Abstract
Considering that stress condition associated with osteoporosis, the hypothalamic-pituitary-adrenal (HPA) axis, which is essential for central stress response system, is implicated in regulating bone mass accrual. Melanocortin 2 receptor (MC2R), the receptor of adrenocorticotropic hormone is expressed in both adrenal gland cells and bone cells. To elucidate the role of HPA axis in bone metabolism, we assessed the skeletal phenotype of MC2R deficient mice (MC2R−/− mice). We first examined bone mineral density and cortical thickness of femur using dual x-ray absorptiometry and micro-computed tomography. We then conducted histomorphometric analysis to calculate the static and dynamic parameters of vertebrae in MC2R−/− mice. The levels of osteoblastic marker genes were examined by quantitative PCR in primary osteoblasts derived from MC2R−/− mice. Based on these observations, bone mineral density of femur in MC2R−/− mice was increasing relative to litter controls. Meanwhile, the thickness of cortical bone of femur in MC2R−/− mice was remarkably elevated. Moreover, serum osteocalcin level was drastically raised in MC2R−/− mice. However, bone histomorphometry revealed that static and dynamic parameters reflecting bone formation and resorption were unchanged in vertebrae of MC2R−/− mice compared to the control, indicating that MC2R function may be specific to appendicular bone than axis bone. Taken together, the HPA axis due to deletion of MC2R is involved in bone metabolism.
Abbreviations: MC2R, melanocortin 2 receptor; HPA axis, hypothalamic-pituitary-adrenal axis; GCs, Glucocorticoids; ACTH, adrenocorticotropic hormone; HSD, hydroxysteroid dehydrogenase; FGD, familial glucocorticoid deficiency
Keywords: Melanocortin 2 receptor, Hypothalamic-pituitary-adrenal axis, Bone metabolism, Osteocalcin
Highlights
-
•
Bone mineral density of femur in MC2R −/− mice was increasing relative to litter controls.
-
•
Static and dynamic parameters were unchanged in MC2R −/− mice.
-
•
The thickness of cortical bone in MC2R −/− mice was remarkably elevated.
-
•
Serum osteocalcin level was drastically raised in MC2R −/− mice.
1. Introduction
The hypothalamic-pituitary-adrenal (HPA) axis is involved in maintaining homeostasis under stress conditions, such as infection and psychological stress (Eskandari et al., 2007). Overstimulation of the HPA axis leads to enhanced susceptibility to infection, suggesting that infectious stress disturbs the maintenance of homeostasis through the HPA axis. The HPA axis is implicated in regulating bone metabolism (Cizza et al., 2009). While increasing plasma cortisol due to the activation of the HPA axis is considered an important causative factor of low bone mass in depression (Altindag et al., 2007; Kahl et al., 2006), other studies documented no differences in plasma cortisol level (Petronijević et al., 2008; Yazici et al., 2003).
Glucocorticoids (GCs) which are the end products of the HPA axis via adrenocorticotropic hormone (ACTH) are crucial factors in the regulation of bone metabolism (Hofbauer and Rauner, 2009). Because of its importance in human health, studying the molecular mechanisms of the GC signaling axis has become a major focus area in biomedical research. GCs bind to a glucocorticoid receptor belonging to a member of the nuclear receptor family (Lu and Cidlowski, 2006), and the local GC activity is regulated by 2 isoforms of 11β-hydroxysteroid dehydrogenase (11β-HSD). 11β-HSD type 1 regenerates corticosterone from 11-dehydrocorticosterone, whereas 11β-HSD type 2 converts active GC to inactive forms (Stewart, 1999). It has been demonstrated that GC signaling is required to maintain normal bone volume and architecture, suggesting that endogenous GCs have an anabolic effect on skeletal metabolism and bone formation in vivo (Sher et al., 2004).
The melanocortin receptor system is classified into 5 receptors including MC1R, MC2R, MC3R, MC4R, and MC5R (Cone et al., 1993). MC2R which is activated by only ACTH leads to a regulation in the release of GCs by the adrenal cortex. In addition to the adrenal cortex, MC2R is expressed in a number of extra-adrenal sites, including bone cells (Boston and Cone, 1996) (Zhong et al., 2005). Functional roles of MC2R in these cells have been investigated in vitro. ACTH promotes osteoblast differentiation via MC2R accompanied by vascular endothelial growth factor production (Zaidi et al., 2010). Moreover, ACTH and 1,25-dihydroxyvitamin D3 enhance human osteogenesis in vitro (Tourkova et al., 2017).
However, the role of the HPA axis associated with MC2R signaling pathway in bone metabolism in vivo is not fully elucidated. Here we characterized the bone phenotype of melanocortin 2 receptor knockout mice (MC2R−/− mice) which exhibited the disruption of MC2R signaling and are deficient of GC (Chida et al., 2007).
2. Materials and methods
2.1. Animals
MC2R−/− mice generated as reported previously were used in this study (Chida et al., 2007). All mice were kept on a normal-chow diet (NCD; 5.1% of the total energy from fat; total energy, 17.5 kJ/g) in an environmentally controlled clean room at the Department of Oral and Maxillofacial Surgery, Saitama Medical University. The experiments were conducted according to the institutional guidelines for ethical animal experiments and the safety guidelines for gene manipulation experiments. MC2R−/− mice on a B6/Balb mixed background and their MC2R+/− littermates were used in this study (Chida et al., 2009). We also used female MC2R+/− littermates for breeding.
2.2. Cell culture
We cultured primary osteoblasts from calvariae of 4-day-old mice in α-MEM (WAKO, Osaka, Japan) containing 10% FBS (BioWest, Nuaillé, France) with 1% penicillin/streptomycin at 37 °C in a 5% CO2 atmosphere in the presence of ascorbic acid (0.1 mg/ml, Sigma-Aldrich, USA) for 3 days. We performed in triplicate wells and repeated more than 2 times.
2.3. Measurement of serum osteocalcin
Serum osteocalcin from 6-month-old mice was measured using a mouse osteocalcin ELISA kit (Biomedical Technologies, USA), according to the manufacturer's instruction. Five mice were examined for each group.
2.4. Bone mineral density measurement and micro-computed tomography scanning
Bone mineral density of the femur was measured using dual x-ray absorptiometry (DXA) (DSC-600EX-IIIR; Aloka, Tokyo, Japan) with 2 distinct energy peaks of 22 KeV and 55 KeV (Kureha, Tokyo, Japan). Micro-computed tomography (Scan X mate-A090; Comscantecno, Kanagawa, Japan) images of the femur were taken (Kureha, Tokyo, Japan). Scanning conditions of 75 kV, 100 μA, and 9.116 μm of thickness were applied to obtain a detailed image of the sample, using TRI/3D-BON (RATOC, Tokyo, Japan). Six mice were examined for each group.
2.5. Histomorphometric analysis
We injected calcein (25 mg/kg, Sigma-Aldrich) intraperitoneally 5 and 2 days before sacrifice. After vertebrae were fixed in 4% paraformaldehyde and dehydrated by ethanol, they were embedded in methylmethacrylate. The plastic blocks were trimmed and 4 or 7 μm-thick sections were prepared with a RM2255 (Leica Microsystems). Undecalcified sections of the lumbar vertebrae were stained for von Kossa, Toluidine Blue, and TRAP staining. Static and dynamic histomorphometric analyses were performed using OsteoMeasure Analysis System (OsteoMetrics, USA) following nomenclature defined by the American Society for Bone and Mineral Research (ASBMR). Bone volume/tissue volume (BV/TV; %), mineral apposition rate (MAR; μm/year), trabecular thickness (Tb.Th; mm), osteoblast surface (Ob.S/BS; %) and osteoclast number/bone perimeter (No.Oc/B·Pm; per 100 mm) were analysed. Six mice were examined for each group.
2.6. Measurement of deoxypyridinoline cross-links
For urine deoxypyridinoline (DPD) measurements, mice were housed in metabolic cages to allow collection of 24-h urine samples. We measured urinary DPD with the METRA DPD-EIA kit (Quidel, San Diego, CA, USA), according to the manufacturer's instructions. We used creatinine values to standardize between samples (Creatinine Assay Kit, Cayman, Michigan, USA). Five mice were examined for each group.
2.7. Whole-body skeletal staining
To assess the development of the skeletal system, we eviscerated embryos from E18.5 and fixed them in 95% ethanol for 24 h. Then, they were rinsed with water and stained with 0.02% Alcian blue 8GX (Sigma-Aldrich) prepared in 70% ethanol and 30% acetic acid for 2 days. After being rinsed with 95% ethanol for several hours, specimens were transferred to 2% KOH for 24 h, and then further stained with 0.01% Alizarin red S (Sigma-Aldrich) in 1% KOH solution for 12 h. Skeletons were kept in 1% KOH/20% glycerol until they were clearly visible, and then were stored in a solution of 50% ethanol and 50% glycerol. Four mice were examined for each group.
2.8. Quantitative RT-PCR analysis
To validate the changes in gene expression levels, quantitative real-time RT-PCR (qPCR) analysis was performed using the Step One Plus Real-Time PCR System according to the manufacturer's instructions (Thermo Scientific). Total RNA (2 μg) was extracted from the cells using ISOGEN. The reverse-transcription reaction was performed with a High Capacity cDNA Reverse Transcription kit (Thermo Scientific). TaqMan Gene Expression Assays for Akp2 (assay identification number Mm01187113_g1) and Bglap (assay identification number Mm03413826_mH) were inventoried products (Thermo Scientific). Mouse GAPDH gene was used as endogenous control (assay identification number Mm03302249_g1). All samples were performed in triplicate. Each reaction was performed in triplicate on three individual samples. Values were normalised to those of GAPDH using the 2-ΔΔCt method.
2.9. Statistical analysis
All values are represented as the mean ± SEM. Comparisons between 2 groups were analysed using Student's t-tests, and comparisons among more than 2 groups were analysed using ANOVA. In all analyses, a 2-tailed probability of less than 5% (P < 0.05) was considered statistically significant.
3. Results
3.1. Increased bone mineral density and augmented cortical bone thickness in MC2R−/− mice
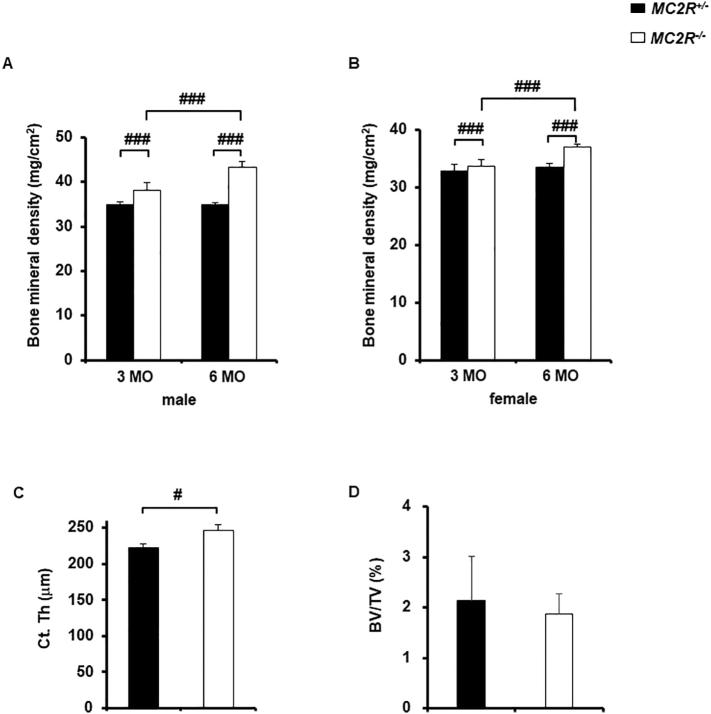
First, we assessed bone mass in femur by using DXA analysis quantitatively. Male MC2R−/− mice showed significantly increased bone mineral density compared to male MC2R+/− mice at both 3 months and 6 months (Fig. 1A). In female MC2R−/− mice, bone mineral density was also elevated compared to female MC2R+/− mice (Fig. 1B).
Fig. 1.
High bone mass in association with thickening of cortical bone in MC2R−/− mice femur
DXA analysis and micro-computed tomography scanning of femur in MC2R+/− and MC2R−/− mice (each N = 6). (A) Bone mineral density in male MC2R+/− and MC2R−/− mice at 3 and 6 months, (B) Bone mineral density in female MC2R+/− and MC2R−/− mice at 3 and 6 months, (C) Cortical bone thickness in male MC2R−/− and MC2R+/− mice at 6 months, (D) Bone volume per tissue volume (BV/TV) in male MC2R−/− and MC2R+/− mice at 6 months. MO. month-old. #, P < 0.05; ###, P < 0.001. Data are expressed as means ± SEM.
To investigate whether cortical thickness of the femur is augmented, we performed micro-computed tomography scanning. We found that cortical thickness in male MC2R−/− mice was significantly increased compared to the control (Fig. 1C). However, BV/TV of the cancellous bone in male MC2R−/− mice was not significantly different (Fig. 1D).
3.2. Normal prenatal skulls in MC2R−/− mice
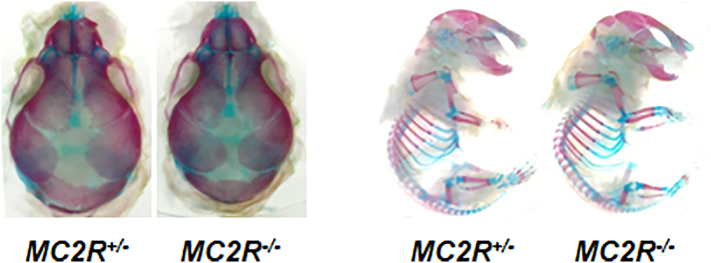
We next examined the bone condition of skulls at prenatal period. The staining of E18.5 MC2R−/− skulls with alizarin red/alcian blue revealed no significant differences compared to the control (Fig. 2), indicating that bone mineral density is increased after birth.
Fig. 2.
Normal skeletal phenotype in prenatal period of MC2R−/− mice
Skeletons from E18.5 embryos of MC2R−/− and MC2R+/− mice (each N = 4) were double-stained with alcian blue and alizarin red. Enlarged head region of the skeleton and embryos.
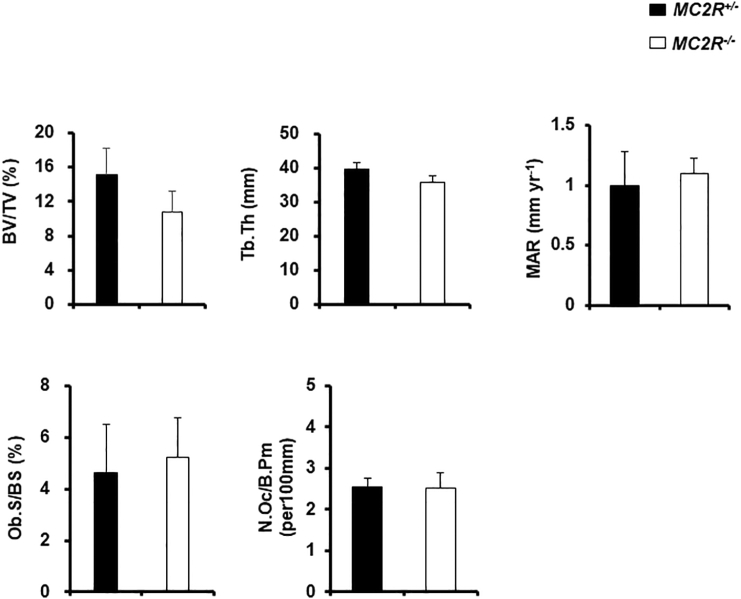
3.3. Normal cancellous bone in MC2R−/− mice
To better characterize the cancellous bone phenotype in male MC2R−/− mice, we performed histomorphometric analyses of vertebrae. All parameters including BV/TV, Tb/Th, MAR, Ob.S/BS, and No.Oc/B·Pm were not significantly different (Fig. 3), suggesting that cancellous bone is not affected in MC2R−/− mice.
Fig. 3.
Normal cancellous bone in MC2R−/− mice
Histomorphometric analysis of the vertebrae of male MC2R+/− and MC2R−/− mice (each N = 6) at 6 months. Bone volume per tissue volume (BV/TV), Trabecular thickness (Tb. Th), Mineral apposition rate (MAR), Osteoblast surface area per bone surface area (Ob.S/BS), Osteoclast number per bone perimeter (No. Oc./BPm).
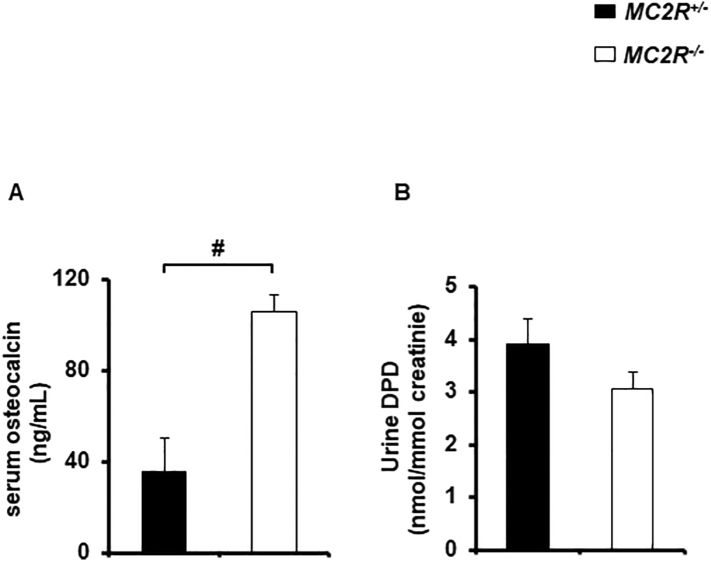
3.4. Bone formation and resorption markers in MC2R−/− mice
To analyse whether osteocalcin, a useful marker of bone formation, was elevated in the serum. We showed that serum osteocalcin was significantly increased in male MC2R−/− mice (Fig. 4A). We also assessed urine DPD, a breakdown product of collagen released systemically during osteoclast resorption. As we expected, urine DPD was were not significantly increased in male MC2R−/− mice compared to the control (Fig. 4B), indicating that osteoclast activity is not increased in MC2R−/− mice.
Fig. 4.
Serum osteocalcin level was increased in MC2R−/− mice
(A) Serum osteocalcin in male MC2R−/− and MC2R+/− mice (each N = 5) at 6 months, (B) Urinary elimination of deoxypyridinoline (DPD) in male MC2R+/− and MC2R−/− mice (each N = 5) at 6 months, #, P < 0.05. Data are expressed as means ± SEM.
3.5. No effect of osteoblastic gene expression on primary osteoblasts from MC2R−/− mice
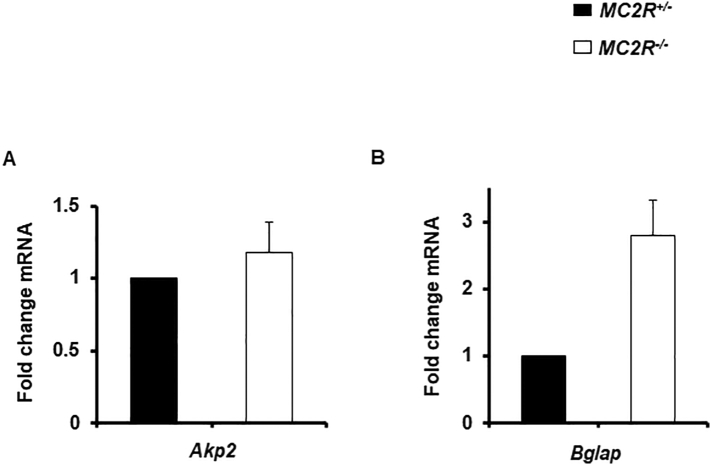
Finally, we examined the characterization of primary osteoblastic cells derived from newborn calvaria. There were no significant differences between MC2R+/− mice and MC2R−/− mice in the expression of osteoblastic genes such as both Akp2 (Fig. 5A) and Bglap (Fig. 5B) in vitro and the morphological changes were not observed in primary osteoblastic cells from MC2R−/− mice (data not shown).
Fig. 5.
No distinguishable effect of osteoblastic gene expression on primary osteoblasts derived from MC2R−/− mice
(A) Gene expression of Akp2 in MC2R+/− and MC2R−/− mice, (B) Gene expression of Bglap in male MC2R+/− and MC2R−/− mice. We performed in triplicate wells and repeated more than 2 times.
4. Discussion
4.1. The effect of GC loss and MC2R signaling disruption in osteoblasts on MC2R−/− mice
Endogenous GC is capable of promoting osteoblast differentiation (Hofbauer and Rauner, 2009) (Eijken et al., 2005). Previously, Yang et al. showed that Col3.6-HSD2 transgenic mice which is a glucocorticoid loss-of-function model revealed that neonatal transgenic skulls had reduced bone mass with thinner parietal bones and enlarged occipital fontanelles (Yang et al., 2010). In the present study, we found that normal prenatal skulls in MC2R−/− mice are normal. We speculate that endogenous GC derived from mother may affect mouse embryos and the postnatal bone phenotype may partially depend on loss of endogenous GC (Chida et al., 2009).
Although several animal models have been developed to analyse the role of endogenous GC in skeletal development in vivo, they have obtained conflicting results. Studies on transgenic mice selectively overexpressing 11β-HSD2 within osteoblasts and osteocytes under the control of the osteocalcin 2 promoter revealed that endogenous GC is not required for normal skeletal development (O'Brien et al., 2004). Moreover, 11β-HSD1 knockout mice exhibited normal bone mass (Justesen et al., 2004). Paradoxically, it was previously demonstrated that cancellous bone mass was lower in the following mice: the transgenic mice overexpressing 11β-HSD2 within the osteoblasts under the control of the collagen type I promoter fragments (Sher et al., 2004) (Sher et al., 2006) (Yang et al., 2010), the conditional knockout mice in which a glucocorticoid receptor is specifically disrupted in the osteoblasts under the control of Runx2 promoter fragments (Rauch et al., 2010), the adrenalectomized rat (Durbridge et al., 1990) (Li et al., 1996). On the other hand, adrenalectomy resulted in an increase in osteocalcin (Patterson-Buckendahl et al., 1988). Similarly, osteocalcin level was increased in MC2R−/− mice.
Accumulating evidences suggest that ACTH promotes osteoblast differentiation and inhibit osteoclast differentiation in vitro (Zaidi et al., 2010) (Tourkova et al., 2017). Sakamoto et al. reported that the osteoblast/osteocyte-specific G-protein α-subunit knockout mice had reduced trabecular bone volume but had increased cortical bone thickness (Sakamoto et al., 2005). As MC2R belongs to G-protein coupled receptors which are coupled to a heterotrimeric G-protein formed from three unique subunits (i.e., α, β, γ) (Cai and Hruby, 2016), the augmentation of cortical bone thickness in MC2R−/− mice may be caused by the disrupted signaling in G-protein coupled receptors in osteoblasts associated with G-protein α-subunit. These reports imply that MC2R signaling has a direct anabolic effect on bone metabolism. However, our study did not show low bone mass in MC2R−/− mice. It is conjectured that loss of endogenous GC leads to increase osteocalcin level and maintains normal cancellous bone mass with augmented cortical thickness. MC2R function may be specific to appendicular bone than axis bone (Seeman et al., 1982). Although Bglap gene expression in osteoblasts from MC2R−/− mice was increased almost 2.5 times, there were no significant differences due to the large variation in the measurement. We speculate that osteocalcin may be derived from not only osteoblasts but mesenchymal stem cells (La Noce et al., 2019).
4.2. Role of the HPA system in human bone metabolism
Recent clinical evidences demonstrate that major depressive disorder has been involved in low bone mineral density (Wu et al., 2018). Depression is associated with the hyperactivity of the HPA system which has also been implicated in regulating bone metabolism (Cizza et al., 2009). Long-term exposure to excess GCs in patients with hypercortisolism results in bone loss. According to the recent study, ACTH might be protective for lumbar bone mineral density in patients with Cushing's disease, indicating ACTH might have a protective effect on bone (Guo et al., 2018). Moreover, familial glucocorticoid deficiency (FGD), or hereditary unresponsiveness to ACTH, is an autosomal recessive disorder resulting from resistance to the action of ACTH on the adrenal cortex (Maharaj et al., 2019). Mutations of MC2R are responsible for 25% of FGD cases. Patients FGD have an advanced bone age than age-matched controls and excessive growth exhibiting tall stature (Elias et al., 2000; Imamine et al., 2005). However, we did not find significant differences of body length between MC2R−/− mice and the controls (data not shown). In this study, we found the augmentation of cortical thickness was due to the enhancement of bone formation. These studies imply the possibility that the HPA system plays a critical role for bone metabolism in humans.
4.3. The limitation of this study and the perspective
In conclusion, the HPA axis is involved in bone metabolism through MC2R. However, our study has the following limitation. As MC2R−/− mice lead to GC deficiency, we cannot analyse the authentic roles of MC2R signaling in bone metabolism. To exclude the effect of GC deficiency, we need to develop osteoblast-specific MC2R−/− mice or to use MC2R−/− mice with controlled release tablet of GC. Further studies are needed to elucidate MC2R signaling in bone metabolism.
Transparency document
Transparency document
CRediT authorship contribution statement
Conception and design of the experiments: TS. Collection, assembly, analysis and interpretation of data: TS, TI, MU, SK, YS, YT, TK, and KI. Drafting the article or revising it critically for important intellectual content: TS, ST, MM, RX and DC.
Declaration of competing interest
The authors declare that there is no conflict of interest.
Acknowledgments
Acknowledgement
None.
Grants
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan (17H04409 to T.S.).
Footnotes
The Transparency document associated this article can be found, in online version.
References
- Altindag O. Relation of cortisol levels and bone mineral density among premenopausal women with major depression. Int. J. Clin. Pract. 2007;61(3):416–420. doi: 10.1111/j.1742-1241.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- Boston B.A., Cone R.D. Characterization of melanocortin receptor subtype expression in murine adipose tissues and in the 3T3-L1 cell line. Endocrinology. 1996;137(5):2043–2050. doi: 10.1210/endo.137.5.8612546. [DOI] [PubMed] [Google Scholar]
- Cai M., Hruby V.J. The melanocortin receptor system: a target for multiple degenerative diseases. Curr. Protein Pept. Sci. 2016;17(5):488–496. doi: 10.2174/1389203717666160226145330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida D. Melanocortin 2 receptor is required for adrenal gland development, steroidogenesis, and neonatal gluconeogenesis. Proc. Natl. Acad. Sci. U. S. A. 2007;104(46):18205–18210. doi: 10.1073/pnas.0706953104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida D. Characterization of mice deficient in melanocortin 2 receptor on a B6/Balbc mix background. Mol. Cell. Endocrinol. 2009;300(1–2):32–36. doi: 10.1016/j.mce.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Cizza G. Depression as a risk factor for osteoporosis. Trends Endocrinol. Metab. 2009;20(8):367–373. doi: 10.1016/j.tem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone R.D. Cloning and functional characterization of a family of receptors for the melanotropic peptides. Ann. N. Y. Acad. Sci. 1993;680:342–363. doi: 10.1111/j.1749-6632.1993.tb19694.x. [DOI] [PubMed] [Google Scholar]
- Durbridge T.C. Progressive cancellous bone loss in rats after adrenalectomy and oophorectomy. Calcif. Tissue Int. 1990;47(6):383–387. doi: 10.1007/bf02555891. [DOI] [PubMed] [Google Scholar]
- Eijken M. 11beta-Hydroxysteroid dehydrogenase expression and glucocorticoid synthesis are directed by a molecular switch during osteoblast differentiation. Mol. Endocrinol. 2005;19(3):621–631. doi: 10.1210/me.2004-0212. [DOI] [PubMed] [Google Scholar]
- Elias L.L. Tall stature in familial glucocorticoid deficiency. Clin. Endocrinol. 2000;53(4):423–430. doi: 10.1046/j.1365-2265.2000.01122.x. [DOI] [PubMed] [Google Scholar]
- Eskandari F. Low bone mass in premenopausal women with depression. Arch. Intern. Med. 2007;167(21):2329–2336. doi: 10.1001/archinte.167.21.2329. [DOI] [PubMed] [Google Scholar]
- Guo W. Effect of hypercortisolism on bone mineral density and bone metabolism: a potential protective effect of adrenocorticotropic hormone in patients with Cushing’s disease. J Int Med Res. 2018;46(1):492–503. doi: 10.1177/0300060517725660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer L.C., Rauner M. Minireview: live and let die: molecular effects of glucocorticoids on bone cells. Mol. Endocrinol. 2009;23(10):1525–1531. doi: 10.1210/me.2009-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamine H. Possible relationship between elevated plasma ACTH and tall stature in familial glucocorticoid deficiency. Tohoku J. Exp. Med. 2005;205(2):123–131. doi: 10.1620/tjem.205.123. [DOI] [PubMed] [Google Scholar]
- Justesen J. Mice deficient in 11beta-hydroxysteroid dehydrogenase type 1 lack bone marrow adipocytes, but maintain normal bone formation. Endocrinology. 2004;145(4):1916–1925. doi: 10.1210/en.2003-1427. [DOI] [PubMed] [Google Scholar]
- Kahl K.G. Bone mineral density, bone turnover, and osteoprotegerin in depressed women with and without borderline personality disorder. Psychosom. Med. 2006;68(5):669–674. doi: 10.1097/01.psy.0000237858.76880.3d. [DOI] [PubMed] [Google Scholar]
- La Noce M. Cytoplasmic interactions between the glucocorticoid receptor and HDAC2 regulate osteocalcin expression in VPA-treated MSCs. Cells. 2019;8(3) doi: 10.3390/cells8030217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. Skeletal response to corticosteroid deficiency and excess in growing male rats. Bone. 1996;19(2):81–88. doi: 10.1016/8756-3282(96)00170-6. [DOI] [PubMed] [Google Scholar]
- Lu N.Z., Cidlowski J.A. Glucocorticoid receptor isoforms generate transcription specificity. Trends Cell Biol. 2006;16(6):301–307. doi: 10.1016/j.tcb.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Maharaj A. Isolated glucocorticoid deficiency: genetic causes and animal models. J. Steroid Biochem. Mol. Biol. 2019;189:73–80. doi: 10.1016/j.jsbmb.2019.02.012. [DOI] [PubMed] [Google Scholar]
- O'Brien C.A. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. 2004;145(4):1835–1841. doi: 10.1210/en.2003-0990. [DOI] [PubMed] [Google Scholar]
- Patterson-Buckendahl P.E. Circulating osteocalcin in rats is inversely responsive to changes in corticosterone. Am. J. Phys. 1988;254(5 Pt 2):R828–R833. doi: 10.1152/ajpregu.1988.254.5.R828. [DOI] [PubMed] [Google Scholar]
- Petronijević M. Low bone mineral density and high bone metabolism turnover in premenopausal women with unipolar depression. Bone. 2008;42(3):582–590. doi: 10.1016/j.bone.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Rauch A. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab. 2010;11(6):517–531. doi: 10.1016/j.cmet.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Sakamoto A. Deficiency of the G-protein alpha-subunit G(s)alpha in osteoblasts leads to differential effects on trabecular and cortical bone. J. Biol. Chem. 2005;280(22):21369–21375. doi: 10.1074/jbc.M500346200. [DOI] [PubMed] [Google Scholar]
- Seeman E. Differential effects of endocrine dysfunction on the axial and the appendicular skeleton. J. Clin. Invest. 1982;69(6):1302–1309. doi: 10.1172/jci110570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher L.B. Transgenic expression of 11beta-hydroxysteroid dehydrogenase type 2 in osteoblasts reveals an anabolic role for endogenous glucocorticoids in bone. Endocrinology. 2004;145(2):922–929. doi: 10.1210/en.2003-0655. [DOI] [PubMed] [Google Scholar]
- Sher L.B. Impaired cortical bone acquisition and osteoblast differentiation in mice with osteoblast-targeted disruption of glucocorticoid signaling. Calcif. Tissue Int. 2006;79(2):118–125. doi: 10.1007/s00223-005-0297-z. [DOI] [PubMed] [Google Scholar]
- Stewart P.M. Cortisol as a mineralocorticoid in human disease. J. Steroid Biochem. Mol. Biol. 1999;69(1–6):403–408. doi: 10.1016/s0960-0760(99)00072-2. [DOI] [PubMed] [Google Scholar]
- Tourkova I.L. Adrenocorticotropic hormone and 1,25-dihydroxyvitamin D(3) enhance human osteogenesis in vitro by synergistically accelerating the expression of bone-specific genes. Lab. Investig. 2017;97(9):1072–1083. doi: 10.1038/labinvest.2017.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q. Depression and risk of fracture and bone loss: an updated meta-analysis of prospective studies. Osteoporos. Int. 2018;29(6):1303–1312. doi: 10.1007/s00198-018-4420-1. [DOI] [PubMed] [Google Scholar]
- Yang M. Col3.6-HSD2 transgenic mice: a glucocorticoid loss-of-function model spanning early and late osteoblast differentiation. Bone. 2010;47(3):573–582. doi: 10.1016/j.bone.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazici K.M. Bone mineral density in premenopausal women with major depressive disorder. Psychiatry Res. 2003;117(3):271–275. doi: 10.1016/s0165-1781(03)00017-9. [DOI] [PubMed] [Google Scholar]
- Zaidi M. ACTH protects against glucocorticoid-induced osteonecrosis of bone. Proc. Natl. Acad. Sci. U. S. A. 2010;107(19):8782–8787. doi: 10.1073/pnas.0912176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q. Multiple melanocortin receptors are expressed in bone cells. Bone. 2005;36(5):820–831. doi: 10.1016/j.bone.2005.01.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document