Abstract
Recent studies have found that anemia and anisocytosis are precipitating factors for certain heart diseases in dogs. This study evaluated the prevalence and correlation of anemia and red blood cell distribution width (RDW) in dogs with heartworm disease (HWD). The study population consisted of 20 healthy control dogs and 86 dogs with HWD: 28 dogs with no clinical signs or pulmonary hypertension (Group 1), 42 dogs with mild clinical signs but no pulmonary hypertension (Group 2), and 16 dogs with severe clinical signs and pulmonary hypertension (Group 3). Along with echocardiographic interrogation of pulmonary hypertension, red blood cell (RBC) profiles were evaluated, including RDW. The total number of red blood cells (tRBCs), hematocrit (HCT), and hemoglobin (HGB) concentration was significantly lower in Group 3 dogs compared to control dogs (P < 0.05), while the RDW was significantly higher in Group 3 dogs than in control dogs (P < 0.05). The RDW was closely correlated to other RBC profiles and the presence of pulmonary hypertension (P < 0.05). The severity of tricuspid regurgitant gradient (TRG) was closely correlated with Hb and tRBC (P < 0.05), but not with the RDW and reticulocyte count. This study finding indicated that anemia and anisocytosis are common complications in dogs with severe clinical signs and pulmonary hypertension caused by heartworm disease (HWD). It would therefore be beneficial for clinicians to routinely check red blood cell (RBC) profiles, including RDW, in order to monitor the progression of heartworm disease in dogs.
Résumé
Des études récentes ont montré que l’anémie et l’anisocytose sont des facteurs précipitants pour certaines conditions cardiaques chez les chiens. La présente étude a évalué la prévalence et la corrélation de l’anémie et de la distribution de la largeur des globules rouges (RDW) chez des chiens avec la maladie des vers du coeur (HWD). La population à l’étude consistait en 20 chiens témoins en santé et de 86 chiens avec HWD : 28 chiens sans signe clinique ou hypertension pulmonaire (Groupe 1), 42 chiens avec signes cliniques légers mais sans hypertension pulmonaire (Groupe 2) et 16 chiens avec signes cliniques sévères et hypertension pulmonaire (Groupe 3). En plus de l’interrogation échocardiographique de l’hypertension pulmonaire, les profils des globules rouges (RBC) furent évalués, incluant la RWD. Le nombre total de globules rouges (tRBCs), l’hématocrite (HCT) et la concentration en hémoglobine (Hb) étaient significativement plus bas chez les chiens du groupe 3 comparativement aux chiens témoins (P < 0,05) alors que la RWD était significativement plus élevée chez les chiens du Groupe 3 que chez les chiens témoins (P < 0,05). La RWD était étroitement corrélée à d’autres profiles de RBC et à la présence d’hypertension pulmonaire (P < 0,05). La sévérité du gradient de régurgitation de la tricuspide (TRG) était étroitement corrélée avec Hb et tRBC (P < 0,05), mais pas avec RWD et le dénombrement de réticulocytes. Les trouvailles de cette étude indiquent que l’anémie et l’anisocytose sont des complications fréquentes chez les chiens avec des signes cliniques sévères et de l’hypertension pulmonaire causés par HWD. Il serait donc bénéfique pour les cliniciens de vérifier de routine les profiles de RBC, incluant RWD, afin de suivre la progression de la maladie des vers du coeur chez les chiens.
(Traduit par Docteur Serge Messier)
Introduction
Heartworm disease (HWD) is the most common infectious heart disease in dogs (1). Clinical signs are generally related to cardiovascular and pulmonary diseases and are largely dependent on worm-burden, host-immune reaction to the clearance of dead worms, duration of infection, and ability to restrict exercise. Anemia and leukocytosis with eosinophilia are common hematological abnormalities in dogs with severe clinical signs of HWD. Recent studies have found that anemia and anisocytosis are precipitating factors for heart failure in humans (2–4). Two recent canine studies also demonstrated an increased prevalence of anemia in dogs with chronic mitral valve insufficiency (CMVI) (5) and that anemia was a significant prognostic factor for mortality in dogs with CMVI (6).
Red blood cell distribution width (RDW) is part of the red blood cell (RBC) profile that indicates the variability (heterogeneity) of RBC volume/size (anisocytosis) (7). It is widely used to differentiate between regenerative anemia and non-regenerative anemia (8). Although reference ranges for RDW in blood analyzers differ widely by manufacturers (9), several human studies have found that RDW was closely associated with heart failure (2,3). Several other human studies have also demonstrated that the degree of anisocytosis was closely and independently associated with clinical outcomes in patients with heart diseases (2,3,10,11). Although elevated RDW is generally associated with nutritional deficiencies, such as iron, folate, or vitamin B12 deficiency, and hemolytic anemia, recent veterinary studies found that RDW levels were weakly to strongly associated with certain heart diseases in dogs and cats (12,13).
Furthermore, a recent canine study found that RDW was significantly greater in dogs with pre-capillary pulmonary hypertension, i.e., CMVI, compared to control dogs, although the RDW was not associated with survival in this study population (14). In contrast, another canine study found that RDW in dogs with pre-capillary and post-capillary pulmonary hypertension, e.g., congenital shunts or pulmonary diseases, was significantly higher than in healthy dogs, although only dogs with severe pulmonary hypertension had significantly increased RDW compared to dogs without it (15).
Because hypoxia-mediated pulmonary hypertension and hemolytic anemia have been well-documented in dogs with heartworm disease (1,16), the authors expected that the degree of anemia and anisocytosis might play a key role in the progression of the disease. This study therefore evaluated the prevalence and correlation of anemia and RDW in dogs with heartworm disease.
Materials and methods
Study population
Approval was given by the animal ethics committee of Kangwon National University before study initiation. The owner’s consent for each dog to participate was obtained before enrollment in the study. A total of 86 dogs with HWD and 20 healthy control dogs were enrolled in this study. The heartworm infection was diagnosed using a commercial enzyme-linked immunosorbent assay (ELISA) kit (SNAP 4Dx Plus Test; IDEXX Laboratory, Westbrook, Maine, USA), according to the manufacturer’s instructions. Control dogs were selected from the screening colony (150 client-owned dogs) and had no evidence of systemic diseases of pulmonary hypertension, i.e., no detectible tricuspid regurgitation or pulmonary regurgitation jets, with right ventricular outflow tract AT (acceleration time) of > 64 ms and AT/ET (acceleration time/ejection time) of ≥ 0.42 on routine laboratory tests and cardiology exams, including echocardiography. The dogs with HWD were sub-divided into 3 groups: Group 1 — asymptomatic and no echocardiographic evidence of pulmonary hypertension; Group 2 — mild clinical signs, e.g., occasional coughing with or without exercise intolerance, but no echocardiographic evidence of pulmonary hypertension; and Group 3 — severe clinical signs and echocardiographic evidence of moderate to severe pulmonary hypertension, i.e., > 50 mmHg systolic pulmonary arterial pressure.
Measurement of echocardiographic markers
Echocardiographic examinations were conducted in accordance with recommended standards for dogs using an ultrasound machine (Acuson X-300; Siemens, Munich, Germany) with a 3–9 MHz cardiac probe. For echocardiographic assessment, pulsed wave-spectra signals for calculating pulmonary arterial flow of ejection time (ET) and acceleration time (AT) were acquired from the right parasternal short-axis view of the pulmonary artery (PA), with the sample volume at the valve level. Continuous wave (CW)-spectra of tricuspid regurgitation (TR), when present, were acquired from the left apical cranial 2-chamber views. The AT and AT/ET were measured off-line by a single observer (Hyun) blinded to dog identification, clinical data, and radiographic results, from the recorded echocardiography data. Tricuspid regurgitant gradient (TRG; ΔP) was calculated by applying the modified Bernoulli equation (ΔP = 4 × velocity2) to the peak velocity of tricuspid regurgitation (TR) jets. Systolic pulmonary arterial pressure (sPAP) was calculated by adding the systolic pressure gradient to the estimated right atrial pressure (ΔP+π right atrium). The estimated right atrial pressure (RAP) was 5 mmHg in dogs without any evidence of right atrial (RA) dilation; 10 mmHg in dogs with evidence of RA dilation, but no signs of right-sided heart failure (R-HF); and 15 mmHg in dogs with evidence of RA dilation and clinical signs of R-HF.
Measurement of red blood cell profiles and urinalysis
Blood samples were collected from either jugular or cephalic vein in EDTA-blood tubes. Red blood cell (RBC) profiles were measured using an automated blood cell counter (ProCyte Dx Hematology Analyzer; IDEXX). Tested RBC profiles were total number of red blood cells (tRBC), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and red blood cell distribution width-corpuscular volume (RDW-CV), along with total number of white blood cells (tWBC). The RDW-CV was calculated by dividing the standard deviation of the mean cell size by the MCV of the red cells and multiplying by 100 to convert to a percentage (7). Urine samples were obtained by cystocentesis for evaluating occult blood in the urine using a urine test strip (Clinistix; Bayer Diagnostic, Leverkusen, Germany).
Statistical analysis
Statistical analyses were done using commercially available statistical software (MedCalc 12.1.3.0 for Windows; Ostend, Belgium). Descriptive statistics were calculated for quantitative variables by study group and analyzed for normality using the Kolmogorov-Smirnov test. Data are reported as the mean ± standard deviation (SD). Differences in continuous data among study groups were determined by the 1-way analysis of variance (ANOVA) test. When the factors were significant, a post-hoc test (Scheffé’s test) with a Bonferroni correction was applied. Associations between continuous variables and RBC profiles were investigated by Pearson’s coefficient correlation. The level of significance was set at P < 0.05.
Results
Characteristics of study population
Mean ± SDs of age and body weight of each group were 5.4 ± 2.1 y and 10.5 ± 5.4 kg in control (n = 20); 4.3 ± 1.5 y and 20.2 ± 7.6 kg in Group 1 (n = 28); 4.5 ± 2.9 y and 17.2 ± 10.7 kg in Group 2 (n = 42), and 5.6 ± 3.5 y and 15.9 ± 10.5 kg in Group 3 (n = 16), respectively. Male was the predominant sex in all groups (16:4 in control, 21:7 in Group 1, 30:12 in Group 2, and 9:7 in Group 3). This is shown in Table I.
Table I.
Red blood cell profiles in this study population.
| Control (n = 20) | Group 1 (n = 28) | Group 2 (n = 42) | Group 3 (n = 16) | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Sex (M/F) | 16/4 | 21/7 | 30/12 | 9/7 | ||||
| Age (y) | 5.4 | 2.1 | 4.3 | 1.5 | 4.5 | 2.9 | 5.6 | 3.5 |
| BW (kg) | 10.5 | 5.4 | 20.2 | 7.6 | 17.2 | 10.7 | 15.9 | 10.5 |
| HCT (%) | 47.68 | 5.20 | 46.16 | 4.94 | 41.89 | 11.01 | 33.65* | 9.59 |
| HGB (g/dL) | 16.49 | 1.35 | 15.79 | 1.55 | 14.56 | 3.92 | 12.05* | 3.23 |
| MCH (pg) | 21.53 | 1.67 | 20.92 | 1.60 | 20.23 | 2.19 | 21.15 | 3.72 |
| MCHC (g/dL) | 34.71 | 1.53 | 34.32 | 2.02 | 34.74 | 2.05 | 36.03 | 1.82 |
| MCV (fL) | 62.08 | 4.57 | 61.21 | 5.98 | 57.55 | 6.33 | 58.70 | 10.28 |
| tRBC (M/μL) | 7.71 | 0.86 | 7.60 | 1.05 | 7.76 | 1.43 | 5.95* | 2.16 |
| RDW-CV | 18.39 | 1.93 | 19.00 | 2.09 | 19.86 | 3.57 | 20.23* | 2.57 |
| tWBC (k/μL) | 13.99 | 4.92 | 16.37 | 3.56 | 12.57 | 3.55 | 18.69* | 10.43 |
BW — body weight; HCT — hematocrit; HGB — hemoglobin; MCH — mean corpuscular hemoglobin; MCHC — mean corpuscular hemoglobin concentration; MCV — mean corpuscular volume; tRBC — total number of red blood cells; RDW-CV — red blood cell distribution width-corpuscular volume; tWBC — total number of white blood cells.
P < 0.05 in control vs groups with heartworm disease (HWD). No statistical difference among groups with HWD.
Prevalence and association of RBC profiles and occult blood in urine with stage of heartworm disease
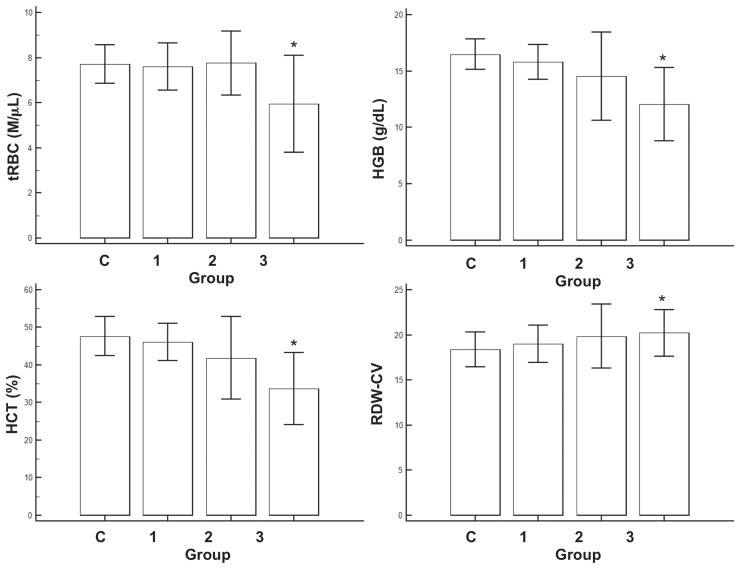
The number of dogs with anemia (either < 5.5 M/μL of tRBC, < 37% HCT) was 0/20 (0%) in control; 2/28 (8%) in Group 1; 6/42 (14%) in Group 2; and 14/16 (88%) in Group 3, respectively, while the number of dogs with RDW-CV higher than reference range was 0/20 (0%) in control; 2/28 (7%) in Group 1; 8/42 (19%) in Group 2; and 4/16 (25%) in Group 3, respectively (Table I and Figure 1). The tRBC and HCT levels were significantly lower in Group 3 compared to control and RDW-CV was significantly higher in Group 3 compared to control (P < 0.05; Table I and Figure 1). The tRBC was loosely but significantly correlated to HCT (R = 0.527), HGB (R = 0.541), MCV (R = −0.454), MCH (R = −0.466), RDW-CV (R = 0.423), and the presence of pulmonary hypertension (R = −0.256; P < 0.05, Table II), while RDW-CV was correlated to MCH (R = −0.555), MCV (R = −0.527), RBC (R = 0.423), and the presence of pulmonary hypertension (R = 0.225; P < 0.05, Table II).
Figure 1.
Mean and standard deviation of total number of red blood cells (tRBC), hematocrit (HCT), hemoglobin (HGB), and red blood cell distribution width-corpuscular volume (RDW-CV) in this study population.
* P < 0.05 in control versus groups with different severity of heartworm disease. No statistically significant difference was found among dogs with different severity of heartworm disease.
Table II.
Correlation of red blood cell profiles and severity of heartworm in this study.
| HCT | HGB | MCH | MCHC | MCV | tRBC | RDW-CV | HWD | tWBC | ||
|---|---|---|---|---|---|---|---|---|---|---|
| HCT | R | 0.968 | −0.233 | −0.233 | −0.045 | 0.527 | 0.188 | −0.442 | −0.017 | |
| P | < 0.0001 | 0.0161 | 0.0162 | 0.6499 | < 0.0001 | 0.0531 | < 0.0001 | 0.8616 | ||
| HGB | R | 0.968 | −0.186 | 0.004 | −0.138 | 0.541 | 0.193 | −0.413 | 0.011 | |
| P | < 0.0001 | 0.0566 | 0.9662 | 0.1580 | < 0.0001 | 0.0474 | < 0.0001 | 0.9103 | ||
| MCH | R | −0.233 | −0.186 | 0.260 | 0.804 | −0.466 | −0.555 | −0.117 | −0.030 | |
| P | 0.0161 | 0.0566 | 0.0071 | < 0.0001 | < 0.0001 | < 0.0001 | 0.2343 | 0.7570 | ||
| MCHC | R | −0.233 | 0.004 | 0.260 | −0.304 | −0.112 | −0.029 | 0.189 | 0.187 | |
| P | 0.0162 | 0.9662 | 0.0071 | 0.0015 | 0.2511 | 0.7667 | 0.0522 | 0.0551 | ||
| MCV | R | −0.045 | −0.138 | 0.804 | −0.304 | −0.454 | −0.527 | −0.231 | −0.102 | |
| P | 0.6499 | 0.1580 | < 0.0001 | 0.0015 | < 0.0001 | < 0.0001 | 0.0173 | 0.2976 | ||
| tRBC | R | 0.527 | 0.541 | −0.466 | −0.112 | −0.454 | 0.423 | −0.256 | −0.166 | |
| P | < 0.0001 | < 0.0001 | < 0.0001 | 0.2511 | < 0.0001 | < 0.0001 | 0.0081 | 0.0884 | ||
| RDW-CV | R | 0.188 | 0.193 | −0.555 | −0.029 | −0.527 | 0.423 | 0.225 | −0.018 | |
| P | 0.0531 | 0.0474 | < 0.0001 | 0.7667 | < 0.0001 | < 0.0001 | 0.0204 | 0.8578 | ||
| HWD | R | −0.442 | −0.413 | −0.117 | 0.189 | −0.231 | −0.256 | 0.225 | 0.084 | |
| P | < 0.0001 | < 0.0001 | 0.2343 | 0.0522 | 0.0173 | 0.0081 | 0.0204 | 0.3944 | ||
| tWBC | R | −0.017 | 0.011 | −0.030 | 0.187 | −0.102 | −0.166 | −0.018 | 0.084 | |
| P | 0.8616 | 0.9103 | 0.7570 | 0.0551 | 0.2976 | 0.0884 | 0.8578 | 0.3944 |
HCT — hematocrit; HGB — hemoglobin; MCH — mean corpuscular hemoglobin; MCHC — mean corpuscular hemoglobin concentration; MCV — mean corpuscular volume; tRBC — total number of red blood cells; RDW-CV — red blood cell distribution width-corpuscular volume; HWD — heartworm disease (severity of); tWBC — total number of white blood cells.
Bold — P < 0.05.
The number of dogs with hypohemoglobinemia (< 13.1 g/dL) was 0/20 (0%) in control, 0/28 (0%) in Group 1, 6/42 (14%) in Group 2, and 14/16 (88%) in Group 3, respectively (Table I). The hemoglobin concentration was significantly lower in Group 3 than in control (P < 0.05; Table I). The hemoglobin concentration was significantly correlated to other hematological parameters and the severity of heartworm disease (Table II). Mean ± SDs of MCV, MCH, and MCHC are summarized in Table I, but were not significantly different among study groups (P > 0.05; Figure 1).
The number of dogs with positive occult blood in the urine anemia was 0/20 (0%) in control, 0/28 (0%) in Group 1, 8/42 (19%) in Group 2, and 14/16 (88%) in Group 3, respectively. All dogs with hypohemoglobinemia were positive on the occult blood in the urine strip test.
Association of RBC profiles and TRG in Group 3
Tricuspid regurgitant gradient (TRG) was closely correlated with Hb (R = 0.6661, P = 0.0180), MCHC (R = 0.5961, P = 0.0408), and tRBC (R = 0.6144, P = 0.0335) and weakly correlated with PCV (R = 0.5470, P = 0.06657). However, TRG was not correlated with RDW (R = −0.1185, P = 0.7137) and MCHC (R = 0.1817, P = 0.95553). The number of reticulocytes was also not correlated with TRG (R = −0.1773, P = 0.5816, data not shown here).
Discussion
The most common hematological abnormalities in dogs with heartworm disease (HWD) are mild nonregenerative anemia, neutrophilia, eosinophilia, basophilia, and thrombocytopenia (1). Hemolytic anemia and hemoglobinuria are common findings in dogs with heavy worm burden (caval syndrome) and traumatic destruction of red blood cells as they pass through the worm mass (1,16). Several retrospective studies found mild-to-moderate anemia in micro-filaremic dogs (17–19). Several studies found higher prevalence of anemia in human (20) and canine (5) heart diseases. Human studies also found that anemia was a precipitating factor for deteriorating clinical signs in heart disease (21,22).
In this study, the overall prevalence of anemia was 25.6% in dogs with HWD and this was higher in dogs with more severe clinical signs (88% in Group 3). Two recent veterinary studies demonstrated the prevalence of anemia to be 14.5% (5) and 15.3% (23) in dogs with chronic mitral valve insufficiency (CMVI). Anemia was also increased in dogs with CMVI that were at the most advanced stage of heart failure (42%) (23), although one recent canine study failed to reveal the correlation between anemia and severity of heart failure (6). Although the pathological mechanism of anemia in dogs with HWD might be different than in dogs with other heart diseases, such as CMVI, the overall prevalence of anemia in dogs with severe HWD was similar to that in dogs at an advanced stage of heart failure. Since one study suggested a pathological link between the pathogenesis of heart failure and anemia (22), this mechanism, other than destroying RBCs and leading to pulmonary hypertension, could aggravate the severity of anemia in dogs seriously infected with HWD.
Most dogs with anemia in this study population were regenerative, which suggests that intravascular hemolysis might be a major contributor to anemia (data not shown here), although hypoxia-mediated pulmonary hypertension might complicate the development of anemia by increasing RBC production. Calvert and Rawlings (24) found that low-grade, nonregenerative anemia was common in dogs with HWD, with 10% of mildly to moderately affected dogs and up to 60% of severely affected dogs developing the condition. In this study, however, regenerative anemia was more common in dogs with severe HWD (~75%, data not shown here). If the dogs in this study had visited the clinic earlier, before the diminished regenerative response to anemia, this might have explained the discrepancy from the previous study (24).
Three major mechanisms that are involved in the development of anemia in dogs are hemorrhage (loss), hemolysis (decreased lifespan), and decreased production, e.g., chronic kidney disease and chronic inflammatory disease (25). Unlike other causes of anemia, the regenerative response is more common in hemolytic anemia, although the previous study found that mild nonregenerative anemia was more common in dogs with severe HWD (24). The mechanisms of anemia involved in dogs with HWD are complicated because several pathomechanisms can contribute to the development of anemia, such as intravascular hemolysis, anemia of chronic inflammatory disease, chronic kidney disease from immune-medicated gromerulonephritis, and possibly nutrient deficiency due to anorexia and cardiac chachexia (25).
Several human studies have demonstrated that reduced hemoglobin concentrations are closely associated with hemodynamic function, severity of heart failure, and peak oxygen consumption (26,27). Another veterinary study found that a hemoglobin concentration of < 12.5 g/dL was a predictor of poor outcome in dogs with CMVI (5). In the present study, the mean hemoglobin concentrations also gradually decreased in dogs with heartworm disease and were the lowest in dogs with severe clinical signs (88% in Group 3). Although intravascular hemolysis is the major cause of hypohemoglobinemia (hypoxemia) in dogs with severe HWD, pathological mechanisms other than mechanical destruction of red blood cells (RBCs) are possibly involved in hypohemoglobinemia in dogs with HWD. As shown in the urine occult blood test, all dogs with positive occult blood in the urine had hypohemoglobinemia, which supports the fact that intravascular hemolysis by mechanical destruction of RBCs is responsible for hypohemoglobinemia and hemoglobinuria in this study population.
This study also found that the severity of tricuspid reguritant gradient (TRG) was closely correlated with hemoglobin (Hb) and mean corpuscular hemoglobin concentration (MCHC), which suggests that hypohemoglobinemia could alter the hemodynamics in dogs with severe infection. The RDW and reticulocyte count were not associated with the severity of TRG, which suggests that the regenerative response to anemia and hypohemoglobinemia might not contribute to the alteration of hemodynamics in dogs with severe heartworm disease (Group 3 in this study), although the severity of anemia was closely correlated with TRG. Because most dogs with high TRG had hemolytic anemia due to intravascular destruction of RBCs, further study using dogs with pulmonary hypertension without hemolytic anemia is warranted in order to clarify the effect of RBC profiles on hemodynamics in dogs with HWD.
One study of dogs with CMVI also demonstrated that the mean hemoglobin concentrations were lowered in dogs with a more advanced stage of heart failure (26). Human studies found less favorable outcomes from anemia and hypohemoglobinemia in coronary heart diseases, asymptomatic left ventricular dysfunction (28), and advanced stages of heart failure (26). Since the myocardial hyperdynamic state from persistent anemia and hypohemoglobinemia (hypoxemia) could cause aberrant ventricular hypertrophy and myocardial injuries, persistent anemia and hypohemoglobinemia might contribute to deteriorating clinical signs in dogs with severe heartworm disease.
Increased anisocytosis, which is indicated by increased red blood cell distribution width (RDW), was observed in dogs with CMVI (24) and dogs with pre-capillary and post-capillary pulmonary hypertension (12,14). Heartworm disease is a major cause of post-capillary pulmonary hypertension in dogs (1). Our study was the first to date to demonstrate the elevated level of RDW in dogs with HWD and its close association with the presence and severity of pulmonary hypertension. Similar to our study, another recent study found that dogs with severe pulmonary hypertension had significantly increased RDW levels compared to dogs without pulmonary hypertension (14). Furthermore, our study demonstrated that the RDW level was weakly correlated to most blood cell indices, i.e., total number of red blood cells, hemoglobin, mean corpuscular volume (MCV), and mean corpuscular hemoglobin concentration (MCHC), and the presence of pulmonary hypertension in this study group.
As hemolytic anemia is a major cause of anisocytosis and a common diagnostic finding in dogs with severe HWD, the pathological causes of elevated RDW levels might be complicated by the response to chronic hypoxic injury, i.e., pulmonary hypertension, and the response of rapid RBC generation to intravascular hemolysis in dogs with HWD, as observed in this study. There could therefore be 2 pathological causes of increased RDW levels in dogs with severe clinical signs of heartworm disease and pulmonary hypertension, which consisted of the dogs in Group 3 in this study. Nevertheless, the RDW might be a useful marker of deterioration of clinical signs in dogs with HWD because higher anisocytosis indicated a regenerative response of RBC production against chronic hypoxia, as observed in human studies (20,26–28).
This study had several limitations. First, dogs with heartworm disease and hemolytic anemia but without pulmonary hypertension were not included in this study because of difficulty in recruitment. These dogs might be useful in identifying the major contributor to increased red blood cell distribution width (RDW) in dogs with HWD. Second, RDW was measured by corpuscular volume in this study, although it might be more accurate to directly estimate RDW using a ratio of standard deviation (SD). A validation study with analyzers measuring RDW-SD is therefore warranted to validate our study results. Third, the influence of nutritional deficiencies, such as iron, folate, or vitamin B12, was not determined in this study population. This may not be a substantial influence, however, based on the regenerative response to anemia and the medical history of these mostly client-owned dogs as a result of proper dietary management. Fourth, the levels of hypoxia in this study population were not initially assessed by blood gas analysis to measure partial pressure of oxygen (PO2) or with pulse oximetry to measure peripheral oxygen saturation (SpO2). Levels of SpO2 were monitored in all dogs in Group 3, however, when worms were surgically removed. The actual state of hypoxia at the beginning of the study was still unknown, however, because these dogs were supplied with 100% oxygen during surgery. One human retrospective study also found that pulse oximetry could produce greater errors at low saturations in humans with low Hb concentrations. Further study is warranted using blood gas analysis to evaluate the level of hypoxia in dogs with severe HWD. Finally, the statistical results of this study may be misinterpreted due to the small size of our study. Although we tried to conduct power calculations of the appropriate size of sample population before the study, no known previous studies have evaluated RDW levels in dogs with HWD. Nevertheless, further study is warranted to validate our study findings.
In conclusion, this study evaluated the prevalence and correlation of anemia and red blood cell distribution width (RDW) in dogs with heartworm disease (HWD) and demonstrated that anemia and anisocytosis are more prevalent in these dogs, especially those with severe clinical signs and pulmonary hypertension. The severity of tricuspid regurgitant gradient (TRG) was closely correlated with hemoglobin (Hb) and the total number of red blood cells (tRBCs), but not with the RDW and reticulocyte count. As most dogs with pulmonary hypertension had hemolytic anemia due to intravascular destruction of RBCs, further study is warranted using dogs with pulmonary hypertension without hemolytic anemia to clarify the effect of RBC profiles on hemodynamics in dogs with HWD. Although further study is also warranted to distinguish the pathological mechanisms of anemia and anisocytosis other than mechanical destruction of RBCs, i.e., intravascular hemolysis, in dogs with HWD, the study herein clearly indicated that anisocytosis and anemia are associated with more severe clinical signs and pulmonary hypertension in dogs with HWD. It would therefore be beneficial for clinicians to routinely check red blood cell (RBC) profiles, including RDW, in order to monitor the progression of heartworm disease in dogs.
References
- 1.Rawlings CA, Calvert CA. Heartworm disease. In: Ettinger SJ, Feldman FC, editors. Textbook of Veterinary Internal Medicine: Diseases of the Dog and Cat. 4th ed. Philadelphia, Pennsylvania: WB Saunders; 1995. pp. 1046–1068. [Google Scholar]
- 2.Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer M. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation. 2008;117:163–168. doi: 10.1161/CIRCULATIONAHA.107.727545. [DOI] [PubMed] [Google Scholar]
- 3.Montagnana M, Cervellin G, Meschi T, Lippi G. The role of red blood cell distribution width in cardiovascular and thrombotic disorders. Clin Chem Lab Med. 2011;50:635–641. doi: 10.1515/cclm.2011.831. [DOI] [PubMed] [Google Scholar]
- 4.Danese E, Lippi G, Montagnana M. Red blood cell distribution width and cardiovascular diseases. J Thorac Dis. 2015;7:E402–E411. doi: 10.3978/j.issn.2072-1439.2015.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu IB, Huang HP. Prevalence and prognosis of anemia in dogs with degenerative mitral valve disease. Biomed Res Int. 2016 doi: 10.1155/2016/4727054. 4727054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinelli E, Locatelli C, Bassis S, et al. Preliminary investigation of cardiovascular-renal disorders in dogs with chronic mitral valve disease. J Vet Intern Med. 2016;30:1612–1618. doi: 10.1111/jvim.14524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lippi G, Plebani M. Red blood cell distribution width (RDW) and human pathology. One size fits all. Clin Chem Lab Med. 2014;52:1247–1249. doi: 10.1515/cclm-2014-0585. [DOI] [PubMed] [Google Scholar]
- 8.Neiger R, Hadley J, Pfeiffer DU. Differentiation of dogs with regenerative and non-regenerative anaemia on the basis of their red cell distribution width and mean corpuscular volume. Vet Rec. 2002;150:431–434. doi: 10.1136/vr.150.14.431. [DOI] [PubMed] [Google Scholar]
- 9.Guglielmini C, Poser H, Pria AD, et al. Red blood cell distribution width in dogs with chronic degenerative valvular disease. J Am Vet Med Assoc. 2013;243:858–862. doi: 10.2460/javma.243.6.858. [DOI] [PubMed] [Google Scholar]
- 10.Balta S, Demirkol S, Aparci M, Arslan Z, Ozturk C. Red cell distribution width in myocardial infarction. Med Princ Pract. 2015;24:584–585. doi: 10.1159/000437355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balta S, Demir M, Demirkol S, Arslan Z, Unlu M, Celik T. Red cell distribution width is related to stroke in patients with heart failure. Clin Appl Thromb Hemost. 2015;21:190. doi: 10.1177/1076029613509479. [DOI] [PubMed] [Google Scholar]
- 12.Martinez C, Mooney CT, Shiel RE, Tang PK, Mooney L, O’Neill EJ. Evaluation of red blood cell distribution width in dogs with various illnesses. Can Vet J. 2019;60:964–971. [PMC free article] [PubMed] [Google Scholar]
- 13.Stanzani G, Cowlam R, English K, Connolly DJ. Evaluation of red blood cell distribution width in cats with hypertrophic cardiomyopathy. J Vet Cardiol. 2015;17:S233–S243. doi: 10.1016/j.jvc.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Swann JW, Sudunagunta S, Covey HL, English K, Hendricks A, Connolly DJ. Evaluation of red cell distribution width in dogs with pulmonary hypertension. J Vet Cardiol. 2014;16:227–235. doi: 10.1016/j.jvc.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 15.de Madron E, King JN, Strehlau G, White RV. Survival and echocardiographic data in dogs with congestive heart failure caused by mitral valve disease and treated by multiple drugs: A retrospective study of 21 cases. Can Vet J. 2011;52:1219–1225. [PMC free article] [PubMed] [Google Scholar]
- 16.Rawlings CA, Tackett RL. Postadulticide pulmonary hypertension of canine heartworm disease: Successful treatment with oxygen and failure of antihistamines. Am J Vet Res. 1990;51:1565–1569. [PubMed] [Google Scholar]
- 17.Mazzotta E, Guglielmini C, Menciotti G, et al. Red blood cell distribution width, hematology, and serum biochemistry in dogs with echocardiographically estimated precapillary and postcapillary pulmonary arterial hypertension. J Vet Intern Med. 2016;30:1806–1815. doi: 10.1111/jvim.14596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma MC, Pachauri SP. Blood cellular and biochemical studies in canine dirofilariasis. Vet Res Commun. 1982;5:295–300. doi: 10.1007/BF02214997. [DOI] [PubMed] [Google Scholar]
- 19.Atwell RB, Buoro IB. Clinical presentations of canine dirofilariasis with relation to their haematological and microfilarial status. Res Vet Sci. 1983;35:364–366. [PubMed] [Google Scholar]
- 20.Komajda M. Prevalence of anemia in patients with chronic heart failure and their clinical characteristics. J Card Fail. 2004;10:1–4. doi: 10.1016/j.cardfail.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Szachniewicz J, Petruk-Kowalczyk J, Majda J, et al. Anemia as an independent predictor of poor outcome in patients with chronic heart failure. Int J Cardiol. 2003;90:303–308. doi: 10.1016/s0167-5273(02)00574-0. [DOI] [PubMed] [Google Scholar]
- 22.Caramelo C, Justo S, Gil P. [Anemia in heart failure: Pathophysiology, pathogenesis, treatment, and incognitae]. Rev Esp Cardiol. 2007;60:848–860. [Article in Spanish] [PubMed] [Google Scholar]
- 23.Kim JS. Evaluation of anemia and red blood cell distribution width in dogs with chronic mitral valve degeneration. [Master thesis] Chuncheon, Korea: Kangwon National University; 2017. [Google Scholar]
- 24.Calvert CA, Rawlings CA. Canine heartworm disease. In: Fox PR, editor. Canine and Feline Cardiology. New York, New York: Churchill Livingstone; 1988. pp. 541–549. [Google Scholar]
- 25.Stokol T. Anemia, erythrocytosis. In: Ettinger SJ, Feldman EC, Côté E, editors. Textbook of Veterinary Internal Medicine. 8th ed e-book. St. Louis, Missouri: Elsevier; 2017. pp. 740–749. [Google Scholar]
- 26.Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Borenstein J. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol. 2002;39:1780–1786. doi: 10.1016/s0735-1097(02)01854-5. [DOI] [PubMed] [Google Scholar]
- 27.Okonko DO, Anker SD. Anemia in chronic heart failure: Pathogenetic mechanisms. J Card Fail. 2004;10:S5–S9. doi: 10.1016/j.cardfail.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 28.McMurray JJ. What are the clinical consequences of anemia in patients with chronic heart failure? J Card Fail. 2004;10:S10–S12. doi: 10.1016/j.cardfail.2004.01.001. [DOI] [PubMed] [Google Scholar]