Abstract
The outbreak of Coronavirus disease of 2019 (COVID-19) caused by the Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2), has posed a serious health threat. The increasing number of COVID-19 cases around the world is overwhelming hospitals and pushing the global death toll to over 746,000, which has pushed the sprint to find new treatment options. In this article, we reviewed the SARS-CoV-2 pathophysiology, transmission, and potential treatment strategies.
Keywords: SARS-CoV-2, Pandemic, 2019 novel coronavirus, Viral inhibitor, ACE-2 receptor, Receptor binding protein
COVID19 pandemic background
Coronavirus Disease 2019 (COVID-19) caused by an infection with the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has caused one of the largest global outbreaks in recent years, and posed a serious threat to the global public health [1, 2]. Considering the rapidly increasing cases of COVID-19 and disease severity, the World Health Organization (WHO) declared a global health emergency on January 30, 2020 [3]. Despite implementing worldwide combined efforts to prevent SARS-CoV-2 further transmission by quarantining the infected persons and their family members, social distancing, and schools closure, the spreading of infection could not be contained; therefore, on March 11, 2020, the WHO declared COVID-19 a pandemic [3]. As of now, around 213 countries and territories outside of the Mainland China have reported SARS-CoV-2 infections [1, 4]. The massive impact of SARS-CoV-2 infection has been seen in the United States of America, Europe, and Asia. As of Aug 12th, 2020, the time of writing this review, SARS-CoV-2 has infected more than 20.54 million people worldwide and resulted in 746,151 deaths (Additional file 1: Figure S1A).
The worldwide date indicates an exponential infection rate of SARS-CoV-2 cases after the first week of March-2020 (Additional file 1: Figure S1B). The mean primary reproduction number (R0) was estimated to range from 2.24 [95% confidence interval (CI) 1.96–2.55] to 3.58 (95% CI 2.89–4.39), and associated with two- to eight-fold increase in the reporting rate as compared to other viral infections (Additional file 1: Figure S1C) [5, 6]. The current statistics are showing that the epidemic doubling time is as low as 6.4 days [5], including potential asymptomatic transmissions. Although the situation is evolving and updated on daily basis, more data is required to confirm these estimations. This data indicates a high potential for the SARS-CoV-2 outbreak and warrants immediate therapeutic interventions.
Outbreaks of coronavirus
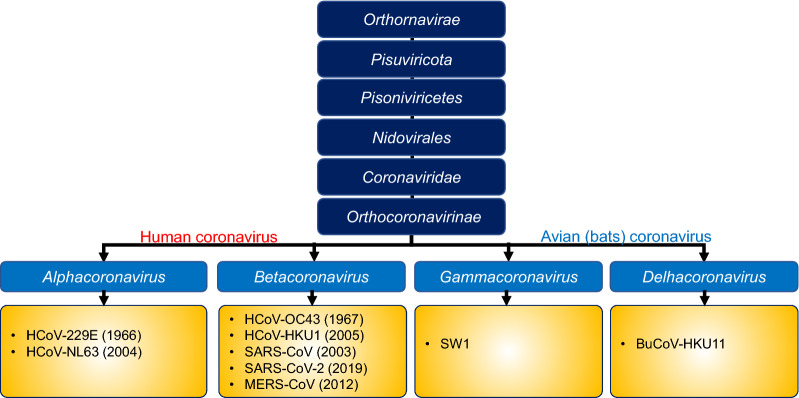
Seven Coronaviruses (CoV) of zoonotic origins have crossed the species barrier so far, to cause infections in humans, and three of them have caused a deadly infection in last two decades, including the Middle East Respiratory Syndrome Coronavirus (MERS-CoV), Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV), and SARS-CoV-2 (Fig. 1) [7–9]. Among these, SARS-CoV originating from bats emerged in Guangdong, China in 2002, and resulted in the 2003 outbreak with about 10% case fatality rate (CFR) [10], while MERS-CoV originating from the dromedary camels was first reported in Saudi Arabia in June 2012, and later in 27 countries, infecting a total of 2494 individuals and resulting in a CFR of about 34.4% [11]. The recent SARS-CoV-2 pandemic is thought to be originated from an animal reservoir, through spillover infection, before being transferred to humans in Wuhan city of China [12]. Although the exact mechanisms of SARS-CoV-2 transmission are not fully understood, human-to-human transmission of SARS-CoV-2 from patients or asymptomatic carriers occurs via two routes [13]. The first is directly through close contact with an infected person (< 2 meters) where respiratory secretions can enter, mouth, eye, nose, or airways. The second route is indirect, via the touching of an object, surface, or hand of an infected person contaminated with respiratory secretions and subsequently touching own’s mouth, eye, or nose [13]. The SARS-CoV-2 infection resulted in highly variable CFR depending on co-morbidity and country—ranging from 0.1 to 9.26% [14].
Fig. 1.
Taxonomy of Coronaviridae. HCoV, human coronavirus; MERS-CoV, Middle East respiratory syndrome coronavirus; SARS-CoV, severe acute respiratory syndrome coronavirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; SW1, Beluga whale coronavirus; and BuCoV-HKU11, bulbul coronavirus HKU11
SARS-CoV-2 Structure and Pathophysiology
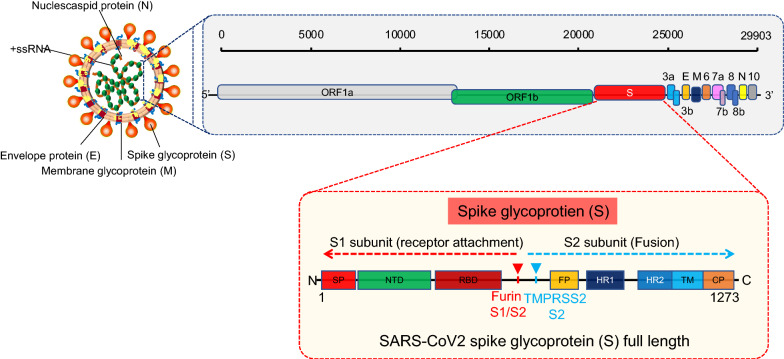
To understand the pathogenic mechanisms of SARS-CoV-2 and to discuss the current therapeutic targets; it is important to describe the viral structure, genome, and replication cycle. CoVs are positive-stranded RNA viruses with a nucleocapsid and envelope [15]. A SARS-CoV-2 virion is approximately 50–200 nm in diameter [16] and has a +ssRNA genome of approximately 29.9 kb in length—the largest known RNA virus with a 5′-cap structure and 3′-poly-A-tail and possess 14 putative open reading frames (ORFs) encoding 27 proteins [17, 18]. The virion has four structural proteins, known as the S (spike), E (envelope), M (membrane), and N (nucleocapsid) proteins; the N protein holds the RNA genome, and the S, E, and M proteins together create the viral envelope [19]. The spike glycoprotein-S facilitates the virus attachment to the angiotensin-converting enzyme 2 (ACE2) receptor and fuses with the membrane of the host cell [19]. SARS-CoV-2 then uses serine proteases TMPRSS2 (transmembrane protease serine 2) for S protein priming, infecting the target cells [20] (Fig. 2). The spike proteins of SARS-CoV-2 contains two subunits; S1 receptor binding subunit and S2 fusion subunit, to mediate the virion binding to receptor protein and initiate membrane fusion. The S1 and S2 subunits are divided by the S cleavage site (Fig. 2). To facilitate virion attachment to receptor and fuses with cells membrane, the spike protein needs to be cleaved by cellular proteases from the S1/S2 cleavage site (Fig. 2). Interestingly, the molecular analysis of S proteins identified an insertion at S1/S2 site, which is absent in other SARS-CoV [21], though the importance of this insertion is still unknown, it seems that this unique insertion is providing a gain-of-function advantage for an easy cell infection and efficient spreading throughout the human host.
Fig. 2.
Schematic representation of the SARS-CoV-2 structure, genome and functional domain of SARS-CoV-2 S protein. The viral surface proteins, envelope membrane and spike, are embedded in a lipid bilayer, while the single-stranded positive-sense viral RNA (SS-RNA) is associated with the nucleocapsid protein. The spike proteins contain S1 and S2 subunits and the cleavage sites for furin and TMPRSS2. The spike proteins mediate the viral attachment to the host cells after activation by the enzyme TMPRSS2. SP, signal peptide; NTD, N-terminal domain; RBD, receptor-binding domain, contains core binding motif in the external subdomain; FP, fusion peptide; HR, heptad repeat 1 and heptad repeat 2; TM, transmembrane domain; CP, cytoplasm domain
The viral RNA hijacks the host cell’s machinery to initiate the viral genome replication and polypeptides chain synthesis and form the replication-transcription complex (RCT) needed to synthesize the sub-genomic RNAs as well as structural proteins (envelope and nucleocapsid) (Fig. 2). The viral envelope has a crucial role in the viral assembly, release, and promoting viral pathogenesis [22]. However, the exact role of the many small viral peptides (e.g., those of glycoprotein subunits) has not yet been described. More research is needed to understand the structural characteristics of SARS-CoV-2 that underlie various pathogenic mechanisms.
Susceptibility to SARS-CoV-2 infection
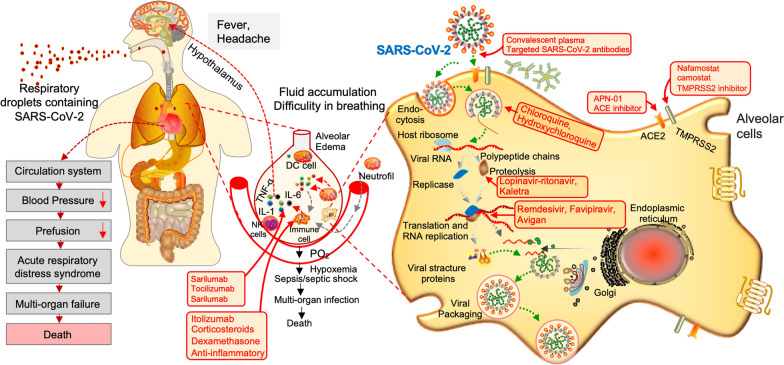
Symptoms of SARS-CoV-2 resemble those of the common cold, including fever, coughing, and shortness of breath [23]. However, the infection can lead to pneumonia, multi-organs failure, severe acute respiratory syndrome, and even death in severe cases (Fig. 3) [24]. Elderly individuals (aged > 60 years) and people with underlying chronic health conditions are more susceptible to severe disease (18.5%) as compared to children and younger healthy adults (6%) [25]. The clinical data collected from the non-survivors patients revealed that the most distinctive comorbidities of SARS-CoV-2 infection were hypertension (24–75%) and diabetes mellitus (16.2–35%) [26, 27]. Notably, the most frequent comorbidities were reported in SARS-CoV-2 patients treated with angiotensin-converting enzyme (ACE) inhibitors [27, 28]. SARS-CoV-2 binds to host cells through the ACE2 receptor, which is expressed by epithelial cells of the lungs, intestines, kidneys, brain, and blood vessels [29]. The expression of ACE2 is substantially increased in diabetic and hypertensive patients, treated with ACE inhibitors and angiotensin II type-I receptor blockers (ARBs) [29], which consequently promotes SARS-CoV-2 infection severity.
Fig. 3.
An infection and replication model of SARS-CoV-2 in host cells and current treatment strategies to interfere with steps in the SARS-CoV-2 replication cycle. SARS-CoV-2 binds to host cells through the ACE2 receptor, and after endocytosis and subsequent uncoating, the components of SARS-CoV-2 use host cells machinery to produce new viruses. Finally, the SARS-CoV-2 virions are released from the host cell by exocytosis. During this process, the viral replication can be inhibited at different stages by repositioned drugs (highlighted in red). On the other hand, SARS-CoV-2 stimulates the host immune system to release the cytokines and subsequent inflammation and immune-dysfunction through activation or impairment of various immune cells, such, dendritic cells, NK cells, macrophages, and neutrophils. This process can lead to sepsis, septic shock, multiple organ failure, and death. SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; TMPRSS2, transmembrane protease serine 2; NK, natural killer; DC, dendritic cell; IL-1, interleukin-1; IL-6, interleukin-6
Therapeutic Strategies for patients infected with SARS-CoV-2
No specific antiviral therapeutic agents or vaccine for SARS-CoV-2 are currently available to save the infected patients, protect health care workers and others at high risk of infection. Therefore, to control the rapidly growing SARS-CoV-2 outbreak, the WHO, announced on March 18, 2020, the launch of SOLIDARITY, which is an unprecedented multinational coordinated effort to collect rapidly robust clinical and scientific data during the SARS-CoV-2 pandemic [30], giving hope and planning to eradicate the SARS-CoV-2 virus. Various antiviral therapies with much broader landscapes are being selected by WHO, including the experimental antiviral drug Remdesivir; the Malaria medication Chloroquine/Hydroxychloroquine; a combination of Human Immunodeficiency Viruses (HIV) drugs such as Lopinavir and Ritonavir; and finally, a combination of HIV drugs added to Interferon-beta.
Remdesivir was originally developed by Gilead Sciences to combat Ebola and other related viruses by inhibiting viral replication. Remdesivir is an adenosine analogue with broad-spectrum antiviral activities [31]. A nucleoside analogue competes with natural nucleosides during replication for the RdRp active site, thus inhibiting the viral replication [32]. This drug is currently being extensively evaluated against SARS-CoV-2 in the United States and Europe, and according to the latest information, the efficacy of Remdesivir is found ambiguous against severely infected patients [33]. Despite its controversial results, the US Food and Drug Administration (FDA) approved the emergency use of the experimental Remdesivir to treat hospitalized SARS-CoV-2 patients [34].
Chloroquine and hydroxy-chloroquine have received intense attention worldwide because of the positive results generated from the preliminary studies of their use to treat SARS-CoV-2 patients. Chloroquine and hydroxychloroquine possibly decrease acidity in endosomes compartments of infected cells and can inactivate the virus (Fig. 3). In addition, chloroquine and hydroxy-chloroquine can also impair the terminal glycosylation of the ACE2 receptor, thus inhibiting the viral penetration into the cells [35]. However, ex-vivo studies performed in the cell culture model have suggested that chloroquine and hydroxy-chloroquine can cripple the SARS-CoV-2 virus, but the effective dose required is usually high, which can cause severe toxicity [36]. After reviewing the safety concerns of antimalarial drugs [37], the WHO temporarily suspended the hydroxy-chloroquine arm of its Solidarity trial [38].
Another underway ‘SOLIDARITY’ trial for SARS-CoV-2 treatment combines two drugs, Lopinavir and Ritonavir. These drugs were originally developed to treat HIV patients by inhibiting the protease enzyme that is needed by the virus to cleave long polypeptides chains during the assembly of new viruses [39]. Lopinavir and Ritonavir effectively inhibit the 3C-like proteinase, which plays a key role in the processing of viral polyproteins [40] and posing a possible potent therapeutic option against SARS-CoV-2. Although the preliminary data from the Chinese study is unclear [41], other clinical trials are underway (Table 1). In addition to the known antiviral drug combinations, some trials are currently exploring these drugs in combination with the anti-interferon-beta, an anti-inflammatory molecule [42].
Table 1.
Emerging investigational therapeutic trials to treat SARS-CoV-2 patients
| Drugs | Company | MoA | Comment/status |
|---|---|---|---|
| Remdesivir | Gilead Sciences, Inc. | Viral transcription inhibitor |
Originally developed for Ebola and MERS Remdesivir found ambiguous against SARS-CoV-2 |
| Chloroquine/hydroxychloroquine and Azithromycin combination | Novartis pharmaceuticals, Mylan NV, Teva Pharma-ceuticals USA | Decrease acidity in endosomes |
Developed for malaria and rheumatoid arthritis SARS-CoV-2 patients showed substantial improvements after treatment WHO suspended the trial of hydroxychloroquine over safety concerns |
| Lopinavir-ritonavir, Kaletra | Abbott Laboratories, AbbVie, Inc. | Protease inhibitor |
Anti-HIV treatment Initial clinical data indicates, no change in time to clinical improvement of SARS-CoV-2 patients |
| Lopinavir-ritonavir plus IFN-β | The University of Hong Kong | Protease inhibitor plus anti-inflammatory |
IFN-β used in regulating inflammation in lessened disease No clinical efficacy data yet, but some doctors feels, it might be risky for patients with severe SARS-CoV-2 patients |
| Favipiravir/Avigan | Fujifilm Toyama Clinical | Viral transcription inhibitor |
Next generation flu drug Drug accelerate the viral clearance and improve the lung conditions |
| Hydroxychloroquine and Nitazoxanide Combination | Tanta University | Blocks maturation of the viral hemagglutinin |
Nitazoxanide has broad-spectrum activity against helminthic, protozoal, and viruses Clinical trial not yet started |
| Nafamostat and camostat | Susanne Arnold, University of Kentucky, University Hospital Padova | Antagonist TMPRSS2, Block the entry of SARS-CoV-2 |
Nafamostat and camostat are approved in Japan for use against pancreatitis Drugs are currently in phase 2/3 clinical trial in different countries |
| Hydroxychloroquine and Famotidine | Northwell Health, NY, USA | Inhibit the entry of SARS-CoV-2 | Drugs are currently in phase 3 clinical trial in USA |
| Ruxolitinib | Novartis Pharmaceuticals | Janus kinase (JAK) inhibitor |
Ruxolitinib was developed for high-risk myelofibrosis No efficacy data available against SARS-CoV-2 |
| Sarilumab and Tocilizumab | Assistance Publique—Hôpitaux de Paris | Antibodies to inhibit IL-6 |
Sarilumab originally developed for rheumatoid arthritis Clinical trial for SARS-CoV-2 not yet started |
| Itolizumab | Biocon Limited | Anti-CD6 IgG monoclonal antibody |
Itolizumab originally developed for chronic plaque psoriasis Clinical trial for SARS-CoV-2 completed and treatment showed substantial improvement in moderate to severe SARS-CoV-2 patients |
| APN-01 | Apeiron biologics | ACE inhibitor |
Originally developed for SARS treatment Clinical trial for SARS-CoV-2 not yet started |
| siRNAs | Alnylam pharmaceuticals and vir biotechnology | Conserved regions of coronavirus RNA. |
siRNAs hit highly conserved regions of SARS-CoV-2 RNA Clinical trial not yet started |
| N-803 | ImmunityBio | Kill the infected cells |
N-803 showed strong positive response in monkeys against HIV Clinical trial not yet started |
| Pirfenidone | Roche | Anti-inflammatory drug, inhibits transforming growth factor-β |
Pirfenidone used for idiopathic pulmonary fibrosis (IPF), a lung fibrosis disease Pirfenidone can improve lung functions in SARS-CoV-2 patients No clear clinical efficacy data yet |
| Umifenovir | Shahid Beheshti University of Medical Sciences, Iran | Binds to viral lipid membrane and inhibits viral entry |
Approved for influenza A and B virus in Russia and china Umifenovir found ambiguous against mild to moderate SARS-CoV-2 |
| Ivermectin | Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins | Destabilize the cell-transport proteins |
Ivermectin is broad-spectrum anti-parasitic drug Administration of Ivermectin in SARS-CoC-2 patients found efficacious Drug is being tested in combination with hydroxy-chloroquine in many countries |
| Corticosteroids | Hospices Civils de Lyon, France | Anti-inflammatory | Corticosteroids are being tested in SARS-CoV-2 patients |
Apart from the ‘SOLIDARITY’ trial, other therapeutic options are also being explored against SARS-CoV-2 to improve the outcomes of critically ill patients. As of Aug 12th, 2020, more than 1000 clinical trials are currently exploring different treatment strategies against SARS-CoV-2 [42], including drug repositioning, novel therapeutic options, and vaccines. Potential treatment strategies that are currently in the testing phase against SARS-CoV-2 or likely to be initiated as clinical trials are summarized in table-1. These include drugs that can reduce inflammation (such as itolizumab that binds to CD6 receptor and blocks the activation of T lymphocytes and suppress the pro-inflammatory cytokines or corticosteroids that decrease the cytokines storm), ACE-2 inhibitor, SARS-CoV-2 specific siRNAs, and immunomodulators. In addition, a number of reposition antiviral drugs such as Favipiravir (a nucleoside analogue inhibiting the RNA polymerase), ribavirin (a guanosine analogue), are also being tested against moderate to severe SARS-CoV-2 patients [43]. The potential viral targets and clinical status of these therapeutic options are shown in Fig. 3 and Table 1.
In addition to the antiviral treatment options, systemic transfusion of convalescent plasma collected from healthy donors who recovered from SARS CoV-2 is being tested in different clinical trials on severely infected SARS-Cov-2 patients to reduce the cytokines storm and to replenish the patient’s own antibodies during the acute phase of the disease. Interestingly, the administration of convalescent plasma containing neutralizing antibodies showed a significant decline in the viral load within few days post-transfusion and a substantial improvement in the clinical conditions of the patients [44]. Several companies and universities, such as Takeda, Mount Sinai, and Hopkins are evaluating the mass-production of monoclonal antibodies. Importantly, the success of the convalescent sera transfusion has given clues on how the immune system combats SARS-CoV-2, and how easily a vaccine can be made. In addition, virus-specific neutralizing antibodies that can accelerate the virus clearance and/or prevent its entry into target cells can serve as the primary mechanism for the restriction and clearance of the virus (Fig. 3).
Vaccine for SARS-CoV-2
With the challenges known to be associated with generating a vaccine against RNA viruses, experts feel that developing an efficacious vaccine for SARS-CoV-2 will be very challenging [45]. RNA viruses are known to be difficult when it comes to vaccine development; however, more than 100 research groups, including biotech companies and research institutes, are currently evaluating different approaches [46]. While some of these vaccines have initiated human trials [44] (Table 2), according to the latest data, some vaccine candidates such as ChAdOxa nCoV-19 (containing spike protein to boost antibodies production against spike protein), and Gam-COVID-Vac Lyo have shown an effective single-dose immune response in clinical trials [44]. However, most experts estimate that a successful vaccine will not be available before 2021.
Table 2.
Emerging investigational vaccine trials to treat SARS-CoV-2 patients
| Vaccines | Company | MoA | Comment/status |
|---|---|---|---|
| Targeted SARS-CoV-19 antibodies | Tsinghua University in Beijing, China | Neutralize SARS-CoV-2 virion to infect cells |
Specific antibodies can inactivate the viral particle, which eventually could be helpful in treating COVID-19 patients No clinical trial yet |
| ChAdOxa nCoV-19 vaccine | Oxford University | Immune system |
Spike protein of SARS-CoV-2 expressed in harmless common cold adenovirus Vaccine candidate showed effective immune response in clinical trial |
| Gam-COVID-Vac Lyo | Gamaleya Research Institute, Russia | Immune system |
Gam-COVID-Vac Lyo”, is a viral vector-based vaccine-fused with the spike protein of SARS-CoV-2 to stimulate the immune response Vaccine showed effective single-dose immune response in clinical trial |
| mRNA-1273 | Moderna, Inc. | Binds to SARS-CoV-2 RNA |
Entered in clinical testing Phase-2 No clinical efficacy data yet |
| DNA vaccines | Inovio | Immune system |
DNA plasmid expressing S (spike) protein Presently at phase-1 trial |
| Virus-like particles | CanSino Biologicals | Immune system |
Developing vaccine by expressing S (spike) protein in adenovirus Presently at the pre-clinical stage |
| Live attenuated vaccine | Soligenix and University of Hawaii | Immune system |
Live inactivated vaccines are challenging to grow and scale-up Presently at pre-clinical stage |
|
Repurposed vaccines Bacille Calmette-Guerin |
Assiut University | Immune system |
Trial underway against SARS-CoV-2 No clinical data yet |
The development of an effective vaccine is the ultimate solution to control this battle; however, once a safe and potent vaccine becomes available, it will be vital to make it accessible to everyone who needs it. Therefore, regulatory agencies need to align with R & D to fast-track the pre-clinical and clinical evaluation, regulatory approvals and mass production of vaccine for worldwide distribution for all populations.
Although several repositioned drugs are being tested against SARS-CoV-2 and most of these drugs have already been approved for another disease. This indicates that these drugs do not act specifically against human SARS-CoV-2 and have not been tested against COVID-19 in animal models, though that would usually require FDA approvals. Another factor should also be considered: the clinical trials performed to get approval for other diseases often does not evaluate combinations with other drugs. So, we feel that the potential for synergistic toxicity needs to be evaluated before such ‘repositioned’ drugs approved for SARS-CoV-2 treatment regimes. Considering the pandemic situation, evaluation of therapeutic molecules and vaccine candidates against this emerging infection is a crucial step in the management of SARS-CoV-2 disease, which seems to be key in combating pandemics; however, regulatory agencies must require to check with developers of vaccine candidates and repositioned drugs for potential efficacy and safety evaluation in animal studies.
Conclusions
Despite the fact that the number of new SARS-CoV-2 cases have started to slow down in many countries, health experts and epidemiologists are warning that we are still in the early stages of the pandemic. A complete return to normal life will only be possible once a vaccine is found and made available to everyone, which seems still at a very early stage of development and will require more time. In the meantime, some effective therapeutic options are urgently required to control the COVID-19 pandemic and give hope to save human lives. We have highlighted here the current status of the therapeutic tools used in the battle against COVID-19. While FDA is approving different clinical trials to fast-track the efficacy assessments of different anti-viral drugs or drugs combination, gathering quality clinical data will be vital to ensure the safety and efficacy. The current battle against COVID-19 pandemic also emphasizes the need for policies for being better equipped for any future pandemic, which includes increased funding to drugs and vaccines development, kits development, testing facilities, and fast-track FDA approval policies.
Supplementary information
Additional file 1: Figure S1. Worldwide reported SARS-CoV-19 cases and deaths. Top 10 massively impacted countries by SARA-Cov-19 (A). Worldwide infection and mortality graph of SARS-CoV-2 (B), estimated infection rates of common viral outbreaks (C).
Abbreviations
- COVID-19
Coronavirus disease of 2019
- CoV
Coronavirus
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- WHO
World Health Organization
- CI
Confidence interval
- MERS-CoV
Middle East Respiratory Syndrome Coronavirus
- SARS-CoV
Severe acute respiratory syndrome coronavirus
- CFR
Case fatality rate
- ORFs
Open reading frames
- S
Spike
- E
Envelope
- M
Membrane
- N
Nucleocapsid
- ACE
Angiotensin-converting enzyme
- ACE2
Angiotensin-converting anzyme 2
- TMPRSS2
Transmembrane protease serine-2
- RCT
Replication transcription complex
- ARBs
Angiotensin II type-I receptor blockers
- HIV
Human Immunodeficiency Viruses
- FDA
Food and Drug Administration
Authors’ contributions
All authors contributed to conceptualization and data curation. MK wrote the first draft. SAK reviewed the data and the final manuscript. All authors read and approved the final manuscript.
Funding
This work was financially supported by funds from Sidra Medicine to Dr. Souhaila Al Khodor.
Availability of data and materials
Not applicable
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have reviewed the final version of the manuscript and approved it for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12967-020-02520-8.
References
- 1.Organization WH: Rolling updates on coronavirus disease (COVID-19). 2020. https://wwww.hoint/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen.
- 2.Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organization WH: WHO Timeline—COVID-19. 2020. https://www.who.int/news-room/detail/27-04-2020-who-timeline—covid-19.
- 4.Koo JR, Cook AR, Park M, Sun Y, Sun H, Lim JT, Tam C, Dickens BL. Interventions to mitigate early spread of SARS-CoV-2 in Singapore: a modelling study. Lancet Infect Dis. 2020. [DOI] [PMC free article] [PubMed]
- 5.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park M, Cook AR, Lim JT, Sun Y, Dickens BL. A systematic review of COVID-19 epidemiology based on current evidence. J Clin Med. 2020;9:4. doi: 10.3390/jcm9040967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 8.Drosten C, Gunther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 9.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong NS, Zheng BJ, Li YM, Xie ZH, Chan KH, Li PH, Tan SY, Chang Q, Xie JP, et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet. 2003;362(9393):1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haagmans BL, Al Dhahiry SH, Reusken CB, Raj VS, Galiano M, Myers R, Godeke GJ, Jonges M, Farag E, Diab A, et al. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis. 2014;14(2):140–145. doi: 10.1016/S1473-3099(13)70690-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO: Coronavirus disease 2019 (COVID-19) situation report–55. In., March 15, 2020 edn. 2020 https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200315-sitrep-55-covid-19.pdf?sfvrsn=33daa5cb_8.
- 14.Khafaie MA, Rahim F. Cross-country comparison of case fatality rates of COVID-19/SARS-COV-2. Osong Public Health Res Perspect. 2020;11(2):74–80. doi: 10.24171/j.phrp.2020.11.2.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Licastro D, Rajasekharan S, Dal Monego S, Segat L, D’Agaro P, Marcello A, Regione FVGLGoC. Isolation and full-length genome characterization of SARS-CoV-2 from COVID-19 cases in Northern Italy. J Virol. 2020. [DOI] [PMC free article] [PubMed]
- 18.Wu A, Peng Y, Huang B, Ding X, Wang X, Niu P, Meng J, Zhu Z, Zhang Z, Wang J, et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27(3):325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu C, Liu Y, Yang Y, Zhang P, Zhong W, Wang Y, Wang Q, Xu Y, Li M, Li X et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020. [DOI] [PMC free article] [PubMed]
- 20.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaimes JA, Millet JK, Whittaker GR. Proteolytic cleavage of the SARS-CoV-2 spike protein and the role of the novel S1/S2 site. IScience. 2020;23(6):101212. doi: 10.1016/j.isci.2020.101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J. 2019;16(1):69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CDC: Coronavirus Disease 2019 (COVID-19). In. Edited by Prevention CfDCa. 2020. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html.
- 24.Vincent JL, Taccone FS. Understanding pathways to death in patients with COVID-19. Lancet Respir Med. 2020. [DOI] [PMC free article] [PubMed]
- 25.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020. [DOI] [PMC free article] [PubMed]
- 26.AA: Chronic illness raises risk from COVID-19: Expert. https://www.aacomtr/en/health/chronic-illness-raises-risk-from-covid-19-expert/1791249. vol. 03.04.2020. Yesim Sert Karaaslan; 2020.
- 27.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guan WJ, Zhong NS. Clinical Characteristics of Covid-19 in China. Reply. N Engl J Med. 2020. [DOI] [PubMed]
- 29.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94:7. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Organization. WH: ‘Solidarity’ clinical trial for COVID-19 treatment. In., March 20, 2020 edn. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments. WHO; 2020.
- 31.Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, Siegel D, Perron M, Bannister R, Hui HC, et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531(7594):381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elfiky AA. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248:117477. doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.SILVERMAN E: New data on Gilead’s remdesivir, released by accident, show no benefit for coronavirus patients. Company still sees reason for hope. Stat News. 2020.
- 34.Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment.
- 35.Savarino A, Di Trani L, Donatelli I, Cauda R, Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6(2):67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kupferschmidt K, Cohen J. Race to find COVID-19 treatments accelerates. Science. 2020;367(6485):1412–1413. doi: 10.1126/science.367.6485.1412. [DOI] [PubMed] [Google Scholar]
- 37.Christian Funck-Brentano J-ES. Chloroquine or hydroxychloroquine for COVID-19: why might they be hazardous? Lancet 2020. [DOI] [PMC free article] [PubMed]
- 38.Organization WH: WHO suspends hydroxychloroquine trials for COVID-19. 2020. https://www.aa.com.tr/en/latest-on-coronavirus-outbreak/who-suspends-hydroxychloroquine-trials-for-covid-19/1853478.
- 39.Chandwani A, Shuter J. Lopinavir/ritonavir in the treatment of HIV-1 infection: a review. Ther Clin Risk Manag. 2008;4(5):1023–1033. doi: 10.2147/tcrm.s3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L, Lin D, Kusov Y, Nian Y, Ma Q, Wang J, von Brunn A, Leyssen P, Lanko K, Neyts J, et al. alpha-Ketoamides as broad-spectrum inhibitors of coronavirus and enterovirus replication: structure-based design, synthesis, and activity assessment. J Med Chem. 2020;63(9):4562–4578. doi: 10.1021/acs.jmedchem.9b01828. [DOI] [PubMed] [Google Scholar]
- 41.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trials C: COVID-19. 2020. https://clinicaltrialsgov/ct2/results?cond=COVID-19&term=drugs&cntry=&state=&city=&dist=&Search=Search.
- 43.Chan KW, Wong VT, Tang SCW. COVID-19: an update on the epidemiological, clinical, preventive and therapeutic evidence and guidelines of integrative Chinese-Western medicine for the management of 2019 novel coronavirus disease. Am J Chin Med. 2020;48(3):737–762. doi: 10.1142/S0192415X20500378. [DOI] [PubMed] [Google Scholar]
- 44.Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, Wang F, Li D, Yang M, Xing L et al: Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020. [DOI] [PMC free article] [PubMed]
- 45.Jiang S. Don’t rush to deploy COVID-19 vaccines and drugs without sufficient safety guarantees. Nature. 2020;579(7799):321. doi: 10.1038/d41586-020-00751-9. [DOI] [PubMed] [Google Scholar]
- 46.Busniness S: LIVE BLOG: R&D response to COVID-19 pandemic. https://sciencebusiness.net/covid-19/news/live-blog-rd-response-covid-19-pandemic. Accessed 13 May 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Worldwide reported SARS-CoV-19 cases and deaths. Top 10 massively impacted countries by SARA-Cov-19 (A). Worldwide infection and mortality graph of SARS-CoV-2 (B), estimated infection rates of common viral outbreaks (C).
Data Availability Statement
Not applicable