Abstract
Giant cell arteritis (GCA) is a rare form of large and medium vessel vasculitis affecting about 20 cases per 100,000 persons older than the age of 50 years. GCA results in inflammation and constriction of the temporal arteries, cranial arteries, the aorta, and its major branches. Patients often present with vague constitutional symptoms and fever of unknown origin. GCA is a medical emergency requiring prompt diagnosis and early treatment with glucocorticoids which is essential to avoid irreversible end organ damage such as loss of vision, stroke and aneurysm formation. We report a case of a 63-year-old male patient presenting to our healthcare facility with sudden loss of vision and an ischemic brain infarct to be finally diagnosed as a case of giant cell arteritis with positron emission tomography-computed tomography imaging used to evaluate the full extent of the involved vasculature. Diagnostic imaging with FDG positron emission tomography-computed tomography can play a crucial role in the diagnosis, evaluation of the full burden of the disease and follow up to the response of therapy.
Keywords: Giant cell arteritis, Takayasu's arteritis, Large vessel vasculitis, FDG Positron emission tomography, Computed tomography, Standardized uptake values
Introduction
Large vessel vasculitis (LVV) comprises of a group of chronic granulomatous non-necrotizing inflammatory diseases affecting large and sometimes medium-sized vessels namely giant cell arteritis and Takayasu's arteritis [1]. GCA frequently co-exists with polymyalgia rheumatica in almost 50% of cases as they are both from the same disease spectrum [2]. While Takayasu's arteritis (TA) leads to progressive inflammatory changes of the aorta and its major branches including the pulmonary and coronary arteries resulting in stenotic occlusions, calcification and aneurysmal formation of the affected vessels [3,4]. It is seen in 2.6 per million annually and is more common in younger age groups of 20-30 years old with female predominance [4]. Giant cell arteritis (GCA) is estimated to be seen in 1 in every 5000 patients usually above the age of 50 [5]. It can be further subdivided into those with extracranial vessel involvement only and those with both cranial and extracranial involvement which carries a worse prognosis [6,7]. The most commonly involved vasculature are the branches of the internal and external carotid arteries [4]. GCA with intracranial involvement should be distinguished from atherosclerosis, primary angiitis of the cerebrum and reversible cerebral vasoconstriction syndrome [8]. We present a case of giant cell arteritis diagnosed by ultrasound guided temporal artery biopsy and histopathology with positron emission tomography-computed tomography (PET-CT) imaging used to determine the full burden of the disease.
Case report
A 63-year-old male patient presented to the emergency department during an episode of painless right eye vision loss of ten minutes duration. He had experienced a similar incident of right eye vision loss the night before, which lasted approximately 30 minutes and had improved on its own. Prior to this, his consultation with an ophthalmologist at an external facility was unremarkable.
He was admitted with a provisional diagnosis of amaurosis fugax versus nonarteritic anterior ischemic optic neuropathy.
He gave a history of a chronic nonproductive cough, unexplained recurrent febrile illness and weight loss of about 10 kg in the last month. He is a non-smoker. His medical history included type II diabetes, hypertension and hyperlipidemia.
On examination pupillary reflexes were normal to direct and indirect light reflex. He had pseudophakia in both eyes and there was no rapid afferent pupillary defect. His right optic disc was pale. Ophthalmologic examination revealed a cherry red spot on the right macula. The rest of his cranial nerve examination was normal. Temporal artery was non-tender and non-pulsatile. Examination of his upper and lower limbs was normal for motor and sensory function.
Blood tests revealed normocytic anemia (Hb 9.6 g/dL), elevated Erythrocyte Sedimentation Rate (117 mm/h) and C-reactive protein (111 mg/L). His HbA1c was 7.2%. Autoimmune screening including cANCA (Neutrophil cytoplasmic Abs Cytoplasmic), pANCA (Neutrophil cytoplasmic Abs Perinuclear), and ANA (Anti-Nuclear Antibodies) were all negative.
Magnetic resonance imaging of the brain reported a small right occipital ischemic infarct. Contrast-enhanced CT of the chest, abdomen and pelvis was performed to search for a possible focus of infection or inflammation and revealed moderately diffuse mural thickening and mild enhancement of the arch of the aorta and its major branches (Fig. 1).
Fig. 1.
(A)Contrast-enhanced CT scan in axial section at the level of the proximal branches of the aortic arch showing mural thickening and mild enhancement of the visualized vasculature. (B)Sagittal reformatted image showing mural thickening of the aortic arch and its major branches with specks of calcifications seen distally (arrow)
Based upon the patient's clinical history of painless vision loss, ischemic stroke, elevated inflammatory serum markers and vascular thickening on CT scan an inflammatory vascular disease was suspected.
Temporal artery sonogram demonstrated bilateral thickened temporal arteries with absent flow in the frontal and parietal arteries. Temporal artery biopsy was performed and histopathology revealed large vessel circumferential mural involvement by necrotizing giant-cell-rich granulomatous inflammation with marked thickening and occlusion of the intima.
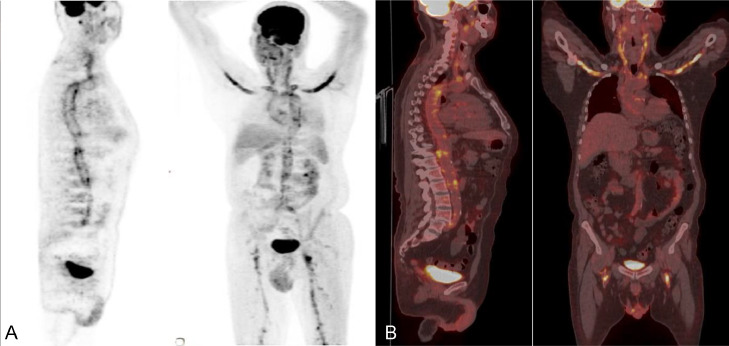
Furthermore, an 18F-FDG PET-CT was performed to delineate the full burden of the disease and revealed avid tracer uptake of the arch of the aorta and its major branches with involvement of the axillary and femoral vessels (Fig. 2).
Fig. 2.
(A) FDG-PET images in AP and lateral views showing tracer uptake in the arch of the aorta and its major branches with avid SUV max 7 uptake seen involving the subclavian, axillary and proximal femoral arteries bilaterally. (B)18F-FDG PET-CT in coronal and sagittal views showing avid tracer uptake in the arch of the aorta and its major branches extending to the axillary and femoral arteries bilaterally
The diagnosis of giant cell arteritis prompted commencing treatment with steroids and steroid sparing immunosuppressive therapy which helped to stabilize his disease. Regular monitoring and follow up of the patient guided the course of management and early treatment of complications.
Discussion
GCA is a medical emergency due to the risk of irreversible damage to end organs. The commonest presenting symptoms are headaches, jaw claudication, painful loss of vision but patients can also present with unexplained fatigue, weight loss, and ischemic brain infarcts. Nevertheless, 17% of patients present as fever of unknown origin and the diagnosis may be difficult and delayed especially if the temporal artery biopsy was inconclusive or negative [1,5,9].
The early diagnosis of large vessel vasculitis is crucial to prevent serious irreversible complications [6,10,11]. It is also important in the prevention of toxicity from unnecessary drug management [8]. Complications such as occlusive stenosis are more commonly seen in TA while aneurysmal dilatation is more commonly seen in patients with GCA [2].
Our patient presented with sudden onset of painless loss of vision in his right eye. Painless loss of vision is an atypical presentation for temporal arteritis. Temporal arteritis patients who suffer visual deficits in one eye have a higher risk (20-50%) of bilateral vision loss if they are not treated with or discontinue their immunosuppressant treatment [10,11].
Both GCA and Takayasu arteritis still lack specific serum biomarkers for a prompt diagnosis [2,3]. The inflammatory markers erythrocyte sedimentation rate and C-reactive protein levels do not always correlate with disease activity and are usually underestimated in patients who are undergoing treatment with steroids [12].
The first imaging modality of choice in GCA diagnosis is temporal artery sonography which may reveal vessel wall edematous changes and thickening ' classic Halo sign' as well as non-compressibility [13,8]. However false positive results may be attributed to severe atherosclerosis and other causes of vasculitis [8].
The European League Against Rheumatism (EULAR) recommended diagnosing large vessel vasculitis on clinical grounds and on diagnostic imaging bases leaving the performance of temporal artery biopsy (TAB) to doubtful diagnosis due to the disadvantage of it being invasive and costly [14].
Furthermore, temporal artery biopsy may also give a false negative result in cases of focal or segmental involvement of the temporal artery, if the patient is on corticosteroids or if there is no involvement of the artery in the disease [6]. Therefore imaging modalities provide a non- invasive, highly sensitive and faster method of diagnosis than TAB [8]. In addition, patients with large vessel extracranial GCA involvement have a lower TAB sensitivity in comparison to those with cranial GCA [8].
Magnetic resonance imaging (MRI) is the preferred imaging modality of choice in Takayasu's arteritis patients where the disease commonly involves the aorta and its major branches keeping in mind the strive for no radiation exposure as these patients present at a younger age group. High resolution MRI findings of vessel wall thickening, structural changes and mural enhancement is well depicted in the diseased vessels [8].
The second modality of choice for diagnosis of LVV is FDG PET-CT [8]. FDG accumulates in active inflammatory cells due to the increased expression of glucose transporters and increased production of glycolytic enzymes [3]. These pathological sites of rapid metabolism indicate an inflammatory reaction while the degree of uptake (SUV max) may be used as a quantitative method of measurement [12].
In a recent study by Soussan et al it described a good overall performance of FDG PET-CT in the diagnosis of LVV showing a high specificity and sensitivity for the disease [6,1]. Tezuka et al recommended the use of FDG-PET for the initial diagnosis and for relapsed cases but not for routine follow-ups [12].
PET performance is said to be more precise in GCA than in TA with positivity criteria of the vascular wall tracer uptake equal or higher than that of the liver [1].
It has a higher sensitivity in depicting early vascular inflammatory changes associated with large vessel vasculitis than MRI [13]. In addition, an increased uptake in the peri-articular and synovial structures can be seen in cases of GCA associated with polymyalgia rheumatica [2].
PET-CT also has the advantage of having a high resolution for anatomical vascular details and can readily detect structural changes such as stenosis, aneurysmal dilatation and dissection to promptly treat acute findings and complications [2].
The differential diagnosis for an increased FDG uptake includes other forms of inflammatory vasculitis, infections and neoplasms [13]. PET has the capacity to identify these changes in tissue metabolism in pathological inflammatory processes before any structural vascular changes are visible [3]. It is superior to the previously used Gallium-citrate scintigraphy in the detection of active inflammation or infection [9]. Therefore, PET-CT can be used to identify the extent of the involved vasculature as with our patient, rule out other causes such as infection or tumors that may be attributed to the patients symptoms and to monitor the disease activity [15].
However, its usefulness in those with cranial involvement in GCA is degraded as superficial cranial arteries are not clearly visualized and as there is a normal physiological FDG uptake by the brain [2,8].
Another major pitfall of FDG PET-CT is the difficulty to distinguish between severe atherosclerotic disease and inflammatory vasculitis. Atherosclerosis is often described on PET-CT as patchy foci with less intense uptake in contrast to vasculitis that shows avid smooth linear tracer distribution [13,3]. Also an increased femoral artery uptake may denote smooth muscle activity and should not confused with active inflammation [3].
The vascular inflammatory uptake in patients with LVV may persist even after the patients are in remission and this may be attributed to a degree of persistent low-grade inflammation, vessel remodeling or neovascular formation [13,3,15].
Our patient had irreversible right visual loss and atypical clinical presentation of temporal arteritis. Although the diagnosis of GCA was initially suspected his atypical presentation proved challenging to confirm the diagnosis and was eventually confirmed on temporal artery biopsy while PET-CT revealed the full disease burden of the involved vasculature. FDG PET-CT has a rising role in the diagnosis and monitoring of disease progression in large vessel vasculitis however no formal consensus or international diagnostic criteria has been set and further prospective studies are highly needed [2,6,1,8].
Footnotes
Patient consent was obtained.
Competing Interests: Authors declare no conflict of interest.
References
- 1.Soussan M, Nicolas P, Schramm C, Katsahian S, Pop G, Fain O. Management of large-vessel vasculitis with FDG-PET: a systematic literature review and meta-analysis. Medicine. 2015;94(14):e622. doi: 10.1097/MD.0000000000000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slart RHJA, Glaudemans AWJM, Chareonthaitawee P, Treglia G, Besson FL, Bley TA. FDG-PET/CT(A) imaging in large vessel vasculitis and polymyalgia rheumatica: joint procedural recommendation of the EANM, SNMMI, and the PET Interest Group (PIG), and endorsed by the ASNC. Eur J Nucl Med Mol Imaging. 2018;45(7):1250–1269. doi: 10.1007/s00259-018-3973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papathanasiou ND, Du Y, Menezes LJ, Almuhaideb A, Shastry M, Beynon H. 18F-Fludeoxyglucose PET/CT in the evaluation of large-vessel vasculitis: diagnostic performance and correlation with clinical and laboratory parameters. Br J Radiol. 2012;85(1014):e188–e194. doi: 10.1259/bjr/16422950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu FP, Luo S, Wang ZJ, Jin ZY, Zhang LJ, Lu GM. Takayasu arteritis: imaging spectrum at multidetector CT angiography. Br J Radiol. 2012;85(1020):e1282–e1292. doi: 10.1259/bjr/25536451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mujukian A, Kay M, Marks JL. Symptomatic lower limb large vessel vasculitis presenting as fever of unknown origin diagnosed on FDG-PET/CT. BMJ Case Reports. 2018;2018 doi: 10.1136/bcr-2017-224019. bcr-2017-224019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hay B, Mariano-Goulart D, Bourdon A, Benkiran M, Vauchot F, De Verbizier D. Diagnostic performance of 18F-FDG PET-CT for large vessel involvement assessment in patients with suspected giant cell arteritis and negative temporal artery biopsy. Ann Nucl Med. 2019;33(7):512–520. doi: 10.1007/s12149-019-01358-5. [DOI] [PubMed] [Google Scholar]
- 7.Croce A, Bellan M, Pedrazzoli R, Sola D, Puta E, Sacchetti G. AB1132 the role of PET/CT in the management of giant cell arteritis. Ann Rheumat Dis. 2019;78(2):2029. [Google Scholar]
- 8.Dejaco C, Ramiro S, Duftner C, Besson FL, Bley TA, Blockmans D. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheumat Dis. 2018;77(5):636–643. doi: 10.1136/annrheumdis-2017-212649. [DOI] [PubMed] [Google Scholar]
- 9.Otsuka Hideki, Morita Naomi, Yamashita Kyo, Nishitani Hiromu. FDG-PET/CT for diagnosis and follow-up of vasculitis. J Med Invest JMI. 2007;54(3,4):345–349. doi: 10.2152/jmi.54.345. [DOI] [PubMed] [Google Scholar]
- 10.Lyons HS, Quick V, Sinclair AJ, Nagaraju S, Mollan SP. A new era for giant cell arteritis. Eye. 2020;34:1013–1026. doi: 10.1038/s41433-019-0608-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pacella F, Mazzeo F, Giorgi D, Cerutti F, Impallara D, Cuozzo G. Giant cell arteritis: the importance of immediate and appropriate diagnosis and treatment for better prognosis. Clin Ophthalmol. 2012;6:909–913. doi: 10.2147/OPTH.S24572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tezuka D, Haraguchi G, Ishihara T, Ohigashi H, Inagaki H, Suzuki J. Role of FDG PET-CT in Takayasu arteritis: sensitive detection of recurrences. JACC Cardiovasc Imaging. 2012;5(4):422–429. doi: 10.1016/j.jcmg.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Pipitone N., Versari A., Salvarani C. Role of imaging studies in the diagnosis and follow-up of large-vessel vasculitis: an update. Rheumatology. 2008;47(4):403–408. doi: 10.1093/rheumatology/kem379. [DOI] [PubMed] [Google Scholar]
- 14.Dejaco C, Ramiro S, Duftner C, Schmidt WA. Response to: ‘The role of temporal artery biopsy in patients with giant cell arteritis is debated’ by Moiseev et al. Ann Rheumat Dis. 2019;78:e32. doi: 10.1136/annrheumdis-2018-213284. [DOI] [PubMed] [Google Scholar]
- 15.Moiseev SV, Smitienko I, Bulanov N, Novikov PI. The role of temporal artery biopsy in patients with giant-cell arteritis is debated. Ann Rheumat Dis. 2019;78(4):e31. doi: 10.1136/annrheumdis-2018-213282. [DOI] [PubMed] [Google Scholar]