Abstract
The treatment of AF has evolved over the past decade with increasing use of catheter ablation in patients refractory to medical therapy. While pulmonary vein isolation using endocardial catheter ablation has been successful in paroxysmal AF, the results have been more controversial in patients with long-standing persistent AF where extrapulmonary venous foci are increasingly recognised in the initiation and maintenance of AF. Hybrid ablation is the integration of minimally invasive epicardial ablation with endocardial catheter ablation, and has been increasingly used in this population with better results. The aim of this article was to analyse and discuss the evidence for the integration of catheter and minimally invasive surgical approaches to treat AF with specific focus on convergent ablation and exclusion of the left atrial appendage using a surgically applied clip.
Keywords: AF, ablation, convergent procedure, AtriClip
AF is the most commonly encountered atrial arrhythmia in clinical practice. Restoration of normal sinus rhythm through catheter- and surgically based approaches has been increasingly used as technologies and outcomes have improved.[1] Success rates for AF ablation vary greatly depending on the duration of AF (more successful for paroxysmal AF, less successful in persistent AF and even less so for long-standing persistent AF). The persistent AF population represents a challenging cohort that frequently requires multiple ablation procedures to maintain sinus rhythm.[2] Additionally, the left atrial appendage (LAA) has been implicated as an independent driver of AF arrhythmogenesis, as well as a site responsible for thromboembolism, and this in turn has increased interest in LAA management.[3] In this article, we discuss how the convergent AF procedure and external surgical LAA ligation can be performed through a multidisciplinary approach to manage conventional treatment-refractory persistent AF patients.
The substrate in paroxysmal AF appears to largely originate from the pulmonary veins, and as a result, pulmonary vein isolation (PVI) has demonstrated effectiveness in eliminating AF recurrence in the majority of patients.[4,5] Nevertheless, the success rates for ablation of paroxysmal AF ablation still warrants improvement. The FIRE and ICE trial yielded an approximate 65% freedom from atrial arrhythmias off antiarrhythmics in both the cryoballoon and the radiofrequency ablation arms at 18 months.[6]
Even more recently, the Cryoballoon versus Irrigated Radiofrequency Catheter Ablation: Double Short versus Standard Exposure Duration (CIRCA-DOSE) trial reported only a 51–54% freedom from atrial arrhythmias at 1 year with loop recorder data utilising the latest iterations of contact force-sensing ablation catheters and the second-generation cryoballoon.[7] If one excludes asymptomatic and shorter-lived recurrences, the successful elimination of AF increases to around 80% for both ablation devices. However, PVI alone does not address AF and other atrial arrhythmias originating from regions outside the pulmonary veins. Frequent extra-PV targets of AF ablation include the posterior left atrium, superior vena cava, ligament of Marshall, coronary sinus, crista teminalis and the left atrial appendage.[8,9]
Patients with persistent AF are thought to have arrhythmogenic substrate outside the pulmonary veins, thus explaining poor outcomes in studies with ablation strategies limited to PVI. Substract and Trigger Ablation for Reduction of AF Trial Part II (STAR AF II) compared the strategies of pulmonary vein isolation, PVI with ablation of complex fractionated electrograms and PVI plus linear ablation, and the freedom from AF at 18 months was 59%, 49% and 46%, respectively.[10] Freedom from any atrial arrhythmias off antiarrhythmic therapy was even lower. The pathophysiological mechanisms for persistent and also long-standing persistent AF are frequently more complex than those of paroxysmal AF.
Although still considered a cornerstone of persistent AF ablation, PVI alone does not sufficiently maintain normal sinus rhythm in this population. In the post-STAR AF II era, operators have struggled to address the persistent AF population. In addition to PVI, a variety of supplemental procedures have found clinical application, such as roof and mitral isthmus lines, posterior wall isolation, rotor mapping, ablation of autonomic ganglia, ablation of low-voltage fibrotic regions, high-dose isoproterenol to elicit focal triggers, vein of Marshall alcohol ablation and left atrial appendage isolation.[3,11–17] Unfortunately, no catheter-based approach has consistently yielded a high rate of success in persistent patients.
Surgical Ablation
The first cut-and-sew maze procedure was performed in 1987.[18] Subsequent revisions culminating in the Cox maze III and Cox maze IV have yielded high success rates, maintaining sinus rhythm in 80–90% of patients off antiarrhythmic therapy.[19] However, the surgical maze procedure requires cardiopulmonary bypass and is associated with significantly higher morbidity compared with a catheter-based approach. Minimally invasive epicardial approaches have attempted to replicate the efficacy of the Cox maze procedure, but with less morbidity. In a pooled analysis of minimally invasive epicardial approaches, only 43% of patients with long-standing persistent AF maintained sinus rhythm, as compared with 75% in paroxysmal AF.[20] In comparison, single-procedure freedom from atrial arrhythmias ± antiarrhythmics for catheter-based ablation of long-standing persistent AF was reported to be ~52% at 1 year by Ganesan et al., and ~37% after one or two procedures in the Hamburg experience.[21,22]
The advantage of surgical ablation lies in the surgeon’s ability to directly visualise and ablate the target structures of interest. In addition to endocardial access, the surgeon also has direct access to epicardial structures, such as the ligament of Marshall and ganglionated plexi, that may serve as drivers of persistent AF. Direct visualisation enables ablation while avoiding complications involving the phrenic nerve and the oesophagus.[23] Moreover, the surgeon can exclude the left atrial appendage, which eliminates associated AF triggers while potentially reducing the patient’s risk of stroke.[24]
The AF Catheter Ablation Versus Surgical Ablation Treatment (FAST) trial compared bilateral thoracoscopic epicardial ablation with endovascular catheter ablation randomising 124 patients with drug refractory AF to either surgical PVI using a bipolar radiofrequency (RF) clamp, LAA staple with additional ganglionated plexi ablation, ligament of Marshall, and optional linear ablations lines or endovascular ablation with antral PVI and additional linear ablation. The overall freedom from AF at 12 months was 66% in the surgical arm, as compared with 37% in the catheter ablation arm. The difference was notable even in patients with persistent AF (56% versus 36%; p=0.341). The surgical arm, however, was associated with more procedural complications.[25]
While the cut-and-sew maze creates a definitive scar to isolate and compartmentalise the regions of the atria, less invasive iterations of the maze procedure depends on achieving transmurality, contiguity and durability of the lesions created with RF or cryothermy. Validation of the lesion set is not readily performed surgically, in contrast to that of catheter-based approaches. Additionally, the surgical environment in most institutions does not provide access to electrophysiological manoeuvres, such as comprehensive lesion validation and non-pulmonary vein trigger mapping. The lack of electrophysiological testing during surgical ablation may explain the varied and poor AF-free outcomes despite extensive lesion sets being performed.
Hybrid Ablation
The desire to create durable transmural lesions, close the appendage, validate the lesions set and address additional arrhythmic substrate has led to the concept of hybrid ablation. Centres have emerged embracing this multidisciplinary approach, and integrate the expertise of electrophysiologists and surgeons. Unfortunately, much of the hybrid experience comes from single-centre observation studies that vary in surgical technique, as well as the endocardial ablation strategy.[26–28]
Hybrid surgical approaches predominately involve either bilateral thoracoscopy using bipolar RF clamps or a unilateral thoracoscopic approach through the right chest alone.[26,27] The surgeon utilises RF energy tools to create block across linear lesions in both atria. The catheter-based portion of the procedure usually follows, validating the surgical work, and addressing additional substrate, triggers and creation of a cavotricuspid isthmus line.
Mahapatra et al. first described their experience with a staged hybrid ablation for patients with persistent AF who had failed antiarrhythmic drug therapy and at least one attempt at catheter ablation.[26] Using bilateral thoracoscopy, they created bilateral antral PVI lesions and isolated the superior vena cava, connected the veins with a roof line, created lesions connecting the right and left superior PVs to the non-coronary commissure of the aortic valve, and a lesion connecting the left superior PV to the LAA followed by LAA closure. Catheter ablation was performed 3–5 days later. They compared these patients with a matched catheter ablation-alone group and found higher freedom from atrial arrhythmia off antiarrhythmic drugs in the hybrid group at 20 months of follow-up (87% versus 53%; p=0.04). There were no complications in this report. Other hybrid procedures followed with single-centre observational reports using variable ablation lesions with sinus rhythm rates, off antiarrhythmic drugs, ranging from 37% to 86%.[27–31]
Posterior Wall Isolation
With the failure of rotor mapping, complex fractionated atrial electrogram ablation and simple linear ablation, there is increasing interest in the isolation of the posterior wall. Cardiac MRI data have implicated the posterior wall as a region with a high prevalence of atrial fibrosis.[32] Additionally, the varied myocardial fibre orientation of the posterior wall and the high prevalence of autonomic ganglionic plexi may also contribute to the AF substrate.[33,34] Debulking of the posterior wall perhaps reduces the AF substrate to a critical level at which AF cannot sustain. This critical mass hypothesis, first suggested in observational studies by Garrey et al. more than a century ago and reproduced in animal studies more recently by Lee et al., may explain the success of ablation lesion strategies that effectively compartmentalise the atria.[35,36]
The current strategies for posterior wall isolation using catheter ablation include a single-ring approach, pulmonary vein isolation and box lesion set or obliteration of posterior wall potentials. The single-ring approach is similar to the Cox maze III procedure, which involves isolating the pulmonary veins and posterior wall, but has had variable success rates, and due to difficulty in achieving complete block in the roof portion of the circle, recurrent conduction can occur and compromise isolation of the posterior wall.[37] Pulmonary vein isolation and a box lesion set uses double circles around the veins as anchors for posterior wall isolation, and an additional roof line to connect the superior PVs and a low posterior line to connect the inferior veins. This technique also showed only modest success rates in observational trials.[38] Endocardial homogenisation of the posterior wall signals may address the shortcomings of linear ablation, but potentially increases the rate of atrioesophageal fistula.[39]
Concern about atrioesophageal fistula frequently limits the amount of ablation that an operator can deliver to the posterior wall. Current catheter-based strategies to address posterior wall substrate include high-power, short-duration application of radiofrequency energy to theoretically limit deep ablation; real-time temperature monitoring; oesophageal deviation; and cryoballoon across the posterior space.[40] Despite the endocardial energy used, oesophageal injury during posterior wall catheter ablation can occur, and although the occurrence of an atrioesophageal fistula is of low relative frequency, the high mortality of this complication necessitates standardised ablation protocols and close post-procedural surveillance.
The posterior wall of the left atrium may also have epicardial connections. This may explain the variability in success with posterior box lesion sets. Multiple reports have demonstrated the ability to have endocardial electrical isolation, yet persistent posterior wall activity.[41–43] Because endocardial catheter ablation alone has limited epicardial efficacy, related to energy penetration restrictions, supplemental epicardial ablation to achieve definitive posterior wall isolation has gained attention.
Convergent Ablation
The convergent procedure is a form of hybrid AF ablation that utilises a pericardioscopic approach from the upper abdomen. As such, the convergent approach distinguishes itself as a compliment to catheter ablation rather than a complex surgical procedure. The convergent procedure simplifies the lesion set to focus on an effective endocardial and epicardial posterior wall and pulmonary vein isolation (Figure 1). Involving an epicardial approach, there may also be utility in the ablation of ganglionated plexi and epicardial fat in the surgical lesion set. Pericardioscopy provides laparoscopic access to the pericardium and epicardial space. Under direct visualisation, the Epi-Sense® (Atricure, Mason, OH, US) unipolar vacuum-assisted linear RF ablation catheter uses vacuum to suck the atrial tissue into apposition with the RF coil. Saline continuously irrigates the electrode to improve energy penetration and limit char. The pericardioscopic access provides optimal access to the posterior left atrium and posterior pulmonary vein antrums. It also enables direct electrocardiogram evaluation before, during and after the procedure to help confirm a complete lesion set. This immediate and comprehensive lesion approach is more difficult with other hybrid procedures.
Figure 1: Epicardial and Endocardial Lesions of the Convergent Procedure.
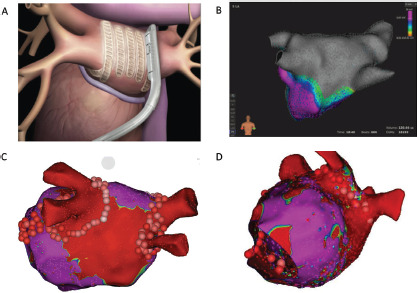
A: The Epi-Sense Coagulation Device applied to the posterior left atrium. B: Posterior wall and pulmonary vein isolation with high-density endocardial mapping of the left atrium after convergent epicardial ablation and cryoballoon isolation of all four pulmonary veins. The grey colour denotes scar, while the purple colour denotes healthy atrial voltage. C: The transmural scar (red) created from the epicardial ablation. Healthy left atrial voltages are seen in purple. Endocardial pulmonary vein isolation was performed with radiofrequency ablation and creation of a lateral mitral isthmus line. The left atrial appendage was closed with an AtriClip and the vein of Marshall was epicardially ablated. D: Absence of the left atrial appendage after closure with an AtriClip is also noted in a lateral view in another patient. Source: Reproduced with permission from AtriCure.
Endocardial ablation follows the epicardial procedure to confirm lesion integrity and supplement the epicardial procedure, which can be performed during the same setting or in a staged fashion – each approach offering distinct advantages.[44]
A concomitant approach has the advantage of providing immediate endocardial confirmation of posterior isolation and provides timely feedback to the surgeon. The concomitant approach requires efficient schedule coordination between the surgeon, electrophysiologist and the staff to offer the patient a same-day procedure. Simultaneous epicardial mapping of the posterior wall scar utilising 3D mapping systems can be performed to demonstrate gaps and allow for surgeons to create additional epicardial lesions. During a staged procedure, the surgeon performs the epicardial procedure, which includes pulmonary vein isolation and posterior wall isolation followed by catheter ablation after days to weeks. This approach offers convenience to both the electrophysiologist and surgeon. Additionally, it gives time for reconnections to develop by the time endocardial ablation is performed, and gaps in the epicardial ablation can be addressed.
Evidence for Convergent Ablation
Kiser et al. reported the initial convergent procedure experience in 28 patients with persistent or long-standing persistent AF.[45] The patients underwent concomitant epicardial radiofrequency ablation and transseptal endocardial ablation to exclude the entire posterior left atrium and isolate the PVs. They reported no deaths. At ≤6 months follow-up, freedom from AF and antiarrhythmic drugs was 76%.
Since then, other observational studies with ≥12 months follow-up have reported similar results of success (Table 1), with freedom from AF at 12 months ranging 73–88% and patients in sinus rhythm ranging 52–88%.[46–48] Gersak et al. reported the longest follow-up on convergent procedures, with 81% of patients being free from AF at 4 years.[49] Among comparison studies of convergent versus endocardial-only ablation, Edgerton et al. in 2009 initiated a prospective study that enrolled 24 patients to a hybrid approach and 35 patients to catheter ablation only.[50] Their hybrid group underwent surgical ablation through a pericardioscopic approach followed immediately by endocardial catheter ablation. They used a unipolar radiofrequency device to perform PVI, posterior box, ablate the ligament of Marshall (without dissection) and the lateral right atrium. The endocardial portion entailed verification and completion of epicardial lines, ablation in the coronary sinus, isolation of the LAA, and ablation of complex fractionated atrial electrograms. At 12-month follow-up, the hybrid group had lower arrhythmia-free survival (24% versus 63%; p<0.001). The complication rates were significantly higher in the hybrid group (21% versus 3%; p=0.036), including three deaths, one tamponade and one phrenic nerve palsy in the hybrid group compared with one tamponade in the catheter ablation group. The authors attributed the deaths and complications to the unipolar RF design; however, their experience was largely with the first iteration of the procedure before the epicardial lesion set was modified, before oesophageal protections were instituted, and involved an extensive endocardial lesion set.
Table 1: Convergent Procedure Studies.
| Studies | Type | Year | Type of AF (PAF, PsAF, LSPAF) | Timeframe of Treatment | No. of Patients | Timing | Mortality | Complications | Follow-up | Freedom from AF ± Antiarrhythmics (%) | Freedom from any Atrial Arrhythmia ± Antiarrhythmics (%) | Freedom from any Atrial Arrhythmia off Antiarrhythmics (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transabdominal without AtriClip | ||||||||||||
| Kiser et al.[46] | Observational | 2011 | PAF, PsAF, LSPAF | Jan 2009-May 2010 | 65 | Concomitant | 5% | 8% | 12 months | – | 88% | 83% |
| Zembala et al.[47] | Observational | 2012 | PsAF, LSPAF | Aug 2009-Dec 2011 | 27 | Staged | 3.7% | 10% | 12 months | 80% | – | – |
| Gehi et al.[48] | Observational | 2013 | PAF, PsAF, LSPAF | Jan 2009-Dec 2011 | 101 | Concomitant | 0% | 6% | 12 months | 73% | – | – |
| Civello et al.[76] | Observational | 2013 | PAF, PsAF, LSPAF | May 2010-Dec 2011 | 104 | Concomitant | 0% | 6% | 12 months | – | 87.5% | 72% |
| Thosani et al.[54] | Observational | 2013 | PsAF, LSPAF | Jun 2010-Feb 2013 | 43 | Concomitant | 0% | 0% | 6 months | – | 89% | – |
| Gersak et al.[51] | Observational | 2014 | PsAF;, LSPAF | Jan 2010-Dec 2011 | 73 | Concomitant | 0% | 7% | 12 months | 73% | – | – |
| Edgerton et al.[50] | Prospective | 2016 | LSPAF | – | 24 | Concomitant | 14% | 7% | 24 months | 19% | – | – |
| Gersak et al.[49] | Observational | 2016 | PAF, PsAF, LSPAF | Jan 2009-Jul 2013 | 76 | Concomitant & staged | 0% | 12% | 48 months | – | 81% | 69% |
| Zembala et al.[55] | Prospective | 2017 | PsAF, LSPAF | Jul 2009-Dec 2014 | 90 | Staged | 1% | 8% | 12 months | – | 82% | 62% |
| Kress et al.[52] | Observational | 2017 | PsAF, LSPAF | Jun 2010-Aug 2014 | 64 | Concomitant | 1.6% | 7.8% | 16 months | 72% | – | |
| Jan et al.[77] | Randomised | 2018 | PAF Jan 2013-Jun 2015 | 24 | Concomitant | 0% | 12.5% | 30.5 months | – | – | 58.3% | |
| Gulkarov et al.[78] | Observational | 2019 | PsAF, LSPAF | Oct 2013-Mar 2017 | 31 | Concomitant | 0% | 12.9% | 24 months | 71% | 52% | – |
| Subxiphoid without AtriClip | ||||||||||||
| Gegechkori et al.[71] | Observational | 2019 | PsAF, LSPAF | Jan 2014-Aug 2016 | 59 | Concomitant | 0% | 1.6% | 12 months | – | 76% | – |
| Sabzwari et al.[79] | Observational | 2019 | LSPAF | Feb 2015-Sept 2017 | 30 | – | 0% | 0% | 12 months | 80% | – | 63% |
| Subxiphoid with AtriClip | ||||||||||||
| Gegechkori et al.[71] | Observational | 2019 | PsAF, LSPAF | Aug 2016-Oct 2019 | 64 | Concomitant | 0% | 7.8% | 12 months | – | 87.5% | – |
| Tonks et al.[72] | Observational | 2019 | PAF, PsAF, LSPAF | Feb 2016-May 2017 | 36 (25/36 subxiphoid, 13/36 with AtriClip) | Concomitant (32/36) | 0% | 16.7% | 12 months | – | 78% | – |
LSPAF = longstanding persistent AF; PAF = paroxysmal AF; PsAF = persistent AF.
Gehi et al. reported results for a transdiaphragmatic pericardiosopic approach in 101 patients with long-standing persistent (n=37), persistent (n=47) and paroxysmal AF (n=17).[48] At 12-month follow-up, 66% with single and 71% with repeat ablation were in normal sinus rhythm. The endocardial procedure was performed immediately after the epicardial portion. Gersak et al. reported both a single-centre and a multicentre pericardioscopic approach with an epicardial lesion set including pulmonary venous antrum and posterior wall followed by similar area ablation endocardially.[51] In their single-centre data on 50 patients with persistent or long-standing persistent AF with implantable loop recorder monitoring, they reported 88% normal sinus rhythm at 1 year. In the multicentre data for persistent (30.1%) and long-standing persistent AF (69.1%) patients (n=73), they reported 80% normal sinus rhythm at 1-year follow-up, which was performed with Holter monitoring and implantable loop recorder. Of these, approximately half of the patients were not taking antiarrhythmic drugs at follow-up.
In a retrospective study of consecutive patients, Kress et al. compared convergent ablation with endocardial-only ablation in 133 patients with persistent and long-standing AF.[52] In this series, cryoballoon was primarily used for endocardial ablation in both procedures. They found the convergent group had fewer recurrences than the endocardial-only group, and 16-month AF-free survival was 72% with convergent ablation compared with 51% for endocardial-only ablation (p=0.01). Complications were not significantly different between groups (7.8% for convergent and 2.9% for endocardial-only ablation; p=0.205).
Evolution of the Convergent Procedure
The first iteration of the convergent procedure emerged as a surgical epicardial ablation performed without incisions in the chest. After evaluating the original nContact ablation technology during open cardiac procedures, Kiser conceived the pericardioscopic cannula, which enabled transabdominal access to the posterior pericardium of the beating heart.[44,53] The first clinical application was a surgical procedure alone, utilising both thoracoscopy and pericardioscopy to create an epicardial lesion set for AF. The results of this pericardioscopic ex-maze procedure were similar to catheter ablation alone; however, chest incisions were still necessary. Kiser assembled a team of international experts in Krakow, Poland, and on 3 January 2009, performed the first convergent epicardial and endocardial ablation procedure.[45]
The early convergent procedure included surgical ablation of the anterior and posterior aspects of the pulmonary veins through a transabdominal approach (Figure 1). A left atrial roof and inferior floor line were also created by curling the catheter along the cannula guidewire. During the same setting, endocardial catheter ablation addressed gaps in the surgical lesion set at the pericardial reflections of the superior and inferior vena cava. Simultaneous catheter ablation addressed the cavotricuspid isthmus, the mitral valve annulus and any other high-frequency activity deemed clinically relevant by the operator. The hybrid catheter and surgical approach saw improved outcomes, but centres also identified oesophageal injuries and associated morbidity and mortality.[49–50,54–56]
In 2011, Kiser et al. evaluated these published and the non-published, but early reported, outcomes and complications of the convergent procedure while examining the predicate iterations of the pericardioscopic approach.[45] As a result, the authors recommended, and subsequent procedural guidelines were instituted, to reduce the risk of oesophageal injury by: attentively positioning the ablation device only towards the epicardium; monitoring oesophageal temperature; using fluoroscopy to identify and avoid the oesophagus; and irrigating the pericardial space with cool saline.
Improvements to the convergent procedure have reduced procedural complexity while further reducing complications (Table 2). Unlike the original description of complicated device manipulation over wires and within the transverse sinus, the procedure was modified in 2012 to keep the epicardial ablation catheter in a straight configuration. The resulting epicardial lesion set sought to homogenise the posterior LA wall rather than create a convoluted linear box lesion. (Figure 2) The procedure also moved to a subxiphoid approach in 2015 (Figure 3).[57] This change eliminated the rare complication of bowel herniation into the thoracic space via the transabdominal, transdiaphragmatic approach, while still allowing the surgeon sufficient access to the posterior left atrium.[58–60] These changes have enhanced efficacy, as well as the safety profile of the procedure.
Table 2: Convergent Procedure Complications.
| Complications | ||
|---|---|---|
| Atrioesophageal fistula | 6/884 | 0.7% |
| Pericardial effusion | 10/884 | 1.1% |
| Pericardial tamponade | 9/884 | 1.0% |
| Cardiac death | 2/884 | 0.2% |
| Unexplained death | 2/884 | 0.2% |
| Major bleeding | 12/884 | 1.4% |
| Hematemesis | 1/884 | 0.1% |
| Stroke | 7/884 | 0.8% |
| TIA | 2/884 | 0.2% |
| Pleural effusion | 3/884 | 0.3% |
| Lung injury | 1/884 | 0.1% |
| Pulmonary vein stenosis | 1/884 | 0.1% |
| Transient phrenic nerve palsy | 3/884 | 0.3% |
| Groin/puncture site complications | 2/884 | 0.2% |
| Infection | 1/884 | 0.1% |
TIA = transient ischaemic attack.
Figure 2: Evolution of the Convergent Procedure.
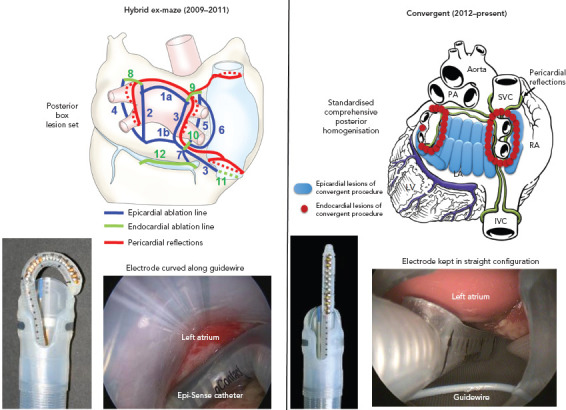
The convergent procedure has evolved from the hybrid ex-maze’s (left panel) complex epicardial (blue lines) and endocardial (green lines) linear ablation set to the current convergent lesion set (right panel), which involves epicardial homogenization of the posterior wall (blue lines), followed by endocardial ablation (red dots) to complete the pulmonary vein isolation and a cavotricuspid isthmus line. IVC = inferior vena cava; LA = left atrium; PA = pulmonary artery; RA = right atrium; SVC = superior vena cava. Source: Reproduced with permission from AtriCure.
Figure 3: Transabdominal and Subxiphoid Approaches.
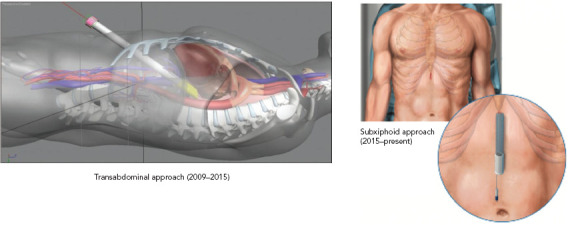
Source: Reproduced with permission from AtriCure.
Concomitant LAA Exclusion Using the AtriClip
The LAA has been implicated in the initiation and perpetuation of AF, particularly in the persistent AF population. The Effect of Empirical Left Atrial Appendage Isolation on Long-term Procedure Outcome in Patients With Persistent or Long-standing Persistent Atrial Fibrillation Undergoing Catheter Ablation (BELIEF) trial examined patients who had long-standing AF and randomised them to ablation with LAA isolation versus ablation without LAA isolation (NCT01362738). Single-procedure freedom at 12 months with LAA isolation was 56% versus 28% without isolation (p=0.001). While endocardial isolation of the LAA is possible, multiple procedures are frequently required to create lasting LAA electrical isolation. A consequence of electrical isolation appears to be an increased incidence of LAA thrombus secondary to mechanical standstill of the appendage.[61] While this may be addressed with a WATCHMAN implant, concomitant endocardial AF ablation and WATCHMAN implantation is cost prohibitive due to reimbursement constraints.
The AtriClip has been utilised in >200,000 patients, predominantly in open-chest surgical procedures to close the appendage. Retrospective data demonstrate that the AtriClip closure is safe, durable and leads to a reduction in thromboembolic events.[62–65] Acute and long-term closure rates have been >95% with residual stumps >10 mm in only 0–5% of cases.[62,63] The AtriClip began to be placed through a left thoracoscopic approach in 2012, and convergent procedures began incorporating the AtriClip in 2017 (Figure 4).[66–69] As the convergent procedure already enlists the assistance of the surgeon, the thoracoscopic addition of the AtriClip is able to be performed in the same procedure setting in a cost-effective manner.
Figure 4: AtriClip Application to Electrically and Mechanically Isolate the Appendage.
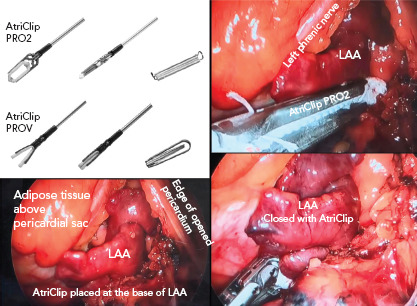
The PRO2 and PROV AtriClips are shown in the top left inset. A multilobed left atrial appendage (LAA) is shown in the bottom left inset, which is closed with a PRO2 AtriClip (right panel). Note the purple colour change of the ligated appendage (bottom right) Source: Reproduced with permission from AtriCure.
The AtriClip seeks to address the LAA as an electrical source of AF triggers and the mechanical risk for stroke. Studies have demonstrated that the AtriClip achieves acute electrical isolation of the appendage, which has earned it a US Food and Drug Administration indication.[70] Additional benefits of incorporating a left thoracoscopic approach to LAA management include the ability to epicardially ablate the vein of Marshall, which may allow for easier creation of a lateral mitral isthmus line.
While recent reports have demonstrated favourable outcomes with the addition of the AtriClip to the convergent procedure, further studies are required.[71,72] Outcomes from the LAA Ligation Adjunctive to PVI for Persistent or Longstanding Persistent AF (aMAZE) trial (NCT02513797), which investigates the antiarrhythmic effect of closing the LAA with Lariat in patients with persistent AF, are anticipated to shed further light on the importance of LAA electrical isolation.[73]
Future Direction
The Epi/Endo Ablation For Treatment of Persistent Atrial Fibrillation (AF) (CONVERGE) trial (NCT01984346) is an investigational device-exempt, prospective, multicentre, open-label, randomised controlled pivotal study to evaluate the safety and efficacy of the Epi-Sense AF Guided Coagulation System (Atricure) for the treatment of symptomatic persistent and long-standing persistent AF in patients refractory to medical therapy. The primary objective is to demonstrate the superiority of the convergent procedure compared with stand-alone endocardial radiofrequency catheter ablation. A total of 153 patients have been randomised in a two-to-one manner to the convergent procedure or endocardial-only ablation and followed for a minimum of 1 year.
Unlike other catheter ablation trials for persistent AF, CONVERGE imposed no limits on the duration of AF and allows left atrial sizes up to 6 cm. As a result, the CONVERGE trial is the only ablation trial thus far to include a substantial portion of patients with long-standing persistent AF. The study finished enrolment in August 2018, and 12-month follow-up for primary effectiveness was completed in August 2019. The results are expected to be reported in 2020. If positive, the CONVERGE trial would mark a major milestone by confirming a superior method for ablation of persistent and long-standing persistent AF. Future trials utilising the convergent procedure are necessary to assess the use of endocardial cryoablation as an alternative to endocardial RF ablation, and to assess the incremental benefit of LAA exclusion and electrical silencing.
Conclusion
The convergent procedure as practiced today has evolved from its original design as a modification of the Cox maze linear lesion set to its current lesion protocol, which prioritises homogenisation of the posterior wall substrate through the pericardium to dovetail the electrophysiologist’s endocardial wide area circumferential pulmonary vein isolation.[74] With iterative procedural refinements in the epicardial access, catheter manipulation and oesophageal protection, the rate of procedural complications has significantly declined. In summary, the convergent hybrid ablation affords endocardial pulmonary vein isolation, epicardial posterior wall isolation and left atrial appendage management via external ligation in either a single or staged procedural setting.[75]
With a cumulative experience in >10,000 patients to date, the convergent procedure now has an established position in the vast array of procedures directed at managing non-paroxysmal AF.
Clinical Perspective
The strategy of pulmonary vein isolation alone in the treatment of patients with persistent AF has been unsatisfactory
Isolation of the posterior wall of the left atrium is a strategy employed in open chest maze procedures, as well as hybrid AF ablations, the convergent AF procedure and endocardial approaches. Operators seek achievement of a durable posterior wall isolation in a safe manner
The convergent AF ablation procedure was developed to achieve endocardial and epicardial isolation of the pulmonary veins and posterior wall. The epicardial portion of the procedure has evolved over time to a more simplified lesion set and from a transabdominal to a subxiphoid approach. Additional safety measures including oesophageal temperature monitoring and saline irrigation in the pericardial space have been implemented, which have minimised the complications with the procedure while maintaining a high success rate
Convergent ablations are now increasingly performed with concomitant application of an AtriClip to electrically and mechanically isolate the left atrial appendage.
Acknowledgments
The authors contributed equally.
References
- 1.Calkins H, Hindricks G, Cappato R et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–444. doi: 10.1016/j.hrthm.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhargava M, Di Biase L, Mohanty P et al. Impact of type of atrial fibrillation and repeat catheter ablation on long-term freedom from atrial fibrillation: results from a multicenter study. Heart Rhythm. 2009;6:1403–12. doi: 10.1016/j.hrthm.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Di LB, Burkhardt JD, Mohanty P et al. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation. 122:109–18. doi: 10.1161/CIRCULATIONAHA.109.928903. [DOI] [PubMed] [Google Scholar]
- 4.Haissaguerre M, Jaïs P, Shah DC et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 5.Pappone C, Augello G, Sala S et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J Am Coll Cardiol. 2006;48:2340–7. doi: 10.1016/j.jacc.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 6.Kuck KH, Brugada J, Fürnkranz A et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374:2235–45. doi: 10.1056/NEJMoa1602014. [DOI] [PubMed] [Google Scholar]
- 7.Andrade JG, Champagne J, Dubuc M et al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation. 2019;140:1779–88. doi: 10.1161/CIRCULATIONAHA.119.042622. [DOI] [PubMed] [Google Scholar]
- 8.Shah D, Haissaguerre M, Jais P, Hocini M. Nonpulmonary vein foci: do they exist? Pacing Clin Electrophysiol. 2003;26:1631–5. doi: 10.1046/j.1460-9592.2003.t01-1-00243.x. [DOI] [PubMed] [Google Scholar]
- 9.Yamada T, Murakami Y, Okada T et al. Non-pulmonary vein epicardial foci of atrial fibrillation identified in the left atrium after pulmonary vein isolation. Pacing Clin Electrophysiol. 2007;30:1323–30. doi: 10.1111/j.1540-8159.2007.00865.x. [DOI] [PubMed] [Google Scholar]
- 10.Verma A, Jiang CY, Betts TR et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–22. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 11.Yokokawa M, Chugh A, Ulfarsson M et al. Effect of linear ablation on spectral components of atrial fibrillation. Heart Rhythm. 2010;7:1732–7. doi: 10.1016/j.hrthm.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 12.Kim JS, Shin SY, Na JO et al. Does isolation of the left atrial posterior wall improve clinical outcomes after radiofrequency catheter ablation for persistent atrial fibrillation?: A prospective randomized clinical trial. Int J Cardiol. 2015;181:277–83. doi: 10.1016/j.ijcard.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 13.Narayan SM, Patel J, Mulpuru S, Krummen DE. Focal impulse and rotor modulation ablation of sustaining rotors abruptly terminates persistent atrial fibrillation to sinus rhythm with elimination on follow-up: a video case study. Heart Rhythm. 2012;9:1436–9. doi: 10.1016/j.hrthm.2012.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Q, Hou Y, Yang S. A meta-analysis of the comparative efficacy of ablation for atrial fibrillation with and without ablation of the ganglionated plexi. Pacing Clin Electrophysiol. 2011;34:1687–94. doi: 10.1111/j.1540-8159.2011.03220.x. [DOI] [PubMed] [Google Scholar]
- 15.Jadidi AS, Lehrmann H, Keyl C et al. Ablation of persistent atrial fibrillation targeting low-voltage areas with selective activation characteristics. Circ Arrhythm Electrophysiol. 2016;9:e002962. doi: 10.1161/CIRCEP.115.002962. [DOI] [PubMed] [Google Scholar]
- 16.Santangeli P, Marchlinski FE. Techniques for the provocation, localization, and ablation of non-pulmonary vein triggers for atrial fibrillation. Heart Rhythm. 2017;14:1087–96. doi: 10.1016/j.hrthm.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 17.Valderrábano M, Liu X, Sasaridis C et al. Ethanol infusion in the vein of Marshall: adjunctive effects during ablation of atrial fibrillation. Heart Rhythm. 2009;6:1552–8. doi: 10.1016/j.hrthm.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox J L, Boineau J P, Schuessler R B et al. Successful surgical treatment of atrial fibrillation. Review and clinical update. JAMA. 1991;266:1976–80. doi: 10.1001/jama.1991.03470140088029. [DOI] [PubMed] [Google Scholar]
- 19.Gaynor SL, Schuessler RB, Bailey MS et al. Surgical treatment of atrial fibrillation: predictors of late recurrence. J Thorac Cardiovasc Surg. 2005;129:104–11. doi: 10.1016/j.jtcvs.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 20.Krul Sébastien PJ, Driessen Antoine HG, Zwinderman Aeilko H et al. Navigating the mini-maze: systematic review of the first results and progress of minimally invasive surgery in the treatment of atrial fibrillation. Int J Cardiol. 2013;166:132–40. doi: 10.1016/j.ijcard.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Ganesan AN, Shipp NJ, Brooks AG et al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2:e004549. doi: 10.1161/JAHA.112.004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tilz RR, Rillig A, Thum AM et al. Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the Hamburg Sequential Ablation Strategy. J Am Coll Cardiol. 2012;60:1921–9. doi: 10.1016/j.jacc.2012.04.060. [DOI] [PubMed] [Google Scholar]
- 23.Lau DH, Schotten U, Mahajan R et al. Novel mechanisms in the pathogenesis of atrial fibrillation: practical applications. Eur Heart J. 2015;37:1573–81. doi: 10.1093/eurheartj/ehv375. [DOI] [PubMed] [Google Scholar]
- 24.Di LB, Burkhardt JD, Mohanty P et al. Left atrial appendage: an under recognized trigger site of atrial fibrillation. Circulation. 2010;122:109–18. doi: 10.1161/CIRCULATIONAHA.109.928903. [DOI] [PubMed] [Google Scholar]
- 25.Boersma LVA, Castella M, van BW et al. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation. 2012;125:23–30. doi: 10.1161/CIRCULATIONAHA.111.074047. [DOI] [PubMed] [Google Scholar]
- 26.Mahapatra S, LaPar DJ, Kamath S et al. Initial experience of sequential surgical epicardial-catheter endocardial ablation for persistent and longstanding persistent atrial fibrillation with long-term follow-up. Ann Thorac Surg. 2011;91:1890–8. doi: 10.1016/j.athoracsur.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bisleri G, Rosati F, Bontempi L et al. Hybrid approach for the treatment of long-standing persistent atrial fibrillation: electrophysiological findings and clinical results. Eur J Cardiothorac Surg. 2013;44:919–23. doi: 10.1093/ejcts/ezt115. [DOI] [PubMed] [Google Scholar]
- 28.Gehi A, Mounsey JP, Cherkur S et al. Hybrid surgical and catheter ablation of atrial fibrillation: comparison of techniques. Circulation. 2014;130(Suppl 2):A12595. [Google Scholar]
- 29.Krul SP, Driessen AH, Van Boven WJ et al. Thoracoscopic video-assisted pulmonary vein antrum isolation, ganglionated plexus ablation, and periprocedural confirmation of ablation lesions: first results of a hybrid surgical-electrophysiological approach for atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:262–70. doi: 10.1161/CIRCEP.111.961862. [DOI] [PubMed] [Google Scholar]
- 30.Pison L, Gelsomino S, Luca F et al. Effectiveness and safety of simultaneous hybrid thoracoscopic and endocardial catheter ablation of lone atrial fibrillation. Ann Cardiothorac Surg. 2014;3:38–44. doi: 10.3978/j.issn.2225-319X.2013.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bulava A, Mokracek A, Hanis J et al. Sequential hybrid procedure for persistent atrial fibrillation. J Am Heart Assoc. 2015;4:e001754. doi: 10.1161/JAHA.114.001754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segerson NM, Daccarett M, Badger TJ et al. Magnetic resonance imaging – confirmed ablative debulking of the left atrial posterior wall and septum for treatment of persistent atrial fibrillation: rationale and initial experience. JACC Clin Electrophysiol. 2010;21:126–32. doi: 10.1111/j.1540-8167.2009.01611.x. [DOI] [PubMed] [Google Scholar]
- 33.Suenari K, Chen YC, Kao YH et al. Discrepant electrophysiological characteristics and calcium homeostasis of left atrial anterior and posterior myocytes. Basic Res Cardiol. 2011;106:65–74. doi: 10.1007/s00395-010-0132-1. [DOI] [PubMed] [Google Scholar]
- 34.Jongbloed MR, Schalij MJ, Poelmann RE et al. Embryonic conduction tissue: a spatial correlation with adult arrhythmogenic areas. J Cardiovasc Electrophysiol. 2004;15:349–55. doi: 10.1046/j.1540-8167.2004.03487.x. [DOI] [PubMed] [Google Scholar]
- 35.Garrey WE. The nature of fibrillary contraction of the heart.-Its relation to tissue mass and form. Am J of Physiol. 1914;33:397–414. doi: 10.1152/ajplegacy.1914.33.3.397. [DOI] [Google Scholar]
- 36.Lee AM, Aziz A, Didesch J et al. Importance of atrial surface area and refractory period in sustaining atrial fibrillation: testing the critical mass hypothesis. J Thorac Cardiovasc Surg. 2013;146:593–8. doi: 10.1016/j.jtcvs.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas SP, Lim TW, McCall R et al. Electrical isolation of the posterior left atrial wall and pulmonary veins for atrial fibrillation: feasibility of and rationale for a single-ring approach. Heart Rhythm. 2007;4:722–30. doi: 10.1016/j.hrthm.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 38.Saad EB, Slater C. Complete isolation of the left atrial posterior wall (box lesion) to treat longstanding persistent atrial fibrillation. J Atr Fibrillation. 2014;7:1174. doi: 10.4022/jafib.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bai R, Di Biase L, Mohanty P et al. Proven isolation of the pulmonary vein antrum with or without left atrial posterior wall isolation in patients with persistent atrial fibrillation. Heart Rhythm. 2016;13:132–40. doi: 10.1016/j.hrthm.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 40.Romero J, Avendano R, Grushko M et al. Oesophageal injury during AF ablation: techniques for prevention. Arrhythm Electrophysiol Rev. 2018;7:24–31. doi: 10.15420/aer.2017.46.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Groot N, van der Does L, Yaksh A et al. Direct proof of endo-epicardial asynchrony of the atrial wall during atrial fibrillation in humans. Circ Arrhythm Electrophysiol. 2016;9:e003648. doi: 10.1161/CIRCEP.115.003648. [DOI] [PubMed] [Google Scholar]
- 42.Glover BM, Hong KL, Baranchuk A et al. Preserved left atrial epicardial conduction in regions of endocardial “isolation”. JACC Clin Electrophysiol. 2018;4:557–8. doi: 10.1016/j.jacep.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 43.Jiang R, Buch E, Gima J et al. Feasibility of percutaneous epicardial mapping and ablation for refractory atrial fibrillation: Insights into substrate and lesion transmurality. Heart Rhythm. 2019;16:1151–9. doi: 10.1016/j.hrthm.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 44.Kiser AC, Cockfield W. Paracardioscopic ex-maze procedure for atrial fibrillation. Multimed Man Cardiothorac Surg. 2010;2010 doi: 10.1510/mmcts.2008.003863. [DOI] [PubMed] [Google Scholar]
- 45.Kiser AC, Landers M, Horton R et al. The convergent procedure: a multidisciplinary atrial fibrillation treatment. Heart Surg Forum. 2010;13:E317–21. doi: 10.1532/HSF98.20091112. [DOI] [PubMed] [Google Scholar]
- 46.Kiser AC, Landers MD, Boyce K et al. Simultaneous catheter and epicardial ablations enable a comprehensive atrial fibrillation procedure. Innovations. 2011;6:243–7. doi: 10.1097/imi.0b013e31822ca15c. [DOI] [PubMed] [Google Scholar]
- 47.Zembala M, Filipiak K, Kowalski O et al. Minimally invasive hybrid ablation procedure for the treatment of persistent atrial fibrillation: one year results. Kardiol Pol. 2012;70:819–28. [PubMed] [Google Scholar]
- 48.Gehi AK, Mounsey JP, Pursell I et al. Hybrid epicardial-endocardial ablation using a pericardioscopic technique for the treatment of atrial fibrillation. Heart Rhythm. 2013;10:22–8. doi: 10.1016/j.hrthm.2012.08.044. [DOI] [PubMed] [Google Scholar]
- 49.Gersak B, Jan M. Long-term success for the convergent atrial fibrillation procedure: 4-year outcomes. Ann Thorac Surg. 2016;102:1550–7. doi: 10.1016/j.athoracsur.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 50.Edgerton Z, Perini AP, Horton R et al. Hybrid procedure (endo/epicardial) versus standard manual ablation in patients undergoing ablation of longstanding persistent atrial fibrillation: results from a single center. J Cardiovasc Electrophysiol. 2016;27:524–30. doi: 10.1111/jce.12926. [DOI] [PubMed] [Google Scholar]
- 51.Gersak B, Zembala MO, Muller D et al. European experience of the convergent atrial fibrillation procedure: multicenter outcomes in consecutive patients. J Thorac Cardiovasc Surg. 2014;147:1411–6. doi: 10.1016/j.jtcvs.2013.06.057. [DOI] [PubMed] [Google Scholar]
- 52.Kress DC, Erickson L, Choudhuri I et al. Comparative Effectiveness of Hybrid Ablation Versus Endocardial Catheter Ablation Alone in Patients With Persistent Atrial Fibrillation. JACC Clin Electrophysiol. 2017;3:341–349. doi: 10.1016/j.jacep.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 53.Kiser AC. Paracardioscopy provides endoscopic visualization of the heart. Innovations. 2009;4:233–5. doi: 10.1097/imi.0b013e3181b03b78. [DOI] [PubMed] [Google Scholar]
- 54.Thosani AJ, Gerczuk P, Liu E et al. Closed chest convergent epicardial-endocardial ablation of non-paroxysmal atrial fibrillation – a case series and literature review. Arrhythm Electrophysiol Rev. 2013;2:65–8. doi: 10.15420/aer.2013.2.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zembala M, Filipiak K, Kowalski O et al. Staged hybrid ablation for persistent and longstanding persistent atrial fibrillation effectively restores sinus rhythm in long-term observation. Arch Med Sci. 2017;13:109–17. doi: 10.5114/aoms.2015.53960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pearman CM, Poon SS, Bonnett LJ et al. Minimally invasive epicardial surgical ablation alone versus hybrid ablation for atrial fibrillation: a systematic review and meta-analysis. Arrhythm Electrophysiol Rev. 2017;6:202–9. doi: 10.15420/aer/2017.29.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kiser AC, Mounsey JP, Cherkur S et al. Trans xyphoid hybrid catheter and surgical ablation with left atrial appendage occlusion. Innovations. 2015;10:S14. doi: 10.14797/mdcj-12-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fahim M, Campbell-Smith T. Strangulated intrapericardial diaphragmatic hernia after hybrid maze procedure. J Coll Physicians Surg Pak. 2019;29:S95–7. doi: 10.29271/jcpsp.2019.12.S95. [DOI] [PubMed] [Google Scholar]
- 59.Delliturri A, Chiba S, Brichkov I, Sherwinter D. Laparoscopic repair of a peritoneopericardial diaphragmatic hernia after a convergent procedure for the treatment of atrial fibrillation. J Thorac Dis. 2017;9:e767–70. doi: 10.21037/jtd.2017.08.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaufman AJ, Kahn ET, Villena-Vargas J et al. Laparoscopic repair of an intrapericardial diaphragmatic hernia after convergent maze procedure. Ann Thorac Surg. 2017;103:e541–3. doi: 10.1016/j.athoracsur.2017.01.033. [DOI] [PubMed] [Google Scholar]
- 61.Rillig A, Tilz RR, Lin T et al. Unexpectedly high incidence of stroke and left atrial appendage thrombus formation after electrical isolation of the left atrial appendage for the treatment of atrial tachyarrhythmias. Circ Arrhythm Electrophysiol. 2016;9:e003461. doi: 10.1161/CIRCEP.115.003461. [DOI] [PubMed] [Google Scholar]
- 62.Caliskan E, Eberhard M, Falk V et al. Incidence and characteristics of left atrial appendage stumps after device-enabled epicardial closure. Interact Cardiovasc Thorac Surg. 2019;29:663–9. doi: 10.1093/icvts/ivz176. [DOI] [PubMed] [Google Scholar]
- 63.van Laar C, Verberkmoes NJ, van Es HW et al. Thoracoscopic left atrial appendage clipping: a multicenter cohort analysis. JACC Clin Electrophysiol. 2018;4:893–901. doi: 10.1016/j.jacep.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 64.Caliskan E, Sahin A, Yilmaz M et al. Epicardial left atrial appendage AtriClip occlusion reduces the incidence of stroke in patients with atrial fibrillation undergoing cardiac surgery. Europace. 2017;20:e105–14. doi: 10.1093/europace/eux211. [DOI] [PubMed] [Google Scholar]
- 65.Ailawadi G, Gerdisch MW, Harvey RL et al. Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. J Thorac Cardiovasc Surg. 2011;142:1002–9. doi: 10.1016/j.jtcvs.2011.07.052. [DOI] [PubMed] [Google Scholar]
- 66.Suwalski P, Witkowska A, Drobinski D et al. Stand-alone totally thoracoscopic left atrial appendage exclusion using a novel clipping system in patients with high risk of stroke-initial experience and literature review. Kardiochir Torakochirurgia Pol. 2015;12:298. doi: 10.5114/kitp.2015.56777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Osmancik P, Budera P, Zdarska J et al. Residual echocardiographic and computed tomography findings after thoracoscopic occlusion of the left atrial appendage using the AtriClip PRO device. Interact Cardiovasc Thorac Surg. 2018;26:919–25. doi: 10.1093/icvts/ivx427. [DOI] [PubMed] [Google Scholar]
- 68.Smith NE, Joseph J, Morgan J et al. Initial experience with minimally invasive surgical exclusion of the left atrial appendage with an epicardial clip. Innovations. 2017;12:28–32. doi: 10.1097/imi.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 69.Bedeir K, Warriner S, Kofsky E et al. Left atrial appendage epicardial clip (AtriClip): essentials and post-procedure management. J Atr Fibrillation. 2019;11 doi: 10.4022/jafib.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Starck CT, Steffel J, Emmert MY Epicardial left atrial appendage clip occlusion also provides the electrical isolation of the left atrial appendage. Interact Cardiovasc Thorac Surg. 2012. pp. 15416–8. [DOI] [PMC free article] [PubMed]
- 71.Gegechkori N, Yang F, Miller A Comparison of hybrid ablation for persistent atrial fibrillation with and without left atrial appendage closure. Report of 1 year follow up (abstract). Presented at Venice Arrythmias 2019, Venice, Italy, 3–5 October 2019
- 72.Tonks R, Lantz G, Mahlow J et al. Short and Intermediate Term Outcomes of the Convergent Procedure: Initial Experience in a Tertiary Referral Center. Ann Thorac Cardiovasc Surg. 2019;20:13–21. doi: 10.5761/atcs.oa.19-00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee RJ, Lakkireddy D, Mittal S et al. Percutaneous alternative to the Maze procedure for the treatment of persistent or longstanding persistent atrial fibrillation (aMAZE trial): Rationale and design. Am Heart J. 2015;170:1184–94. doi: 10.1016/j.ahj.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 74.Lee LS. Subxiphoid minimally invasive epicardial ablation (convergent procedure) with left thoracoscopic closure of the left atrial appendage. Operative Techniques in Thoracic and Cardiovascular Surgery. 2018;23:152–65. doi: 10.1053/j.optechstcvs.2019.04.002. [DOI] [Google Scholar]
- 75.Umbrain V, Verborgh C, Chierchia GB et al. One-stage approach for hybrid atrial fibrillation treatment. Arrhythm Electrophysiol Rev. 2017;6:210–6. doi: 10.15420/2017.36.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Civello KC, Smith CA, Boedefeld W. Combined endocardial and epicardial ablation for symptomatic atrial fibrillation: single center experience in 100+ consecutive patients. Journal of Innovations in Cardiac Rhythm Management. 2013. pp. 1–7.
- 77.Jan M, Zizek D, Gersak ZM et al. Comparison of treatment outcomes between convergent procedure and catheter ablation for paroxysmal atrial fibrillation evaluated with implantable loop recorder monitoring. J Cardiovasc Electrophysiol. 2018;29:1073–80. doi: 10.1111/jce.13625. [DOI] [PubMed] [Google Scholar]
- 78.Gulkarov I, Wong B, Kowalski M et al. Convergent ablation for persistent atrial fibrillation: single center experience. J Card Surg. 2019;34:1037–43. doi: 10.1111/jocs.14204. [DOI] [PubMed] [Google Scholar]
- 79.Sabzwari SRA, Garg L, Mehta N Convergent ablation for the treatment of long-standing persistent atrial fibrillation – a single center experienc. Presented at American Heart Association Scientific Sessions, Philadelphia, PA 16–18 November 2019


