Abstract
In this study, we investigated whether β-glucan from Saccharomyces cerevisiae exerts beneficial effects on mucosal immunity in an ovine ruminal explant (ORE) model. Once the ORE model was established, viability was assessed through histological change, E-cadherin expression, CK-18 and Ki-67 distribution. Then, the OREs were co-cultured with β-glucan, following which, gene and protein expression levels of sheep β-defensin-1 (SBD-1), pro-inflammatory interleukin (IL)-6, and anti-inflammatory IL-10 were detected using quantitative real-time polymerase chain reaction (qPCR) and enzyme-linked immunosorbent assay (ELISA). Hematoxylin & eosin staining, qPCR, and immunohistochemistry showed that the overall ORE structure was intact after 96 hours in culture, but explants cultured for more than 24 hours showed epithelial degradation. Therefore, we performed the follow-up test within 24 hours. qPCR and ELISA revealed that the gene and protein expression levels of SBD-1, IL-6, and IL-10 in the OREs significantly increased (P < 0.05) after treatment with β-glucan compared with controls. This study identified the feasibility and optimal conditions of ORE culture and demonstrated that β-glucan activates SBD-1, IL-6, and IL-10 secretion in OREs to promote mucosal immunity.
Résumé
Dans la présente étude nous avons examiné si le β-glucane de Saccharomyces cerevisiae amène des effets bénéfiques sur l’immunité mucosale dans un modèle d’explant ruminal ovin (ORE). Une fois que le modèle ORE fut établi, la viabilité fut évaluée via les changements histologiques, l’expression d’E-cadhérine et la distribution de CK-18 et Ki-67. Puis, les OREs furent co-cultivés avec du β-glucane, après quoi, les degrés d’expression des gènes et des protéines β-défensine-1 ovine (SBD-1), interleukine (IL)-6 pro-inflammatoire et IL-10 anti-inflammatoire furent détectés en utilisant une réaction d’amplification en chaîne par la polymérase quantitative en temps réel (qPCR) et une épreuve immuno-enzymatique (ELISA). Une coloration à l’hématoxyline et éosine, le qPCR et l’immunohistochimie ont montré que la structure globale d’ORE était intacte après 96 heures en culture, mais des explants cultivés pour plus de 24 heures présentaient une dégradation épithéliale. Par conséquent, nous avons effectué les tests de suivi en dedans de 24 heures. Les analyses par qPCR et ELISA ont révélé que les degrés d’expression des gènes et des protéines SBD-1, IL-6 et IL-10 dans les OREs augmentèrent de manière significative (P < 0,05) après un traitement avec du β-glucane comparativement aux témoins. Cette étude a identifié la faisabilité et les conditions optimales pour la culture d’ORE et a démontré que le β-glucane active la sécrétion de SBD-1, IL-6 et IL-10 dans les OREs afin de promouvoir l’immunité mucosale.
(Traduit par Docteur Serge Messier)
Introduction
Knowledge regarding how organisms resist other organisms has accumulated as the biological sciences have progressed. Defensins are antimicrobial peptides (AMPs) that have a non-enzymatic inhibitory effect on a broad spectrum of microorganisms. Since the need for self-defense is universal, AMPs are universally present in organisms in various forms. β-defensins, a type of defensin, are widely distributed in vertebrates. Sheep β-defensins are small cationic AMPs synthesized by the epithelium to counteract bacterial adherence and invasion (1). Only 2 kinds of β-defensins, sheep β-defensin-1 (SBD-1) and SBD-2, have been identified in ovines (2). SBD-1 is an inducible peptide that is widely expressed in adult ovines, is synthesized and secreted by the epithelium, and has strong antibacterial activity (2,3). Unlike SBD-1, which is more widely expressed, SBD-2 is expressed mainly in the intestine and has sporadic mRNA expression in other tissues (4). Recently, some probiotic yeasts have been shown to increase the secretion of β-defensins, but the yeasts are not affected by the antimicrobial effect of β-defensins (5).
Saccharomyces cerevisiae is a non-pathogenic yeast probiotic that is widely used to promote the intestinal health of humans and animals. Studies have shown that live yeast cultures and prebiotics, such as mannan-oligosaccharides (MOS), complex carbohydrates, mannose (mannoproteins), β-glucan, and proteins derived from the cell wall of S. cerevisiae, can stabilize rumen pH and the number of anaerobic cellulolytic bacteria and enhance the health of the ruminal epithelium (6,7). These additives have been identified as ruminant immune system modulators (8). In addition to its nutritional value, there is evidence that β-glucan can adhere to enteric pathogens, thereby reducing their ability to adhere and invade host cells (9).
Recent studies also found that β-glucan stimulates defensin and cytokine expression in vivo. Cohen et al (10) reported that β-glucan can stimulate IL-8 and C-C motif chemokine ligand-2 (CCL-2) secretion by human intestinal epithelial cells. Marel et al (11) demonstrated that oral administration of β-glucan can upregulate the expression levels of β-defensin-2 and β-defensin genes in common carp gills and skin. Schmitt et al (12) found that zymosan can increase the production of cathelicidin and cytokine IL-1β in the intestinal epithelial RTgutGC cell line from rainbow trout at the transcript and protein levels. In 2018, Jin et al (13) showed that S. cerevisiae significantly increased the expression of SBD-1 in ovine ruminal epithelial cells (ORECs). However, little is known regarding whether β-glucan, a cell wall component of S. cerevisiae, induces SBD-1 expression in ovine ruminal explants (OREs). Therefore, we first established an ORE model and then, investigated whether β-glucan could induce the expression of SBD-1 and its effect on the expression of pro-inflammatory cytokine IL-6 and anti-inflammatory cytokine IL-10.
Materials and methods
Animal sample collection
Healthy adult ovines at 7 to 12 mo of age were obtained from a local abattoir. The experimental animals were euthanized with approval from the Animal Ethics Committee of the Inner Mongolia Agriculture University. Immediately after euthanasia, the rumen was separated from the reticulum and 20 cm2 segments of rumen were harvested. The rumen was flushed with ice-cold phosphate-buffered saline (PBS; Sigma, Munich, Germany) supplemented with 5% penicillin/streptomycin (Sigma). The tissue was placed in a beaker with chilled PBS and transported to a biosafety cabinet for processing.
ORE culture
The protocol for establishing explants was based on previous reports (14–16) with modifications. All tissue manipulations were performed on a refrigerated surface. All visible contents were removed using PBS supplemented with 1 mg/mL penicillin, 500 μg/mL streptomycin, 100 μg/mL gentamicin, and 50 μg/mL amphotericin. The mucosa and submucosa were gently separated from the muscularis propria using forceps and discarded. A surgical scalpel (No. 20) was used to cut the ruminal mucosa into 4 mm2 strips. The pieces were placed nipple side up in 6-well culture plates. The pieces were partially submerged by adding Dulbecco’s Modified Eagle’s Medium/F-12 culture media (Gibco DMEM/F-12; Thermo Fisher Scientific, Grand Island, New York, USA) supplemented with one of the following combinations: mix 1 [200 μg/mL penicillin, 100 μg/mL streptomycin, 2.5 μg/mL amphotericin B, 2 μg/mL insulin-transferrin-selenium additive, 2 mM L-glutamine, and 10% heat-inactivated fetal bovine serum (FBS)] or mix 2 (mix 1 without 10% heat-inactivated FBS). The 6-well culture plates were then incubated in a Forma CO2 incubator (Thermo Fisher Scientific, Waltham, Massachusetts, USA) at 37°C.
Histological analysis
Media were replaced every 24 h (replacement media were pre-warmed to 37°C). Explants were harvested after culture for 0, 4, 8, 12, 24, 48, 72, or 96 h, fixed in 10% buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin & eosin (H&E) to evaluate tissue architecture, cell integrity, and necrosis over the culture period.
Immunohistochemistry (IHC)
After the sections were dewaxed and rehydrated, antigen retrieval treatment was carried out by microwave heating in 0.01 M citrate buffer (pH 6.0) and equilibration in PBS (pH 7.4) for 3 × 5 min. Endogenous peroxidase activity was quenched by incubating sections in reagent A from the UltraSensitive S-P (Rat/Rabbit) Kit (Fuzhou Maixin Biotech, Fujian, China) for 10 min and rinsing in water for 3 × 3 min. The sections were blocked with reagent B for 10 min at 37°C, followed by incubation overnight in a humidified chamber at 4°C with anti-CK-18 antibody (1:400; Abcam, Cambridge, United Kingdom) or anti-Ki-67 antibody (1:500; Abcam) and washing in PBS for 3 × 5 min. Next, the sections were incubated for 10 min with biotin-labeled goat anti-mouse/rabbit IgG (reagent C), washed in PBS for 3 × 5 min, incubated for 10 min with reagent D according to the manufacturer’s instructions, and washed again with PBS for 20 min. Immunoreactivity was detected with DAB (Abcam) solution for 3 min, followed by washing in distilled water for 3 × 5 min. Finally, sections were mildly counterstained with hematoxylin and observed under a light microscope. Equivalent concentrations of mouse monoclonal IgGl (Abcam) and rabbit polyclonal IgG (Abcam) were used as isotype controls for CK-18 and Ki-67, respectively. All images within each experiment were taken under the same conditions.
Preparation of β-glucan
The β-glucan (Sigma) was diluted with PBS to a concentration of 10 mg/mL, mixed thoroughly, and vortexed for an additional 30 s. The β-glucan suspension was added to the cultured OREs at 10, 50, 100, 200, and 400 μg/mL concentrations. Suspensions were freshly prepared for each treatment.
Induction tests
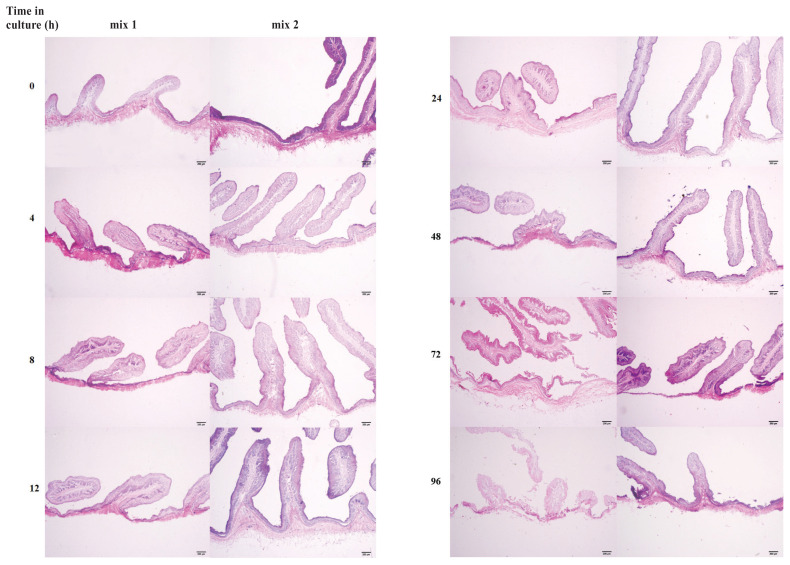
Hematoxylin & eosin staining, quantitative real-time polymerase chain reaction (qPCR), and IHC results showed that the overall structure of the cultured OREs was preserved and the ruminal epithelial cell layer remained intact (Figure 1). Although cultures did remain viable for several days, those that were cultured longer than 24 h exhibited some epithelial degradation. Therefore, we used the system for experiments lasting < 24 h. OREs were placed nipple side up in cell culture plates containing 1 mL DMEM/F12 medium with mix 2. The explants were incubated at 37°C under 5% CO2 in air for stabilization. After a 30-minute stabilization period, the explants were randomly divided into 2 groups: the β-glucan-treated group and the control group. The treatment group was exposed to a range of concentrations (10, 50, 100, 200, and 400 μg/mL) of β-glucan at 37°C and 5% CO2 for 8 h, and the control group explants were cultured in DMEM/F12 medium without β-glucan. Total RNA was extracted from the explants and SBD-1, IL-6, and IL-10 expressions were detected. Then, the OREs were stimulated with the optimal concentration of β-glucan for 2, 4, 8, 12, and 24 h. Finally, the total RNA was extracted from those explants following induction for 2, 4, 8, 12, and 24 h.
Figure 1.
Histological analysis of hematoxylin & eosin stained ovine ruminal explants cultured for different durations. Mix 1 (left) is a medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) and mix 2 (right) is a medium not supplemented with 10% heat-inactivated FBS.
Explant viability assay
Toxicity of the β-glucan was assessed by reducing MTT dye (Sigma) to a methanol-soluble formazan product. The treatment of OREs was performed for 8 h using β-glucan at concentrations of 10, 50, 100, 200, and 400 μg/mL. Additionally, OREs were stimulated with 400 μg/mL β-glucan for 2, 4, 8, 12, and 24 h. Medium alone was used as the control. Then, the explants were washed with PBS 5 times and cultured in DMEM/F12 containing MTT (250 mg/mL) for 3 h at 37°C. Explants were removed from the medium and submerged overnight in 1 mL of methanol to extract the formazan. The optical density of the formazan was determined using the Hybrid Microplate Reader (BioTek, Winooski, Vermont, USA) at 570 nm. The effect of different concentrations of β-glucan on explant viability was determined by calculating the ratio between treated and untreated explants.
RNA extraction, cDNA synthesis, and qPCR
Explant RNA was extracted from samples cryopreserved with liquid nitrogen using an RNA extraction kit (Axygen Scientific, Union City, California, USA) following the manufacturer’s instructions. RNA was reverse transcribed into single-stranded cDNA using the PrimeScript RT reagent Kit with gDNA Eraser (TaKaRa, Otsu, Japan). qPCR thermal cycling was performed as follows: 95°C for 30 s, 45 cycles of 5 s at 95°C, 30 s at 60°C, followed by a 46-step melting-curve analysis (95°C for 5 s, 60°C for 34 s, and 95°C for 15 s). All reactions were conducted in triplicate. Analysis of gene expression data was performed using the 2−ΔΔCt relative expression method with β-actin as the reference gene (17). qPCR assay primers for β-actin, E-cadherin, SBD-1, IL-6, and IL-10 are provided in Table I.
Table I.
Primer sequences.
| Gene | GenBank accession number | Fragment size (bp) | Primer pair sequences (5′-3′) |
|---|---|---|---|
| β-actin | U39357 | 208 | F: GTCACCAACTGGGACGACA R: AGGCGTACAGGGACAGCA |
| E-cadherin | AY508164.1 | 187 | F: CCCCCTGTCGGTGTTTTTATTAT R: ACTGGGGCTTGTTGTCATTCTG |
| SBD-1 | U75250 | 133 | F: GCTCTTCTTCGTGGTCCTGT R: ACAGGTGCCAATCTGTCTCA |
| IL-6 | NM_001009392.1 | 113 | F: AGCAGACTACTTCTGACCACTCCA R: TTTTCACACTCGTCATTCTTCTCAC |
| IL-10 | U11421.1 | 143 | F: GGTGATGCCACAGGCTGAGAAC R: GCTCCACCGCCTTGCTCTTG |
SBD — sheep β-defensin; IL — interleukin.
Reverse transcription PCR (RT-PCR)
Reverse transcription-PCR was carried out in a thermal cycler (GeneAmp PCR System 9700; Thermo Fisher Scientific, Waltham, Massachusetts, USA). cDNA (1 μL) was coamplified in a reaction mixture containing, in a final volume of 50 μL, 25 μL Premix Taq DNA Polymerase (TaKaRa Taq Version 2.0 plus dye), 22 μL RNase-Free dH2O, and 20 μM primers for β-actin or E-cadherin (Table I). Reaction conditions were as follows: 94°C denaturation for 5 min, 35 cycles of 94°C denaturation for 30 s, 60°C annealing for 30 s, 72°C extension for 30 s, and a final extension at 72°C for 7 min. The PCR products were analyzed by electrophoresis on 1% agarose gel with ethidium bromide.
Determination of cytokines by enzyme-linked immunosorbent assay (ELISA)
At the conclusion of the experiment, supernatants were collected from the control group, from OREs treated with different concentrations of β-glucan, and from OREs stimulated with an optimal concentration of β-glucan for 2, 4, 8, 12, or 24 h. SBD-1, IL-6, and IL-10 proteins were quantified in the supernatants by commercially available ELISA kits (Wuhan Xinqidi Biological Technology, Wuhan, China) according to the supplier’s protocols.
Statistical analysis
Data of at least 3 experiments are reported as mean ± standard deviation. Statistical analysis was performed using IBM SPSS Statistics 20.0 (Armonk, New York, USA). Comparisons among multiple groups were conducted by analysis of variance. Duncan’s multiple range test was used to assess the differences in data normality and variance uniformity. Statistical significance of the results was determined using P < 0.05.
Results
Morphological observation of OREs
In order to investigate the effect of the DMEM/F12 culture media supplemented with 10% FBS and the culture duration on the integrity of OREs, explants were divided into 2 groups: mix 1 with 10% FBS and mix 2 without FBS. Analysis of H&E stained sections showed that the rumen epithelial cell layer of the OREs cultured for 0 to 12 h in both media remained intact. However, after culture for 24 to 96 h, the explant epithelium cultured with mix 1 began to degrade and the structural integrity was deteriorated compared with that of the explant cultured with mix 2 (Figure 1). Therefore, the medium without FBS is more suitable for the culture of OREs.
Ruminal explant viability assessment
Expression of E-cadherin
E-cadherin is an important marker of intercellular adhesion and epithelial cell differentiation and plays a crucial role in maintaining the structural integrity and metabolism of epithelial cells. Therefore, total RNA was extracted from all OREs at a given time point to detect E-cadherin expression. Melting curve analysis showed that β-actin and E-cadherin had single peaks (Figures 2A, B), indicating that the amplification product of qPCR was a single specific product. The qPCR results showed that the E-cadherin mRNA expression of β-glucan-treated OREs, which were cultured for 4, 8, 12, and 24 h, was not significantly different (P > 0.05) compared with that of the untreated control group. However, the expression of E-cadherin was significantly decreased after 24 to 96 h of culture (Figure 2C). RT-PCR resulted in approximately 187 bp and 208 bp products that were visualized with electrophoresis using a 1% agarose gel with ethidium bromide (Figure 2D).
Figure 2.
Relative gene expression of E-cadherin in ovine ruminal explants cultured for different durations. qPCR melt curve peak of (A) β-actin and (B) E-cadherin genes. C — Relative gene expression of E-cadherin in explants at different durations relative to the expression of β-actin in the same explants at the respective sampling day. D — Gel electrophoresis of β-actin and E-cadherin RT-PCR products.
Immunohistochemistry
Immunohistochemical (IHC) results showed that the epithelial marker CK-18 and the cell proliferation marker Ki-67 were expressed at 0 h and at 24 h in cultured samples (Figure 3A). The Ki-67-positive cells were mainly distributed in the stratum basale of the mucosa epithelium at 0 h, while in explants cultured for 24 h, Ki-67 was mainly distributed in the stratum spinosum of the mucosa epithelium (Figure 3B). Immunostaining of CK-18 validated the integrity of the ruminal epithelial layer and Ki-67 staining revealed continued proliferation of epithelial cells in the OREs.
Figure 3.
Immunohistochemical staining of CK-18 and Ki-67. CK-18-positive cells exist in explants cultured for (A1) 0 h and (A3) 24 h. A2, A4 — Isotype controls do not show CK-18 positivity. The majority of explants’ Ki-67-positive cells are in (B1) the stratum basale of the mucosa at 0 h and in (B3) the stratum granulosum of the mucosa at 24 h. B2, B4 — Isotype controls do not show Ki-67 positivity. Bar = 50 μm.
β-glucan induced SBD-1, IL-6, and IL-10 expression in OREs
Ovine ruminal explants were co-cultured with β-glucan (0, 10, 50, 100, 200, and 400 μg/mL) for 8 h to determine if it could induce SBD-1, IL-6, and IL-10 expression in OREs. As shown in Figure 4, SBD-1, IL-6, and IL-10 mRNA and protein expression were concentration-dependently enhanced, and maximum SBD-1 mRNA and protein expression were observed in medium containing 400 μg/mL β-glucan (P < 0.01) (Figure 4A). Meanwhile, IL-6 mRNA and protein expression were highest after 100 μg/mL β-glucan treatment (P < 0.01) (Figure 4B). As shown in Figures 4C and C1, IL-10 mRNA and protein expression increased after 10 μg/mL and 100 μg/mL β-glucan treatment (P < 0.05), respectively. These results indicate that β-glucan can upregulate SBD-1, IL-6, and IL-10 mRNA and protein expression in OREs in vitro. To determine the toxicity of β-glucan, OREs were co-cultured with different concentrations of β-glucan (0, 5, 10, 20, 50, and 100 μg/mL) for 8 h. An MTT assay confirmed that the different concentrations of β-glucan were not toxic to the explants (P > 0.05) (Figure 4D).
Figure 4.
Concentration-dependent effect of β-glucan treatment (0, 10, 50, 100, 200, and 400 μg/mL) on mRNA and protein expression of SBD-1, IL-6, and IL-10 in the OREs in vitro. qPCR and ELISA results are shown for (A, A1) SBD-1, (B, B1) IL-6, and (C, C1) IL-10. D, D1 — OREs were treated with β-glucan at varying dosages of 10, 20, 50, 100, 200, and 400 μg/mL for 8 h. The % loss in viability of OREs following β-glucan treatment was determined using an MTT leakage assay. Data are represented as mean ± standard deviation (n = 3).
* Significant difference (P < 0.05) compared with the control group.
** Significant difference (P < 0.01) compared with the control group.
SBD-1, IL-6, and IL-10 expression levels were upregulated in OREs by β-glucan at different time points
Concentrations of β-glucan were selected for stimulation of OREs to examine the time-dependent effect on expression of SBD-1, IL-6, and IL-10. As shown in Figures 5A and A1, SBD-1 mRNA expression increased at 2 h and protein expression was upregulated at 8 h (P < 0.01) after 50 μg/mL and 100 μg/mL β-glucan treatment, respectively. As illustrated in Figures 5B and B1, 50 μg/mL β-glucan induced IL-6 mRNA expression from 2 to 12 h and increased the protein level from 4 to 24 h (P < 0.05), respectively. As indicated in Figures 5C and C1, significant increases were observed in the expression of IL-10 mRNA (from 2 to 24 h) and protein (from 8 to 24 h) with 50 μg/mL and 200 μg/mL β-glucan (P < 0.05), respectively. In order to determine the toxicity of β-glucan, OREs were stimulated with 400 μg/mL β-glucan from 0 to 24 h. The MTT assay showed that explants survived for all time periods with 400 μg/mL β-glucan (P > 0.05) (Figure 5D).
Figure 5.
Effect of β-glucan treatment duration on mRNA and protein expression of SBD-1, IL-6, and IL-10 in the OREs in vitro. qPCR and ELISA results are shown for (A, A1) SBD-1, (B, B1) IL-6, and (C, C1) IL-10. (D) OREs were treated with 400 μg/mL β-glucan for 0, 2, 4, 8, 12, and 24 h. The % loss in viability of OREs following β-glucan treatment was determined using an MTT leakage assay. Data are represented as mean ± standard deviation (n = 3).
* Significant difference (P < 0.05) compared with the control group.
** Significant difference (P < 0.01) compared with the control group.
Discussion
For nearly 2 decades, commercially available yeast probiotics or their cell wall components have been used as feed supplements in the livestock industry to improve animal production, promote health, and reduce the need for antibiotics. β-glucan is a carbohydrate polymer with multiple biological activities, including immunoregulation (18). Although β-glucan has been shown to induce the expression of immune factors in a variety of animals and enhance disease resistance (10–12), there are currently no available data regarding whether β-glucan exerts beneficial effects on the mucosal immune system in an ORE model.
Our group has demonstrated that β-glucan can induce SBD-1 expression in ORECs; however, in vitro cell culture models are usually focused on recreating a specific characteristic of an organ and do not represent the multiple interactions that occur between different cells. In vitro organ culture methods not only overcome these limitations but also provide the advantages of a controlled environment (19). Therefore, we chose to culture OREs for subsequent studies, but it is essential to prolong cell viability during culture.
The maintenance of normal structures and the presence of proliferating cells and differentiated cells serve as markers for evaluating the success of explant culture (20). In this study, we observed the degradation of morphological features when OREs were exposed to 10% FBS. These findings are consistent with those of Reiss et al (21) and Costa et al (14), who reported that FBS can disrupt the structural integrity of mouse and porcine colon explants. FBS has the ability to promote cell proliferation and differentiation (22), but this effect will instead accelerate the degradation of cultured explants. Thus, the addition of FBS to the basal medium appeared to be more advantageous to maintain the morphological structure of the explants.
Ki-67 is a nuclear protein associated with cell proliferation and ribosomal RNA transcription (23). In this study, the distribution of Ki-67 in cultured OREs was analyzed by IHC. The results revealed the expression of Ki-67 in the spinous layer of the OREs after 24 h culture; other authors have reported the presence of Ki-67-positive cells in human and porcine colon organ cultures after 24 h and 120 h, respectively (14,24). The expression of Ki-67 indicates that the explants not only maintained their cell population but also established new cells as the culture proceeded. These cells migrated from the stratum basale to the surface, replacing older cells that have sloughed.
Although we have demonstrated the feasibility of cultivating OREs through these tests, we must address the limitations of this culture system. The system does not support multi-day experiments and therefore, is best suited for tests involving an immediate response to environmental disturbances. Improvements in physicochemical conditions, nutrient supply, and growth factors will likely overcome this defect in future trials, but any in vitro organ culture is temporally limited and inevitably, cell migration and multi-organ communication are lost.
AMPs, as important members of the innate immune system, have a critical role in the resistance to infections by external pathogens (25). Evidence has suggested that β-glucan could inhibit Salmonella enteritidis colonization in the intestine through the induction of AMPs, such as avian β-defensins in broiler chickens (26). SBD-1 can also be induced in ORECs in a concentration- and time-dependent manner (13). Our results suggest that β-glucan similarly induced SBD-1 expression in a concentration- and time-dependent manner in OREs. The SBD-1 mRNA and protein levels were highest after the OREs were induced for 2 h and 8 h at 50 μg/mL and 100 μg/mL β-glucan, respectively.
IL-6 is a cytokine featuring pleiotropic activity and is a key inflammatory cytokine that endorses cell-mediated immunity (27). Thus far, most yeasts and β-glucan have been shown to stimulate the production of IL-6 both in vitro and in vivo (28,29), and it is believed that IL-6 is mainly produced during the immune response of dendritic cells and macrophages to pathogenic microorganisms. In addition to immune-mediated cells, other cell types such as epithelial cells can produce IL-6 in response to various stimuli (30). Rajput et al (31) demonstrated that IL-6 expression levels were high in the jejunum of broilers that were given S. boulardii in feed. Meanwhile, our findings revealed that β-glucan, a cell wall component of S. cerevisiae, could also increase the production of IL-6 in OREs. In addition, researchers found that probiotic-induced IL-6 production could promote the production of IgA by B-cells independently of T-cells (32). Therefore, probiotics can protect the epithelium from pathogen attachment by inducing the production of IL-6.
Recent studies have demonstrated that the expression of proinflammatory and anti-inflammatory cytokines in epithelial cells exposed to probiotics is related to the beneficial effects of these bacterial strains (33). IL-10 is an anti-inflammatory cytokine that regulates both innate and adaptive immunity. It is pleiotropic in immune regulation and inflammation and is an essential immunomodulator in the intestinal tract (34). Therefore, in addition to IL-6, the expression of IL-10 was measured in the present study. The results showed that β-glucan could upregulate the expression of IL-10 compared with that in the control group. Upregulation of IL-6 and IL-10 in the β-glucan stimulated group can be explained by the immune balance and mutual regulation between pro-inflammatory and anti-inflammatory cytokines, suggesting that β-glucan has the effect of maintaining immune balance and preventing further activation of the immune system following a strong innate immune response to pathogen challenge. Similar to our results, Sonck et al (35) showed that β-glucan significantly increased the expression levels of IL-6 and IL-10 in porcine dendritic cells. In contrast, Li et al (36) reported that dietary β-glucan attenuated the increase in plasma IL-6 and TNF-α and enhanced the increase in plasma IL-10 when pigs were challenged with lipopolysaccharide. In addition, a recent study showed that diet supplementation with yeast-derived products downregulated the expression of IL-10 and IL-4 in the cecal tonsils (37). The variations in immunomodulatory properties of β-glucan might be related to the different immunization periods, animals, and sampling sites used in these studies. Nonetheless, these findings indicate that β-glucan plays a role in regulating immune homeostasis and may exhibit different immunomodulatory effects in challenged and unchallenged conditions.
In conclusion, taken together, we have developed and assessed a successful culture protocol for OREs for up to 24 h and demonstrated that β-glucan can improve the production of SBD-1 and cytokines IL-6 and IL-10 in OREs to promote mucosal immunity; however, the molecular immunological mechanism remains to be clarified.
Acknowledgments
We thank Editage for their assistance in editing our article and for language revision, Professor Gui-fang Cao for providing us with laboratory space for the experiments, and the National Natural Science Foundation of China for the support (Grant No. 31560682 and Grant No. 31360593).
References
- 1.Li Q, Bao F, Zhi D, et al. Lipopolysaccharide induces SBD-1 expression via the P38 MAPK signaling pathway in ovine oviduct epithelial cells. Lipids Health Dis. 2016;15:127. doi: 10.1186/s12944-016-0294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huttner KM, Lambeth MR, Burkin HR, Burkin DJ, Broad TE. Localization and genomic organization of sheep antimicrobial peptide genes. Gene. 1998;206:85–91. doi: 10.1016/s0378-1119(97)00569-6. [DOI] [PubMed] [Google Scholar]
- 3.Ackermann MR, Gallup JM, Zabner J, et al. Differential expression of sheep beta-defensin-1 and -2 and interleukin 8 during acute Mannheimia haemolytica pneumonia. Microb Pathog. 2004;37:21–27. doi: 10.1016/j.micpath.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Meyerholz DK, Gallup JM, Grubor BM, et al. Developmental expression and distribution of sheep beta-defensin-2. Dev Comp Immunol. 2004;28:171–178. doi: 10.1016/s0145-305x(03)00105-8. [DOI] [PubMed] [Google Scholar]
- 5.Gácser A, Tiszlavicz Z, Németh T, Seprényi G, Mándi Y. Induction of human defensins by intestinal Caco-2 cells after interactions with opportunistic Candida species. Microbes Infect. 2014;16:80–85. doi: 10.1016/j.micinf.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Silberberg M, Chaucheyras-Durand F, Commun L, et al. Repeated acidosis challenges and live yeast supplementation shape rumen microbiota and fermentations and modulate inflammatory status in sheep. Animal. 2013;7:1910–1920. doi: 10.1017/S1751731113001705. [DOI] [PubMed] [Google Scholar]
- 7.Garcia Diaz T, Ferriani Branco A, Jacovaci FA, Cabreira Jobim C, Bolson DC, Pratti Daniel JL. Inclusion of live yeast and mannan-oligosaccharides in high grain-based diets for sheep: Ruminal parameters, inflammatory response and rumen morphology. PLoS One. 2018;13:e0193313. doi: 10.1371/journal.pone.0193313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li GH, Ling BM, Qu MR, You JM, Song XZ. Effects of several oligosaccharides on ruminal fermentation in sheep: An in vitro experiment. Revue Méd Vét. 2011;162:192–197. [Google Scholar]
- 9.Akbari MR, Haghighi HR, Chambers JR, Brisbin J, Read LR, Sharif S. Expression of antimicrobial peptides in cecal tonsils of chickens treated with probiotics and infected with Salmonella enterica serovar typhimurium. Clin Vaccine Immunol. 2008;15:1689–1693. doi: 10.1128/CVI.00242-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen-Kedar S, Baram L, Elad H, Brazowski E, Guzner-Gur H, Dotan I. Human intestinal epithelial cells respond to β-glucans via Dectin-1 and Syk. Eur J Immunol. 2014;44:3729–3740. doi: 10.1002/eji.201444876. [DOI] [PubMed] [Google Scholar]
- 11.Marel Mv, Adamek M, Gonzalez SF, et al. Molecular cloning and expression of two β-defensin and two mucin genes in common carp (Cyprinus carpio L.) and their up-regulation after β-glucan feeding. Fish Shellfish Immunol. 2012;32:494–501. doi: 10.1016/j.fsi.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Schmitt P, Wacyk J, Morales-Lange B, et al. Immunomodulatory effect of cathelicidins in response to a β-glucan in intestinal epithelial cells from rainbow trout. Dev Comp Immunol. 2015;51:160–169. doi: 10.1016/j.dci.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Jin X, Zhang M, Zhu XM, et al. Modulation of ovine SBD-1 expression by Saccharomyces cerevisiae in ovine ruminal epithelial cells. BMC Vet Res. 2018;14:134. doi: 10.1186/s12917-018-1445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costa MO, Harding JC, Hill JE. Development and evaluation of a porcine in vitro colon organ culture technique. In Vitro Cell Dev Biol Anim. 2016;52:942–952. doi: 10.1007/s11626-016-0060-y. [DOI] [PubMed] [Google Scholar]
- 15.Tsilingiri K, Rescigno M. Should probiotics be tested on ex vivo organ culture models? Gut Microbes. 2012;3:442–448. doi: 10.4161/gmic.20885. [DOI] [PubMed] [Google Scholar]
- 16.Abner SR, Guenthner PC, Guarner J, et al. A human colorectal explant culture to evaluate topical microbicides for the prevention of HIV infection. J Infect Dis. 2005;192:1545–1556. doi: 10.1086/462424. [DOI] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Qi C, Cai Y, Gunn L, et al. Differential pathways regulating innate and adaptive antitumor immune responses by particulate and soluble yeast-derived β-glucans. Blood. 2011;117:6825–6836. doi: 10.1182/blood-2011-02-339812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White RL. What in vitro models of infection can and cannot do. Pharmacotherapy. 2001;21:292S–301S. doi: 10.1592/phco.21.18.292s.33906. [DOI] [PubMed] [Google Scholar]
- 20.Dame MK, Veerapaneni I, Bhagavathula N, Naik M, Varani J. Human colon tissue in organ culture: Calcium and multi-mineral-induced mucosal differentiation. In Vitro Cell Dev Biol Anim. 2011;47:32–38. doi: 10.1007/s11626-010-9358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reiss B, Williams GM. Conditions affecting prolonged maintenance of mouse and rat colon in organ culture. In Vitro. 1979;15:877–890. doi: 10.1007/BF02618044. [DOI] [PubMed] [Google Scholar]
- 22.Wu MF, Stachon T, Seitz B, Langenbucher A, Szentmáry N. Effect of human autologous serum and fetal bovine serum on human corneal epithelial cell viability, migration and proliferation in vitro. Int J Ophthalmol. 2017;10:908–913. doi: 10.18240/ijo.2017.06.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bullwinkel J, Baron-Lühr B, Lüdemann A, Wohlenberg C, Gerdes J, Scholzen T. Ki-67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells. J Cell Physiol. 2006;206:624–635. doi: 10.1002/jcp.20494. [DOI] [PubMed] [Google Scholar]
- 24.Yissachar N, Zhou Y, Ung L, et al. An intestinal organ culture system uncovers a role for the nervous system in microbe-immune crosstalk. Cell. 2017;168:1135–1148. doi: 10.1016/j.cell.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin W, Liu S, Hu L, Zhang S. Characterization and bioactivity of hepcidin-2 in zebrafish: Dependence of antibacterial activity upon disulfide bridges. Peptides. 2014;57:36–42. doi: 10.1016/j.peptides.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 26.Shao Y, Wang Z, Tian X, Guo Y, Zhang H. Yeast β-d-glucans induced antimicrobial peptide expressions against Salmonella infection in broiler chickens. Int J Biol Macromol. 2016;85:573–584. doi: 10.1016/j.ijbiomac.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 27.Rajput IR, Li WF. Potential role of probiotics in mechanism of intestinal immunity. Pak Vet J. 2012;32:303–308. [Google Scholar]
- 28.Silva VO, Pereira LJ, Murata RM. Oral microbe-host interactions: Influence of β-glucans on gene expression of inflammatory cytokines and metabolome profile. BMC Microbiol. 2017;17:53. doi: 10.1186/s12866-017-0946-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan J, Han Z, Qu Y, et al. Structure elucidation and immunomodulatory activity of a β-glucan derived from the fruiting bodies of Amillariella mellea. Food Chem. 2018;240:534–543. doi: 10.1016/j.foodchem.2017.07.154. [DOI] [PubMed] [Google Scholar]
- 30.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- 31.Rajput IR, Ying H, Yajing S, et al. Saccharomyces boulardii and Bacillus subtilis B10 modulate TLRs and cytokines expression patterns in jejunum and ileum of broilers. PLoS One. 2017;12:e0173917. doi: 10.1371/journal.pone.0173917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boirivant M, Strober W. The mechanism of action of probiotics. Curr Opin Gastroenterol. 2007;23:679–692. doi: 10.1097/MOG.0b013e3282f0cffc. [DOI] [PubMed] [Google Scholar]
- 33.Villena J, Kitazawa H. Modulation of intestinal TLR4-inflammatory signaling pathways by probiotic microorganisms: Lessons learned from Lactobacillus jensenii TL2937. Front Immunol. 2014;4:512. doi: 10.3389/fimmu.2013.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8:1095–1104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 35.Sonck E, Devriendt B, Goddeeris B, Cox E. Varying effects of different β-glucans on the maturation of porcine monocyte-derived dendritic cells. Clin Vaccine Immunol. 2011;18:1441–1446. doi: 10.1128/CVI.00080-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Xing J, Li D, et al. Effects of beta-glucan extracted from Saccharomyces cerevisiae on humoral and cellular immunity in weaned piglets. Arch Anim Nutr. 2005;59:303–312. doi: 10.1080/17450390500247832. [DOI] [PubMed] [Google Scholar]
- 37.Alizadeh M, Rodriguez-Lecompte JC, Rogiewicz A, Patterson R, Slominski BA. Effect of yeast-derived products and distillers dried grains with solubles (DDGS) on growth performance, gut morphology, and gene expression of pattern recognition receptors and cytokines in broiler chickens. Poult Sci. 2016;95:507–517. doi: 10.3382/ps/pev362. [DOI] [PubMed] [Google Scholar]