Highlights
-
•
Cure rates are high for choriocarcinoma, however chemoresistant disease often leads to death.
-
•
High expression of PD-L1 suggests a role for checkpoint inhibitors in choriocarcinoma.
-
•
Pembrolizumab should be considered for salvage therapy for chemoresistant choriocarcinoma.
Keywords: Gestational trophoblastic neoplasia, Choriocarcinoma, Chemotherapy resistance, Immunotherapy
1. Introduction
Choriocarcinoma is a highly malignant form of gestational trophoblastic neoplasia. Given its rarity, incidence rates range from 3.3 per 40,000 pregnancies in Southeast Asia to 1 in 40,000 pregnancies in North America and Europe (Goldstein et al., n.d.). Management of these tumors is primarily based on its high sensitivity to chemotherapy and its unique production of hCG (Seckl et al., 2010). While variations in treatment schedules exist, complete remission has been observed following single-agent therapy in 85% of low-risk patients (Alazzam et al., 2009). If resistance to single therapy is observed, approximately 15–20% of patients will respond to second-line combination therapy (Powles et al., 2007). There are limited studies evaluating combination salvage therapies in unresectable drug-resistant patients.
We propose a biologic basis for immune checkpoint therapy in refractory trophoblastic tumors. To facilitate an environment where the developing fetus evades the maternal immune response, placental tissue expresses programmed death-ligand 1 (PD-L1) which binds programmed-death-1 receptor (PD-1) on lymphocytes via immune checkpoint activity. This is the first report of a lasting clinical response in a patient treated with anti-PD1 therapy with over 30 months of disease remission (Huang et al., 2017, Ghorani et al., 2017).
2. Case presentation
A 30-year-old woman presented with a 15-week twin pregnancy consisting of a normal fetus and a suspected molar gestation. Dilation and evacuation was performed with karyotype 46XX and pathology confirming choriocarcinoma. The patient underwent chest imaging which demonstrated several nodules concerning for metastatic disease. The patient had low-risk disease based on World Health Organization’s (WHO) score of 1 and was stage III by Federation of Gynecologic and Oncology (FIGO) criteria. The patient received single agent methotrexate (50 mg/m2), however this was changed to actinomycin-D (1.25 mg/m2) due to plateauing hCG levels. Within 6 weeks of stopping treatment, hCG levels increased and she received 4 cycles of etoposide, methotrexate, actinomycin-D, cyclophosphamide, and vincristine (EMA/CO).
Two years later, the patient’s hCG remained undetectable and she underwent intrauterine inseminations which resulted in an inappropriate rise in her hCG level. A dilation and curettage was performed without products of conception heightening the concern for recurrent choriocarcinoma. Her hCG was 3849 mIU/mL and CT imaging, magnetic resonance imaging (MRI), and positron emission tomography-computerized tomography (PET-CT) were all negative. The hCG level continued to rise and due to high clinical suspicion, she completed nine cycles of methotrexate (50 mg/m2) with an hCG plateau followed by four cycles of single agent actinomycin-D. The patient’s hCG remained elevated and a PET/CT confirmed increased FDG-avidity in the uterine fundus and pulmonary lesions. She completed six cycles of EMA/CO with PET/CT confirming complete response. Despite this, her hCG rapidly rose again to 369 mIU/mL within one month of completing therapy and proceeded with third-line multi-agent chemotherapy.
After completion of five cycles of EMA/EP, her hCG normalized and she desired a pause in treatment and pursued holistic modalities at this time. Within three months, her hCG increased to 766 mIU/mL. A CT of the chest demonstrated an enlarging isolated right anterior lung nodule and she underwent a video-assisted thoracoscopic wedge resection of a 1.5 cm tumor confirming metastatic disease. Following the confirmation of pulmonary metastasis, the patient strongly desired oocyte preservation and underwent ovulation induction with a Reproductive Endocrinologist. Her hCG increased from 12,000 mIU/ML to 96,952 mIU/mL. Given evidence of chemotherapy resistance, she was started on cisplatin (60 mg/m2), etoposide (150 mg/m2), and paclitaxel (135 mg/m2), despite 6 cycles her hCG continued to increase. Imaging studies at this time demonstrated a uterine mass and 2 small pulmonary nodules (0.5 × 0.3 cm, 0.6 × 0.2 cm). The pulmonary nodules were thought to be amenable to stereotactic radiotherapy and she underwent a robotic-assisted total laparoscopic hysterectomy and bilateral salpingo-oophorectomy. Surgical pathology of the uterus demonstrated a 3.2 cm tumor consistent with recurrent choriocarcinoma involving the endometrium and superficial myometrium, the cervix and bilateral fallopian tubes showed no evidence of malignancy. At the patient’s post-operative visit however, she reported intermittent headaches and an MRI confirmed two metastatic lesions. The patient did not receive the planned stereotactic radiotherapy directed to the pulmonary lesions however did receive whole brain radiation in 15 fractions of 37.5 Gy (Fig. 1) and her hCG continued to rise from 569 mI/mL to 4527 mIU/mL.
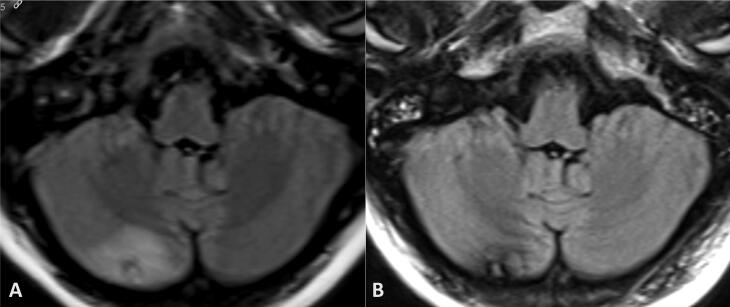
Fig. 1.
Magnetic resonance imaging (MRI) demonstrates a right cerebellar hemisphere lesion (A) with interval resolution of perilesional edema following 37.5 Gy whole brain radiation in 15 fractions, with corresponding hemosiderin/blood products (B).
The patient’s disease was refractory to ifosfamide (904 mg/m2), carboplatin (AUC of 4), etoposide (55 mg/m2) (ICE) and single agent bevacizumab (15 mg/kg). Molecular profiling was performed with findings of PD-L1 and TOP2A biomarker expression. Her immunohistochemical results can be seen in Fig. 2. Given high PD-L1 expression, the patient was started on pembrolizumab dosed at 200 mg intravenous over 30 min every three weeks. After ten cycles her hCG was undetectable at <5mIU/mL, Table 1. Her serum hCG has remained undetectable 31 months after initiating pembrolizumab. Recent CT imaging of the head, chest, abdomen, and pelvis showed several pulmonary nodules continuing to decrease in size and no evidence of disease progression, as seen in Fig. 3.
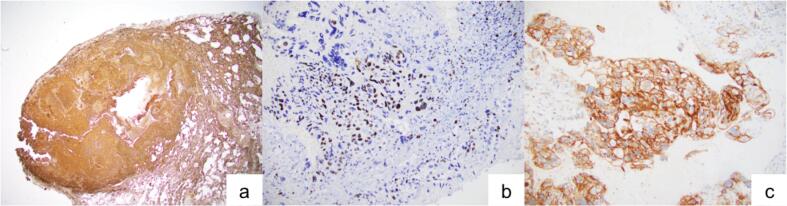
Fig. 2.
Immunohistochemical studies (panel of three IHCs) demonstrate that tumor cells of pulmonary metastasis are (A) diffusely positive for beta hCG, (B) Ki67 confirms a high proliferative index (65%) compatible with choriocarcinoma, and (C) PD-L1 shows strong membranous and cytoplasmic positivity.
Table 1.
Trend of serum hCG levels measured during treatment and maintenance periods. The shaded boxes represent treatment regimens (a) methotrexate, (b) actinomycin-D, (c), EMA-CO, (d) EMA-EP, (e) Cis/Taxol/Etoposide, (f) Ifosfomide/Etoposide, (1) video-assisted thorascopic wedge resection, (2) robotic-assisted total laparoscopic hysterectomy, bilateral salpingectomy, and now on maintenance treatment with pembrolizumab.
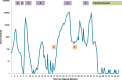 |
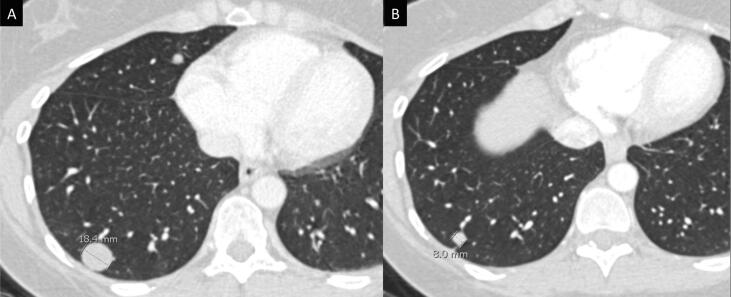
Fig. 3.
Computed tomography (CT) imaging demonstrates an interval decrease in the size of pulmonary metastatic nodules following treatment with 25 cycles of pembrolizumab (A, pre-treatment; B, pembrolizumab therapy).
3. Discussion
As high as 20–30% of high-risk GTD patients will have an incomplete response to first-line chemotherapy and will require salvage therapy (Matsui et al., 2004). Additional treatment for resistant GTN include paclitaxel, cisplatin, etoposide (TP/TE), bleomycin, etoposide, cisplatin (BEP), paclitaxel, ifosfamide, cisplatin (TIP), etoposide, ifosfamide, and cisplatin (VIP), or ifosfamide, carboplatin, and etoposide (ICE) that can lead to remission (Lurain, 2002). The combination of gemcitabine and cisplatin has also been described in achieving remission after in heavily treated patients (Pandian et al., 2004). Given the highly resistant nature of this disease, these varying combinations of chemotherapy can result in significant toxicity and morbidity to the patient.
Several recent studies have confirmed high expression of programmed death-ligand 1 (PD-L1) expression in normal and trophoblastic tumors suggesting a potential role for immune checkpoint inhibitors to limit evasion from immune regulation (Veras et al., 2017, Hajri et al., 2017, Tumors, 2016). Currently, there are only two other published cases of patients with metastatic GTN having received immune checkpoint inhibitors as salvage therapy (Ghorani et al., 2017, Huang et al., 2017). There are five cumulative patients included in these published reports, all of who received treatment for approximately six months with undetectable serum hCG levels. In contrast, our patient remains in remission at over 31 months on pembrolizumab treatment and continues to have undetectable serum hCG levels.
It has been postulated that normal trophoblastic cells behave similarly to malignant cells in their ability to evade the immune system and invade normal tissue. Veras et al. have demonstrated high PD-L1 expression in trophoblastic cells of both normal placentas in addition to gestational trophoblastic tumors (Veras et al., 2017). Binding of PD-1 to its ligand, PD-L1, can initiate a downstream signal inhibition of T-cell activation. The presence of the PD-L1 protein can be detected using immunohistochemistry and this is reported as a percentage of total cells showing protein expression, with a higher percentage representing a higher degree of immune-reactivity. Within normal placental tissue, there was high expression of PD-L1 in syncytiotrophoblasts as compared to cytotrophoblasts. This expression pattern was mimicked in choriocarcinoma with high PD-L1 expression among trophoblastic tumors (Tumors, 2016). The high expression of PD-L1 in syncytiotrophoblasts of both term placentas and choriocarcinomas suggests a mechanism for the immunosuppressive environment necessary for the formation of a fetal-maternal interface. Given paternal antigen expression during pregnancy, PD-L1 expression allows for fetal tolerance to evade maternal immune recognition. The significance of the PD-1/PD-L1 pathway in achieving a fetal-maternal interface has also been demonstrated in mouse models. Guleria et al. showed that PD-L1 deficient mice or mice treated with PD-L1 inhibitors had decreased rates of fetal survival and smaller litter sizes suggesting a role in the development of fetal-maternal immune tolerance (Guleria et al., 2005).
With the findings of high PD-L1 expression among trophoblastic tumors, it becomes necessary to evaluate the clinical implications of expression patterns. Bolze et al. sought to assess the significance of PD-L1 expression among patients with pre-malignant and malignant trophoblastic disease. Expression of PD-L1 was high in all trophoblastic subtypes and 80% of choriocarcinoma specimens analyzed, again suggesting a role for the PD-L1/PD-1 pathway inhibition.
Pembrolizumab is a highly sensitive monoclonal IgG antibody against PD-1 that prevents the engagement with its ligand PD-L1, leading to downstream inhibition of T-cell activity. This immune inhibition results in tumor-specific antigen recognition by cytotoxic T-cells, triggering increased immune response and tumor death. Currently, the US Food and Drug Administration (FDA) has approved pembrolizumab for use in unresectable melanoma, non-small cell lung cancer, recurrent or metastatic head and neck squamous cell cancers, classical Hodgkins lymphoma, in any solid tumor with microsatellite instability, and in PD-L1 positive cervical cancer (FDA’s label for pembrolizumab, 2017). It has an excellent safety profile with discontinuation rates of only 8–20% due to toxicity, however, the question of optimal treatment duration is one that has not yet been answered in regards to immunotherapy (FDA’s label for pembrolizumab, 2017). Given the known serologic marker of hCG for GTN, the longevity of action after discontinuation of immunotherapy may be better understood in these patients compared to other disease sites.
4. Conclusion
While the majority of patients with choriocarcinoma will achieve a complete response from single-agent chemotherapy alone, limited treatment options are available for those who develop unresectable disease or resistance to chemotherapy. The high expression of PD-L1 by normal trophoblasts and trophoblastic tumors such as choriocarcinoma provide a potential mechanism for immune evasion and increased survival of tumor cells. It becomes imperative that further investigation of PD-L1 and PD-1 inhibitors as potential therapeutic in trophoblastic tumors be pursued.
CRediT authorship contribution statement
Kiran H. Clair: Investigation, Writing - original draft. Nicolas Gallegos: Formal analysis, Investigation, Immunohistochemistry and pathology review. Robert E. Bristow: Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Alazzam, M., Tidy, J., Bw, H., Osborne, R., 2009.First line chemotherapy in low risk gestational trophoblastic neoplasia (Review). [DOI] [PubMed]
- FDA’s label for pembrolizumab, 2017.
- Ghorani E., Kaur B., Fisher R.A., Short D., Joneborg U., Carlson J.W., Akarca A., Marafioti T., Quezada S.A., Sarwar N., Seckl M.J. Pembrolizumab is effective for drug-resistant gestational trophoblastic neoplasia. Lancet. 2017;390:2343–2345. doi: 10.1016/S0140-6736(17)32894-5. [DOI] [PubMed] [Google Scholar]
- Goldstein, R., Donald, P. Berkowitz, n.d. Current management of gestational trophoblastic neoplasia. [DOI] [PubMed]
- Guleria I., Khosroshahi A., Ansari M.J., Habicht A., Azuma M., Yagita H., Noelle R.J., Coyle A., Mellor A.L., Khoury S.J., Sayegh M.H. A critical role for the programmed death ligand 1 in fetomaternal tolerance. Brief Definit. Rep. 2005;202:231–237. doi: 10.1084/jem.20050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajri Þ.T., Bolze P., Patrier Þ.S., Abbas F., Schott Þ.A.M., Allias Þ.F., Devouassoux-shisheboran M., Freyer Þ.G., Þþ Þ. PD-L1 Expression in premalignant and malignant trophoblasts from gestational trophoblastic diseases is ubiquitous and independent of clinical outcomes. Int J Gynecol Cancer. 2017;27:554–561. doi: 10.1097/IGC.0000000000000892. [DOI] [PubMed] [Google Scholar]
- Huang M., Pinto A., Castillo R.P., Slomovitz B.M. Complete serologic response to pembrolizumab in a woman with chemoresistant metastatic choriocarcinoma. J. Clin. Oncol. 2017;35:3172–3174. doi: 10.1200/JCO.2017.74.4052. [DOI] [PubMed] [Google Scholar]
- Lurain J.R. Advances in management of high-risk gestational trophoblastic tumors. J. Reprod. Med. 2002;47:451–459. [PubMed] [Google Scholar]
- Matsui H., Iitsuka Y., Suzuka K., Yamazawa K., Mitsuhashi A., Sekiya S. Salvage chemotherapy for high-risk gestational trophoblastic tumor. J. Reprod. Med. 2004;49:438–442. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15283050 [PubMed] [Google Scholar]
- Pandian Z., Seckl M.J., Smith R., Lees D.A.R. Gestational choriocarcinoma: an unusual presentation with response to gemcitabine and surgery. Int. J. Obstetr. Gynecol. 2004;111:382–384. doi: 10.1111/j.1471-0528.2004.00065x.x. [DOI] [PubMed] [Google Scholar]
- Powles, T., Stebbing, J., Short, D., Young, A., Bower, M., Pappin, C., Schmid, P., 2007. A comparison of patients with relapsed and chemo-refractory gestational trophoblastic neoplasia, 732–737. doi: 10.1038/sj.bjc.6603608. [DOI] [PMC free article] [PubMed]
- Seckl M.J., Sebire N.J., Berkowitz R.S. Gestational trophoblastic disease. Lancet. 2010;376:717–729. doi: 10.1016/S0140-6736(10)60280-2. [DOI] [PubMed] [Google Scholar]
- Tumors, T., 2016. Comprehensive immunohistochemical study of, 40 1133–1142. [DOI] [PMC free article] [PubMed]
- Veras E., Kurman R.J., Wang T., Shih I., Ph D. PD-L1 Expression in human placentas and gestational trophoblastic diseases. Int. J. Gynecol. Pathol. 2017:146–153. doi: 10.1097/PGP.0000000000000305. [DOI] [PMC free article] [PubMed] [Google Scholar]