Abstract
Salmonella enterica serovar Typhimurium (S. Typhimurium) is one of the most significant zoonotic pathogens that poses a threat to humans. Previous studies have identified that Salmonella-secreted effector K3 (SseK3) is a novel translated and secreted protein of S. Typhimurium. The objective of this study was to determine whether deletion of the sseK3 gene can attenuate the virulence of S. Typhimurium. To do this, we constructed an sseK3 deletion mutant using the double-exchange allele of the suicide plasmid pRE112ΔsseK3 and assessed the virulence and intracellular proliferation of the mutant. The sseK3 deletion mutant exhibited adhesion and invasion properties similar to those of wild-type (WT) S. Typhimurium, although the virulence and intracellular proliferation of the mutant were significantly reduced compared to that of the WT strain. Furthermore, the observed increase in the median lethal dose (LD50) reflects a decrease in the pathogenicity of the sseK3 deletion mutant in a murine model. In summary, we concluded that disruption of sseK3 can attenuate the intracellular proliferation and reduce the virulence of S. Typhimurium.
Résumé
Salmonella enterica serovar Typhimurium (S. Typhimurium) est un des agents pathogènes zoonotiques les plus importants qui représente une menace pour les humains. Des études antérieures ont identifié que l’effecteur K3 secrété par Salmonella (SseK3) est une nouvelle protéine traduite et secrétée par S. Typhimurium. L’objectif de la présente étude était de déterminer si une délétion du gène sseK3 pouvait atténuer la virulence de S. Typhimurium. Nous avons construit un mutant avec la délétion de sseK3 en utilisant l’allèle d’échange double du plasmide suicide pRE112ΔsseK3 et avons évalué la virulence et la prolifération intracellulaire du mutant. Le mutant de délétion démontrait des propriétés d’adhésion et d’invasion similaires à celles du type sauvage (WT) de S. Typhimurium, bien que la virulence et la prolifération intracellulaire du mutant étaient considérablement réduites comparativement à celles de la souche WT. De plus, l’augmentation observée de la dose létale médiane (LD50) reflète une diminution dans la pathogénicité de ce mutant de délétion sseK3 dans un modèle murin. En résumé, nous concluons qu’une perturbation de sseK3 peut atténuer la prolifération intracellulaire et réduire la virulence de S. Typhimurium.
(Traduit par Docteur Serge Messier)
Introduction
Salmonella Typhimurium is an important infectious disease pathogen that is not only widely distributed in nature but also poses a serious hazard to animals. After being contaminated by Salmonella, animal products can be transmitted to humans through the food chain, threatening human health and potentially leading to death. A host is infected with S. Typhimurium through intestinal epithelial cells, which causes severe multiple systemic diseases, although the pathogenesis of S. Typhimurium is complicated and remains unclear (1–3). The incidence of typhoid fever caused by S. Typhimurium is high in some developing countries with poor water and sanitation (4). In general, investigation of the virulence mechanism of its pathogenesis would contribute to prevention of S. Typhimurium and control of disease.
Multiple virulence proteins of S. Typhimurium exert enormous stress during infection and colonization of a host (5) and the Salmonella pathogenicity island-2 (SPI-2) encoded type-III secretion system (T3SS) plays an obligatory role in its virulence by enabling S. Typhimurium to transport effector (virulence) proteins in Salmonella-containing vacuoles (SCVs) into host cells (6). Numerous virulence proteins collectively disrupt normal cellular processes and allow bacteria to enter and persist in host cells. In addition, some virulence proteins activate cellular inflammatory signals and programmed cell death (6,7).
Among the virulence proteins secreted by the SPI-2 T3SS are SseK1, SseK2, and SseK3, which constitute the Salmonella-secreted effector K (SseK) family. These 3 proteins are highly similar Salmonella effectors (8,9). Amino acid sequence comparison shows that SseK1 and SseK2 are similar to attaching and effacing pathogen-secreted proteins (8). SseK3 was discovered and classified as a member of the SseK family by comparing the genomes of S. Typhimurium strains SL1344 and LT2. SseK3 shares 60% homology with SseK1 and 75% homology with SseK2 (9). Although many studies have explored the pathogenesis of Salmonella virulence, results to date on the role of sseK3 are contradictory (10,11).
The virulence of Salmonella is usually evaluated by morphology, cell invasiveness, and mouse median lethality (12). In this study, the suicide plasmid system was used to successfully construct an S. Typhimurium-specific sseK3 deletion mutant (ΔsseK3) in the wild-type (WT) S. Typhimurium and the biological characteristics of the sseK3 deletion mutant were then analyzed. We detected the effect of sseK3 gene deletion on the proliferation of S. Typhimurium within cells, as well as the effect on its virulence. The results show that sseK3 disruption may attenuate the virulence of S. Typhimurium and reduce intracellular proliferation of the pathogen.
Materials and methods
Animals, bacterial strains, plasmids, cells, and culture
Specific pathogen-free (SPF) BALB/c mice (6- to 8-weeks old; body weight, 20 ± 2 g) were obtained from the experimental animal center at the Henan University of Science and Technology (Luoyang, China). The bacterial strains, plasmids, and primers used in this study are shown in Table I. Salmonella Typhimurium SL1344 and pRE112 plasmid using the method described in previous studies (13,14). Bacterial liquid cultures were maintained in Luria-Bertani (LB) broth. Bacteria were prepared during the exponential growth phase, cultured at 37°C for 8 to 10 h, and streaked to LB agar (Sigma-Aldrich, Cleveland, Ohio, USA). The bacteria were washed twice with phosphate-buffered saline (PBS) and suspended in PBS (15).
Table I.
Bacterial strains, plasmids, and primers used in this study.
| Strain or plasmid | Characteristics/description | Source or reference |
|---|---|---|
| Strains | ||
| SL1344 | Serovar Typhimurium, wild-type | (13) |
| SL1344ΔsseK3 | sseK3 deletion mutant | In this study |
| χ7213 | χ7213, containing pRE sseK3, Cmr plasmid | In this study |
| Plasmids | ||
| pBluescriptIISK(+) | Phagemid cloning vector, oriCOLE1 oriF1(+) bla lacZα | Laboratory stock |
| pBSK-sseK3 | pBluescripIIKSt, sseK3 | In this study |
| pRE112 | pGP704 suicide plasmid, pir dependent, oriT, oriV, sacB, Cmr | (14) |
| pREΔsseK3 | pRE112 derivative containing sseK3 fused in-frame, Cmr | In this study |
| pBR322 | oriColE1, Ampr Tcr | Laboratory stock |
| pBR322-sseK3 | pBR322 carrying the full sseK3 gene (Ampr) | In this study |
| Primers | ||
| ΔsseK3-up-F | 5′-TCTAGACCGCGAGTGACATCAATATTA-3′(Xba I site underlined) | In this study |
| ΔsseK3-up-R | 5′-GGATCCTTTTCAACCCTTACGCTA-3′ (Bam HI site underlined) | In this study |
| ΔsseK3-down-F | 5′-CTCGAGTTATTTGCAAACGTATG-3′ (Xho I site underlined) | In this study |
| ΔsseK3-down-R | 5′-GGTACCTACCTGGAACAATGCAGGTT-3′ (Kpn I site underlined) | In this study |
| ΔsseK3-F | 5′-GCCCCCCCTAACCAAGTAAAAACTAT-3′ | In this study |
| ΔsseK3-R | 5′-CTAAATATTCAGGCGGGTTTATTACCC-3′ | In this study |
| pBR-ΔsseK3-F | 5′-AAGCTTATGTTTTCTCGAGTCAGA-3′ (Hind III site underlined) | In this study |
| pBR-ΔsseK3-R | 5′-GGATCCTTATCTCCAGGAGCTGATA-3′ (Bam HI site underlined) | In this study |
Murine peritoneal macrophages were obtained from BALB/c mice from the experimental animal center at the Henan University of Science and Technology (Luoyang, China). HeLa cells were obtained from the American Type Culture Collection (ATCC) (Manassas, Virginia, USA). Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM)/high-glucose medium (HyClone, Logan, Utah, USA) containing 10% fetal calf serum (FCS) in an incubator at 37°C and 5% carbon dioxide (CO2).
Construction of the ΔsseK3 mutant and its complemented strain
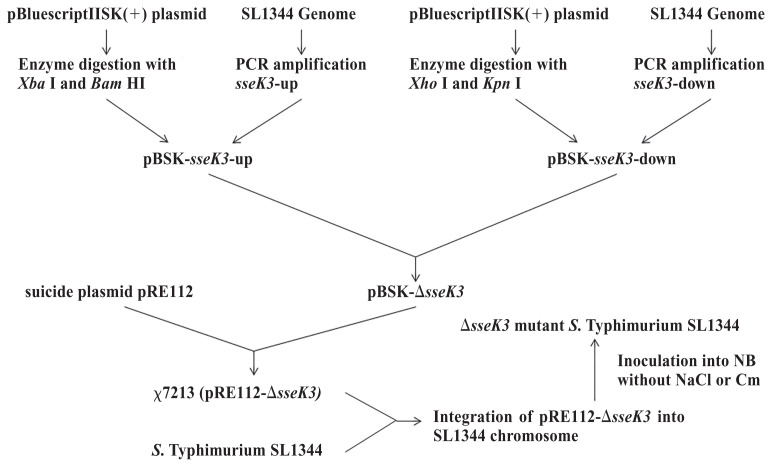
The ΔsseK3 mutant was constructed using the method described in previous reports (16,17). The primers used, which were designed according to the nucleotide sequence of SL1344, are described in Table I. The 1002-bp fragment of the sseK3 upstream deoxyribonucleic acid (DNA) and the 1019-bp fragment of the sseK3 downstream DNA were cloned into the pBluescriptIISK(+) plasmid to produce the recombinant plasmid pBSK-sseK3. After verification by Sanger sequencing at Sangon Biotech, Shanghai, China, the gene fragment lacking sseK3 was excised with the restriction enzymes Xba I and Kpn I and the fragment was cloned into the suicide plasmid pRE112 to produce plasmid pREΔsseK3, which was transformed into Escherichia coli ϰ 7213 (λpir). We identified the ΔsseK3 mutation in WT SL1344 by using pREΔsseK3 and double-crossover allelic exchange. Colonies of the ΔsseK3 mutant were selected by polymerase chain reaction (PCR) and identified by double-digestion with Xba I and Kpn I. The ΔsseK3 mutant was determined by PCR using the primers sseK3-F and sseK3-R and the fragments were subjected to DNA sequencing to confirm deletion of the sseK3 gene. The specific steps are shown in Figure 1. The pBR322 cloning vector contains an ampicillin/tetracycline resistance marker encoded by the bla gene, which is under the control of the AmpR promoter, the high-copy-number pBR322 origin of replication, and genetic elements required for replication (18). The sseK3 gene was cloned into the pBR322 plasmid for complementation studies.
Figure 1.
Flowchart of major steps in deletion of sseK3.
Biofilm formation assay
The biofilm formation assay was conducted as described in previous studies (19,20). Briefly, cultures of the WT, ΔsseK3, and sseK3-complemented strains were adjusted to the same concentration [1 × 106 colony-forming units (CFUs)] and 3 replicate wells per strain with biofilm formation of WT, the ΔsseK3 mutant, and the sseK3-complemented strain were tested at 28°C for 72 h using a 96-well cell culture plate, without shaking, in a wet box to maintain a humidified environment. The 96-well plate was washed 3 times with PBS (pH 7.0) and dried at 37°C for 0.5 h, after which 100 μL of crystal violet (10 mg/mL; Sigma-Aldrich) was added to each well and incubated for 0.5 h. Subsequently, 100 μL of absolute ethanol was added to each well and optical density was measured at 570 nm (OD570). Statistical significance was calculated using 1-way analysis of variance (ANOVA) (Bonferroni’s multiple-comparison test).
Morphotype assay
The morphotype assay was conducted as described in a previous study (19). To determine whether the ΔsseK3 mutant produced less curli and cellulose, 10 μL of the 3 strains cultured overnight (WT, ΔsseK3 mutant, and sseK3-complemented strains) were dropped onto LB agar in the absence of sodium chloride (NaCl) and supplemented with Congo red (40 mg/L; Sigma Aldrich) and Coomassie brilliant blue G (20 mg/L; Sigma Aldrich). The morphology of the colonies was observed after incubation for 48 h at 28°C, with 3 repeats.
Adherence and invasion assay
Adhesion and invasion of HeLa cells by S. Typhimurium were assessed as described in previous studies (21–23). A 24-well cell culture plate was inoculated with 1 × 105 HeLa epithelial cells per well and incubated for 16 h. Wild-type (WT) S. Typhimurium, ΔsseK3 mutant, and sseK3-complemented strains were coincubated with HeLa epithelial cells at a multiplicity of infection (MOI) of 100:1, with 3 replicate wells per strain. Statistical significance was calculated using ANOVA (Bonferroni’s multiple-comparison test).
To allow the bacteria to fully contact the HeLa epithelial cells, the plates were centrifuged and incubated with 5% CO2 for 2 h at 37°C. For the adherence assay, the supernatants were aspirated and the cells were washed 3 times with PBS. Subsequently, the cells were digested with 0.25% trypsin and plated in a gradient dilution and counted. For the invasion assay, the supernatants were aspirated, the cells were washed 3 times with PBS, and gentamicin-containing medium (100 μg/mL) was added and incubated at 37°C with 5% CO2. After incubation, the supernatants were aspirated and the cells were washed 3 times with PBS. Subsequently, the cells were lysed using 0.1% Triton X-100 and plated with a gradient dilution and counted. Statistical significance was calculated using ANOVA (Bonferroni’s multiple-comparison test).
Intracellular proliferation assay
Bacterial proliferation in macrophages was determined as previously described (24–26). Approximately 5 × 105 macrophages were seeded into 24-well plates. Wild-type (WT) S. Typhimurium, ΔsseK3 mutant, and sseK3-complemented strains were added to each well at an MOI of 100:1 and incubated for 0.5 h, with 3 replicate wells per strain. After incubation, gentamicin-containing medium (100 μg/ mL) was added to the cells and incubated for 2 h, after which the infected cells were lysed using 1 mL 0.1% Triton X-100 for 10 min. The numbers of colony-forming units (CFUs) were determined by plating the cell lysates onto Salmonella-Shigella (SS) agar. Statistical significance was calculated using ANOVA (Bonferroni’s multiple-comparison test).
LD50 assay
The median lethal dose (LD50) assay was carried out as described in previous studies (27–29). A total of 120 (N = 120) specific pathogen-free (SPF) BALB/c mice were divided randomly into 5 subgroups, each subgroup containing 12 mice, half male and half female. Based on the results of the preliminary study, mice in the ΔsseK3 mutant group were each given 0.2 mL of 5 × 109, 1 × 109, 1 × 108, 1 × 107, and 1 × 106 CFUs and the mice in the WT group were each given 0.2 mL of 1 × 108, 1 × 107, 1 × 106, 1 × 105, and 1 × 104 CFUs. Twelve of the mice were inoculated orally with 0.2 mL of PBS as a negative control. Using the approach of Bliss, the LD50 was calculated on the 35th day post-infection (28). Kaplan-Meier survival curves of mice infected with the WT or ΔsseK3 mutant strain were generated and data were analyzed by the log-rank test.
Statistical analysis
The data are presented as the mean ± standard deviation (SD), as based on triplicates assays. All statistical analyses were conducted using SPSS statistical software version 10.0 (SPSS, Chicago, Illinois, USA). P-values of < 0.05 or < 0.01 were considered statistically significant. One-way analysis of variance (ANOVA) with a post-hoc test (Bonferroni’s multiple-comparison test) was used to compare and assess significance of the differences among all groups and Kaplan-Meier survival curves were analyzed by the log-rank test using GraphPad Prism software version 5.0 (San Diego, California, USA).
Results
Construction of the ΔsseK3 mutant of S. Typhimurium
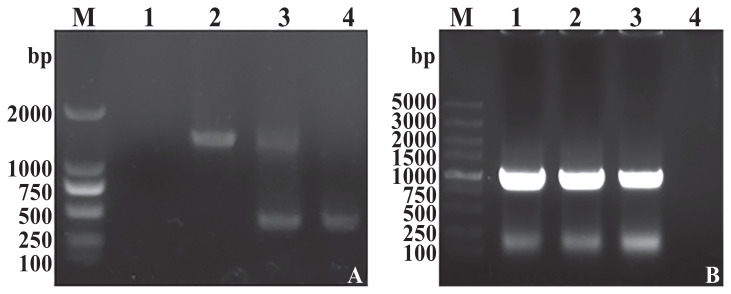
The ΔsseK3 mutant S. Typhimurium SL1344 was constructed using allelic exchange. The in-frame deletion of the sseK3 gene was cloned into plasmid pRE112 to construct the target plasmid (30). The ΔsseK3 mutant was successfully confirmed by polymerase chain reaction (PCR) amplification of a 455-bp fragment that was subjected to DNA sequencing (Figure 2A). We also constructed the sseK3-complemented strain in the ΔsseK3 mutant (Figure 2B).
Figure 2.
A — Identification of the ΔsseK3 in-frame mutation by PCR. M — Marker DL2000; 1 — negative control; 2 — double-crossover ΔsseK3 mutant; 3 — single-crossover ΔsseK3 mutant; and 4 — wild-type (WT). B — Verification of pBR322-sseK3 electroporated into SL1344ΔsseK3. M — Marker DL5000; 1 to 3 — SL1344CΔsseK3; and 4 — negative control.
Analysis of biofilm formation
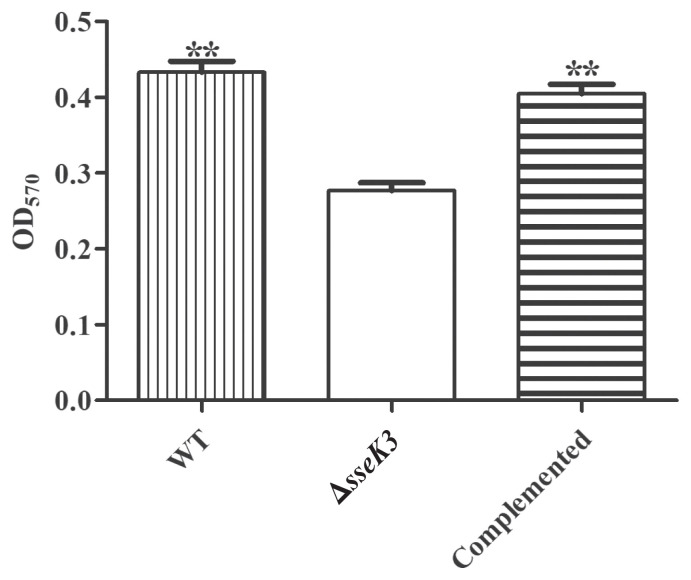
To investigate biofilm formation of the ΔsseK3 mutant of S. Typhimurium, the ΔsseK3 mutant, WT, and sseK3-complemented strains were tested for biofilm-related characteristics using a 96-well plate and absorbance at 570 nm with a microplate reader. The results showed that deletion of the sseK3 gene significantly affected biofilm formation compared to WT (**P < 0.01) (Figure 3). Conversely, biofilm formation was restored in the sseK3-complemented strain (Figure 3). Thus, sseK3 is necessary for the formation of S. Typhimurium biofilms.
Figure 3.
Biofilm formation assay among wild-type (WT), ΔsseK3 mutant, and sseK3-complemented strains. Data for all groups are presented as mean ± SD. Optical density at 570 nm (OD570) values of quantitative microtiter plate among WT, ΔsseK3 mutant, and sseK3-complemented strains with crystal violet staining. Statistical significance was calculated using ANOVA (Bonferroni’s multiple-comparison test) (**P < 0.01). The asterisk (*) indicates that the ΔsseK3 mutant differed significantly from the WT and sseK3-complemented strains.
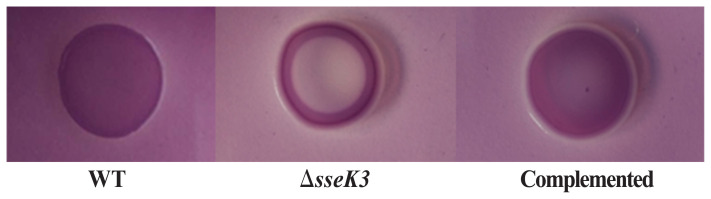
The main components of S. Typhimurium biofilms are cellulose and curli (31). The ΔsseK3 mutant strain colonies were morphologically different than the colonies of the WT strain, with the former strain producing smooth and white (SAW) colonies, indicating a lack of curli and cellulose (Figure 4). Additionally, the sseK3-complemented strain regained the colony morphology phenotype of the WT strain. Therefore, deletion of S. Typhimurium sseK3 may lead to the lack of expression of curli and cellulose, which may result in poor biofilm formation.
Figure 4.
Biofilm morphology among wild-type (WT), ΔsseK3 mutant, and sseK3-complemented strains.
Virulence of ΔsseK3 Salmonella mutant is attenuated in vitro
Adherence to and invasion of intestinal epithelial cells of the host and proliferation of peritoneal macrophages are hallmarks of S. Typhimurium virulence (20,21,32,33). Compared to WT, the ΔsseK3 mutant exhibited similar adhesion to and invasion of HeLa epithelial cells (Table II). The sseK3-complemented strain regained the adherence and invasion ability of WT, however, which suggests that sseK3 does not play an important role in the adherence to and invasion of HeLa cells by S. Typhimurium.
Table II.
Role of ΔsseK3, wild-type (WT), and sseK3-complemented strains in adherence to and invasion of HeLa epithelial cells.
| Strains | Percentage adherence (Number adhered/ Number inoculated) | Percentage invasion (Number invaded/ Number inoculated) |
|---|---|---|
| WT | 5.12 ± 0.02 | 2.36 ± 0.10 |
| ΔsseK3 | 4.67 ± 0.04 | 2.26 ± 0.03 |
| Complemented | 5.07 ± 0.06 | 2.28 ± 0.03 |
Values are mean ± SD.
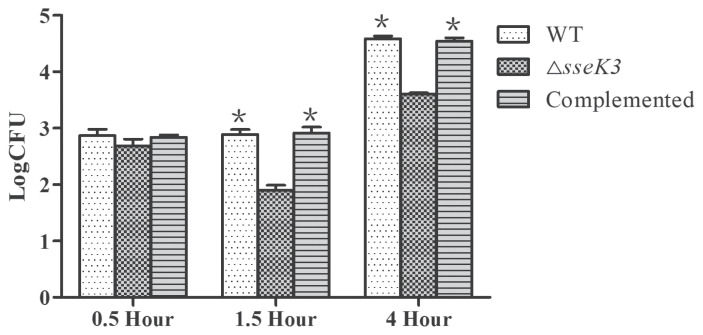
Survival and replication in cells are well-established as important virulence factors of S. Typhimurium. In the intracellular proliferation process, ΔsseK3 exhibited proliferation characteristics in mouse peritoneal macrophages that differed from those of the WT strain (Figure 5). The ΔsseK3 mutant load was decreased by 0.71 logs over 1.5 h, but increased by 1.54 logs over 4 h. The WT and sseK3-complemented load was increased by 1.69 logs over 4 h. These data indicate that sseK3 is necessary for intracellular survival and replication of S. Typhimurium.
Figure 5.
Analysis of intracellular survival and replication of wild-type (WT), ΔsseK3 mutant, and sseK3-complemented strain in macrophages. The WT, ΔsseK3 mutant, and sseK3-complemented strains were coincubated with cells and the number of bacteria was counted at 0.5 h, 1.5 h, and 4 h. Data for all groups are presented as mean ± SD. Statistical significance was calculated using ANOVA (Bonferroni’s multiple-comparison test). The asterisk (*) indicates a statistically significant difference among the WT, ΔsseK3, and complemented strains at 4 h compared with 1.5 h (*P < 0.05).
Virulence of ΔsseK3 Salmonella mutant is attenuated in vivo
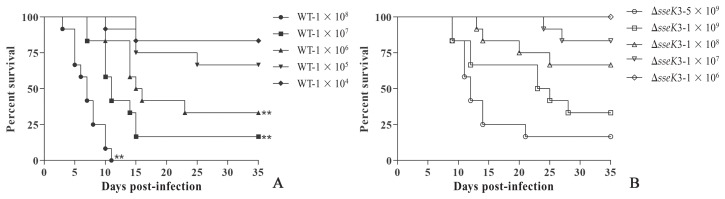
A mouse model of infection was established to determine whether sseK3 deletion alters the virulence characteristics of S. Typhimurium in vivo, whereby differences in virulence among WT, ΔsseK3, and the sseK3-complemented strains were measured using 6-wk-old BALB/c mice. The results of this experiment are shown in Figure 6. The ΔsseK3 Salmonella mutant is attenuated in mice, with all the mice surviving in the 1 × 106 group and 33.33% mortality in mice infected with 1 × 108 CFUs of the ΔsseK3 Salmonella mutant. In contrast, 100% mortality was seen in the mice infected with 1 × 108 CFUs and mortality was 66.67% in mice infected with 1 × 106 CFUs of WT, which indicates a significant attenuation in the ΔsseK3 Salmonella mutant strain compared to the WT strain. Furthermore, the LD50 of the ΔsseK3 strain and WT strain was 2.96 × 108 and 2.86 × 105 CFUs, respectively.
Figure 6.
Percent survival of mice infected with wild-type (WT) (A) or ΔsseK3 mutant strain (B). The mice were inoculated by intraperitoneal injection and mortality was monitored over 5 wk (n = 12/group). Kaplan-Meier survival curves of mice infected with WT or ΔsseK3 mutant strain. Data were analyzed using the log-rank test. P-values for 1 × 106, 1 × 107, and × 108 were **P < 0.01 when comparing the WT to the ΔsseK3 mutant.
Discussion
Many secreted proteins of Salmonella exert different virulence functions (5). Salmonella pathogenicity island-2 (SPI-2) encodes type-III secretion system 2 (T3SS2), which helps Salmonella transport virulence (effector) proteins in Salmonella-containing vacuoles (SCVs) into host cells (34). Salmonella Typhimurium possesses the SseK family of proteins, including SseK2 (STM2137), SseK1 (STM4157), and SseK3 (SB26), which are T3SS effectors. SseK3 is an important protein during Salmonella infection of cells (35). One study identified SseK3-targeted host proteins and found that SseK3 binds to the host E3 ubiquitin ligase, TRIM32, and inhibits the nuclear factor-KappaB (NF-κB) signalling pathway. SseK1 and SseK3 block the NF-κB pathway by modifying the substrate specificities of effectors (35,36). These results indicate that, while SseK3 plays a role in the natural host immune process, the mechanism of action of SseK3 in the virulence of Salmonella in host cells is unclear.
To determine the role of the sseK3 gene in the virulence of S. Typhimurium, we therefore constructed an ΔsseK3 mutant and assessed the virulence of the WT, ΔsseK3 mutant, and sseK3-complemented strains. Biofilm formation is essential for proliferation in host cells and is an essential factor in the virulence of S. Typhimurium (33,37,38). The biofilm formation assay results showed a significant reduction in biofilm formation between the ΔsseK3 mutant and WT, although biofilm formation was restored in the sseK3-complemented strain (Figure 3). This result indicates that sseK3 is necessary for the formation of S. Typhimurium biofilms. Because biofilms are associated with virulence (39–41), sseK3 is therefore associated with the virulence of S. Typhimurium virulence. We did not observe any statistically significant difference in adhesion to and invasion of HeLa cells between the ΔsseK3 mutant and the WT strain, however, which indicates that Salmonella invasion and adhesion are not controlled by sseK3, at least in vitro. This result indicates that sseK3 may not play an important role in the adherence to and invasion of S. Typhimurium in HeLa cells. However, the ΔsseK3 mutant showed significantly lower intracellular proliferation than WT in the macrophages.
The apparent discrepancy between the effect of sseK3 deletion on biofilm formation and its lack of effect on invasion and adhesion may be because sseK3 is located in SPI-2 (42). A major function of SPI-2 is to enable intracellular bacterial replication, whereas the principal role of the SPI-1 encoded secretion system is to facilitate the bacterial invasion of epithelial cells (43). After the ΔsseK3 mutant strains enter a host cell, T3SS2 cannot secrete the SseK3 protein, which may affect virulence and inhibit proliferation within cells.
Furthermore, previous reports have shown that SseK3 is an arginine glycosyltransferase that modifies a conserved arginine residue in the death domain of several mammalian immune signalling proteins (11,34). Through this mechanism, sseK3 may disrupt formation of the death receptor signalling complex during cell death, thereby blocking the transmission of host death receptor signalling and promoting the colonization of S. Typhimurium in the intestine (44). Thus, sseK3 plays an important role in S. Typhimurium in cells. In addition, the LD50 of the ΔsseK3 mutant was 1.035 × 103 times lower than that of the WT strain in the mouse experimental model. This indicates that the sseK3 gene is essential for virulence of S. Typhimurium.
In summary, we constructed an sseK3 mutant of S. Typhimurium and the lack of sseK3 affected the pathogenicity of S. Typhimurium by attenuating the intracellular proliferation and reducing the virulence in vitro and in vivo. These results show that the sseK3 gene is essential for maintaining intracellular proliferation and virulence of S. Typhimurium. This research provides a new and effective avenue for exploring attenuated Salmonella candidate strains.
Acknowledgments
The authors thank Roy Curtiss III at the Department of Biology, Washington University, St. Louis, Missouri, USA for generously donating strains and plasmids for use in this study. The study was funded by grants from the National Natural Science Foundation of Henan (182300410078), National Natural Science Foundation of China (31572489 and 31802159), Henan Science and Technology Key Project (182102110061), and the PhD Start-up Fund of Henan University of Science and Technology (13480071) in Luoyang, China.
References
- 1.Hendriksen RS, Mikoleit M, Carlson VP, et al. WHO Global Salm-Surv external quality assurance system for serotyping of Salmonella isolates from 2000 to 2007. J Clin Microbiol. 2009;47:2729–2736. doi: 10.1128/JCM.02437-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsolis RM, Kingsley RA, Townsend SM, Ficht TA, Adams LG, Bäumler AJ. Of mice, calves, and men. Comparison of the mouse typhoid model with other Salmonella infections. Adv Exp Med Biol. 1999;473:261–274. [PubMed] [Google Scholar]
- 3.Takeuchi A. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella Typhimurium. Am J Pathol. 1967;50:109–136. [PMC free article] [PubMed] [Google Scholar]
- 4.Wang M, Feng P, Chen X, et al. YgaE regulates out membrane proteins in Salmonella enterica serovar Typhi under hyperosmotic stress. Sci World J. 2014;2014 doi: 10.1155/2014/374276. 374276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ibarra JA, Steele-Mortimer O. Salmonella — the ultimate insider. Salmonella virulence factors that modulate intracellular survival. Cell Microbiol. 2009;11:1579–1586. doi: 10.1111/j.1462-5822.2009.01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jennings E, Thurston TLM, Holden DW. Salmonella SPI-2 type III secretion system effectors: Molecular mechanisms and physiological consequences. Cell Host Microbe. 2017;22:217–231. doi: 10.1016/j.chom.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 7.LaRock DL, Chaudhary A, Miller SI. Salmonellae interactions with host processes. Nat Rev Microbiol. 2015;13:191–205. doi: 10.1038/nrmicro3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kujat Choy SL, Boyle EC, Gal-Mor O, et al. SseK1 and SseK2 are novel translocated proteins of Salmonella enterica serovar Typhimurium. Infect Immun. 2004;72:5115–5125. doi: 10.1128/IAI.72.9.5115-5125.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown NF, Coombes BK, Bishop JL, et al. Salmonella phage ST64B encodes a member of the SseK/NleB effector family. PLoS One. 2011;6:e17824. doi: 10.1371/journal.pone.0017824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kidwai AS, Mushamiri I, Niemann GS, Brown RN, Adkins JN, Heffron F. Diverse secreted effectors are required for Salmonella persistence in a mouse infection model. PLoS One. 2013;8:e70753. doi: 10.1371/journal.pone.0070753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S, Zhang L, Yao Q, et al. Pathogen blocks host death receptor signalling by arginine GlcNAcylation of death domains. Nature. 2013;501:242–246. doi: 10.1038/nature12436. [DOI] [PubMed] [Google Scholar]
- 12.Chen S, Zhang C, Liao C, et al. Deletion of invasion protein B in Salmonella enterica serovar Typhimurium influences bacterial invasion and virulence. Curr Microbiol. 2015;71:687–692. doi: 10.1007/s00284-015-0903-x. [DOI] [PubMed] [Google Scholar]
- 13.Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella Typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 14.Edwards RA, Keller LH, Schifferli DM. Improved allelic exchange vectors and their use to analyze 987P fimbria gene expression. Gene. 1998;207:149–157. [Google Scholar]
- 15.Dong H, Peng D, Jiao X, Zhang X, Geng S, Liu X. Roles of the spiA gene from Salmonella enteritidis in biofilm formation and virulence. Microbiology. 2011;157:1798–1805. doi: 10.1099/mic.0.046185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang HY, Dozois CM, Tinge SA, Lee TH, Curtiss R., 3rd Transduction-mediated transfer of unmarked deletion and point mutations through use of counterselectable suicide vectors. J Bacteriol. 2002;184:307–312. doi: 10.1128/JB.184.1.307-312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu C, Meng X, Duan X, et al. SEF14 fimbriae from Salmonella enteritidis play a role in pathogenitic to cell model in vitro and host in vivo. Microb Pathog. 2013;64:18–22. doi: 10.1016/j.micpath.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Balbás P, Soberón X, Merino E, et al. Plasmid vector pBR322 and its special-purpose derivatives — A review. Gene. 1986;50:3–40. doi: 10.1016/0378-1119(86)90307-0. [DOI] [PubMed] [Google Scholar]
- 19.Anriany Y, Sahu SN, Wessels KR, McCann LM, Joseph SW. Alteration of the rugose phenotype in waaG and ddhC mutants of Salmonella enterica serovar Typhimurium DT104 is associated with inverse production of curli and cellulose. Appl Environ Microbiol. 2006;72:5002–5012. doi: 10.1128/AEM.02868-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y, Yu C, Ding K, et al. Role of the sseK1 gene in the pathogenicity of Salmonella enterica serovar enteritidis in vitro and in vivo. Microb Pathog. 2018;117:270–275. doi: 10.1016/j.micpath.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 21.Shippy DC, Eakley NM, Bochsler PN, Chopra AK, Fadl AA. Biological and virulence characteristics of Salmonella enterica serovar Typhimurium following deletion of glucose-inhibited division (gidA) gene. Microb Pathog. 2011;50:303–313. doi: 10.1016/j.micpath.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Shippy DC, Eakley NM, Mikheil DM, Fadl AA. Role of the flagellar basal-body protein, FlgC, in the binding of Salmonella enterica serovar Enteritidis to host cells. Curr Microbiol. 2014;68:62–628. doi: 10.1007/s00284-014-0521-z. [DOI] [PubMed] [Google Scholar]
- 23.He Y, Xu T, Fossheim LE, Zhang XH. FliC, a flagellin protein, is essential for the growth and virulence of fish pathogen Edwardsiella tarda. PLoS One. 2012;7:e45070. doi: 10.1371/journal.pone.0045070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchmeier NA, Heffron F. Intracellular survival of wild-type Salmonella Typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect Immun. 1989;57:1–7. doi: 10.1128/iai.57.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Best A, Price C, Ozanic M, Santic M, Jones S, Kwaik YA. A Legionella pneumophila amylase is essential for intracellular replication in human macrophages and amoebae. Sci Rep. 2018;8:6340. doi: 10.1038/s41598-018-24724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehrt S, Shiloh MU, Ruan J, et al. A novel antioxidant gene from Mycobacterium tuberculosis. J Exp Med. 1997;186:1885–1896. doi: 10.1084/jem.186.11.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toth LA, Kregel K, Leon L, Musch TI. Environmental enrichment of laboratory rodents: The answer depends on the question. Comp Med. 2011;61:314–321. [PMC free article] [PubMed] [Google Scholar]
- 28.van der Velden AW, Bäumler AJ, Tsolis RM, Heffron F. Multiple fimbrial adhesins are required for full virulence of Salmonella Typhimurium in mice. Infect Immun. 1998;66:2803–2808. doi: 10.1128/iai.66.6.2803-2808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mezal EH, Bae D, Khan AA. Detection and functionality of the CdtB, PltA, and PltB from Salmonella enterica serovar Javiana. Pathog Dis. 2014;72:95–103. doi: 10.1111/2049-632X.12191. [DOI] [PubMed] [Google Scholar]
- 30.Reyrat JM, Pelicic V, Gicquel B, Rappuoli R. Counterselectable markers: Untapped tools for bacterial genetics and pathogenesis. Infect Immun. 1998;66:4011–4017. doi: 10.1128/iai.66.9.4011-4017.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zogaj X, Nimtz M, Rohde M, Bokranz W, Römling U. The multicellular morphotypes of Salmonella Typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol Microbiol. 2010;39:1452–1463. doi: 10.1046/j.1365-2958.2001.02337.x. [DOI] [PubMed] [Google Scholar]
- 32.Abshire KZ, Neidhardt FC. Growth rate paradox of Salmonella Typhimurium within host macrophages. J Bacteriol. 1993;175:3744–3748. doi: 10.1128/jb.175.12.3744-3748.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakimoto N, Nishi J, Sheikh J, et al. Quantitative biofilm assay using a microtiter plate to screen for enteroaggregative Escherichia coli. Am J Trop Med Hyg. 2004;71:687–690. [PubMed] [Google Scholar]
- 34.Günster RA, Matthews SA, Holden DW, Thurston TLM. SseK1 and SseK3 type III secretion system effectors inhibit NF-κB signalling and necroptotic cell death in Salmonella-infected macrophages. Infect Immun. 2017;85:e00010–17. doi: 10.1128/IAI.00010-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Z, Soderholm A, Lung TW, et al. SseK3 is a Salmonella effector that binds TRIM32 and modulates the host’s NF-κB signalling activity. PLoS One. 2015;10:e0138529. doi: 10.1371/journal.pone.0138529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hueffer K, Galán J. Salmonella-induced macrophage death: Multiple mechanisms, different outcomes. Cell Microbiol. 2004;6:1019–1025. doi: 10.1111/j.1462-5822.2004.00451.x. [DOI] [PubMed] [Google Scholar]
- 37.Donlan RM, Costerton JW. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Wang Y, Sun L, Grenier D, Yi L. Streptococcus suis biofilm: Regulation, drug-resistance mechanisms, and disinfection strategies. Appl Microbiol Biotechnol. 2018;102:9121–9129. doi: 10.1007/s00253-018-9356-z. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez-Escobedo G, Gunn JS. Identification of Salmonella enterica serovar Typhimurium genes regulated during biofilm formation on cholesterol gallstone surfaces. Infect Immun. 2013;81:3770–3780. doi: 10.1128/IAI.00647-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez-Escobedo G, Marshall JM, Gunn JS. Chronic and acute infection of the gall bladder by Salmonella Typhi: Understanding the carrier state. Nat Rev Microbiol. 2011;9:9–14. doi: 10.1038/nrmicro2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joseph B, Otta SK, Karunasagar I, Karunasagar I. Biofilm formation by Salmonella spp. on food contact surfaces and their sensitivity to sanitizers. Int J Food Microbiol. 2001;64:367–372. doi: 10.1016/s0168-1605(00)00466-9. [DOI] [PubMed] [Google Scholar]
- 42.Lawley TD, Chan K, Thompson LJ, Kim CC, Govoni GR, Monack DM. Genome-wide screen for Salmonella genes required for long-term systemic infection of the mouse. PloS Pathog. 2006;2:e11. doi: 10.1371/journal.ppat.0020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Nhieu GT, Romero S. Common themes in cytoskeletal remodeling by intracellular bacterial effectors. 2017;235:207–235. doi: 10.1007/164_2016_42. [DOI] [PubMed] [Google Scholar]
- 44.Esposito D, Günster RA, Martino L, et al. Structural basis for the glycosyltransferase activity of the Salmonella effector SseK3. J Biol Chem. 2018;293:5064–5078. doi: 10.1074/jbc.RA118.001796. [DOI] [PMC free article] [PubMed] [Google Scholar]