Dear Editor,
Critically ill patients develop acute respiratory distress syndrome (ARDS)[1] and despite the study of genomics of ARDS, there is little progress.[2] The drop in the cost of sequencing has refocused genetic studies from DNA to RNA sequencing and methods to analyze this data have improved.[3] The objective of this investigation is to utilize RNA sequencing data and analysis to identify novel gene targets in ARDS.
A description of the methods is in the eSupplement. The human cohort generated from the GTEx consortium consisted of 25 deceased patients with ARDS identified by the presence of diffuse alveolar damage (DAD, this limitation is described in the supplement), and 74 deceased patients evaluated to not have DAD. The mouse ARDS cohort included C57BL/6 mice ages 10–12 in a model previously described and compared to controls.[4, 5]
Alternatively spliced RNA arise from co/post-transcriptional events facilitated by the spliceosome, introns are removed to form the mature RNA from which protein isoforms are translated. Alternatively transcribed genes are the product of changes in promoter usage, polyadenylation signals, and RNA polymerase II interactions with DNA which can lead to changes in isoform usage similar to alternative splicing events. These are identified from the analysis of RNA sequencing data (see supplement). Significant differentially alternatively transcribed genes and alternative spliced genes were identified (see supplement) and were overlapped with genes previously reported as ARDS related (Figure 1A). Of 89 reported ARDS related genes[2], 38 were confirmed in at least one differential category confirming that our use of humans and mice with DAD/ARDS is appropriate and robust (p=1.25e-14). Eleven previously reported genes were present in all categories (Figure 1A). These eleven genes were evaluated for the change in alternative splicing and alternative transcription (Figure 1B). GO term enrichment analysis was performed on the 11 overlapping genes, revealing 20 significant biological processes including ontology related to aging, and response to abiotic/environmental stimuli (Figure 1C). There are 1639 genes that show overlap in alternative splicing and alternative transcription that were not previously in the literature (Figure 1A). These genes were assessed for directionality alternative splicing and alternative transcription and GO terms (eTable 1,2 in the supplement) should provide the foundation for future work in ARDS.
Figure 1:
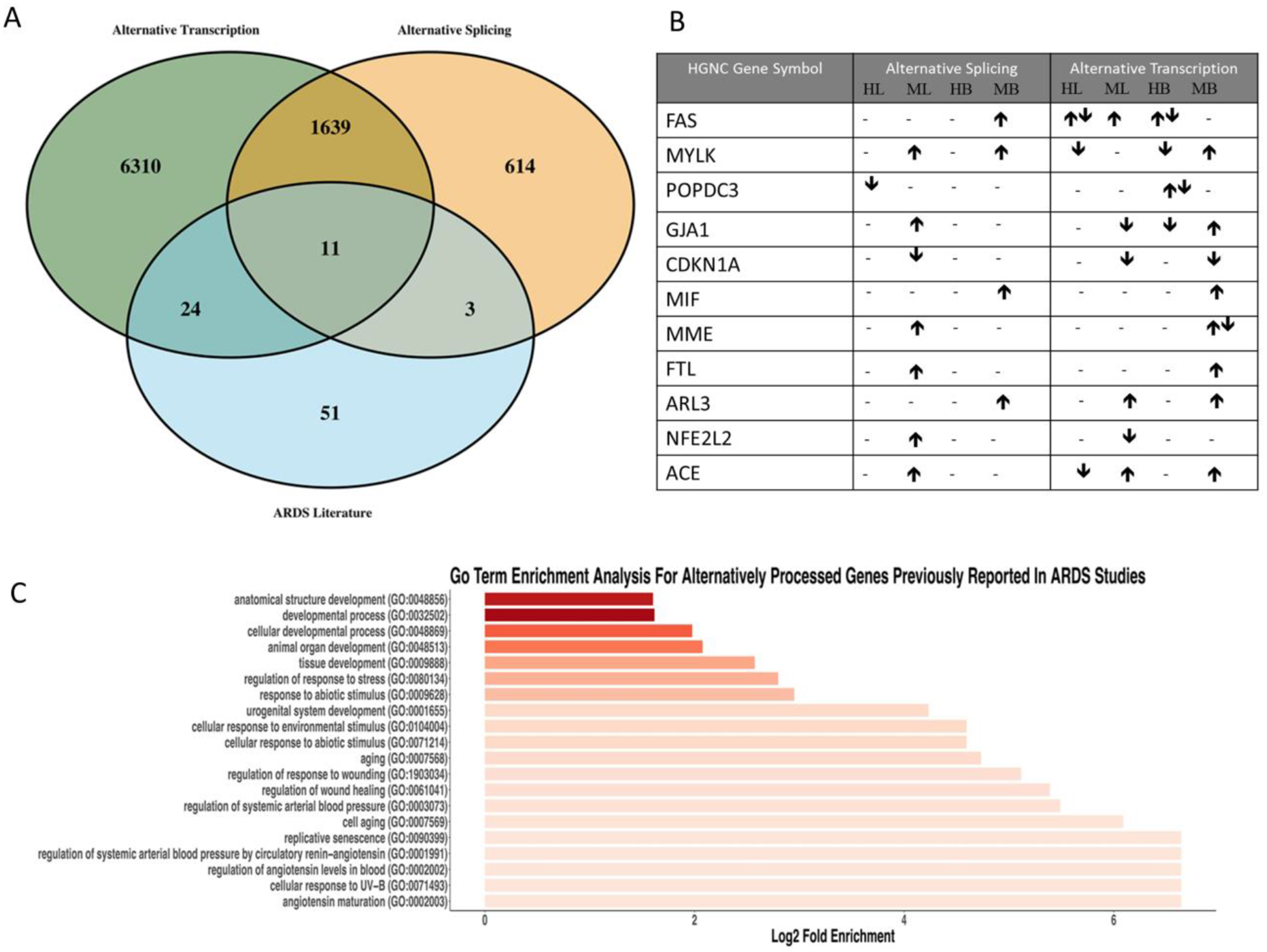
Overlap of Differentially Processed Genes in ARDS Correlates with the Literature Suggesting ARDS Models are Robust. A) Consolidated list of genes reported to be associated with ARDS over the past 50 years[2] were overlapped with the significant genes from the differential expression and alternative processing analyses. Of the 89 genes reported the 11 genes were found to overlap in all three categories. B) Directionality of the change in expression of the eleven genes was then assessed. Arrows indicate whether each gene is enriched (up) or depleted (down) in each sample (HL=human lung, ML=mouse lung, HB=human blood, MB=mouse blood) for each category (Alternative Splicing, Alternative Transcription). Two arrows indicate multiple isoforms were found to be significant in different directions. C) GO terms analysis was performed on the overlapping genes resulting in twenty significantly enriched biological processes.
Studying the underlying changes in RNA processing (alternative splicing and alternative transcription start/end) not only expands basic knowledge of pathogenicity, but also provides additional targets for therapeutics. The most enriched GO term from the alternative splicing set (eFigure 1B), carboxy-terminal domain protein kinase complex (GO:0032806) refers to phosphorylation of the CTD of RNA polymerase II, which is vital in the regulation of transcription and RNA processing. In addition RNA polymerase complex binding (GO:0000993), and transport of the SLBP Independent/Dependent mature mRNA (R-HSA-159227; R-HSA-159230) are among the most enriched (eFigure 1B). This suggests alternative pre-mRNA splicing plays the dominate role in isoform usage in genes where expressions levels do not change, whereas alternative transcription may regulate isoform usage in genes that are more dynamically expressed during critical illness. Although it is possible the enrichment reflects down regulation through inhibitory genes, these data support the hypothesis that alternative splicing and alternative transcription may have separate roles in DAD/ARDS by regulating different genes to perform distinctive functions.
In this analysis of RNA sequencing data from deceased patients with ARDS identified by the presence of DAD and a clinically relevant mouse model of ARDS, novel genes were identified. Future research is needed using on the mechanism of alternative RNA splicing and alternative transcription start/end seen in ARDS.
Supplementary Material
Acknowledgements
This was supported by This work was funded in part by NIH-NIGMS P20GM103652 (S.F.M.); NIH-NIGMS R35GM118097 (A.A.), the C. James Carrico, MD, FACS, Faculty Research Fellowship for the Study of Trauma and Critical Care from the American College of Surgeons (S.F.M.); NIH-NIGMS R01GM127472 (W.G.F.)
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Declaration of Interests
None
References
- 1.Thompson BT, Chambers RC, Liu KD, (2017) Acute Respiratory Distress Syndrome. New England Journal of Medicine 377: 562–572 [DOI] [PubMed] [Google Scholar]
- 2.Reilly JP, Christie JD, Meyer NJ, (2017) Fifty Years of Research in ARDS. Genomic Contributions and Opportunities. American journal of respiratory and critical care medicine [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stark R, Grzelak M, Hadfield J, (2019) RNA sequencing: the teenage years. Nature reviews Genetics [DOI] [PubMed] [Google Scholar]
- 4.Ayala A, Chung CS, Lomas JL, Song GY, Doughty LA, Gregory SH, Cioffi WG, LeBlanc BW, Reichner J, Simms HH, Grutkoski PS, (2002) Shock-induced neutrophil mediated priming for acute lung injury in mice: divergent effects of TLR-4 and TLR-4/FasL deficiency. The American journal of pathology 161: 2283–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lomas-Neira JL, Heffernan DS, Ayala A, Monaghan SF, (2016) Blockade of Endothelial Growth Factor, Angiopoietin-2, Reduces Indicies of ARDS and Mortality in Mice Resulting form the Dual-Insults of Hemorrhagic Shock and Sepsis. Shock (Augusta, Ga) 45: 157–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


