Abstract
Catheter ablation is a rapidly expanding and evolving field. The advent of interventional techniques and advances in technology have allowed catheter ablation to supplant antiarrhythmic surgery for ventricular arrhythmia treatment. However, issues related to access and energy delivery limit the use of catheter ablation in some cases. Hybrid catheter-based and surgical techniques represent a novel approach to overcome these limitations. The hybrid technique combines the strengths and minimises the limitations of either catheter or surgical ablation alone. There is a growing body of evidence in the literature supporting the safety and efficacy of the hybrid surgical technique. This review aims to provide an overview of hybrid surgical-catheter ablation for ventricular arrhythmia.
Keywords: Ventricular tachycardia, hybrid surgical ablation, epicardial access, subxiphoid approach, limited anterior thoracotomy, left ventricular assist device
Catheter-based ablation has been a well-established tool in the treatment of ventricular tachycardia (VT). However, the effectiveness of catheter ablation may be limited by its ability to access sites of arrhythmogenic tissue and achieve adequate lesion size in target areas without risking collateral damage. Antiarrhythmic surgery would be an effective alternative in such situations. Despite the potential usefulness of arrhythmia surgery, major drawbacks include invasiveness, prolonged hospital stays, higher morbidity and potential mortality.[1] These limitations have been partially overcome with the development of minimally invasive surgical approaches and the integration of surgical and catheter-based approaches. We define hybrid ablation as an approach that combines surgical intervention for access and/or ablation, along with catheter mapping and ablation. In this article, we review hybrid techniques for VT ablation, discuss the role of hybrid ablation techniques in the contemporary management of VT and review the outcomes of hybrid approaches. We specifically review issues regarding patient selection, specific procedural considerations, safety and future directions.
A Historical Perspective of Interdisciplinary Collaboration
In the 1970s and 1980s, cardiac electrophysiological (EP) testing was extensively used for diagnosis and mapping of arrhythmias before or during cardiac surgery.[2,3] With advancing EP knowledge and techniques, antiarrhythmic surgery was revolutionised (Figure 1). The foundation of this collaboration began with the Wolff-Parkinson-White (WPW) syndrome in which EP testing assisted in confirming the arrhythmia mechanism and determining the number and approximate location of accessory pathways.[4,5] This expanded to include the encircling endocardial ventriculotomy introduced in 1978 for post-MI VT, the subendocardial resection procedure in 1979 for recurrent VT and the right ventricular disconnection procedures developed in the early 1980s for arrhythmogenic right VT.[6–8] Furthermore, the introduction of steerable catheters in the 1980s and the development of 3D navigation systems in the last two decades allowed detailed EP mapping in a shorter time, which significantly improved preoperative planning for patients undergoing surgical approaches.
Figure 1: Timeline of Advances in Arrhythmia Diagnosis and Management.

Patient Selection
Hybrid ablation combines the advantages of percutaneous endocardial and epicardial catheter-based procedures and those of arrhythmia surgery. In 1996, Sosa et al. initially described a subxiphoid percutaneous epicardial approach for epicardial mapping and ablation in patients with Chagas cardiomyopathy and associated VT secondary to epicardial substrates.[9] The procedure has since been widely adopted in the management of ventricular arrhythmias in complex substrates. Epicardial approaches are often necessary for mapping/ablation of epicardial substrates, such as in patients with non-ischaemic cardiomyopathy including arrhythmogenic right ventricular cardiomyopathy, but also in order to provide an additional vantage point for creation of larger ablation lesions in patients with intramural substrate. However, safe percutaneous epicardial access may not be feasible in some patients. Some of the clinical scenarios where a hybrid approach may be necessary are listed below. These include situations where safe epicardial access may not be feasible and situations where power delivery to the area of interest would be insufficient.
Patients with extracardiac anatomical challenges, such as overlaying bowel loops, severe pectus deformity, rendering epicardial access prohibitively high risk.
Patients with extensive adhesions from previous open-heart surgery, pericarditis or epicardial procedures.[10] Even with successful percutaneous access in patients with previous cardiac surgery, success rates are lower and the risk of complications can be significant.[11] In addition to the limited catheter manipulation by adhesions, the adhesions themselves are sometimes vascular and catheter manipulation may lead to intrapericardial bleeding.
The myocardial tissue that needs to be targeted is in close proximity to critical structures, particularly the proximal coronary arteries making catheter ablation too high risk (Figure 2).
Figure 2: Coronary Angiography View Demonstrating an Externally-Irrigated Ablation Catheter Tip.
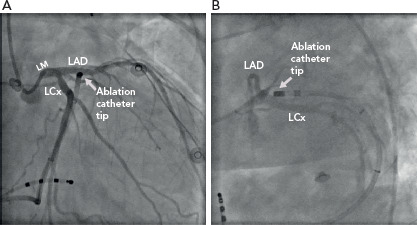
The catheter tip is seen in the great cardiac vein – anterior interventricular vein junction at the site of earliest activation for the culprit ventricular arrhythmia. Note the proximity to the coronary arteries in both the right anterior oblique (A) and left anterior oblique (B) views. LAD = left anterior descending coronary artery; LCx = left circumflex coronary artery; LM = left main coronary artery.
Need for epicardial mapping/ablation in patients with prior coronary artery bypass grafting (CABG). Even though percutaneous epicardial access in these patients is feasible, catheter manipulation and ablation carry a risk of graft disruption.[12] Also, in patients with coronary artery disease, catheter manipulation during mapping and ablation can disrupt the bridging veins that traverse the pericardium to the myocardium as natural bypasses. Thus, the procedure in those patients carries a risk of infarction.[12]
Arrhythmogenic substrate or arrhythmia origin deep within the myocardium and below epicardial fat may not be amenable to catheter ablation via traditional percutaneous epicardial access. Direct visualisation is possible with surgical access and can permit epicardial fat dissection and surgical ‘unroofing’ of the target myocardium.
Inaccessible left ventricle (LV) due to mechanical aortic and mitral valves. While percutaneous transapical, transventricular septal or atrioventricular septal puncture can be performed, these approaches are limited to endocardial mapping and ablation. Given that such patients tend to have non-ischaemic substrate with perivalvular/epicardial substrate, an approach for epicardial mapping and ablation would be desirable.[13]
Surgical Approach
The surgical approach should be individualised and based on the likely origin/exit of the arrhythmia as determined by scar location on preprocedural imaging (typically MRI), non-invasive mapping, or prior invasive mapping. In particular, delineation of the arrhythmogenic substrate with preprocedural delayed-enhancement MRI has been associated with improved procedural and long-term outcomes in patients with non-ischaemic cardiomyopathy undergoing catheter ablation and this may also apply to hybrid procedures.[14] While median sternotomy can provide wide access to the epicardial surface, this may be unnecessary and is associated with increased procedural time and morbidity (Figure 3). Therefore, in the absence of need for concomitant cardiac surgery, two main approaches are currently in use: subxiphoid window and limited anterior thoracotomy (Figure 4). Patient selection for each approach is critical due to the difference in LV epicardial exposure between the two.
Figure 3: Picture Demonstrating Median Sternotomy with Direct Cryoprobe Application Over an Epicardial Region of Scar in a Patient with Ventricular Tachycardia.

The patient had undergone concomitant mitral valve repair.
Figure 4: Schematic Demonstration of Hybrid Surgical Ablation Via a Sub-xiphoid Window (A) and Limited Anterior Thoracotomy (B).

Source: Mayo Foundation for Medical Education and Research. Reproduced with permission from Mayo Foundation for Medical Education and Research. All rights reserved.
Soejima et al. described a subxiphoid approach for VT mapping and ablation that provides access to the inferior and inferolateral LV.[15] After dissecting down to the diaphragmatic pericardial surface, the pericardium is opened and under direct visualisation, lysis of any adhesions is performed with blunt dissection to fully expose the diaphragmatic and posterior epicardium. This approach is suitable for patients with predominately inferior or basal inferolateral scars.
Limited anterior thoracotomy provides access to the anterior, mid to apical anterolateral wall and the true apex. As such, it is suitable for individuals with previous scarring in the left anterior descending coronary artery territory. Typically, the procedure is performed under general anaesthesia. A limited left anterior incision is performed at the target intercostal space and extended through the subcutaneous tissue and fascia.[16] The pericardium is then dissected off the LV laterally to maximally expose the area of interest.
A case report described a successful VT ablation using a transabdominal endoscopic approach using an incision through the central tendon of the diaphragm with concomitant use of an Impella haemodynamic support device (Abiomed) which was felt to be less invasive than performing a subxiphoid window.[17] Another minimally invasive option that provides broad access to the epicardium includes a lateral thoracoscopic approach using one-sided threeport thoracoscopy.[18,19]
After the epicardium has been surgically exposed, the surgeon or electrophysiologist can proceed with direct catheter mapping and ablation with the guidance of a recording system and a 3D electroanatomic mapping system.
Depending on institutional practice and resources, such hybrid procedures can be performed either in the EP laboratory or in the operating room. This was demonstrated in a study of 14 patients who underwent hybrid surgical epicardial ablation with surgical access in the EP laboratory.[16] However, whether this practice can be widely adopted depends on the availability of resources and expertise.
Electroanatomical Mapping
Surgical access allows epicardial mapping of ventricular arrhythmias during the hybrid surgical procedure. LV access could be obtained via the transseptal or retrograde aortic approach in cases of concomitant endocardial mapping. 3D electroanatomical mapping systems integrate anatomy with electrophysiology. These systems enable the display of catheter position in real time and permit geometrical reconstruction of the chamber of interest. Signals for voltage and activation mapping can be annotated simultaneously during baseline rhythm or tachycardia. Since their inception, 3D electroanatomical systems have facilitated the reconstruction of complex anatomical and EP considerations during procedures and increased safety, efficacy and efficiency of ablation compared with only using fluoroscopy.[20] Of note, electroanatomic mapping and EP recording systems may not be readily available in the surgical suite. Portable systems are available but require knowledge and experience to set up and for troubleshooting.
Safety and Outcomes of Hybrid Ventricular Tachycardia Ablation
Several case reports and series have been published demonstrating the feasibility of the hybrid procedures.[10,15,16,21] In 2004, Soejima et al. described their experience using the subxiphoid surgical window in six people with VT with prior cardiac surgery or failed percutaneous pericardial access.[15] The study demonstrated the safety and feasibility of the approach. Access to the pericardium was successful in all patients. No complications were reported apart from transient chest pain consistent with pericarditis. After a follow-up period ranging from 106 to 675 days, two of the six patients had recurrent, though infrequent, VT.
In 2010, a multicentre study assessed the safety of epicardial VT ablation in 134 patients. Percutaneous subxiphoid approach failed in 10% of the cases (n=15). A surgical window for epicardial exposure was performed via a subxiphoid approach in 14 patients with no specific complications reported in this group.[10] On follow-up, 95 of the 134 patients (71%) achieved freedom from recurrence after 23 (±21) months.
A recent study involved five patients who underwent hybrid ablation for recurrent sustained VT using combined endocardial and epicardial mapping and radiofrequency ablation in a hybrid operating room. Surgical approaches included anterolateral thoracotomy, one-sided three-port lateral thoracoscopy and sternotomy. After a mean follow-up of 18 months, three patients remained VT free with two of them using antiarrhythmics. One patient with recurrent VT required increasing amiodarone dose for arrhythmia control and one patient required a redo ablation 21 months after the initial procedure. There were no periprocedural complications.[18]
While these published experiences demonstrate the feasibility and safety of hybrid ablation in centres with experienced operators, no randomised studies have assessed whether this approach is superior to traditional catheter-based ablation in terms of long-term arrhythmia control. In a propensity-matched comparison of 38 patients who underwent hybrid epicardial ablation compared with patients who underwent percutaneous epicardial ablation, there was no significant difference in long-term outcomes.[22]
Endoscopic Robotic Ventricular Tachycardia Ablation
While robotically assisted endoscopic coronary artery bypass surgery was established as an alternative to the standard median sternotomy approach over the past two decades, its application in arrhythmia surgery had to await the evolution of technology. One of the most challenging scenarios electrophysiologists might face is the approach to arrhythmias arising from the LV summit. This region comprises the most superior portion of the LV epicardium, near the bifurcation of the left main coronary artery into the left anterior descending and left circumflex coronary artery. The complexity of the relationship between the LV summit and surrounding structures may limit the feasibility of safe ablation in this area. Access to the most basal aspect of the LV summit is also frequently limited by epicardial fat. As first described by Mulpuru et al., robotically assisted surgery could be used for these complex arrhythmias as they describe a successful case of resistant VT originating from the LV summit with a robotically assisted endoscopic mapping followed by a mini-thoracotomy and cryoablation.[23] This was expanded upon by Aziz et al. with a totally endoscopic LV summit premature ventricular complex ablation performed using a duodecapolar catheter for mapping coupled with an externally irrigated radiofrequency ablation catheter.[24] Such approaches allow for excellent visualisation of the coronary arteries in this sensitive area; furthermore, surgical dissection may permit the surgeon to mobilise adjacent arteries to allow safer delivery of ablative energy. This approach can offer an alternative option for targeting these challenging arrhythmias when standard approaches have failed. However, considerable operator skill and experience is required, especially if it is performed ‘off-pump’.
VT Ablation in the Setting of Left Ventricular Assist Device
People with advanced heart failure have a high incidence of ventricular arrhythmias. While left ventricular assist device (LVAD) implantation is associated with increased survival compared with conventional therapy in carefully selected patients, postoperative VT is common and may occur de novo in one-third of patients, which may be related to the new substrate associated with the apical core incisions but also due to preexisting substrate.[25] In LVAD patients, VT is associated with increased morbidity and mortality.[26] Practical considerations regarding VT ablation in LVAD patients include crossing the aortic valve in the absence of complete valve opening due to loss of significant flow across the valve, or in some cases, over-sewing of valve leaflets.[27] This, however, can be largely circumvented by accessing the ventricle using a transseptal approach.
While feasible, endocardial catheter ablation in patients with LVAD can be high risk. Therefore, mapping and ablation at the time of LVAD implantation surgery represent a novel approach to the management of this high-risk population. A large number of these patients have had previous endocardial mapping and this information can be used in conjunction with epicardial mapping intraoperatively. While mapping and ablation can typically be performed at the time of the surgery, often the sternum is not immediately closed after LVAD implantation.[28] This period represents another opportunity for epicardial mapping and ablation in patients with suspected epicardial VT or substrate.[29] Although adhesions limit percutaneous pericardial access after LVAD implantation, case reports have described ablation after LVAD implantation using lateral thoracotomy or subxiphoid approaches as has been described following non-LVAD cardiac surgery.[30,31]
Procedural Considerations for Hybrid Ablation
A thorough evaluation and multidisciplinary approach including cardiac electrophysiologists, cardiothoracic surgeons, anaesthesiologists, perfusionists and allied health staff are critical in the care of patients before hybrid ablation. These important procedural aspects should be considered and addressed.
Preprocedural imaging is critical in order to define the distribution of the arrhythmogenic substrate (scar) and help determine the optimal hybrid approach. In most cases, cardiac MRI with delayed enhancement protocol will offer the most sensitive evaluation of the extent and location of scarring (Figure 5). Contrast cardiac CT scans can also be used.
Figure 5: Cardiac MRI Short-Axis Views Demonstrating Transmural Delayed Enhancement.

Transmural delayed enhancement involves the apical anterior and lateral walls with extension along the subendocardium in the extreme apex into the inferior wall (bottom panels). Subepicardial and mesocardial scarring is noted with subendocardial sparing along the apical septum and inferior wall. Additionally, confluent subepicardial scarring is noted involving the basal (top panel) and mid (middle panel) ventricular anterolateral and inferolateral walls, with more discrete transmural scarring involving the basal inferolateral wall.
Patients with a history of CABG should be evaluated with CT or invasive coronary angiography to determine the vessel course to assess the anatomic relationship to the selected surgical approach. CT may be effective in excluding large vessel stenosis/occlusion such as in graft vessels. However, its diagnostic value may be more limited for native vessels and invasive coronary angiography may be required.
In cases where an anterior thoracotomy is used, precordial ECG lead placement may be displaced from the standard position due to the incision line and this could affect ECG interpretation during mapping. The leads should be placed as close to the normal position as possible.With the limited anterior thoracotomy approach, single lung ventilation may be needed to allow for the appropriate exposure. Therefore, patients with severe respiratory lung disease may not be suitable candidates.
With severe pericardial adhesions, the surgeon may need to re-enter the thorax at a different intercostal space to extend exposure.[16]
In cases using minimally invasive access, such as robotic access, air accumulation in the pericardial space may increase the defibrillation threshold significantly. As such, one should be ready to deliver implantable cardioverter defibrillators (ICD) shocks, if available, or to evacuate air promptly to permit external defibrillation.[32]
The metal retractors used for surgical access can interfere with the electroanatomic mapping systems and consequently prohibit mapping in certain areas of the heart.
Ablation tools commonly used in the surgical setting use either cryoablation energy or radiofrequency. Cryothermy uses either Argon gas (CryoFlex, Medtronic) or nitrous oxide (cryoICE, AtriCure) to achieve rapid cooling temperature and deliver deep lesions. Flexible cryoprobes have the advantage of delivering tailored lesions over a wider area compared with radiofrequency. Some radiofrequency ablation devices, such as Cardioblate iRF (Medtronic) and Isolator (AtriCure) allow for mapping as well as ablation at the same time, which is not feasible with large cryoprobes.
Haemodynamic decompensation from prolonged procedural time or during activation mapping of VT is a major limitation. While vasopressor and inotropic support are often used, these are frequently insufficient.[33] Therefore, preprocedural risk stratification to assess for the need of potential mechanical circulatory support (MCS) is essential. Patients with advanced age, marked left ventricular dysfunction and comorbidities such as chronic obstructive pulmonary disease and diabetes are at particular risk for haemodynamic decompensation.[34] MCS can be instituted at the beginning of the procedure, especially when activation mapping of unstable VTs is considered necessary. Alternatively, MCS may be established ad hoc, though it should be noted that the outcomes of urgent or emergent MCS for haemodynamic compromise during VT ablation procedures are poor. Short-term MCS options include intra-aortic balloon pump, Impella, TandemHeart (TandemLife) and extracorporeal membrane oxygenation (Table 2).[35–37]
Table 2: Overview of Mechanical Circulatory Support Devices for Haemodynamic Support During Ventricular Tachycardia Ablation.
| Device | Mechanism | Advantages | Disadvantages |
|---|---|---|---|
| IABP | Diastolic aortic augmentation with LV afterload reduction |
|
|
| Impella | LV to ascending aorta pump |
|
|
| TandemHeart | LA (via transseptal puncture) to femoral artery bypass |
|
|
| ECMO | Cardiopulmonary bypass |
|
|
ECMO = extra-corporeal membrane oxygenation; IABP = intra-aortic balloon pump; LV: left ventricle; RV: right ventricle; VT: ventricular tachycardia.
Post-operative pain is a major issue and may be related to the route of access – such as sternotomy, intercostal access or rib retraction – tissue injury from ablation with associated inflammation and surgical drain sites. Inadequate management of post-operative pain prolongs the rehabilitation period and worsens patient-reported outcomes.[38] As such, post-operative pain assessment and appropriate management are crucial.
Management includes multimodal analgesia such as non-steroidal anti-inflammatory drugs (NSAIDs), acetaminophen, as well as opioid and non-opioid analgesics; occasionally, regional anaesthesia may be used for refractory and prolonged pain.[39] Pericarditis is also common following epicardial ablation and measures aimed at reducing pericardial inflammation should be instituted; these predominantly include NSAIDs and colchicine. In certain circumstances, intrapericardial or systemic steroid use and intrapericardial lidocaine may be used.[40–42]
Emerging Technologies in Ventricular Tachycardia Ablation
Though hybrid surgical ablation has provided another tool for the treatment of ventricular arrhythmias, emerging techniques may reduce the reliance on this approach. These remain off label and are mainly relegated to use in tertiary referral centres. Such techniques include needle-based catheter ablation, radiotherapy ablation, bipolar ablation and transcoronary or retrograde venous ethanol ablation (Table 1).[43–53]
Table 1: Emerging Technologies in Ventricular Arrhythmia Ablation.
| Procedures | Technique | Advantages | Disadvantages |
|---|---|---|---|
| Needle-based catheter ablation | Needle deployed through irrigated ablation catheter – distance determined by operator[1] Mapping and ablation via the needle tip can be performed |
Targeting deep intramyocardial or epicardial substrate | Limited experience – still under investigation Potential for extensive myocardial damage and cardiac perforation[2] |
| High-power bipolar ablation | A second ablation catheter is used as the grounding connection in place of a grounding patch thereby theoretically preventing radiofrequency energy dispersal | Allows the delivery of high-power radiofrequency energy creating deep lesions in between the two catheters[3] Reportedly achieves larger lesions and transmurality[4] |
Trial showed higher adverse events[5] |
| Radiotherapy ablation | Delivery of radiation with stereotactic body radiation therapy to induce myocardial cell death | Non-invasive Short duration – permits outpatient therapy |
Dose-dependent adverse effects on left ventricular function[6] Limited long-term studies[7,8] |
| Transcoronary ethanol ablation | Ethanol injection into the coronary branch supplying the arrhythmogenic substrate Retrograde venous ethanol ablation is an alternative to avoid instrumentation of coronary arteries[9] |
Useful for septal ventricular tachycardia with no endocardial origin and also in some cases of epicardial inaccessibility[10] | Limited by coronary anatomy Risk of complete heart block[11] Limited studies[12] |
Conclusion
The evolution of VT ablation techniques is associated with expanding indications. The choice should be guided primarily by the clinical scenario. Hybrid surgical ablation may be viewed as a form of synergism that combines the advantages of EP and surgery. There is now a significant body of evidence suggesting the safety and feasibility of hybrid surgical ablation of VT. Appropriate patient selection is key. Therefore, providers should be fully aware of the indications and caveats associated with this technique.
Clinical Perspective
An increasing body of evidence suggests that hybrid surgical ablation is a safe, effective and feasible technique.
Hybrid ablation should be considered in patients with pericardial adhesions, deep myocardial substrate, proximity to critical structures, inaccessible LV and extra-cardiac anatomic challenges.
A multidisciplinary approach including cardiac electrophysiologists, cardiothoracic surgeons, anaesthesiologists, perfusionists and allied health staff is critical in the care of these patients.
References
- 1.Niebauer MJ, Kirsh M, Kadish A et al. Outcome of endocardial resection in 33 patients with coronary artery disease: correlation with ventricular tachycardia morphology. Am Heart J. 1992;124:1500–6. doi: 10.1016/0002-8703(92)90063-2. [DOI] [PubMed] [Google Scholar]
- 2.Josephson ME, Horowitz LN, Farshidi A et al. Recurrent sustained ventricular tachycardia. 2. Endocardial mapping. Circulation. 1978;57:440–7. doi: 10.1161/01.CIR.57.3.440. [DOI] [PubMed] [Google Scholar]
- 3.Waspe LE, Brodman R, Kim SG et al. Activation mapping in patients with coronary artery disease with multiple ventricular tachycardia configurations: occurrence and therapeutic implications of widely separate apparent sites of origin. J Am Coll Cardiol. 1985;5:1075–86. doi: 10.1016/S0735-1097(85)80007-3. [DOI] [PubMed] [Google Scholar]
- 4.Gallagher JJ, Pritchett EL, Sealy WC et al. The preexcitation syndromes. Prog Cardiovasc Dis. 1978;20:285–327. doi: 10.1016/0033-0620(78)90015-4. [DOI] [PubMed] [Google Scholar]
- 5.Josephson ME, Horowitz LN, Farshidi A, Kastor JA. Recurrent sustained ventricular tachycardia. 1. Mechanisms. Circulation. 1978;57:431–40. doi: 10.1161/01.CIR.57.3.431. [DOI] [PubMed] [Google Scholar]
- 6.Guiraudon G, Fontaine G, Frank R et al. Encircling endocardial ventriculotomy: a new surgical treatment for life-threatening ventricular tachycardias resistant to medical treatment following myocardial infarction. Ann Thorac Surg. 1978;26:438–44. doi: 10.1016/S0003-4975(10)62923-2. [DOI] [PubMed] [Google Scholar]
- 7.Josephson ME, Harken AH, Horowitz LN. Endocardial excision: a new surgical technique for the treatment of recurrent ventricular tachycardia. Circulation. 1979;60:1430–9. doi: 10.1161/01.CIR.60.7.1430. [DOI] [PubMed] [Google Scholar]
- 8.Guiraudon GM, Klein GJ, Gulamhusein SS et al. Total disconnection of the right ventricular free wall: surgical treatment of right ventricular tachycardia associated with right ventricular dysplasia. Circulation. 1983;67:463–70. doi: 10.1161/01.CIR.67.2.463. [DOI] [PubMed] [Google Scholar]
- 9.Sosa E, Scanavacca M, d’Avila A, Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996;7:531–6. doi: 10.1111/j.1540-8167.1996.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 10.Sacher F, Roberts-Thomson K, Maury P et al. Epicardial ventricular tachycardia ablation a multicenter safety study. J Am Coll Cardiol. 2010;55:2366–72. doi: 10.1016/j.jacc.2009.10.084. [DOI] [PubMed] [Google Scholar]
- 11.Killu AM, Asirvatham SJ. Percutaneous pericardial access for electrophysiological studies in patients with prior cardiac surgery: approach and understanding the risks. Expert Rev Cardiovasc Ther. 2019;17:143–50. doi: 10.1080/14779072.2019.1561276. [DOI] [PubMed] [Google Scholar]
- 12.Killu AM, Ebrille E, Asirvatham SJ et al. Percutaneous epicardial access for mapping and ablation is feasible in patients with prior cardiac surgery, including coronary bypass surgery. Circ Arrhythm Electrophysiol. 2015;8:94–101. doi: 10.1161/CIRCEP.114.002349. [DOI] [PubMed] [Google Scholar]
- 13.Soejima K, Nogami A, Sekiguchi Y et al. Epicardial catheter ablation of ventricular tachycardia in no entry left ventricle. Circ Arrhythm Electrophysiol. 2015;8:381–9. doi: 10.1161/CIRCEP.114.002517. [DOI] [PubMed] [Google Scholar]
- 14.Siontis KC, Kim HM, Sharaf Dabbagh G et al. Association of preprocedural cardiac magnetic resonance imaging with outcomes of ventricular tachycardia ablation in patients with idiopathic dilated cardiomyopathy. Heart Rhythm. 2017;14:1487–93. doi: 10.1016/j.hrthm.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Soejima K, Couper G, Cooper JM et al. Subxiphoid surgical approach for epicardial catheter-based mapping and ablation in patients with prior cardiac surgery or difficult pericardial access. Circulation. 2004;110:1197–201. doi: 10.1161/01.CIR.0000140725.42845.90. [DOI] [PubMed] [Google Scholar]
- 16.Michowitz Y, Mathuria N, Tung R et al. Hybrid procedures for epicardial catheter ablation of ventricular tachycardia: value of surgical access. Heart Rhythm. 2010;7:1635–43. doi: 10.1016/j.hrthm.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Buchta P, Zembala M, Hawranek M et al. Hybrid ablation of haemodynamically unstable ventricular tachycardia using a transabdominal minimally-invasive approach and percutaneous left ventricular assist device. Kardiol Pol. 2017;75:1210. doi: 10.5603/KP.2017.0219. [DOI] [PubMed] [Google Scholar]
- 18.Aksu T, Erdem Guler T, Yalin K. Successful ablation of an epicardial ventricular tachycardia by video-assisted thoracoscopy. EP Europace. 2015;17:1116. doi: 10.1093/europace/euv012. [DOI] [PubMed] [Google Scholar]
- 19.Vroomen M, Maesen B, La Meir M et al. Hybrid ablation of ventricular tachycardia: a single-centre experience. J Atr Fibrillation. 2019;11:2118. doi: 10.4022/jafib.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medical Advisory Secretariat Advanced electrophysiologic mapping systems: an evidence-based analysis. Ont Health Technol Assess Ser. 2006;6:1–101. [PMC free article] [PubMed] [Google Scholar]
- 21.Maury P, Leobon B, Duparc A et al. Epicardial catheter ablation of ventricular tachycardia using surgical subxyphoid approach. Europace. 2007;9:212–5. doi: 10.1093/europace/eum016. [DOI] [PubMed] [Google Scholar]
- 22.Li A, Hayase J, Do D et al. Hybrid surgical vs percutaneous access epicardial ventricular tachycardia ablation. Heart Rhythm. 2018;15:512–9. doi: 10.1016/j.hrthm.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Mulpuru SK, Feld GK, Madani M, Sawhney NS. A novel, minimally-invasive surgical approach for ablation of ventricular tachycardia originating near the proximal left anterior descending coronary artery. Circ Arrhythm Electrophysiol. 2012;5:e95–7. doi: 10.1161/CIRCEP.112.975284. [DOI] [PubMed] [Google Scholar]
- 24.Aziz Z, Moss JD, Jabbarzadeh M et al. Totally endoscopic robotic epicardial ablation of refractory left ventricular summit arrhythmia: First-in-man. Heart Rhythm. 2017;14:135–8. doi: 10.1016/j.hrthm.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed A, Amin M, Boilson BA et al. Ventricular arrhythmias in patients with left ventricular assist device (LVAD) Curr Treat Options Cardiovasc Med. 2019;21:75. doi: 10.1007/s11936-019-0783-7. [DOI] [PubMed] [Google Scholar]
- 26.Garan AR, Iyer V, Whang W et al. Catheter ablation for ventricular tachyarrhythmias in patients supported by continuous-flow left ventricular assist devices. ASAIO J. 2014;60:311–6. doi: 10.1097/MAT.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 27.Herweg B, Ilercil A, Kristof-Kuteyeva O et al. Clinical observations and outcome of ventricular tachycardia ablation in patients with left ventricular assist devices. Pacing Clin Electrophysiol. 2012;35:1377–83. doi: 10.1111/j.1540-8159.2012.03509.x. [DOI] [PubMed] [Google Scholar]
- 28.Richenbacher WE, Naka Y, Raines EP et al. Surgical management of patients in the REMATCH trial. Ann Thorac Surg. 2003;75:S86–92. doi: 10.1016/S0003-4975(03)00485-5. [DOI] [PubMed] [Google Scholar]
- 29.Patel M, Rojas F, Shabari FR et al. Safety and feasibility of open chest epicardial mapping and ablation of ventricular tachycardia during the period of left ventricular assist device implantation. J Cardiovasc Electrophysiol. 2016;27:95–101. doi: 10.1111/jce.12839. [DOI] [PubMed] [Google Scholar]
- 30.Mathuria NS, Vaseghi M, Buch E, Shivkumar K. Successful ablation of an epicardial ventricular tachycardia using a surgical ablation tool. Circ Arrhythm Electrophysiol. 2011;4:e84–6. doi: 10.1161/CIRCEP.111.965467. [DOI] [PubMed] [Google Scholar]
- 31.Whang W, Patel MR, Iyer V et al. Epicardial catheter ablation through subxiphoid surgical approach in a patient with implanted left ventricular assist device and cannula-related ventricular tachycardia. Circ Heart Fail. 2014;7:868–9. doi: 10.1161/CIRCHEARTFAILURE.114.001487. [DOI] [PubMed] [Google Scholar]
- 32.Yamada T, McElderry HT, Platonov M et al. Aspirated air in the pericardial space during epicardial catheterization may elevate the defibrillation threshold. Int J Cardiol. 2009;135:e34–5. doi: 10.1016/j.ijcard.2008.03.074. [DOI] [PubMed] [Google Scholar]
- 33.Miller MA, Dukkipati SR, Mittnacht AJ et al. Activation and entrainment mapping of hemodynamically unstable ventricular tachycardia using a percutaneous left ventricular assist device. J Am Coll Cardiol. 2011;58:1363–71. doi: 10.1016/j.jacc.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 34.Santangeli P, Muser D, Zado ES et al. Acute hemodynamic decompensation during catheter ablation of scar-related ventricular tachycardia. Circ Arrhythm Electrophysiol. 2015;8:68–75. doi: 10.1161/CIRCEP.114.002155. [DOI] [PubMed] [Google Scholar]
- 35.Peura JL, Colvin-Adams M, Francis GS et al. Recommendations for the use of mechanical circulatory support: device strategies and patient selection. Circulation. 2012;126:2648–67. doi: 10.1161/CIR.0b013e3182769a54. [DOI] [PubMed] [Google Scholar]
- 36.Health Quality Ontario Percutaneous ventricular assist devices: a health technology assessment. Ont Health Technol Assess Ser. 2017;17:1–97. [PMC free article] [PubMed] [Google Scholar]
- 37.Hajjar LA, Teboul JL. Mechanical circulatory support devices for cardiogenic shock: state of the art. Critical Care. 2019;23:76. doi: 10.1186/s13054-019-2368-y. doi: 10.1186/s13054-019-2368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braun LA, Stanguts C, Casanelia L et al. Massage therapy for cardiac surgery patients – a randomized trial. J Thorac Cardiovasc Surg. 2012;144:1453–9, 1459.e1. doi: 10.1016/j.jtcvs.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 39.White PF. Multimodal analgesia: its role in preventing postoperative pain. Curr Opin Investig Drugs. 2008;9:76–82. [PubMed] [Google Scholar]
- 40.Dyrda K, Piers SRD. Taxis CFvHv, et al. Influence of steroid therapy on the incidence of pericarditis and atrial fibrillation after percutaneous epicardial mapping and ablation for ventricular tachycardia. Circ: Arrhythm Electrophysiol. 2014;7:671–6. doi: 10.1161/CIRCEP.113.001148. [DOI] [PubMed] [Google Scholar]
- 41.Weibel S, Jelting Y, Pace NL et al. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery in adults. Cochrane Database Syst Rev. 2018;6:CD009642–CD009642. doi: 10.1002/14651858.CD009642.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adler Y, Charron P, Imazio M et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2015;36:2921–64. doi: 10.1093/eurheartj/ehv318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sapp JL, Beeckler C, Pike R et al. Initial human feasibility of infusion needle catheter ablation for refractory ventricular tachycardia. Circulation. 2013;128:2289–95. doi: 10.1161/CIRCULATIONAHA.113.003423. [DOI] [PubMed] [Google Scholar]
- 44.Sapp JL, Beeckler C, Pike R et al. Initial human feasibility of infusion needle catheter ablation for refractory ventricular tachycardia. Circulation. 2013;128:2289–95. doi: 10.1161/CIRCULATIONAHA.113.003423. [DOI] [PubMed] [Google Scholar]
- 45.Loo BW Jr, Soltys SG, Wang L et al. Stereotactic ablative radiotherapy for the treatment of refractory cardiac ventricular arrhythmia. Circ Arrhythm Electrophysiol. 2015;8:748–50. doi: 10.1161/CIRCEP.115.002765. [DOI] [PubMed] [Google Scholar]
- 46.Zei PC, Soltys S. Ablative radiotherapy as a noninvasive alternative to catheter ablation for cardiac arrhythmias. Curr Cardiol Rep. 2017;19:79. doi: 10.1007/s11886-017-0886-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cuculich PS, Schill MR, Kashani R et al. Noninvasive Cardiac Radiation for Ablation of Ventricular Tachycardia. N Engl J Med. 2017;377:2325–36. doi: 10.1056/NEJMoa1613773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hohmann S, Deisher AJ, Suzuki A et al. Left ventricular function after noninvasive cardiac ablation using proton beam therapy in a porcine model. Heart Rhythm. 2019;16:1710–9. doi: 10.1016/j.hrthm.2019.04.030. [DOI] [PubMed] [Google Scholar]
- 49.Sauer WH, Steckman DA, Zipse MM et al. High-power bipolar ablation for incessant ventricular tachycardia utilizing a deep midmyocardial septal circuit. Heart Rhythm Case Rep. 2015;1:397–400. doi: 10.1016/j.hrcr.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koruth JS, Dukkipati S, Miller MA et al. Bipolar irrigated radiofrequency ablation: a therapeutic option for refractory intramural atrial and ventricular tachycardia circuits. Heart Rhythm. 2012;9:1932–41. doi: 10.1016/j.hrthm.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 51.Tokuda M, Sobieszczyk P, Eisenhauer AC et al. Transcoronary ethanol ablation for recurrent ventricular tachycardia after failed catheter ablation. Circ Arrhythm Electrophysiol. 2011;4:889–96. doi: 10.1161/CIRCEP.111.966283. [DOI] [PubMed] [Google Scholar]
- 52.Kay GN, Epstein AE, Bubien RS et al. Intracoronary ethanol ablation for the treatment of recurrent sustained ventricular tachycardia. J Am Coll Cardiol. 1992;19:159–68. doi: 10.1016/0735-1097(92)90068-X. [DOI] [PubMed] [Google Scholar]
- 53.Tokuda M, Sobieszczyk P, Eisenhauer AC et al. Transcoronary ethanol ablation for recurrent ventricular tachycardia after failed catheter ablation: an update. Circ Arrhythm Electrophysiol. 2011;4:889–96. doi: 10.1161/CIRCEP.111.966283. [DOI] [PubMed] [Google Scholar]


