation, which also increases the uptake and transport of biphalin in iPSC-BMECs. Modified under the terms of cc-by license from [40].
By contrast, there are receptors responsible for the uptake of large molecules, such as hormones, immunoglobulins, insulin, transferrin, and growth factors, via receptor-mediated transcytosis. Some therapeutic agents can be conjugated to the ligands of these receptors and move with them from the blood to the brain. One such receptor is the transferrin receptor (TfR) which is abundantly expressed at the BBB. Transferrin is a protein that delivers iron to the cells via its specific receptor, located at the luminal side of the BBB. Drugs can be targeted to the TfR by conjugation to transferrin or an engineered antibody that targets TfR. Zhang et al. used antibody conjugation techniques to deliver the high-molecular-weight enzyme, β-galactosidase, to the mouse brain and showed tenfold higher uptake by the brain [41]. Transferrin was also attached to liposomal magnetic nanoparticles (NPs) to increase their transmigration through the BBB. These results indicated that multifunctional nanocarriers increased the transmigration up to twofold without disturbing the BBB integrity [42]. The neuroprotective effect of brain-derived neurotrophic factor (BDNF) was also increased significantly in ischemic injury when conjugated with a murine antibody against TfR, whereas several unconjugated neurotrophins have failed in clinical trials owing to their low BBB permeability [43]. In another study, transferrincoupled liposomes were used for the delivery of vascular endothelial growth factor (VEGF) to ischemic brain. The results showed that this vector enhances the expression of VEGF specifically in brain tissue after a single intravenous administration and improved both the neuroprotection as well as neovascularization in an experimental stroke model [44]. Erythropoietin (EPO) was also conjugated to a TfR monoclonal antibody to create IgG-EPO and the effects were compared with EPO alone in a mouse stroke model. The engineered EPO fusion protein decreased stroke volume and neuronal deficits significantly owing to the higher permeability compared with EPO alone [45].
Insulin receptors of the brain have also been exploited for drug delivery to the CNS. Coloma et al. engineered a chimeric antibody as a vector that targets insulin receptors and its affinity for the receptors was high enough to cause elevated concentrations of the molecules in a primate model [46]. However, the use of these ligands has drawbacks because the administration of the insulin-conjugated drug can trigger hypoglycemia because of the effect of insulin on peripheral tissues. Furthermore, transferrin-conjugated drugs might fail to bind the TfR because of competition between endogenous transferrin and the designed drug. In addition, the capacity of these transporters is limited, and they might fail to deliver a high enough therapeutic payload over time.
It has also been shown that the expression of P-glycoprotein (P-gp) efflux transporters increases at the BBB after cerebral ischemia, which could impede the delivery of therapeutics to the brain. Therefore, either application of inhibitors of these transporters should be taken into account or the drug structure should be manipulated chemically to prevent P-gp-mediated efflux [47]. The other alternative for manipulating P-gp is to interfere with the signaling pathways that regulate the activity and expression of this transporter. For instance, sphingolipid signaling, which reduces P-gp efflux activity, has been targeted by using a sphingosine-1-phosphate analog to enhance the CNS uptake of drugs such as loperamide [48]. However, these strategies should be used cautiously because the long-term application of P-gp inhibitors impairs the protective function of P-gp and increases the risk of toxicity by reducing the drug and xenobiotic elimination capacity of the brain. It has also been shown that one of the pathophysiological adaptions of the BBB during ischemia and/or stroke is elevated endocytic vesicle formation, which contributes to the transcellular entry of the molecules into the brain [49]. This enhanced pinocytosis activity following brain injury can be used to augment drug passage into the brain. Enhanced pinocytotic activity could provide an opportunity for ischemic brain drug delivery, but would need to be exercised with caution because augmentation of this activity could negatively impact vasogenic brain edema in stroke. More investigations into utilizing transcellular transport augmentation are warranted, given that studies also suggest that this mechanism is controlled by intravascular pressure in cerebral endothelial cells [50].
Invasive approaches
Invasive approaches aim to maximize the concentration of the drug that reaches the brain along with minimizing the peripheral tissue exposure and adverse effects. In this method, either drugs are administered selectively into the specific regions of the brain by injection or surgery, or penetration-enhancing techniques are used to temporarily increase the permeability of the drugs through the BBB [51].
Intracerebral, intraventricular, and intrathecal delivery methods include bypassing the BBB and delivering the neurotherapeutics directly into the parenchymal space, cerebral ventricles, and cisterna magna of the brain, respectively [52]. The therapeutic agent can be administered into cerebrospinal fluid (CSF), core, or penumbra of the damaged region or, as a less invasive method, to the epicortical area by bolus injection or continuous infusion, such as convection-enhanced delivery (CED), which can deliver large volumes of drug solutions [53]. These invasive methods have some disadvantages, such as difficulty in placing the catheter in the target region of the brain and the variable distribution of the drugs in the brain because diffusion decreases as the distance from the catheter increases. Furthermore, drug diffusion in the brain depends on the concentration and the physicochemical properties of the drug. A higher amount of drug is required to reach the desired concentration because the diffusion coefficient is limited in the brain. Brain capillary clearance and enzymatic metabolism can also limit the tissue exposure of a drug. Moreover, a common drawback of therapeutic brain infusion is the reflux of drugs along the catheter track, which can prevent continuous infusion, affecting the final distribution volume [54]. The efficacy and feasibility of CED in the delivery of therapeutics to the ischemic brain in rats has been evaluated. It was found that alterations in the brain following ischemic injury, such as cellular or cytotoxic edema, drastically decreased the extracellular space volume and affected the distribution of neurotherapeutic agents delivered by CED. According to these reports, edematous tissue significantly affected the tissue concentration and distribution of the infused drug solution so that infusions occupied a smaller volume of therapeutic space and spread more rapidly to larger regions in the brain, leading to a low average target tissue concentration compared with the control group [55]. Thus, the optimization of techniques, such as image-guided delivery platforms, could aid the delivery of therapeutics to the CNS through invasive methods to further facilitate precise drug delivery techniques.
Another invasive method of brain drug delivery is to increase the permeability of BBB transiently to enhance access to the CNS. This can be achieved by using chemical substances, such as mannitol, which increases the osmotic pressure, shrinking the endothelial cells and loosening the tight junctional proteins, thus increasing the paracellular passage of the drugs to the brain for a few hours [56]. Rapoport et al. injected hypertonic mannitol and arabinose solution by intracarotid infusion into experimental animals and reported a significant increase in the apparent permeability of the substances because of the higher diffusion and bulk flow across the BBB [57]. In another study, the combination of mannitol and stem cells, with their neurotrophic factors, has been suggested as a therapeutic option for ischemic stroke because of the safety and effectiveness of stem cell therapy in animal models of ischemic stroke. Mannitol acts as an adjuvant agent, increases the BBB passage of stem cells, and does not require the invasiveness of other surgical drug delivery procedures, such as craniectomy [58]. Interestingly, this method has also been shown to be effective during the chronic stages of ischemic stroke and increased the permeability of peripherally transplanted stem cells into the brain, which significantly enhanced neurogenesis and improved behavioral outcomes in stroke animals [59].
BBB disruption can also be achieved by ultrasonic and electromagnetic radiations. Ultrasonic waves create microbubbles that induce a mechanical force on the BBB and lead to its disruption; this could be used for site-specific drug delivery to the brain. The combination of ultrasound waves and the subsequent microbubbles has been shown to be a novel neuroprotective therapy in ischemic stroke. Ultrasound-treated animals showed smaller infarct sizes in the brain following middle cerebral artery occlusion (MCAO) injury. This treatment did not alter the BBB integrity or apoptotic cell death, and levels of inflammatory factors also remained constant. Given that ultrasound-mediated microbubble thrombolysis does not have any harmful effects on ischemic stroke, it can be used to permeate the BBB for CNS drug delivery [60]. In another method, femtosecond-pulsed laser irradiation has been used to transiently and reversibly permeabilize targeted blood vessels, causing extravasation of the blood plasma along with the injected drug to the surrounding tissue, such as brain. However, in this minimally invasive method, transcranial delivery of the drugs is not feasible because the skull is not opened and a large amount of drug is needed because of dilution by plasma; thus, the risk of adverse effects increases accordingly [61].
The integrity of the BBB is already dissipated during and after ischemic stroke, increasing the paracellular permeability of compounds; however, the goal of BBB-disrupting techniques is to extend and control the therapeutic time window and entry of drugs to the CNS. However, these methods can be unfavorable because they expose the brain to toxins and infections, and mimic vasogenic edema outcomes by causing astrogliosis and neuronal damage because of the reaction between plasma albumin and astrocytes or neurons. Also, these approaches are not patient friendly because of high costs and need for hospitalization. In addition, complex instruments and expert medical staff are often required for their application. Thus, such methods have remained in preclinical stages of development and create challenges for clinical approval.
Pharmacological and chemical approaches
Pharmacological and chemical approaches involve either manipulating CNS-affecting drug molecules to increase their transport across the BBB without disturbing CNS activity or loss of function or converting them to substrates for efflux pumps. Small uncharged lipophilic molecules can cross the BBB passively and, therefore, any attempts to produce these properties in drug molecules are advantageous.
One of the most common chemical approaches for modification of the drug molecule structure is lipidization. In this method, lipid analogs are added to the polar groups of the drug molecule to improve its permeability and passive diffusion across the BBB. For example, chlorambucil was conjugated to aromatic and aliphatic lipophilic esters to increase its brain penetration. Results showed higher brain penetration of the modified molecules compared with parent drug [62,63].
However, lipophilic analogs change the pharmacokinetic (PK) parameters of therapeutics by higher plasma protein binding, increasing the volume of distribution and rapid plasma clearance. Lipidized drugs also penetrate all biological membranes easily and accumulate in different organs. Therefore, the amount of drug available at the BBB that can then accumulate in the brain is reduced [64,65]. Thus, for optimum brain delivery, the lipophilicity and brain-penetrating properties of the derivative need to be balanced.
Among myriad pharmacological and chemical approaches, nanotechnology has attracted attention because of its potential to augment drug delivery across the BBB. Physicochemical properties of NPs, such as the small size of the carriers, which creates a large surface area to load the desired amount of drug molecules, enhanced solubility, optimum hydrophobicity, and other characteristics that can be controlled to improve the ability of these particles to enhance the stability of therapeutic agents in blood circulation make them suitable candidates for brain drug delivery. By exploiting NPs as carriers of poorly distributed, unstable, or BBB-impermeable therapeutics, a greater amount of drug can reach the desired tissue, without severe toxicities or adverse effects. [66]. These particles can circulate intact and penetrate the BBB using different receptor-mediated endocytic mechanisms and release the drug in the brain microenvironment.
Different nanosystems have been used for this purpose, including polymeric NPs, solid lipid NPs, nanogels, nano emulsions, and nanoliposomes (Table 2). These particles are modified to increase the entrapment and decrease the toxicity of the drug, or to enhance the colloidal stability. They can also be modified to create a sustained-release delivery system and, ultimately, to improve the PK profile and biodistribution of the therapeutics [67]. Therefore, nanotechnology-based drug delivery in stroke is a promising method for both the treatment and diagnosis of this disease. It can be used as a platform for efficient encapsulation of the thrombolytic agent, tPA, reducing its hemorrhagic adverse effect rates as well as improving drug delivery. Al-Jamal and colleagues used functionalized carbon nanotubes for the delivery of caspase-3 small interfering (si)RNA in an endothelin stroke model to evaluate the effect of caspase-3 deactivation in recovery after ischemic insult of the brain. The results showed that nanotube-based effective and direct delivery of siRNA to the CNS alleviated neurodegeneration, offering a new therapeutic method for the treatment of neuropathological disorders [68]. Liposomes, lipid-based vesicular systems, have been used to encapsulate neurotrophic factors to increase their stability against enzymatic degradation and their permeability across the BBB [69]. Similarly, tacrolimus-loaded PEGylated nanoliposomes have been shown to be neuroprotective in ischemia-reperfusion injury because liposomalization both decreased the required dose and improved the therapeutic efficacy by selective delivery of the drug to the brain tissue [70]. PEGylated liposomes have also been used as carriers for asialo-EPO, which is a promising cerebroprotective agent in ischemic stroke and improves neurological deficits but has an extremely short half-life in blood and requires a continuous infusion or multiple doses. Modified liposomes prolonged the blood circulation time and increased the accumulation of this drug in the ischemic area even after a single administration [71]. As a novel nanoantioxidant therapy for targeting specific ROS and RNS that are produced excessively during ischemic stroke and are responsible for oxidative stress and inflammation, synthesized PEGylated melanin NPs have demonstrated neuroprotection with negligible adverse effects [72]. In another antioxidant therapy, various NPs, such as poly(lactide-co-glycolide) (PLGA) and polybutylcyanoacrylate (PBCA), were loaded with superoxide dismutase (SOD) enzyme and tagged with antibodies to target ROS and RNS generation in ischemic stroke. The results indicated that targeted NPs displayed superior protection as well as specific localization to the hippocampus [73]. By contrast, anti-inflammatory treatment after stroke has shown variable consequences because of undesired PK and adverse effects of anti-inflammatory compounds. These limitations can be overcome by using encapsulating therapeutics, such as dexamethasone phosphate in liposomes, which results in desired PK and reduces the toxicity of the drugs. Interestingly, injecting these drugs in combination with thrombolytic agents resulted in enhanced therapeutic efficacy, improved neurological outcome, and diminished stroke injury [74].
TABLE 2.
Nanocarriers and/or nanomaterials for drug delivery to ischemic stroke
| Investigational therapeutic(s) | Finding(s) | Physiological outcome(s) | Refs |
|---|---|---|---|
| Polymers (PEG, dextran, PLGA) | |||
| Triiodothyronine (T3) | Improved neuroprotective efficacy | Significant reduction in tissue infarction and brain edema | [147] |
| Ceria/cerium oxide | Improved stability and half-life, decreased non-specific binding and uptake | Reducing ROS production and apoptotic cell death | [148,149] |
| Superoxide dismutase | Improved half-life and BBB permeability | Site-specific accumulation of drug within injured region, protecting the NVU | [150] |
| 3-n Butylphthalide | Significant reduction of the required dose, targeted delivery to ischemic region | Significant improvements in brain injury and neurological deficits | [151] |
| NR2B9C | ROS-stimulated controlled release of drug, drastic prolongation of systemic circulation | Great protective effect against cytotoxicity in cells, reduced brain injury | [152] |
| EPO | Significant dose reduction | Reduced functional deficit and infarct volume | [153] |
| tPA | Increased circulation time and efficacy | Improved thrombolytic activity | [154] |
| Melanin | Improved potency and safety | Enhanced anti-inflammatory and antioxidative activity | [72] |
| Exosomes | |||
| Cure um in | Site-specific accumulation within injured cells | Strong anti-inflammatory and antiapoptotic activity | [155] |
| miR-17-92 gene | Feasible in vivo delivery of gene | Increased neural plasticity and neurogenesis, functional recovery | [156] |
| Enkephalin | Transporting sufficient amount of drug to brain | Improved brain neuron density and neurological score | [157] |
| microRNA-210 | Safe and efficient delivery | Increased angiogenesis and expression of VEGF | [158] |
| Liposomes | |||
| Allium cepa | Controlled release and distribution, improved BBB permeability, significant reduction of administered dose | Improved cognitive/sensorimotor function, decreased oxidative stress and infarct size | [159] |
| Antiapoptotic bcl2 gene | High level and widespread protein expression in injured area | Significant reduction of apoptotic cells and infarct volume | [160] |
| Simvastatin | Improved bioavailability and reduced adverse effects | Improved therapeutic efficiency | [161] |
| Fasudil | Controlled release and distribution, reduction of administered dose | Improved neurological outcomes and significant reduction in infarct size | [162] |
| Xenon | Improved delivery to ischemic area | Reduced neuronal cell death | [163] |
| Luteolin | Targeted delivery to ischemic region | Balancing the pro/antioxidant status of injured tissue | [164] |
| Lycopene | Improved stability and absorption | Regulation of iron metabolism and improved neuroprotection | [165] |
| Cyclosporine A | Reduced systemic side effects and toxicity | Reduced inflammatory responses | [166] |
| Minocycline | Reduced required dose and cytotoxicity | Efficient targeting of matrix metalloproteinases | [167] |
| Citicoline | Enhanced brain bioavailability | Significant reduction in lesion volume | [168] |
| C-Phycocyanin | Extended neuroprotective time window | Increased cellular expression of antioxidants, inflammatory compounds and glia proteoglycans | [169] |
| Nanoemulsions | |||
| Tanshinone IIA | Improved solubility, stability and BBB permeability | Reduced infarct volume and augmented motor function | [170] |
| Quercetin | Enhanced bioavailability | Improved neurobehavioral activity and reduced infarct volume | [171] |
| Cilostazol | Improved PK properties | Ameliorate neurological deficits | [172] |
| Safranal, thymoquinone | Enhanced bioavailability and absorption | Significant improvement in neurobehavioral and antioxidant activity | [173,174] |
| Nanogels | |||
| Urokinase | Targeted pH-sensitive delivery to ischemic region | Reduced neurological deficits and neurotoxicity | [175] |
| Osteopontin | Extended therapeutic time window | Reduced infarct volume | [176] |
| Solid lipid NPs | |||
| Baicalin | Enhanced bioavailability and improved brain uptake | Reduced excitotoxic neuronal injury | [177] |
| Cure um in | Improved brain delivery | Improved neurological score and cognition | [178] |
| Ferulic acid | Improved bioavailability and efficiency | Promising antioxidant and neuroprotective effects | [179] |
Nanomaterials can also be engineered according to the brain condition during injury to release drug content in a desired time and region. For instance, the pH of ischemic penumbra can be used as an external stimulus for designing pH-sensitive nanocarriers, because this region has been shown to be slightly acidic after ischemic stroke. pH-responsive polymeric NPs have proven to be efficient magnetic resonance imaging (MRI) probes for the imaging and diagnosis of pathological tissues with acidic pH [75]. This technology should be tested as a site-specific drug delivery technique to deliver therapeutics to penumbral brain tissue. Future nanocarriers will need to be nontoxic, biocompatible, and biodegradable, should not aggregate in the blood, and their production process should be fairly simple and efficient. Furthermore, the surface charge of the nanocarriers could affect the integrity and permeability of the BBB, which also has a direct effect on stroke injury. Cationic and positively charged NPs that have higher zeta potential result in immediate toxicity and barrier disruption, whereas neutral and negatively charged particles are not attracted toward the negatively charged cell membrane and demonstrate fewer effects on the BBB [76].
In addition, the cytotoxicity of NPs should also be considered when developing nanocarriers for brain drug delivery. It is possible that NPs might adversely affect the normal physiology of targeted organs and result in nanocytotoxicity, such as proinflammatory gene activation and oxidative stress [77,78]. Other factors that need to be considered include the extent of tissue exposure and distribution, the administered dose, and the route of administration. The latter is important because it defines the metabolism, accumulation, and biodistribution of NPs in the body. Despite the protective role of the BBB, NPs can penetrate tight junctions and expose the brain to cytotoxic nanomaterial; therefore, reliable data of the toxicity of NPs are essential to prevent unfavorable adverse effects [79].
Exosomes
Exosomes are membrane-based microvesicles that are involved in various biological functions, including intercellular communication, and can be secreted by different cells. They contain specific proteins and genes that reveal their cellular sources as well as bioactive molecules that facilitate exosomal intercellular communication, which is mostly by means of transferring different RNA types to target cells. These nanosized vesicles are taken up by cells via various mechanisms, such as endocytosis, phagocytosis, pinocytosis, or fusion to the plasma membrane. Exosomes have been utilized as carriers for therapeutic agents to travel across multiple biological membranes, such as BBB. The main advantage of exosomes, compared with liposomes or polymeric NPs, is that they are non-immunogenic. Therefore, proteins, small molecules, and nucleic acids, such as siRNA, can be loaded into them and remain stable in the circulation until they reached the desired targets. Zhuang et al. encapsulated the anti-inflammatory compound, curcumin, in exosomes and evaluated its efficacy in an lipopolysaccharide (LPS)-induced inflammation model, utilizing the nasal to brain delivery route. Results indicated that intranasally (IN) treated animals with curcumin-loaded exosomes were protected from brain inflammation because of the fast transport of these carriers to the brain [80].
The molecular features and functional characteristics of exosomes are attributed to the cells from which they are secreted [81]. Multipotent mesenchymal stromal cells (MSC) have also been used to derive exosomes, which can be injected intravenously into experimental stroke animals. Exosomes promoted functional recovery, angiogenesis, neurogenesis, and neurovascular remodeling after MCAO in rats and, thus represent a promising treatment strategy for ischemic stroke [82] (Fig. 4). Furthermore, it has also been suggested that MSCs migrate to the diseased brain area (i.e., ischemic brain) and exert their therapeutic effects via secreted exosomes [81]. Xin et al. confirmed that delivery of MSCs transferred miRNAs to brain parenchyma via secreted exosomes, improving neurite growth and branching in post-MCAO brains [83].
FIGURE 4.

Mesenchymal stem cell (MSC)-derived exosomes as a therapeutic strategy in stroke. MSC-derived exosomes are injected either intravenously or directly into the brain parenchyma through other delivery approaches, such as the intranasal route, to circumvent the blood–brain barrier (BBB). Stem cells themselves can also be administered to the brain parenchyma, where exosomes are secreted with therapeutic characteristics. Different properties of exosomes influence their transport across the BBB; however, the surface proteins can be modified to increase brain uptake. The most probable mechanism of exosome transport to the brain is transcytosis. Additionally, breakdown of tight junction proteins following inflammation under pathological conditions further increases the paracellular movement of these vesicles across endothelial cells of the BBB. These exosomes merge with different cells in brain parenchyma and transfer their therapeutic content to the cells, therefore improving functional outcome after stroke. Created with BioRender.
Prodrugs
Another common pharmacological approach for circumventing the BBB as well as improving the PK profile, selectivity, and membrane permeability is to mask the polar functional groups of the drug molecule and convert it to a hydrophobic/lipophilic inert prodrug, which is activated at the desired target [84]. The designed prodrug should be stable physiologically and be transported across the BBB and then released in the brain after being metabolized or activated chemically or by specific enzymes. To convert a prodrug to the related parent drug, different strategies can be utilized. These include esterase activation, in which the ester group of the prodrug is sensitive to brain esterase only, or oxidase activation, given that there are several oxidative enzymes in the CNS, such as monoamine oxidase, which performs oxidative deamination of amine groups. Furthermore, during the prodrug design process, drugs can be conjugated to either specific ligands or antibodies that can be recognized by target tissue receptors or enzymes for more site-specific drug delivery. The latter is known as antibody-directed enzyme prodrug therapy, in which drug molecules are targeted to the site of action by the conjugated antibody and deliver large amounts of drug to the desired site. Cyclodextrin has also been used as a conjugated ligand that entraps lipophilic drugs and increases the solubility as well as brain uptake of therapeutics [85]. Additionally, β-cyclodextrin NPs can be used as drug carriers because they have high BBB permeability and act as a sustained drug delivery system, which is useful for treating chronic diseases of the brain [86].
The ideal goal of the prodrug strategy is to increase the accumulation of the drug in the brain. For instance, poor permeability, ionization of the carboxyl functional group at physiological pH, and substrate for efflux transporters hinder the CNS delivery and accumulation of nonsteroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen and ketoprofen, which are reported to be good candidates for reducing the risk of CNS disease. However, coupling ketoprofen with diacetyl glyceride and ibuprofen with ascorbic acid on their carboxylic acid groups increased the permeability and transport ability of these NSAIDs, respectively [87,88].
Honokiol, an herb-derived phytochemical agent with demonstrated neuroprotective activity, was converted to its phosphate prodrug to improve its poor aqueous solubility and oral bioavailability. The prodrug maintained efficacy in reducing stroke induced-injury and infarction volume and showed improved absorption compared with the parent drug [89]. By exploiting properties of L-carnitine, an endogenous fatty acid transporter that passes easily through the BBB and cell membranes, a series of carnitine esters was developed to improve the lipophilicity of carnitine. Carnitine esters were used as carriers for the delivery of thrombolytic and neuroprotective agents, and of tetra methylpyrazine (TMP) and its active metabolite to the ischemic brain. These novel twin compounds had longer half-lives and better systemic availability and also protected the ischemic brain by reducing the inflammatory responses and oxidative stress as well as by supporting the mitochondrial structure [90].
Other novel approaches
Intranasal
IN drug delivery could be a safe, non-invasive approach for the delivery of neurotherapeutics, such as peptides, chemical drugs, stem cells, and DNA, through the nasal cavity, which has a layer of mucosa with high blood flow. This method bypasses the BBB and first-pass hepatic metabolism, two of the major obstacles when drugs are administered via the non-invasive oral route. Also, when administered IN, therapeutic agents can enter the CNS instead of the systemic blood circulation, which also results in reduced peripheral tissue drug exposure. In addition, because of rapid absorption into the brain, this method could be a suitable approach for the treatment of conditions where quick intervention is required (i.e., seizures). Moreover, a large amount of intact drug can be delivered into the brain through nasal administration compared with the intravenous route because it eliminates plasma protein binding [91]. Although the exact mechanisms and pathways of drug transport from the nasal cavity to various parts of the brain have not yet been fully interpreted, some of the mechanisms are well studied. Two such mechanisms are the perineural and perivascular pathways. For a drug molecule to be delivered to widespread regions within the brain after IN administration, it should first travel across the olfactory epithelium in the nasal cavity. The nasal cavity is connected to the brain via nerves and blood vessels. Thus, drug molecules follow the vascular space around neurons and blood vessels to distribute in different regions of the brain. Given that drugs are on the brain side of the blood vessels, they do not need to cross the BBB to distribute in the brain. To follow the neural route of transport, molecules use either intracellular, axonal transport (endocytosis–exocytosis) or extracellular (perineural) pathways to reach the olfactory bulb. The CSF distributes molecules from the olfactory bulb to different regions of the brain [92,93].
IN drug delivery does not essentially require any alterations to therapeutic agents and, being a non-invasive route of administration, is more comfortable for patients. This route of administration was used preclinically to deliver curcumin-loaded NPs to ischemic brain to improve the solubility and absorption of curcumin [92]. A caspase-1 inhibitor has also been administered intranasally in a global cerebral ischemia model as a neuroprotective strategy. Results of the study revealed that IN delivery of caspase-1 inhibitor attenuated hippocampal apoptotic neuronal death and reduced neuroinflammatory responses by blocking proinflammatory factor production [93]. Insulin-like growth factor-I (IGF-I) has been shown to be neuroprotective in stroke when injected directly into the rat brain. Although IGF-I does not cross the BBB, when administered via the IN route, it significantly reduced infarct size by 63% and improved neurological deficits following brain injury [94]. In a promising and novel approach to treat neonatal ischemic brain injury, MSCs were transplanted intranasally 2 weeks after hypoxic injury and the effects were evaluated at Day 28 after injury in mice. This treatment reduced the lesion size significantly compared with the control group. It also increased the production and upregulation of different cell repair-promoting factors, such as fibroblast growth factor, in the brain to reduce cell death. Therefore, IN administration of stem cells can reliably be applied for the treatment of hypoxic brain injuries [95]. This novel, cell-based delivery strategy might also hold promise for neuroregeneration.
However, some limitations also exist with IN delivery. Potent drugs are the best candidates, because they can be administered at a lower volume. Unfortunately, IN delivery is not suitable for the delivery of hydrophilic agents, which require larger doses to be clinically efficient. There is also a chance of enzymatic degradation by nasal enzymes or active mucociliary clearance, and the slightly acidic pH of the nasal epithelium might create unwanted conditions for brain drug delivery [96]. In addition, the diffusion distance with IN delivery is limited, which can be challenging for the drug to reach penumbral brain tissue, and the axonal transport process can be slow across the olfactory nucleus. Moreover, the olfactory epithelium has a small surface area compared with the BBB, which also has various transporters for targeted delivery.
Gene delivery
Gene therapy is a promising and novel strategy for treating cerebrovascular disorders in which the principal objective is to express a deficient gene or to increase the expression of a therapeutic gene. For this purpose, genes are transferred either to cerebral blood vessels with systemic injection or directly to the brain parenchyma by different carriers and vectors. Given that brain diseases, such as ischemic stroke, are associated mainly with neuronal damage, overexpression of growth factors such as BDNF or VEGF, by means of gene therapy, showed improved neuroprotection and enhanced angiogenesis and neurogenesis. Moreover, neuroprotection in acute ischemic stroke can also be achieved by the targeting and inhibition of proinflammatory mediators [97,98].
Two major types of carrier used for gene delivery to the brain are viral and nonviral vectors. These vectors enter cells by endocytosis, which is more efficient than other diffusion methods. Viral vectors are single or double-stranded RNA or DNA viruses, such as lentivirus, adenovirus or adeno-associated virus (AAV), that acclimatize the desired gene inside their genome and transfer it to the target region. Zhang et al. generated BDNF gene-carrying recombinant AAV and injected it intracerebroventricularly in focal ischemic animal models. Two weeks after vector infusion, the neuronal growth was improved and BDNF overexpression protected neurons from cell death and apoptosis [99]. It has also been demonstrated that BDNF and nerve growth factor-loaded recombinant AAV significantly improved ischemia-induced motor impairments along with neuronal cell death mitigation when administered to the striatum for a couple of weeks before injury [100]. Recently, Chen et al. used AAV vectors to deliver a single genetic neural factor, NeuroD1, to ischemic brain for the conversion of active astrocytes to neurons to regenerate and protect lost and injured neurons. This in vivo reprogramming approach showed significant regeneration of neurons compared with a glial-to-neuron conversion technique and improved post-stroke recovery and motor function after injury [101].
One of the advantages of gene delivery to neurons is that a single injection of the designed vector would be sufficient to reverse the cell damage because neurons are nondividing postmitotic cells and, thus, a lower amount of therapeutic is necessary to create a functional drug delivery approach. However, there are some limitations related to gene delivery with viral vectors, such as stimulation of immune responses in the body after administration of the viral carrier, which can be avoided by coating the vector with polymers. Also, the packaging capacity of some viruses is low, and they cannot accommodate high-molecular-weight genes, which limits their therapeutic targets. Permanent or long-term overexpression of some growth factors is not always favorable for treatment because these growth factors cause tumor growth; in addition, for the recovery of ischemic stroke, only transient expression is required. For example, the lentivirus genome has stable integration with the host genome and might enable oncogenic recombination [102]. Overall production costs of these drug delivery systems and long-term safety concerns also remain a challenge.
The second type of gene carriers are nonviral vectors, such as polymers and liposomes. Unlike viral carriers, these are nonimmunogenic and can easily be modified to target specific regions of the brain to increase the therapeutic efficacy. Additionally, nonviral vectors can be loaded with drug molecules as well as genes for combination therapy. Hyun et al. used a combinational approach for the delivery of the heme oxygenase-1 gene and dexamethasone through conjugation with polyethyleneimine polymers for the treatment of ischemic stroke. This NP complex decreased inflammatory factors and the infarction size more efficiently than either alone and showed synergistic effects both in vitro and in vivo [103].
Liposomes have also been used as nonviral vectors for gene delivery. An interesting example of this approach was observed when plasmid-loaded liposomes were injected into stroke animal models. These vectors delivered the fibroblast growth factor gene to macrophages in the blood; plasmid-transfected macrophages accumulated in the infarct border and expressed the growth factor, which increased neurogenesis in injured brains [104]. In the process of nonviral vector selection and design, the charge density of the gene-carrier complex should be optimized. To form a nonviral vector, the carrier should carry a cationic (positive) charge to establish an ideal and stable complex with the negatively-charged plasmid. However, cationic carriers can be cytotoxic because of their interaction and binding with other negatively charged cellular elements and can interfere with their normal functions. By contrast, low charge-density carriers cannot produce stable complexes because of weak interactions with the plasmid. Hence, a balance between stable complex formation and the toxicity of carriers should be considered when developing carriers for gene delivery. However, these problems can be solved by using biodegradable positively-charged carriers that degrade after gene delivery. Nevertheless, there needs to be enough charge to create the vector complex so that, at later stages, this carrier will be eliminated from the body via biodegradation [105].
Thrombectomy
An alternative treatment for patients with ischemic stroke, especially those who are ineligible for thrombolytic therapy with tPA, is reopening the vessels with mechanical intervention (i.e., endovascular thrombectomy). This method has recently emerged as a novel and promising approach to treat acute ischemic stroke.
In a clinical trial, the safety and effectiveness of thrombectomy in patients with stroke were evaluated and the results showed that thrombectomy within 8 h of stroke onset attenuated the post-stroke disability and improved clinical outcomes [106]. Albers and colleagues also provided evidence that thrombectomy along with normal medical therapy in ischemic but not infarcted tissue led to superior functional outcomes compared with thrombolytic therapy alone [107]. Thus, this method offers an extended time frame for treatment and improves revascularization. There are two major categories of thrombectomy in stroke: (i) stent-based thrombectomy with retrievable stents; and (ii) aspiration-based thrombectomy with aspiration catheters [108]. Among several thromboaspiration methods, the A Direct Aspiration, First Pass Technique (ADAPT) has been shown to be a simple, yet effective and fast approach in ischemic stroke thrombectomy. However, it has been reported that, despite successful revascularization by thrombectomy, there can be poor neurological recovery and clinical outcomes in some patients with ischemic stroke, which creates a demand for pharmacological treatments as an adjuvant therapy with neurothrombectomy [109]. Combining neurotherapeutic agents with mechanical revascularization promotes localized reperfusion and drug delivery in the damaged region, decreases hemorrhage rate, and increases the time window for efficient recanalization. Moreover, direct intra-arterial delivery of therapeutic agents before or after clot extraction, reduces the risk of systemic toxicity, and higher concentrations can be delivered to the desired region of the injured brain. The ADAPT method has the option of using an adjuvant treatment in case of recanalization failure [110]. With the goal of demonstrating the feasibility of combining neuroprotective compound administration and endovascular thrombectomy in ischemic stroke, Fraser et al. sought to evaluate the effects of intra-arterial verapamil, an L-type calcium channel blocker, administered immediately following thrombectomy and reperfusion in a mouse model of ischemia. Verapamil is already used in subarachnoid hemorrhage to treat subsequent vasospasm. According to their results, which were also supported by a preclinical study, intra-arterial administration of verapamil, along with thrombectomy and removal of the thrombus, was feasible and safe even in humans. Furthermore, it also decreased the risk of hemorrhage and prevented neuronal death and adverse ischemic effects [111]. Higher revascularization rates as well as low procedural complications in clinical use and improved clinical outcomes are advantages of The Solitaire Flow Restoration device, a stent-based mechanical thrombectomy method reported by Pereira et al. for acute ischemic stroke therapy [112]. Additionally, the safety of the Solitaire AB device, a completely retrievable stent, was evaluated and it was shown to be an effective remover of clots from large arteries because it revascularized 90% of treated vessels [113]. Along with the numerous advantages of endovascular therapies, there are some limitations, such as difficulty in guiding the catheter to the occluded site without damaging the nearby arteries, breakup and distal movement of the clots, risks related to the patient hospitalization, anesthesia, and complications associated with hemorrhage [114].
Bioengineering
Bioengineering is the implementation of engineering techniques to improve the performance of a task or set of tasks performed by a biological system. Some of the limitations of existing ischemic stroke therapies include reduced drug availability in the target region and limited therapeutic efficacy. With the advance of technology, it is now possible to utilize novel drug delivery strategies in ischemic stroke therapy that can mimic biological systems to enhance site-specific drug delivery, therapeutic efficacy, and neuronal repair [115].
By considering two of the major strategies in stroke therapy, dissolving the thrombus by thrombolytic agents and protecting neurons with a neuroprotectant, a thrombin-responsive nanoplatelet platform was developed as a combinational approach. These nanoplatelets behave like a platelet in the blood circulation and are recruited to the thrombus at the site of injury, releasing both tPA and a neuroprotective agent sequentially after being degraded by thrombin. In this approach, the intrinsic properties of the clot are used to engineer a biomimetic carrier that releases the load at the specific and desired site as well as increasing the availability and therapeutic efficacy of a poorly BBB-permeable neuroprotectant agent [116].
In another biomimetic engineering approach, the surface of PLGA NPs was coated with the membrane of neuronal stem cells and engineered to overexpress the CXCR4 chemokine. Surface engineering enables the particles to migrate to the ischemic region, which is enriched with the ligand of this chemokine. This developed carrier was used to carry glyburide, an antiedema agent, for stroke management. According to the published data, this approach efficiently improved the circulation and chemotactic interaction of the NPs with the target, which enhanced the accumulation of the particles in the injured brain [117].
To avoid the off-CNS and systemic adverse effects of poorly permeable BDNF, a depot system based on a hyaluronic acid hydrogel was developed. Hydrogel can be injected into the ischemic cavity and delivers BDNF over weeks for neurological promotion and chronic recovery after stroke. The hydrogel-BDNF induced the migration of immature neurons to the penumbral area and enhanced axonal sprouting within the cortical system [118]. These biomaterial hydrogels can be injected in liquid form and they solidify in the brain to form a sustained-release platform with similar mechanical properties to the brain tissue [119].
Concluding remarks
The significant challenge of improving efficient drug delivery methods to the CNS is evident when considering the lack of effective neurotherapeutics for CNS diseases. Although different approaches have been developed to transfer and deliver drugs into the injured brain, none have recently been demonstrated to be satisfactory in the case of CNS disorders such as stroke, or have been successfully translated for clinical use in patients with stroke. This is because of the complicated physiology of the brain, which presents inimitable challenges for drug development by the strict regulation of therapeutic entry or distribution within the brain tissue. Therefore, detailed understanding and consideration of the cellular behavior, pathophysiology of brain diseases, site and mechanism of action of the drug, as well as numerous drug administration technologies, will help to overcome the challenges associated with creating a proficient drug delivery technique to resolve neurovascular disorders, such as stroke. Moreover, novel delivery strategies are also expected to facilitate the diagnosis of brain diseases.
In this review, we described current approaches and new techniques to deliver neurotherapeutics, both small and large molecules, to the brain, with a focus on acute ischemic stroke therapy. Although shortcomings remain for all drug delivery strategies, the advantages of non-invasive techniques compared with invasive methods include their safety and ease of administration, which also improves patient compliance. The most promising technologies appear to be those in which physiological approaches have been applied to take advantage of overexpressed endogenous receptors of the BBB during injury. However, the role of other cells of the NVU, including astrocytes, neurons, and microglia cells, should also be considered when designing drug delivery approaches. The development of delivery approaches targeting specific regions of the brain and specific cells involved in the pathophysiology of stroke is needed to improve stroke outcomes without off-target adverse effects. Hence, exploring the potential of multifunctional carriers to deliver therapeutics to injured neurons after crossing the BBB could improve stroke therapy [120]. The ultimate goal is to achieve more effective and precise drug concentrations in the ischemic brain, thus improving clinical outcomes and patient recovery after stroke.
Acknowledgements
Research and concepts described in this review were supported by the National Institute on Drug Abuse (NIDA) grants R01DA049737 and R01DA029121 and the National Institute of Neurological Disorders and Stroke (NINDS) grant R01NS106879.
Biography
 Saeideh Nozohouri is currently studying for a PhD in pharmaceutical sciences at Texas Tech University Health Sciences Center (TTUHSC), Amarillo, TX. She was awarded a Pharm. D. by Tabriz University of Medical Sciences, Iran in 2016. Currently, she is working as a research assistant in the research group of Thomas Abbruscato on the development of therapeutics for ischemic stroke. Her fields of interest are neurosciences, drug development and delivery to the brain, as well as neuroprotection in ischemic stroke.
Saeideh Nozohouri is currently studying for a PhD in pharmaceutical sciences at Texas Tech University Health Sciences Center (TTUHSC), Amarillo, TX. She was awarded a Pharm. D. by Tabriz University of Medical Sciences, Iran in 2016. Currently, she is working as a research assistant in the research group of Thomas Abbruscato on the development of therapeutics for ischemic stroke. Her fields of interest are neurosciences, drug development and delivery to the brain, as well as neuroprotection in ischemic stroke.
 Ali Sifat was awarded a B. Pharm and M. Pharm by the University of Dhaka, Bangladesh. He is currently a graduate research assistant at TTUHSC, studying for a PhD in pharmaceutical science. His research interests include electronic cigarettes, brain glucose utilization in cerebral ischemia, neonatal hypoxic-ischemic brain injury, and cognitive function.
Ali Sifat was awarded a B. Pharm and M. Pharm by the University of Dhaka, Bangladesh. He is currently a graduate research assistant at TTUHSC, studying for a PhD in pharmaceutical science. His research interests include electronic cigarettes, brain glucose utilization in cerebral ischemia, neonatal hypoxic-ischemic brain injury, and cognitive function.
 Bhuvaneshwar Vaidya is a research assistant professor at TTUHSC School of Pharmacy. He was awarded a PhD in pharmaceutical sciences from Dr Hari Singh Gour University, Sagar, India. His area of research includes novel drug delivery approaches for improved treatment of life-threatening disorders, including cardio/cerebrovascular diseases and cancer.
Bhuvaneshwar Vaidya is a research assistant professor at TTUHSC School of Pharmacy. He was awarded a PhD in pharmaceutical sciences from Dr Hari Singh Gour University, Sagar, India. His area of research includes novel drug delivery approaches for improved treatment of life-threatening disorders, including cardio/cerebrovascular diseases and cancer.
 Thomas Abbruscato is Chair and university distinguished professor of Pharmaceutical Sciences at TTUHSC, Jerry H. Hodge School of Pharmacy. Research in his group focuses on the central nervous system (CNS) entry of drugs and nutrients with respect to their ability to cross the blood–brain barrier (BBB). Their goal is to exploit strategies to offset metabolic degradation, utilize BBB transport systems to gain CNS access, and target receptors, transporters, and/or carriers that would offset stroke injury. Current research is supported by National Institute of Neurological Disorders and Stroke and National Institute on Drug Abuse.
Thomas Abbruscato is Chair and university distinguished professor of Pharmaceutical Sciences at TTUHSC, Jerry H. Hodge School of Pharmacy. Research in his group focuses on the central nervous system (CNS) entry of drugs and nutrients with respect to their ability to cross the blood–brain barrier (BBB). Their goal is to exploit strategies to offset metabolic degradation, utilize BBB transport systems to gain CNS access, and target receptors, transporters, and/or carriers that would offset stroke injury. Current research is supported by National Institute of Neurological Disorders and Stroke and National Institute on Drug Abuse.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Benjamin EJ et al. (2018) Heart Disease and Stroke Statistics-2018 Update: a report from the American Heart Association. Circulation 137, e67–e492 [DOI] [PubMed] [Google Scholar]
- 2.Brzica H et al. (2017) Role of transporters in central nervous system drug delivery and blood–brain barrier protection: relevance to treatment of stroke. J. Cent. Nerv. Syst. Dis. 9, 1179573517693802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onwuekwe I et al. (2012) Ischemic stroke and neuroprotection. Ann. Med. Health Sci. Res. 2, 186–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson BJ and Ronaldson PT (2014) Drug delivery to the ischemic brain. Adv. Pharmacol. 71, 165–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sifat AE et al. (2017) Blood-brain barrier protection as a therapeutic strategy for acute ischemic stroke. AAPS J. 19, 957–972 [DOI] [PubMed] [Google Scholar]
- 6.Chamorro Á et al. (2016) Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 15, 869–881 [DOI] [PubMed] [Google Scholar]
- 7.Tsuji K et al. (2005) Tissue plasminogen activator promotes matrix metalloproteinase-9 upregulation after focal cerebral ischemia. Stroke 36, 1954–1959 [DOI] [PubMed] [Google Scholar]
- 8.Sutherland BA et al. (2012) Neuroprotection for ischaemic stroke: translation from the bench to the bedside. Int. J. Stroke 7, 407–418 [DOI] [PubMed] [Google Scholar]
- 9.Manzanero S et al. (2013) Neuronal oxidative stress in acute ischemic stroke: sources and contribution to cell injury. Neurochem. Int. 62, 712–718 [DOI] [PubMed] [Google Scholar]
- 10.Maples KR et al. (2001) Comparison of the radical trapping ability of PBN, S-PPBN and NXY-059. Free Radic. Res. 34, 417–426 [DOI] [PubMed] [Google Scholar]
- 11.Lapchak PA et al. (2002) Neuroprotective effects of the spin trap agent disodium-[(tert-butylimino) methyl] benzene-1, 3-disulfonate N-oxide (generic NXY-059) in a rabbit small clot embolic stroke model: combination studies with the thrombolytic tissue plasminogen activator. Stroke 33, 1411–1415 [DOI] [PubMed] [Google Scholar]
- 12.Diener H-C et al. (2008) NXY-059 for the treatment of acute stroke: pooled analysis of the SAINT I and II Trials. Stroke 39, 1751–1758 [DOI] [PubMed] [Google Scholar]
- 13.Yang J et al. (2015) Edaravone for acute stroke: meta-analyses of data from randomized controlled trials. Dev. Neurorehabil. 18, 330–335 [DOI] [PubMed] [Google Scholar]
- 14.Stroke Therapy Academic Industry, R (1999) Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 30, 2752–2758 [DOI] [PubMed] [Google Scholar]
- 15.Fisher M et al. (2009) Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 40, 2244–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang PF et al. (2014) Polyinosinic-polycytidylic acid has therapeutic effects against cerebral ischemia/reperfusion injury through the downregulation of TLR4 signaling via TLR3. J. Immunol. 192, 4783–4794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Z et al. (2015) Combination of the immune modulator fingolimod with alteplase in acute ischemic stroke: a pilot trial. Circulation 132, 1104–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang Q and Wang XL (2003) Effects of chiral 3-n-butylphthalide on apoptosis induced by transient focal cerebral ischemia in rats. Acta Pharmacol. Sin. 24, 796–804 [PubMed] [Google Scholar]
- 19.Liao S-J et al. (2009) Enhanced angiogenesis with dl-3n-butylphthalide treatment after focal cerebral ischemia in RHRSP. Brain Res. 1289, 69–78 [DOI] [PubMed] [Google Scholar]
- 20.Xue LX et al. (2016) Efficacy and safety comparison of DL-3-n-butylphthalide and Cerebrolysin: effects on neurological and behavioral outcomes in acute ischemic stroke. Exp. Ther. Med. 11, 2015–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davignon JJC (2004) Beneficial cardiovascular pleiotropic effects of statins. Circulation 109 (23_suppl_1), III-39–III-43 [DOI] [PubMed] [Google Scholar]
- 22.Gutierrez-Fernandez M et al. (2012) CDP-choline treatment induces brain plasticity markers expression in experimental animal stroke. Neurochem. Int. 60, 310–317 [DOI] [PubMed] [Google Scholar]
- 23.Marei HE et al. (2018) Potential of stem cell-based therapy for ischemic stroke. Front. Neurol. 9, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaidya B et al. (2018) The neuroprotective role of the brain opioid system in stroke injury. Drug Discov. Today 23, 1385–1395 [DOI] [PubMed] [Google Scholar]
- 25.Kilic E et al. (2006) TAT fusion proteins against ischemic stroke: current status and future perspectives. Front. BioSci. 11, 1716–1721 [DOI] [PubMed] [Google Scholar]
- 26.Blanchette M and Daneman R (2015) Formation and maintenance of the BBB. Mech. Dev. 138, 8–16 [DOI] [PubMed] [Google Scholar]
- 27.Haseloff RF et al. (2015) Transmembrane proteins of the tight junctions at the blood–brain barrier: structural and functional aspects. Semin. Cell Dev. Biol. 38, 16–25 [DOI] [PubMed] [Google Scholar]
- 28.Hawkins BT and Davis TP (2005) The blood-brain barrier/neurovascular unit in health and disease. Pharm. Rev. 57, 173–185 [DOI] [PubMed] [Google Scholar]
- 29.Sharma H and Dey P (1981) Impairment of blood-brain barrier (BBB) in rat by immobilization stress: role of serotonin (5-HT). Indian J. Physiol. Pharm. 25, 111–122 [PubMed] [Google Scholar]
- 30.Gabathuler R (2010) Approaches to transport therapeutic drugs across the blood–brain barrier to treat brain diseases. Neurobiol. Dis. 37, 48–57 [DOI] [PubMed] [Google Scholar]
- 31.Pardridge WM (1997) Drug delivery to the brain. J. Cereb. Blood Flow Metab. 17, 713–731 [DOI] [PubMed] [Google Scholar]
- 32.Gabathuler R (2010) Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol. Dis. 37, 48–57 [DOI] [PubMed] [Google Scholar]
- 33.Girardin F et al. (2006) Membrane transporter proteins: a challenge for CNS drug development. Dialog. Clin. Neurosci. 8, 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh VK and Subudhi BB (2016) Development and characterization of lysine-methotrexate conjugate for enhanced brain delivery. Drug Deliv. 23, 2327–2337 [DOI] [PubMed] [Google Scholar]
- 35.Allen DD et al. (2003) Active transport of high-affinity choline and nicotine analogs into the central nervous system by the blood-brain barrier choline transporter. J. Pharmacol. Exp. Ther. 304, 1268–1274 [DOI] [PubMed] [Google Scholar]
- 36.Yeh W-L et al. (2008) Enhancement of glucose transporter expression of brain endothelial cells by vascular endothelial growth factor derived from glioma exposed to hypoxia. Mol. Pharmacol. 73, 170–177 [DOI] [PubMed] [Google Scholar]
- 37.Vemula S et al. (2009) A functional role for sodium-dependent glucose transport across the blood-brain barrier during oxygen glucose deprivation. J. Pharmacol. Exp. Ther. 328, 487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carman AJ et al. (2011) Adenosine receptor signaling modulates permeability of the blood-brain barrier. J. Neurosci. 31, 13272–13280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson BJ et al. (2014) Hypoxia/reoxygenation stress signals an increase in organic anion transporting polypeptide 1a4 (Oatp1a4) at the blood-brain barrier: relevance to CNS drug delivery. J. Cereb. Blood Flow Metab. 34, 699–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albekairi TH et al. (2019) Brain delivery of a potent opioid receptor agonist, biphalin during ischemic stroke: role of organic anion transporting polypeptide (OATP). Pharmaceutics 11, 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y et al. (2005) Delivery of b-galactosidase to mouse brain via the blood-brain barrier transferrin receptor. J. Pharmacol. Exp. Ther. 313, 1075–1081 [DOI] [PubMed] [Google Scholar]
- 42.Ding H et al. (2014) Enhanced blood-brain barrier transmigration using a novel transferrin embedded fluorescent magneto–liposome nanoformulation. Nanotechnology 25, 055101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu DJN (2005) Neuroprotection in experimental stroke with targeted neurotrophins. NeuroRx 2, 120–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao H et al. (2011) Postacute ischemia vascular endothelial growth factor transfer by transferrin-targeted liposomes attenuates ischemic brain injury after experimental stroke in rats. Hum. Gene Ther. 22, 207–215 [DOI] [PubMed] [Google Scholar]
- 45.Fu A et al. (2011) Neuroprotection in stroke in the mouse with intravenous erythropoietin-Trojan horse fusion protein. Brain Res. 1369, 203–207 [DOI] [PubMed] [Google Scholar]
- 46.Coloma MJ et al. (2000) Transport across the primate blood-brain barrier of a genetically engineered chimeric monoclonal antibody to the human insulin receptor. Pharm. Res. 17, 266–274 [DOI] [PubMed] [Google Scholar]
- 47.Spudich A et al. (2006) Inhibition of multidrug resistance transporter-1 facilitates neuroprotective therapies after focal cerebral ischemia. Nat. Neurosci. 9, 487. [DOI] [PubMed] [Google Scholar]
- 48.Cannon RE et al. (2012) Targeting blood-brain barrier sphingolipid signaling reduces basal P-glycoprotein activity and improves drug delivery to the brain. Proc. Natl. Acad. Sci. U. S. A. 109, 15930–15935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knowland D et al. (2014) Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron 82, 603–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cipolla MJ et al. (2004) Transcellular transport as a mechanism of blood-brain barrier disruption during stroke. Front. Biosci. 9, 777–785 [DOI] [PubMed] [Google Scholar]
- 51.Hynynen K et al. (2008) Ultrasound for drug and gene delivery to the brain. Adv. Drug Deliv. Rev. 60, 1209–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alam MI et al. (2010) Strategy for effective brain drug delivery. Eur. J. Pharm. Sci. 40, 385–403 [DOI] [PubMed] [Google Scholar]
- 53.Rogawski MAJN (2009) Convection-enhanced delivery in the treatment of epilepsy. Neurotherapeutics 6, 344–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fiandaca MS et al. (2008) Image-guided convection-enhanced delivery platform in the treatment of neurological diseases. Neurotherapeutics 5, 123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haar PJ et al. (2010) Quantification of convection-enhanced delivery to the ischemic brain. Physiol. Meas. 31, 1075. [DOI] [PubMed] [Google Scholar]
- 56.Wang M et al. (2007) Enhanced disruption of the blood brain barrier by intracarotid mannitol injection during transient cerebral hypoperfusion in rabbits. J. Neurosurg. Anesthesiol. 19, 249–256 [DOI] [PubMed] [Google Scholar]
- 57.Rapoport SI (2000) Osmotic opening of the blood-brain barrier: principles, mechanism, and therapeutic applications. Cell. Mol. Neurobio.l 20, 217–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gonzales-Portillo GS et al. (2014) Mannitol-enhanced delivery of stem cells and their growth factors across the blood-brain barrier. Cell. Transplant. 23, 531–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tajiri N et al. (2016) Breaking the blood-brain barrier with mannitol to aid stem cell therapeutics in the chronic stroke brain. Cell Transplant 25, 1453–1460 [DOI] [PubMed] [Google Scholar]
- 60.Fatar M et al. (2005) Neuroprotective effect of combined ultrasound and microbubbles in a rat model of middle cerebral artery infarction. AIP Conf. Proc. 754, 62–64 [Google Scholar]
- 61.Choi M et al. (2011) Minimally invasive molecular delivery into the brain using optical modulation of vascular permeability. Proc. Natl. Acad. Sci. U. S. A. 108, 9256–9261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X et al. (2014) Scopine as a novel brain-targeting moiety enhances the brain uptake of chlorambucil. Bioconj. Chem. 25, 2046–2054 [DOI] [PubMed] [Google Scholar]
- 63.Greig NH et al. (1990) Physicochemical and pharmacokinetic parameters of seven lipophilic chlorambucil esters designed for brain penetration. Cancer Chemother. Pharmacol. 25, 311–319 [DOI] [PubMed] [Google Scholar]
- 64.Lu C–T et al. (2014) Current approaches to enhance CNS delivery of drugs across the brain barriers. Int. J. Nanomedicine 9, 2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pardridge WM (2007) Drug targeting to the brain. Pharm. Res. 24, 1733–1744 [DOI] [PubMed] [Google Scholar]
- 66.Stockwell J et al. (2014) Novel central nervous system drug delivery systems. Chem. Biol. Drug Des. 83, 507–520 [DOI] [PubMed] [Google Scholar]
- 67.Nozohouri S et al. (2019) A multilayer hollow nanocarrier for pulmonary co-drug delivery of methotrexate and doxorubicin in the form of dry powder inhalation formulation. Mater. Sci. Eng. C 99, 752–761 [DOI] [PubMed] [Google Scholar]
- 68.Al-Jamal KT et al. (2011) Functional motor recovery from brain ischemic insult by carbon nanotube-mediated siRNA silencing. Proc. Natl. Acad. Sci. U. S. A 108, 10952–10957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu S et al. (2014) Transport of glial cell line-derived neurotrophic factor into liposomes across the blood-brain barrier: in vitro and in vivo studies. Int. J. Mol. Sci. 15, 3612–3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ishii T et al. (2013) Treatment of cerebral ischemia-reperfusion injury with PEGylated liposomes encapsulating FK506. FASEB J. 27, 1362–1370 [DOI] [PubMed] [Google Scholar]
- 71.Ishii T et al. (2012) A single injection of liposomal asialo-erythropoietin improves motor function deficit caused by cerebral ischemia/reperfusion. Int. J. Pharm. 439, 269–274 [DOI] [PubMed] [Google Scholar]
- 72.Liu Y et al. (2017) Comprehensive insights into the multi-antioxidative mechanisms of melanin nanoparticles and their application to protect brain from injury in ischemic stroke. J. Am. Chem. Soc. 139, 856–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yun X et al. (2013) Nanoparticles for targeted delivery of antioxidant enzymes to the brain after cerebral ischemia and reperfusion injury. J. Cereb. Blood Flow Metab. 33, 583–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tiebosch IA et al. (2012) Combined treatment with recombinant tissue plasminogen activator and dexamethasone phosphate-containing liposomes improves neurological outcome and restricts lesion progression after embolic stroke in rats. J. Neurochem. 123, 65–74 [DOI] [PubMed] [Google Scholar]
- 75.Yang HY et al. (2016) pH-Responsive biodegradable polymeric micelles with anchors to interface magnetic nanoparticles for MR imaging in detection of cerebral ischemic area. Nanoscale 8, 12588–12598 [DOI] [PubMed] [Google Scholar]
- 76.Lockman PR et al. (2004) Nanoparticle surface charges alter blood-brain barrier integrity and permeability. J. Drug Target 12, 635–641 [DOI] [PubMed] [Google Scholar]
- 77.Hussain S et al. (2005) In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol. In Vitro 19, 975–983 [DOI] [PubMed] [Google Scholar]
- 78.Xia T et al. (2006) Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 6, 1794–1807 [DOI] [PubMed] [Google Scholar]
- 79.Yildirimer L et al. (2011) Toxicology and clinical potential of nanoparticles. Nano Today 6, 585–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhuang X et al. (2011) Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 19, 1769–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perets N et al. (2019) Golden exosomes selectively target brain pathologies in neurodegenerative and neurodevelopmental disorders. Nano Lett. 19, 3422–3431 [DOI] [PubMed] [Google Scholar]
- 82.Xin H et al. (2013) Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cereb. Blood Flow Metab. 33, 1711–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xin H et al. (2012) Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 30, 1556–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pavan B et al. (2008) Progress in drug delivery to the central nervous system by the prodrug approach. Molecules 13, 1035–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nonaka N et al. (2008) Delivery of galanin-like peptide to the brain: targeting with intranasal delivery and cyclodextrins. J. Pharmacol. Exp. Ther. 325, 513–519 [DOI] [PubMed] [Google Scholar]
- 86.Gil ES et al. (2012) beta-cyclodextrin-poly(beta-amino ester) nanoparticles for sustained drug delivery across the blood-brain barrier. Biomacromolecules 13, 3533–3541 [DOI] [PubMed] [Google Scholar]
- 87.Deguchi Y et al. (2000) Improved brain delivery of a nonsteroidal anti-inflammatory drug with a synthetic glyceride ester: a preliminary attempt at a CNS drug delivery system for the therapy of Alzheimer’s disease. J. Drug Target 8, 371–381 [DOI] [PubMed] [Google Scholar]
- 88.Zhao Y et al. (2014) Design, synthesis and biological evaluation of brain targeting l-ascorbic acid prodrugs of ibuprofen with ‘lock-in’ function. Eur. J. Med. Chem. 82, 314–323 [DOI] [PubMed] [Google Scholar]
- 89.Xu G et al. (2018) Synthesis, characterization and in vivo evaluation of honokiol bisphosphate prodrugs protects against rats’ brain ischemia–reperfusion injury. Asian J. Pharm. Sci. 14, 640–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Z et al. (2017) Therapeutic potential of novel twin compounds containing tetramethylpyrazine and carnitine substructures in experimental ischemic stroke. Oxid. Med. Cell. Longev. 2017, 7191856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chapman CD et al. (2013) Intranasal treatment of central nervous system dysfunction in humans. Pharm. Res. 30, 2475–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ahmad N et al. (2016) PNIPAM nanoparticles for targeted and enhanced nose-to-brain delivery of curcuminoids: UPLC/ESI-Q-ToF-MS/MS-based pharmacokinetics and pharmacodynamic evaluation in cerebral ischemia model. Drug Deliv. 23, 2095–2114 [DOI] [PubMed] [Google Scholar]
- 93.Zhao N et al. (2017) Intranasal delivery of a caspase-1 inhibitor in the treatment of global cerebral ischemia. Mol. Neurobiol. 54, 4936–4952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu XF et al. (2001) Intranasal administration of insulin-like growth factor-I bypasses the blood-brain barrier and protects against focal cerebral ischemic damage. J. Neurol. Sci. 187, 91–97 [DOI] [PubMed] [Google Scholar]
- 95.Van Velthoven CT et al. (2010) Nasal administration of stem cells: a promising novel route to treat neonatal ischemic brain damage. Pediatr. Res. 68, 419. [DOI] [PubMed] [Google Scholar]
- 96.Lochhead JJ and Thorne RG (2012) Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 64, 614–628 [DOI] [PubMed] [Google Scholar]
- 97.Toyoda K et al. (2003) Gene therapy for cerebral vascular disease: update 2003. Br. J. Pharmacol. 139, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shimamura M et al. (2011) Experimental and clinical application of plasmid DNA in the field of central nervous diseases. Curr. Gene Ther. 11, 491–500 [DOI] [PubMed] [Google Scholar]
- 99.Zhang J et al. (2011) rAAV-mediated delivery of brain-derived neurotrophic factor promotes neurite outgrowth and protects neurodegeneration in focal ischemic model. Int. J. Clin. Exp. Pathol. 4, 496. [PMC free article] [PubMed] [Google Scholar]
- 100.Andsberg G et al. (2002) Neuropathological and behavioral consequences of adeno-associated viral vector-mediated continuous intrastriatal neurotrophin delivery in a focal ischemia model in rats. Neurobiol. Dis. 9, 187–204 [DOI] [PubMed] [Google Scholar]
- 101.Chen YC et al. (2019) A NeuroD1 AAV-based gene therapy for functional brain repair after ischemic injury through in vivo astrocyte-to-neuron conversion. Mol. Ther. 2019 10.1016/j.ymthe.2019.09.003 Published online September 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Papale A et al. (2009) Viral vector approaches to modify gene expression in the brain. J. Neurosci. Methods 185, 1–14 [DOI] [PubMed] [Google Scholar]
- 103.Hyun H et al. (2011) Combinational therapy of ischemic brain stroke by delivery of heme oxygenase-1 gene and dexamethasone. Biomaterials 32, 306–315 [DOI] [PubMed] [Google Scholar]
- 104.Tanaka S et al. (2004) Infiltrating macrophages as in vivo targets for intravenous gene delivery in cerebral infarction. Stroke 35, 1968–1973 [DOI] [PubMed] [Google Scholar]
- 105.Kim I-D et al. (2010) Neuroprotection by biodegradable PAMAM ester (e-PAM-R)-mediated HMGB1 siRNA delivery in primary cortical cultures and in the postischemic brain. J. Control. Release 142, 422–430 [DOI] [PubMed] [Google Scholar]
- 106.Jovin TG et al. (2015) Thrombectomy within 8 hours after symptom onset in ischemic stroke. N. Engl. J. Med. 372, 2296–2306 [DOI] [PubMed] [Google Scholar]
- 107.Albers GW et al. (2018) Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N. Engl. J. Med. 378, 708–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wei D et al. (2017) The use and utility of aspiration thrombectomy in acute ischemic stroke: a systematic review and meta-analysis. Am. J. Neuroradiol. 38, 1978–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Amar AP et al. (2015) Combined neurothrombectomy or thrombolysis with adjunctive delivery of 3K3A-activated protein C in acute ischemic stroke. Front. Cell. Neurosci. 9, 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Turk AS et al. (2014) Initial clinical experience with the ADAPT technique: a direct aspiration first pass technique for stroke thrombectomy. J. Neurointerv. Surg. 6, 231–237 [DOI] [PubMed] [Google Scholar]
- 111.Fraser JF et al. (2017) Intra-arterial verapamil post-thrombectomy is feasible, safe, and neuroprotective in stroke. J. Cereb. Blood Flow Metab. 37, 3531–3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pereira VM et al. (2013) Prospective, multicenter, single-arm study of mechanical thrombectomy using Solitaire Flow Restoration in acute ischemic stroke. Stroke 44, 2802–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Castano C et al. (2010) Mechanical thrombectomy with the Solitaire AB device in large artery occlusions of the anterior circulation: a pilot study. Stroke 41, 1836–1840 [DOI] [PubMed] [Google Scholar]
- 114.Tomsick TA et al. (2010) Equipoise among recanalization strategies. Neurology 74, 1069–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Modo M et al. (2013) Bioengineering solutions for neural repair and recovery in stroke. Curr. Opin. Neurol. 26, 626–631 [DOI] [PubMed] [Google Scholar]
- 116.Xu J et al. (2019) Sequentially site-specific delivery of thrombolytics and neuroprotectant for enhanced treatment of ischemic stroke. ACS Nano 13, 8577–8588 [DOI] [PubMed] [Google Scholar]
- 117.Ma J et al. (2019) Targeted drug delivery to stroke via chemotactic recruitment of nanoparticles coated with membrane of engineered neural stem cells. Small 15, 1902011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cook DJ et al. (2017) Hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke. J. Cereb. Blood Flow Metab. 37, 1030–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nih LR et al. (2016) Hydrogels for brain repair after stroke: an emerging treatment option. Curr. Opin. Biotechnol. 40, 155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Luo Y et al. (2019) Dual and multi-targeted nanoparticles for site-specific brain drug delivery. J. Control. Release 317, 195–215 [DOI] [PubMed] [Google Scholar]
- 121.Avgerinos KI et al. (2019) Intravenous magnesium sulfate in acute stroke. Stroke 50, 931–938 [DOI] [PubMed] [Google Scholar]
- 122.Maniskas ME et al. (2016) Stroke neuroprotection revisited: Intra-arterial verapamil is profoundly neuroprotective in experimental acute ischemic stroke. J. Cereb. Blood Flow Metab. 36, 721–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ballarin B and Tymianski M (2018) Discovery and development of NA-1 for the treatment of acute ischemic stroke. Acta Pharmacol. Sin. 39, 661–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xiong X-Y et al. (2018) Refocusing neuroprotection in cerebral reperfusion era: New challenges and strategies. Front. Neurol. 9, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shuaib A et al. (2007) NXY-059 for the treatment of acute ischemic stroke. N. Engl. J. Med. 357, 562–571 [DOI] [PubMed] [Google Scholar]
- 126.Amaro S et al. (2015) Uric acid improves glucose-driven oxidative stress in human ischemic stroke. Ann. Neurol. 77, 775–783 [DOI] [PubMed] [Google Scholar]
- 127.Qin C et al. (2017) Fingolimod protects against ischemic white matter damage by modulating microglia toward M2 polarization via STAT3 pathway. Stroke 48, 3336–3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Elkins J et al. (2017) Safety and efficacy of natalizumab in patients with acute ischaemic stroke (ACTION): a randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 16, 217–226 [DOI] [PubMed] [Google Scholar]
- 129.Minnerup J et al. (2008) Meta-analysis of the efficacy of granulocyte-colony stimulating factor in animal models of focal cerebral ischemia. Stroke 39, 1855–1861 [DOI] [PubMed] [Google Scholar]
- 130.Minnerup J and Schäbitz W-R. (2009) Multifunctional actions of approved and candidate stroke drugs. Neurotherapeutics 6, 43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Siren AL et al. (2009) Therapeutic potential of erythropoietin and its structural or functional variants in the nervous system. Neurotherapeutics 6, 108–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Maurer MH et al. (2007) Transplantation of adult neural progenitor cells transfected with vascular endothelial growth factor rescues grafted cells in the rat brain. Int. J. Biol. Sci. 4, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ferrer I et al. (2001) Brain-derived neurotrophic factor reduces cortical cell death by ischemia after middle cerebral artery occlusion in the rat. Acta Neuropathol. 101, 229–238 [DOI] [PubMed] [Google Scholar]
- 134.Bath PM et al. (2019) Prehospital transdermal glyceryl trinitrate for ultra-acute intracerebral hemorrhage: data from the RIGHT-2 trial. Stroke 50, 3064–3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jiang WW et al. (2017) Sodium hydrosulfide attenuates cerebral ischemia/reperfusion injury by suppressing overactivated autophagy in rats. FEBS Open Biol. 7, 1686–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mateos L et al. (2016) Angiotensin II type-2 receptor stimulation induces neuronal VEGF synthesis after cerebral ischemia. Biochim. Biophys. Acta 1862, 1297–1308 [DOI] [PubMed] [Google Scholar]
- 137.Zheng Y et al. (2014) Inhibition of autophagy contributes to melatonin-mediated neuroprotection against transient focal cerebral ischemia in rats. J. Pharmacol. Sci. 124, 354–364 [DOI] [PubMed] [Google Scholar]
- 138.Hou Y et al. (2018) Resveratrol provides neuroprotection by regulating the JAK2/STAT3/PI3K/AKT/mTOR pathway after stroke in rats. Genes Dis. 5, 245–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang Y et al. (2013) Activated protein C analog protects from ischemic stroke and extends the therapeutic window of tissue-type plasminogen activator in aged female mice and hypertensive rats. Stroke 44, 3529–3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Martin RH et al. (2016) Alias (albumin in acute ischemic stroke) trials: analysis of the combined data from parts 1 and 2. Stroke 47, 2355–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hong JM et al. (2018) Safety and Optimal Neuroprotection of neu2000 in acute Ischemic stroke with reCanalization: study protocol for a randomized, double-blinded, placebo-controlled, phase-II trial. Trials 19, 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Malhotra K et al. (2018) Minocycline for acute stroke treatment: a systematic review and meta-analysis of randomized clinical trials. J. Neurol. 265, 1871–1879 [DOI] [PubMed] [Google Scholar]
- 143.Adibhatla RM and Hatcher JF (2002) Citicoline mechanisms and clinical efficacy in cerebral ischemia. J. NeuroSci. Res. 70, 133–139 [DOI] [PubMed] [Google Scholar]
- 144.Simonyi A et al. (2005) Polyphenols in cerebral ischemia. Mol Neurobiol 31, 135–147 [DOI] [PubMed] [Google Scholar]
- 145.Tahir RA and Pabaney AH (2016) Therapeutic hypothermia and ischemic stroke: a literature review. Surg Neurol. Int. 7 (Suppl. 14), S381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pan J et al. (2016) Remote ischemic conditioning for acute ischemic stroke: dawn in the darkness. Rev. Neurosci. 27, 501–510 [DOI] [PubMed] [Google Scholar]
- 147.Mdzinarishvili A et al. (2013) Engineering triiodothyronine (T3) nanoparticle for use in ischemic brain stroke. J. Drug Deliv. Transl. Res. 3, 309–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim CK et al. (2012) Ceria nanoparticles that can protect against ischemic stroke. Angew. Chem. Int. Ed. Engl. 51, 11039–11043 [DOI] [PubMed] [Google Scholar]
- 149.Gao Y et al. (2018) A facile approach for synthesis of nano-CeO2 particles loaded co-polymer matrix and their colossal role for blood-brain barrier permeability in cerebral ischemia. J. Photochem. Photobiol. B 187, 184–189 [DOI] [PubMed] [Google Scholar]
- 150.Jiang Y et al. (2015) SOD1 nanozyme salvages ischemic brain by locally protecting cerebral vasculature. J. Control. Release 213, 36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lu YM et al. (2014) Targeted therapy of brain ischaemia using Fas ligand antibody conjugated PEG-lipid nanoparticles. Biomaterials 35, 530–537 [DOI] [PubMed] [Google Scholar]
- 152.Lv W et al. (2018) Bioengineered boronic ester modified dextran polymer nanoparticles as reactive oxygen species responsive nanocarrier for ischemic stroke treatment. ACS Nano 12, 5417–5426 [DOI] [PubMed] [Google Scholar]
- 153.Chen H et al. (2012) Nanoerythropoietin is 10-times more effective than regular erythropoietin in neuroprotection in a neonatal rat model of hypoxia and ischemia. Stroke 43, 884–887 [DOI] [PubMed] [Google Scholar]
- 154.Zamanlu M et al. (2019) Enhanced thrombolysis using tissue plasminogen activator (tPA)-loaded PEGylated PLGA nanoparticles for ischemic stroke. J. Drug Deliv. Sci. 53, 101165 [Google Scholar]
- 155.Tian T et al. (2018) Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 150, 137–149 [DOI] [PubMed] [Google Scholar]
- 156.Xin H et al. (2017) MicroRNA cluster miR-17–92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke 48, 747–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu Y et al. (2019) Rapid enkephalin delivery using exosomes to promote neurons recovery in ischemic stroke by inhibiting neuronal p53/Caspase-3. Biomed. Res. Int. 2019, 4273290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang H et al. (2019) Exosome-mediated targeted delivery of miR–210 for angiogenic therapy after cerebral ischemia in mice. J. Nanobiotechnol 17, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Singh V et al. (2018) Amelioration of ischaemia reperfusion-induced cerebral injury in mice by liposomes containing Allium cepa fraction administered intranasally. Artif. Cells Nanomed. Biotechnol. 46 (Supp. 3), S982–S992 [DOI] [PubMed] [Google Scholar]
- 160.Cao Y et al. (2002) Liposome-mediated transfer of the bcl-2 gene results in neuroprotection after in vivo transient focal cerebral ischemia in an animal model. Gene Ther. 9, 415. [DOI] [PubMed] [Google Scholar]
- 161.Campos–Martorell M et al. (2016) Charge effect of a liposomal delivery system encapsulating simvastatin to treat experimental ischemic stroke in rats. Int. J. Nanomed. 11, 3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Takanashi Y et al. (2001) Neuroprotection by intrathecal application of liposome-entrapped fasudil in a rat model of ischemia. Neurol. Med. Chir. 41, 107–114 [DOI] [PubMed] [Google Scholar]
- 163.Peng T et al. (2013) Therapeutic time window and dose dependence of xenon delivered via echogenic liposomes for neuroprotection in stroke. CNS Neurosci. Ther. 19, 773–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhao G et al. (2011) Postischemic administration of liposome-encapsulated luteolin prevents against ischemia-reperfusion injury in a rat middle cerebral artery occlusion model. J. Nutr. Biochem. 22, 929–936 [DOI] [PubMed] [Google Scholar]
- 165.Zhao Y et al. (2018) Nano-liposomes of lycopene reduces ischemic brain damage in rodents by regulating iron metabolism. Free Radic. Biol. Med. 124, 1–11 [DOI] [PubMed] [Google Scholar]
- 166.Partoazar A et al. (2017) Nanoliposome containing cyclosporine A reduced neuroinflammation responses and improved neurological activities in cerebral ischemia/reperfusion in rat. Fundam. Clin. Pharmacol. 31, 185–193 [DOI] [PubMed] [Google Scholar]
- 167.Xing C et al. (2012) Delivering minocycline into brain endothelial cells with liposome-based technology. J. Cereb. Blood Flow Metab. 32, 983–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ramos-Cabrer P et al. (2011) Serial MRI study of the enhanced therapeutic effects of liposome-encapsulated citicoline in cerebral ischemia. Int. J. Pharm. 405, 228–233 [DOI] [PubMed] [Google Scholar]
- 169.Chung GY et al. (2018) Chitosan-coated C-phycocyanin liposome for extending the neuroprotective time window against ischemic brain stroke. Curr. Pharm. Des. 24, 1859–1864 [DOI] [PubMed] [Google Scholar]
- 170.Li Y et al. (2018) An Angiopep-2 functionalized nanoformulation enhances brain accumulation of tanshinone IIA and exerts neuroprotective effects against ischemic stroke. New J. Chem. 42, 17359–17370 [Google Scholar]
- 171.Ahmad N et al. (2018) Intranasal delivery of quercetin-loaded mucoadhesive nanoemulsion for treatment of cerebral ischaemia. Artif. Cells Nanomed. Biotechnol. 46, 717–729 [DOI] [PubMed] [Google Scholar]
- 172.Nagai N et al. (2015) Intravenous administration of cilostazol nanoparticles ameliorates acute ischemic stroke in a cerebral ischemia/reperfusion-induced injury model. Int. J. Mol. Sci. 16, 29329–29344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ahmad N et al. (2016) Quantification and evaluation of thymoquinone loaded mucoadhesive nanoemulsion for treatment of cerebral ischemia. Int. J. Biol. Macromol. 88, 320–332 [DOI] [PubMed] [Google Scholar]
- 174.Ahmad N et al. (2017) The effect of safranal loaded mucoadhesive nanoemulsion on oxidative stress markers in cerebral ischemia. Artif. Cells Nanomed. Biotechnol. 45, 775–787 [DOI] [PubMed] [Google Scholar]
- 175.Cui W et al. (2016) pH gradient difference around ischemic brain tissue can serve as a trigger for delivering polyethylene glycol-conjugated urokinase nanogels. J. Control. Release 225, 53–63 [DOI] [PubMed] [Google Scholar]
- 176.Joachim E et al. (2014) Gelatin nanoparticles enhance the neuroprotective effects of intranasally administered osteopontin in rat ischemic stroke model. Drug Deliv. Transl. Res. 4, 395–399 [DOI] [PubMed] [Google Scholar]
- 177.Liu Z et al. (2015) Effect of Baicalin-loaded PEGylated cationic solid lipid nanoparticles modified by OX26 antibody on regulating the levels of baicalin and amino acids during cerebral ischemia–reperfusion in rats. Int. J. Pharm. 489, 131–138 [DOI] [PubMed] [Google Scholar]
- 178.Kakkar V et al. (2013) Curcumin loaded solid lipid nanoparticles: an efficient formulation approach for cerebral ischemic reperfusion injury in rats. Eur. J. Pharm. Biopharm. 85, 339–345 [DOI] [PubMed] [Google Scholar]
- 179.Hassanzadeh P et al. (2018) Ferulic acid-loaded nanostructured lipid carriers: a promising nanoformulation against the ischemic neural injuries. Life Sci. 193, 64–76 [DOI] [PubMed] [Google Scholar]