Abstract
Background
Active surveillance for transfusion reactions is critically important among pediatric patients undergoing chemotherapy. Among pediatric-adolescent-young-adult (AYA) hematology/oncology patients, who have been typically excluded from transfusion reaction studies, this profile remains poorly characterized.
Methods
We assessed the incidence and clinical characteristics of transfusion reactions (n = 3246 transfusions) in this population (n = 201 patients) at our center.
Findings
The incidence of adjudicated transfusion reactions was 2·04%. The incidence was higher for platelet (2·78%) compared to packed red blood cell transfusions (1·49%) (p = 0·0149). The majority (61·4%) of all reactions were classified as febrile non-haemolytic transfusion, while 35·7% were considered allergic, and 2·9% were classified as transfusion-associated circulatory overload. The incidence of transfusion reactions in patients who were pre-medicated was higher (2·51%) than in patients who were not (1·52%) (p = 0·0406). Sub-set analysis revealed a 3·95% incidence of adjudicated transfusion reactions among recipients of immune effector cells (IECs) (n = 3), all of which occurred during the potential window for cytokine release syndrome; two-thirds of these reactions were severe/potentially life-threatening.
Interpretation
The incidence of transfusion reactions among pediatric-AYA hematology/oncology patients may be lower than the general pediatric population. Patients with a prior history of transfusion reactions and those receiving platelet transfusions may be at higher risk for reaction. From our limited sample, IEC recipients may be at risk for severe transfusion reactions. Large multi-center prospective studies are needed to characterize transfusion reactions in this population. Appropriate characterization of reactions in this population may inform risk stratification and mitigate missed opportunities for prompt recognition and appropriate management.
Funding
None.
Research in context.
Evidence before this study
Transfusions are frequently required in pediatric and adolescent-young adult (AYA) hematology/oncology patients. Transfusion reactions can be a severe and/or life-threatening complication of blood product administration. There is a striking paucity of data and understanding of transfusion reaction incidence and clinical characteristics in this pediatric-AYA hematology/oncology population. We searched PubMed using the terms “transfusion reactions,” “pediatric,” “hematology,” “oncology,” “immune effector cell,” with no date restrictions, for reports in any written language. A paucity of transfusion reaction data exists in pediatric-AYA hematology/oncology populations. This prompted us to investigate transfusion reactions in this population.
Added value of this study
This is the first study to evaluate transfusion reaction incidence and characterization in pediatric-AYA hematology/oncology patients. The incidence of transfusion reactions among pediatric-AYA hematology/oncology patients was 2•04%, which may be lower than the general pediatric population. Two-thirds of the transfusion reactions were febrile nonhaemolytic transfusion reactions in this immunocompromised population, compared to approximately one-third in general pediatric populations. Patients with a prior history of transfusion reactions and those receiving platelet transfusions may be at higher risk for reaction. From our limited sample, immune effector cell recipients may be at high risk for severe transfusion reactions.
Implications of all the available evidence
Appropriate characterization of transfusion reactions in this population may inform risk stratification and improve prompt recognition and appropriate management. Large multi-center prospective studies are needed to further characterize transfusion reactions in this population.
Alt-text: Unlabelled box
1. Introduction
Patients receiving cytotoxic and in particular, myeloablative chemotherapy as used for conditioning in haematopoietic cell transplant (HCT), require frequent transfusions to prevent complications of therapy-induced pancytopenia [1]. Novel immunotherapies including immune effector cells (IEC) have been associated with impressive disease-free outcomes but also prolonged cytopenias that may require transfusion support. IEC patients may develop overlapping symptoms as a result of unique associated toxicities such as cytokine release syndrome (CRS). Haemovigilance is important for detection of various types of transfusion reactions such as febrile nonhaemolytic transfusion reactions (FNHTRs), allergic transfusion reactions, transfusion-associated circulatory overload (TACO), transfusion-related acute lung injury (TRALI), septic transfusion reactions, and haemolytic transfusion reactions among others [2]. The clinical characteristics and management of these transfusion reactions are listed in Table 1.
Table 1.
Transfusion reaction signs/symptoms and management [3].
| Transfusion Reaction Type | Signs/Symptoms | Management |
|---|---|---|
| Febrile nonhaemolytic transfusion reaction (FNHTR) | Increase in temperature by ≥1 °C and/or temperature ≥38 °C +/- transient hypertension, chills, rigors, and discomfort |
Stop transfusion, initiate transfusion reaction work-up looking for signs of infection and haemolysis, provide antipyretic/supportive care |
| Allergic transfusion reaction |
Mild: rash, pruritus, urticaria, localized angioedema Severe: anaphylaxis (bronchospasm, respiratory distress, hypotension in addition to mild symptoms) |
Mild: Stop transfusion. Administer antihistamine +/- corticosteroid, if symptoms resolve transfusion can be restarted. Severe/anaphylactic: Stop transfusion. Rapidly administer intramuscular epinephrine +/- antihistamine (H1 and H2), bronchodilator, corticosteroid. Do not resume transfusion. |
| Transfusion associated circulatory overload (TACO) | New onset or worsening of ≥3 of the following within 6 h of transfusion cessation:
|
Stop transfusion. Administer supplemental oxygen and diuretics as needed. Consider prolonging the time of transfusion for future transfusions. Consider prophylactically administering diuretics before or after future transfusions. |
| Transfusion-related acute lung injury (TRALI) | Dyspnea, tachypnea, hypoxemia, +/- fever, hypothermia, rigors, hypotension, hypertension, or tachycardia. (Symptoms typically within 6 h of end of transfusion, though delayed cases are possible) Transient leukopenia may be observed. Bilateral interstitial infiltrates on imaging. |
Stop transfusion. Supportive management with supplemental oxygen, mechanical ventilation if needed (restrictive tidal volume), and restrictive fluid strategy. |
| Septic transfusion reaction | Fevers (increase in temperature by ≥1 °C and/or temperature ≥38 °C; increase by ≥2 °C heightens clinical suspicion), rigors, hypotension. Diagnosis requires isolation of organism from blood product and patient. | Stop transfusion. Blood cultures should be obtained from the patient and from the blood product. Broad-spectrum antibiotics should be started. |
| Acute haemolytic transfusion reaction | Fever, chills, back/flank pain, hypotension, dyspnea. May have haemoglobinuria, haemoglobinemia, acute renal failure, disseminated intravascular coagulation, shock, death. | Stop transfusion. Supportive management. |
Transfusion reactions may be categorized as non-severe, severe, life-threatening, or resulting in death. For non-severe reactions, medical intervention may be required but the lack of intervention does not result in permanent damage. Severe reactions are defined as requiring inpatient hospitalization or prolongation of hospitalization as a result of the adverse transfusion reaction. If no medical intervention occurs, these reactions may lead to permanent damage. Life-threatening transfusion reactions are defined as those requiring major intervention such as vasopressors, intubation, or transfer to the intensive care unit to prevent death. These biovigilance definitions have been generated by the Center for Disease Control and Prevention to provide a consistent standard nationwide [3].
With active haemovigilance, the reported incidence of transfusion reactions across the general adult population is approximately 2% [4], while general pediatric patients may experience 1·9–2·6 times more reactions than adults [5,6]. We hypothesized that immunocompromised pediatric-adolescent-young adult (AYA) hematology/oncology and HCT/IEC patients may have a different transfusion reaction profile from the general population. The therapeutic armamentarium for young hematology/oncology and HCT/IEC patients is rapidly evolving and there is a dearth of information regarding the incidence or clinical characteristics of transfusion reactions in these patients. Improved characterization and recognition of transfusion reactions in this population is important as overlapping toxicity profiles may complicate adjudication and hinder preventative and/or appropriate interventions when indicated.
2. Methods
We conducted a retrospective review of all transfusions and transfusion reactions in patients aged less than 25 years at our cancer center over a six-month period from July 1, 2019 to January 1, 2020. Approval for this study was obtained through the Institutional Review Board. Data was accessed using the electronic medical record and Haemovigilance Unit data.
Haemovigilance and adjudication occurred as followed: bedside staff generated a report for any of the following signs or symptoms during or within 6 h following transfusion: fever, chills/rigors, nausea/vomiting, anxiety, diarrhea, feeling of impending doom, loss of consciousness, hypotension, hypertension, tachycardia, edema, rash, flushing, urticaria, pruritus, cyanosis, infusion site pain, abdominal pain/cramps, chest pain/chest tightness, flank pain, low back pain, new-onset headache, discoloration of urine, dyspnea/labored breathing, wheezing, stridor, shortness of breath, hypoxemia, cough, tachypnea, bleeding, swollen lips/tongue/mucous membranes, difficulty speaking, or any other concerning symptoms.
All transfusion reaction reports created by bedside staff were then assessed by expert adjudication (Table 2), whereby Transfusion Medicine physicians used the National Healthcare Safety Network Biovigilance Component Haemovigilance Module Surveillance Protocol guidelines [3] to determine if the patient's symptoms met criteria for a specific transfusion reaction. Imputability and severity were also determined using these guidelines. In addition to reporting by bedside staff, there was also active, remote Haemovigilance Unit monitoring and investigation for changes in vital signs and temperature for all patients receiving blood product transfusions. Discussion with primary treatment team to assist with adjudication occurred as appropriate.
Table 2.
Transfusion reaction adjudication.
| Report/Adjudication | Examples |
|---|---|
| Transfusion Reaction Diagnosis | Febrile nonhaemolytic transfusion reaction, allergic transfusion reaction, transfusion-associated circulatory overload, transfusion-related acute lung injury, septic transfusion, acute haemolytic transfusion reaction, etc. |
| Testing Results | Gram stain and culture of product, blood culture of patient, urinalysis, direct Coombs, lactate dehydrogenase, total bilirubin, chest radiograph, etc. |
| Transfusion Reaction Severity | Non-severe, severe, life-threatening, death, not determined |
| Transfusion Reaction Imputability | Definite, possible, doubtful, ruled out, not determined |
| Outcome | Minor or no sequelae, major or long term sequelae, death, not determined |
| Recommendations | Treatment and monitoring recommendations advised |
Statistical analysis was performed using Wilcoxon Rank Sum Test for two-group comparisons and Kruskal-Wallis Rank Sum Test for multiple-group comparisons when assessing differences in average number of transfusions per patient in each category. When comparing the transfusion reaction rates between different groups, Fisher's exact test was used for assessing p-values. A p-value less than 0·05 was considered significant. Statistical analyses were conducted using R package [7].
2.1. Role of the funding source
No funding involved in this study.
3. Results
3.1. Patient characteristics
The median age of our study population (n = 201) was 17 (range 0–24) years. This was a racially/ethnically diverse group with 36·8% Caucasian, 32·8% Hispanic/Latino, 13·4% Black, 12·5% Asian, and 4·5% other/unknown. Almost half (42·8%) were female. Underlying diagnosis and transplant status were also varied with 46·3% solid tumors, 29·4% leukemia/lymphoma patients, 18·8% HCT/IEC patients, and 5·5% benign hematology conditions (Table 3).
Table 3.
Demographics and transfusion information.
| Number of Patients | Percentage of Patients (%) (Total N = 201 Patients) | Number of Transfusion Events | Percentage (%) (Total N = 3426 Transfusion Events) | Average Transfusions per Person | P value | |
|---|---|---|---|---|---|---|
| Sex | 0·5164 | |||||
| Female | 86 | 42·8 | 1857 | 54·2 | 21·6 | |
| Male | 115 | 57·2 | 1569 | 45·8 | 13·6 | |
| Age | 0·0314 | |||||
| 0–10 years | 60 | 29·8 | 465 | 13·6 | 7·8 | |
| 11–18 years | 57 | 28·4 | 999 | 29·1 | 17·5 | |
| 19–25 years | 84 | 41·8 | 1962 | 57·3 | 23·4 | |
| Race/Ethnicity | 0·6084 | |||||
| Caucasian (non-Hispanic/Latino) | 74 | 36·8 | 1086 | 31·7 | 14·7 | |
| Hispanic/Latino | 66 | 32·8 | 1415 | 41·3 | 21·5 | |
| Black | 27 | 13·4 | 492 | 14·4 | 18·2 | |
| Asian | 25 | 12·5 | 204 | 5·9 | 8·2 | |
| Other/Unknown | 9 | 4·5 | 229 | 6·7 | 25·4 | |
| Underlying diagnosis | <0·0001 | |||||
| Haematopoietic cell transplant | 37 | 18·8 | 1171 | 34·2 | 31·6 | |
| Autologous | 8 | 4·0 | 255 | 7·4 | 31·9 | |
| Immune effector cell therapy | 7 | 3·5 | 76 | 2·2 | 10·9 | |
| Allogeneic | 22 | 11·0 | 840 | 24·5 | 38·2 | |
| Matched related donor | 6 | 3·0 | 120 | 3·5 | 20·0 | |
| Matched unrelated donor | 8 | 4·0 | 272 | 7·9 | 34·0 | |
| Haploidentical | 4 | 2·0 | 117 | 3·4 | 29·3 | |
| Mismatched unrelated donor | 1 | 0·5 | 23 | 0·7 | 23·0 | |
| Umbilical cord blood | 3 | 1·5 | 308 | 9·0 | 102·7 | |
| Bone marrow source | 11 | 5·5 | 325 | 9·5 | 29·5 | |
| Peripheral blood stem cells | 8 | 4·0 | 207 | 6·0 | 25·9 | |
| Myeloablative | 18 | 8·9 | 728 | 21·2 | 40·4 | |
| Reduced toxicity conditioning | 4 | 2·0 | 112 | 3·3 | 28·0 | |
| Non-haematopoietic cell transplant | 164 | 81·6 | 2255 | 65·8 | 13·8 | |
| Leukemia/lymphoma | 60 | 29·8 | 1422 | 41·5 | 24·1 | |
| Acute lymphoblastic leukemia | 35 | 17·4 | 573 | 16·7 | 16·4 | |
| Acute myeloid leukemia | 16 | 8·0 | 795 | 23·2 | 49·7 | |
| Lymphoma | 9 | 4·5 | 66 | 1·9 | 7·3 | |
| Solid tumors | 93 | 46·3 | 651 | 19·0 | 7·0 | |
| Ewing sarcoma | 14 | 7·0 | 153 | 4·5 | 10·9 | |
| Osteosarcoma | 15 | 7·5 | 132 | 3·9 | 8·8 | |
| Rhabdomyosarcoma | 14 | 7·0 | 58 | 1·7 | 4·1 | |
| Brain tumors | 16 | 8·0 | 104 | 3·0 | 6·5 | |
| Other | 34 | 16·9 | 204 | 6·0 | 6·0 | |
| Benign haematologic conditions | 11 | 5·5 | 170 | 5·0 | 15·5 | |
| Product | ||||||
| Platelets | – | – | 1726 | 50·4 | – | |
| Red blood cells | – | – | 1476 | 43·1 | – | |
| Fresh frozen plasma | – | – | 87 | 2·5 | – | |
| Cryoprecipitate | – | – | 3·3 | – | ||
| Granulocytes | – | – | 24 | 0·7 | – | |
| Premedication status | – | |||||
| Any premedication | – | – | 1789 | 52·2 | – | – |
| Premedication for fever (acetaminophen and/or hydrocortisone) | – | – | 1608 | 46·9 | – | |
| Premedication for allergic reaction (diphenhydramine and/or hydrocortisone) | – | – | 1715 | 50·1 | – | |
| Transfusion reactions reported by bedside staff | 52 | 25·9 | 93 | 2·71 | – | – |
| Transfusion reactions adjudicated by Transfusion Medicine | 45 | 22·4 | 70 | 2·04 | – | – |
3.2. Transfusion history
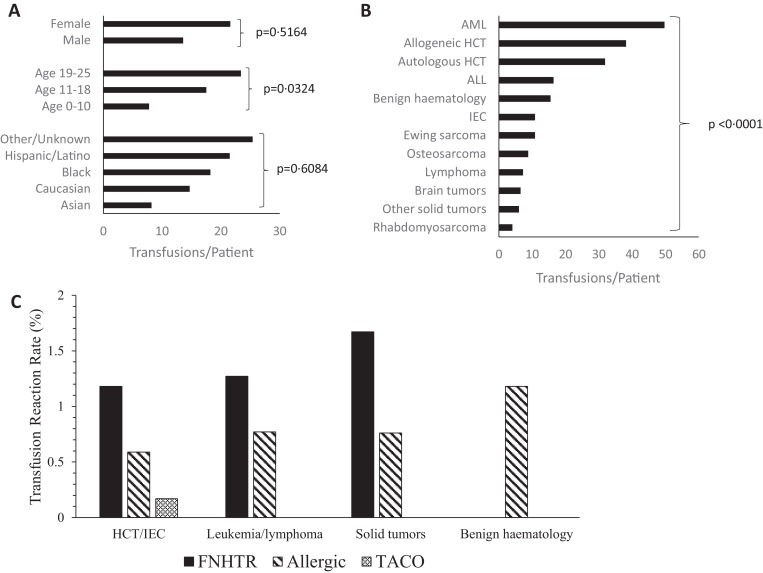
During the study period, 3426 blood products were transfused. Of those, 50·4% were platelets, 43·1% were packed red blood cells (pRBC), 3·3% were cryoprecipitate, 2·5% were thawed plasma, and 0·7% were granulocyte infusions. The average number of transfusions per person in the entire cohort was 17·0 blood product administrations/patient. Females were transfused an average of 21·4 transfusions/patient and males received 13·6 transfusions/patient (p = 0·5164). There were significant differences in transfusion burden based on age, with older patients receiving more transfusions (23·4 transfusions/patient) compared to younger children (7·8 transfusions/patient) (p = 0·0314). As shown in Fig. 1A, transfusion burden did not vary significantly by race/ethnicity (p = 0·6084). There was a significant difference in transfusion rate based on underlying diagnosis/treatment (Fig. 1B). Patients undergoing allogeneic HCT and those with a diagnosis of acute myeloid leukemia (AML) had the average highest rates of transfusion at 38·2 transfusions/patient and 49·7 transfusions/patient respectively (p = 0·0007).
Fig. 1.
(A) Average number of transfusions per patient in each demographic category. (B) Average number of transfusions per patient with each underlying diagnosis or transplant status. (C) Transfusion reaction rate of each type of reaction within the diagnostic categories listed.
3.3. Haemovigilance and incidence of transfusion reactions
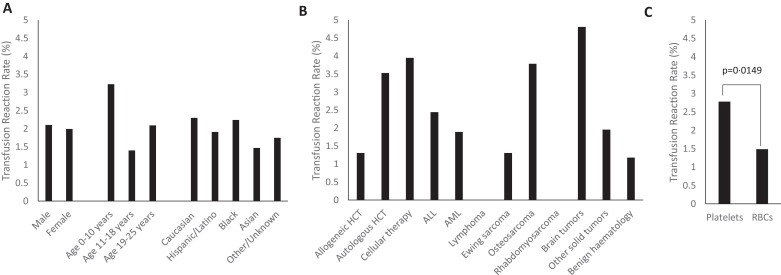
With haemovigilance, 93 transfusion reaction reports were generated by bedside staff (2·71% of all transfusions). The incidence of true transfusion reactions (n = 70) as determined by expert adjudication was 2·04% (Table 4). Adjudicated transfusion reactions occurred in 22·3% (n = 45) of our patients. Median age at the time of reaction was 19 years (range 1–24 years). Males and females had similar rates of transfusion reactions respectively. Younger children aged less than 10 years had a slightly higher transfusion reaction rate at 3·23% compared to those aged 11–18 years or 19–25 years with rates of 1·40%, and 2·09% respectively, though this difference did not reach statistical significance in our cohort (p = 0·0757) (Fig. 2A).
Table 4.
Transfusion reaction characterization.
| Number of adjudicated transfusion reactions | Percentage (%) of total adjudicated transfusion reactions (N = 70) | Percentage (%) of total transfusions in each group | P value | |
|---|---|---|---|---|
| Sex | 0·9036 | |||
| Female | 37 | 52·9 | 1·99 | |
| Male | 33 | 47·1 | 2·10 | |
| Age | 0·0757 | |||
| 0–10 years | 15 | 21·4 | 3·23 | |
| 11–18 years | 14 | 20·0 | 1·40 | |
| 19–25 years | 41 | 58·6 | 2·09 | |
| Race/Ethnicity | 0·9359 | |||
| Caucasian (non-Hispanic/Latino) | 25 | 35·7 | 2·30 | |
| Hispanic/Latino | 27 | 38·6 | 1·91 | |
| Black | 11 | 15·7 | 2·24 | |
| Asian | 3 | 4·3 | 1·47 | |
| Other/Unknown | 4 | 5·7 | 1·75 | |
| Product | 0·0318 | |||
| Platelets | 48 | 68·6 | 2·78 | |
| Red blood cells | 22 | 31·4 | 1·49 | |
| Thawed plasma | 0 | 0 | 0 | |
| Cryoprecipitate | 0 | 0 | 0 | |
| Granulocytes | 0 | 0 | 0 | |
| Underlying diagnosis | ||||
| Haematopoietic cell transplant | 23 | 32·9 | 1·96 | 0·7940 (major diagnosis categories) |
| Autologous | 9 | 12·9 | 3·53 | 0·1047 (diagnosis subtypes) |
| Immune effector cell therapy | 3 | 4·3 | 3·95 | |
| Allogeneic | 11 | 15·7 | 1·31 | |
| Matched related donor | 3 | 4·2 | 2·50 | |
| Matched unrelated donor | 3 | 4·2 | 1·10 | |
| Haploidentical | 0 | 0 | 0 | |
| Mismatched unrelated donor | 0 | 0 | 0 | |
| Umbilical cord blood | 5 | 14·2 | 1·62 | |
| Bone marrow source | 4 | 5·7 | 1·23 | |
| Peripheral blood stem cells | 2 | 2·9 | 0·97 | |
| Myeloablative | 11 | 15·7 | 1·51 | |
| Reduced toxicity conditioning | 0 | 0 | 0 | |
| Non-haematopoietic cell transplant | 47 | 67·1 | 2·08 | |
| Leukemia/lymphoma | 29 | 41·4 | 2·04 | |
| Acute lymphoblastic leukemia | 14 | 20·0 | 2·44 | |
| Acute myeloid leukemia | 15 | 21·4 | 1·89 | |
| Lymphoma | 0 | 0 | 0 | |
| Solid tumors | 16 | 22·9 | 2·46 | |
| Ewing sarcoma | 2 | 2·9 | 1·31 | |
| Osteosarcoma | 5 | 7·1 | 3·79 | |
| Rhabdomyosarcoma | 0 | 0 | 0 | |
| Brain tumors | 5 | 7·1 | 4·81 | |
| Other | 4 | 5·7 | 1·96 | |
| Benign haematologic conditions | 2 | 2·9 | 1·18 | |
| Transfusion reaction type | ||||
| Febrile non-haemolytic transfusion reaction | 43 | 61·4 | – | – |
| Premedication with acetaminophen and/or hydrocortisone | (24/43) | (55·8) | ||
| Allergic | 25 | 35·7 | – | |
| Premedication with diphenhydramine and/or hydrocortisone | (19/25) | (76·0) | ||
| Transfusion-associated circulatory overload | 2 | 2·9 | – | |
| Transfusion-associated acute lung injury | 0 | 0 | – | |
| Haemolytic transfusion reaction | 0 | 0 | – | |
| Severity | ||||
| Non-severe | 62 | 88·6 | ||
| Severe/Life-threatening | 5 | 7·1 | ||
| Death | 0 | 0 | ||
| Not determined | 3 | 4·3 |
Fig. 2.
(A) Transfusion reaction rate by demographic characteristics. (B) Transfusion reaction rate by underlying diagnosis. (C) Transfusion reaction rate by blood product.
3.4. Transfusion reaction classification
The majority (61·4%) of transfusion reactions were classified as febrile non-haemolytic transfusion reactions (FNHTR), while 35·7% were considered allergic and 2·9% were diagnosed as transfusion-associated circulatory overload (TACO). Both of the TACO transfusion reactions occurred in HCT/IEC patients, one after double cord blood allogeneic transplantation, and one after IEC administration. In both of these patients, there was evidence of fluid volume overload with increasing respiratory distress, positive fluid balance, pulmonary edema and new oxygen requirement. Neither of these patients had a prior history of TACO or were pre-medicated with diuretics. Of the transfusions administered to patients with benign haematologic diseases, none resulted in FNHTR and two resulted in an allergic reaction. The HCT/IEC, leukemia/lymphoma, and solid tumor groups had similar rates of NHFTRs and allergic transfusion reactions (Fig. 1C). Of the 70 total reactions, five were potentially life-threatening (7·1% of reactions). One severe allergic reaction occurred in an IEC patient, two severe FNHTRs occurred in pediatric oncology patients (ALL and brain tumor) and two TACOs occurred in HCT/IEC patients (one cord blood recipient and one IEC recipient). None of the transfusion reactions were associated with death.
3.5. Transfusion reactions characterized by underlying diagnosis/treatment
An underlying diagnosis of leukemia was associated with 41·4% and HCT/IEC was associated with 32·9% of all transfusion reactions respectively. Of the 23 reactions in HCT/IEC patients, 11 occurred in allogeneic HCT recipients, nine were in autologous HCT recipients, and three were in patients receiving IEC. The reactions that occurred in IEC patients (three reactions out of 76 transfusions, incidence 3·95%) were distinguished from cytokine release syndrome (CRS: fever, hypotension, hypoxia, fluid retention, and rashes among other symptoms) [8]; all three reactions occurred during the potential window for CRS (2–6 days post IEC infusion) and adjudication was done in consultation with the primary treatment team. True reactions occurred in two patients who had received tumor infiltrating lymphocytes (TIL) with interleukin-2 (IL-2). Adjudicated true reactions in this sub-group included TACO (n = 1), FNHTR (n = 1) and severe allergic reaction (n = 1). The patient who developed TACO had signs of fluid volume overload with weight gain, pulmonary edema, pleural effusions, and increasing oxygen requirement during a transfusion on day +3 following IEC infusion. This same patient experienced a severe allergic reaction during a transfusion three days later as characterized by rash, angioedema, and dyspnea. Adjudication of transfusion reaction was complicated by concurrent CRS from day +3 to day +8 following IEC infusion. CRS was managed conservatively without medication management. The IEC patient with the FNHTR had a temperature increase of 1·6 °C to 38·6 °C approximately 4 h after completion of a platelet transfusion, on day +5 following IEC infusion. This patient too had signs/symptoms possibly consistent with CRS, including fevers from day +5 to day +7 following IEC infusion. There was one “false positive” (negatively-adjudicated) transfusion reaction in this subgroup in a CD19 chimeric antigen receptor (CAR) T-cell patient with dyspnea that was attributed to CRS. Transfusion reaction rates across all underlying diagnoses/treatments are summarized in Fig. 2B.
3.6. Transfusion reactions characterized by product type
Platelets accounted for the majority (68·6%) of positively adjudicated reactions, while pRBCs were associated with 31·4%. Thus, of all platelet transfusions (n = 1726), reactions were documented in 2·78%, and of all RBC transfusions (n = 1476), reactions were documented in 1·49%, suggesting a higher reaction rate with platelets than with RBC transfusions (p = 0·0149) (Fig. 2C). There were no reactions identified with cryoprecipitate, thawed plasma, or granulocyte infusions, though these products comprised only 6·5% of all transfusions (n = 224).
3.7. Transfusion reactions characterized by pre-medication status
The majority of transfusion reactions occurred in the setting of pre-medication. More than half (55·8%) of FNHTRs occurred in patients who were pretreated with acetaminophen and/or hydrocortisone; and 76% of allergic transfusion reactions occurred in patients who were pre-treated with diphenhydramine and/or hydrocortisone. The incidence of transfusion reactions in patients who were pre-medicated with anti-pyretics and/or anti-histamines was significantly higher (2·51%) compared to patients who were not pre-medicated (1·52%) (p-value 0·0406).
3.8. Characteristics of negatively-adjudicated reactions
Almost one quarter (24·7%) of bedside reports of transfusion reactions were subsequently adjudicated as negative; an alternative attribution was determined and appropriate medical intervention occurred. Of the 23 reports that were not adjudicated as true transfusion reactions, 19 (83%) were generated for fever, two for tachycardia, one for dyspnea, and one for hypotension; all were deemed unrelated to the transfusion and subsequently attributed to other causes, most commonly underlying infection and/or neutropenic fever.
4. Discussion
To our knowledge, this study is the first to report the incidence and characterize the spectrum of transfusion reactions seen in immune-compromised pediatric-AYA, hematology/oncology and HCT/IEC patients. These findings are important as, historically, there has been concern for underreporting of transfusion reactions in oncology patients [9,10]. In our population, the incidence of transfusion reactions among pediatric-AYA hematology/oncology patients (2·04%) was similar to the reported general adult and lower than the general pediatric population incidence. Understanding the incidence and characteristics associated with transfusion reactions in this population may improve our ability to detect and promptly manage these potentially life-threatening complications.
The incidence of transfusion reactions varies widely depending on the study design and type of haemovigilance system used—active versus passive [4,[11], [12], [13], [14], [15], [16], [17], [18]]. Active haemovigilance reporting systems have observed higher rates of transfusion reactions compared to passive systems. Hendrickson et al. using systematic active surveillance and expert adjudication, identified a transfusion reaction rate of approximately 2% [4]. However, in a study which reviewed the transfusion reactions reported to the National Healthcare Safety Network Haemovigilance Module, only 5136 reactions were reported among 2144,723 components transfused (0·24%) [19].
In our study, platelet transfusions and pre-medication were associated with higher incidence of transfusion reactions. Younger children appeared to have a higher incidence of reactions, but in our relatively older cohort, we may not have been powered to detect significance. Febrile non-haemolytic transfusion reactions were the most common reaction observed. Potentially life-threatening reactions were rare but occurred most commonly in IEC recipients. These findings may inform future haemovigilance prediction systems for this population.
Cellular and immunotherapies (i.e., immune effector cells and checkpoint inhibitors) have revolutionized the field of oncology but are associated with unique toxicities and potentially overlapping symptoms associated with transfusion reactions [8,20]. Haemovigilance adjudication protocols in this population should include input from primary treatment teams; patients receiving these therapies are at risk for haemodynamic compromise and transfusion reactions may easily be incorrectly attributed to CRS and/or other expected toxicities. Prompt recognition of transfusion reactions and initiation of appropriate management among these patients may be life-saving. One patient in our IEC cohort developed a severe transfusion reaction and another patient developed TACO concurrent with CRS. The observed symptoms during both transfusions were associated with clinical deterioration compared to pre-transfusion baseline and after consultation with the primary treatment team were subsequently adjudicated as true transfusion reactions. Active haemovigilance was associated with conservative management in this clinically labile patient.
Interestingly, prior reports have found a higher incidence of transfusion reactions and in particular allergic transfusion reactions among pediatric patients as compared to adults [5,6]. We observed a lower incidence of reactions in our immune-compromised population compared to historical reports among all pediatric patients. Yet, we observed a higher incidence of FNHTR than reported among adult and general pediatric patients [5,6]. The differences in observations in our population may be related to different physiology, pre-medication rates, and/or intensity of monitoring [6]. It is possible that comorbidities including frequent infections (resulting in fevers) and immune dysfunction/dysregulation may influence transfusion reaction presentation in this pediatric-AYA hematology/oncology population. Of note, the majority of reactions that were negatively-adjudicated were also related to fever, which was deemed unrelated to the transfusion. Prospective studies are needed to investigate this further. Our study was limited by its retrospective nature and the intrinsic biases that are involved in this study design.
In summary, the incidence of transfusion reactions among pediatric-AYA hematology/oncology patients may be similar to the reported general adult and lower than the general pediatric population incidence. Patients with a prior history of transfusion reactions and those receiving platelet transfusions may be at higher risk for reaction. IEC recipients may be at high risk for severe transfusion reactions and large multi-center prospective studies are needed to further characterize this sub-population. Emerging data may allow development of optimized risk stratification, early and more accurate detection of transfusion reactions and facilitate appropriate management.
Funding
No sources of funding to declare.
Author contributions
M.A.K. completed data collection, analysis, and wrote the manuscript; S.J.K., P.T., D.P, J.M.K. and K.M.M treated the patients and assisted in editing the manuscript; B.M. assisted in data collection and editing the manuscript; J.W. assisted with biostatistical analyses and editing the manuscript; K.M.M and J.M.K conceptualized the study, analyzed the data and wrote the manuscript; All co-authors reviewed the manuscript and made significant contributions.
Data sharing statement
Datasets available upon request to corresponding author.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgements
We wish to acknowledge our patients and their caregivers; we also thank our nursing unit and Haemovigilance Unit staff.
References
- 1.Lieberman L., Liu Y., Portwine C., Barty R.L., Heddle N.M. An epidemiologic cohort study reviewing the practice of blood product transfusions among a population of pediatric oncology patients. Transfusion. 2014;54(10 Pt 2):2736–2744. doi: 10.1111/trf.12677. [DOI] [PubMed] [Google Scholar]
- 2.Delaney M., Wendel S., Bercovitz R.S. Transfusion reactions: prevention, diagnosis, and treatment. Lancet. 2016;388(10061):2825–2836. doi: 10.1016/S0140-6736(15)01313-6. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Centers for Disease Control and Prevention. The National Healthcare Safety Network (NHSN) Manual: biovigilance Component v2.5. Atlanta GDoHQP, National Center for Emerging and Zoonotic Infectious Diseases. Available at: http://www.cdc.gov/nhsn/PDFs/Biovigilance/BV-HV-protocol-current.pdf. Accessed 3 June 2020.
- 4.Hendrickson J.E., Roubinian N.H., Chowdhury D. Incidence of transfusion reactions: a multicenter study utilizing systematic active surveillance and expert adjudication. Transfusion. 2016;56(10):2587–2596. doi: 10.1111/trf.13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oakley F.D., Woods M., Arnold S., Young P.P. Transfusion reactions in pediatric compared with adult patients: a look at rate, reaction type, and associated products. Transfusion. 2015;55(3):563–570. doi: 10.1111/trf.12827. [DOI] [PubMed] [Google Scholar]
- 6.Vossoughi S., Perez G., Whitaker B.I., Fung M.K., Stotler B. Analysis of pediatric adverse reactions to transfusions. Transfusion. 2018;58(1):60–69. doi: 10.1111/trf.14359. [DOI] [PubMed] [Google Scholar]
- 7.R Core Team . R Foundation for Statistical Computing V; Austria: 2013. R: a language and environment for statistical computing.http://www.R-project.org/ URL. [Google Scholar]
- 8.Lee D.W., Santomasso B.D., Locke F.L. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transp. 2019;25(4):625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huh Y.O., Lichtiger B. Transfusion reactions in patients with cancer. Am J Clin Pathol. 1987;87(2):253–257. doi: 10.1093/ajcp/87.2.253. [DOI] [PubMed] [Google Scholar]
- 10.Narvios A.B., Lichtiger B., Neumann J.L. Underreporting of minor transfusion reactions in cancer patients. MedGenMed. 2004;6(2):17. [PMC free article] [PubMed] [Google Scholar]
- 11.Lieberman L., Maskens C., Cserti-Gazdewich C. A retrospective review of patient factors, transfusion practices, and outcomes in patients with transfusion-associated circulatory overload. Transfus Med Rev. 2013;27(4):206–212. doi: 10.1016/j.tmrv.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Chung K.W., Harvey A., Basavaraju S.V., Kuehnert M.J. How is national recipient hemovigilance conducted in the United States. Transfusion. 2015;55(4):703–707. doi: 10.1111/trf.12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolton-Maggs P.H., Cohen H. Serious Hazards of Transfusion (SHOT) haemovigilance and progress is improving transfusion safety. Br J Haematol. 2013;163(3):303–314. doi: 10.1111/bjh.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleinman S., Chan P., Robillard P. Risks associated with transfusion of cellular blood components in Canada. Transfus Med Rev. 2003;17(2):120–162. doi: 10.1053/tmrv.2003.50009. [DOI] [PubMed] [Google Scholar]
- 15.Piccin A., Cronin M., Brady R., Sweeney J., Marcheselli L., Lawlor E. Transfusion-associated circulatory overload in Ireland: a review of cases reported to the National Haemovigilance Office 2000 to 2010. Transfusion. 2015;55(6):1223–1230. doi: 10.1111/trf.12965. [DOI] [PubMed] [Google Scholar]
- 16.Raval J.S., Mazepa M.A., Russell S.L., Immel C.C., Whinna H.C., Park Y.A. Passive reporting greatly underestimates the rate of transfusion-associated circulatory overload after platelet transfusion. Vox Sang. 2015;108(4):387–392. doi: 10.1111/vox.12234. [DOI] [PubMed] [Google Scholar]
- 17.Narick C., Triulzi D.J., Yazer M.H. Transfusion-associated circulatory overload after plasma transfusion. Transfusion. 2012;52(1):160–165. doi: 10.1111/j.1537-2995.2011.03247.x. [DOI] [PubMed] [Google Scholar]
- 18.Rogers M.A., Rohde J.M., Blumberg N. Haemovigilance of reactions associated with red blood cell transfusion: comparison across 17 Countries. Vox Sang. 2016;110(3):266–277. doi: 10.1111/vox.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harvey A.R., Basavaraju S.V., Chung K.W., Kuehnert M.J. Transfusion-related adverse reactions reported to the National Healthcare Safety Network Hemovigilance Module, United States, 2010 to 2012. Transfusion. 2015;55(4):709–718. doi: 10.1111/trf.12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramos-Casals M., Brahmer J.R., Callahan M.K. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6(1):38. doi: 10.1038/s41572-020-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]