Abstract
Background: Anthracycline-based chemotherapy is an effective treatment used for early-stage breast cancer patients. However, anthracycline use is limited due to its cardiotoxic effects. Recent studies have shown that Platycodon grandiflorum (PG) protects the heart from anthracycline-induced cardiotoxicity. However, no randomized, placebo-controlled clinical trial has been performed to investigate the clinical use of PG to prevent anthracycline-induced cardiotoxicity. This study aimed to evaluate the cardioprotective effects and safety of PG in early breast cancer patients receiving anthracycline-based chemotherapy. Methods: A total of 125 early breast cancer patients receiving anthracycline-based chemotherapy were enrolled and randomized into a PG group or placebo group in a 1:1 ratio. Results: Only 2 (3.1%) participants in the placebo group and 1 (1.6%) participant in the PG group experienced NYHA (New York Heart Association) class III or IV heart failure. There were no significant differences observed between the 2 groups. However, compared with the placebo group, patients in the PG group showed a lower incidence of subclinical heart failure (21.9% vs 8.2%, respectively, P = .033), as well as lower cardiac troponin T levels (48.4% vs 31.1%, respectively, P = .002). Importantly, there were no differences observed in the antitumor effects of anthracycline between the 2 groups (disease-free survival: hazards ratio = 1.09, 95% confidence interval = 0.45-2.62, P = .84; overall survival: hazards ratio = 1.46, 95% confidence interval = 0.33-6.43, P = .62). Conclusion: PG prevents anthracycline-induced acute and chronic cardiac injury in early-stage breast cancer patients without compromising the antitumor effects of chemotherapy.
Keywords: anthracycline, breast cancer, cardiotoxicity, Platycodon grandiflorum, clinical trial, heart failure
Introduction
Anthracycline (ANT)-based chemotherapy remains the backbone for adjuvant treatment against breast cancer.1,2 However, toxic side effects caused by ANT therapy, such as hair loss, myelosuppression, and cardiotoxicity, limit its clinical application. Cardiotoxicity is considered the most serious side effect of ANT.3 In 2003, Swain and colleagues4 showed that a cumulative dose of doxorubicin greatly increased the probability of heart failure and that a cumulative dose of 500 mg/m2 of doxorubicin led to a heart failure incidence rate of 26%.
While it has been accepted that iron-mediated production of reactive oxygen species (ROS) lead to myocardial oxidative stress, the molecular basis of ANT-induced cardiotoxicity is unknown.5 Despite great efforts made over the last 50 years, the best approach that should be used to prevent ANT-mediated cardiotoxicity remains controversial.6 Indeed, the use of traditional antioxidants such as vitamin C, vitamin E, coenzyme Q10, and acetylcysteine, or cardioprotective drugs such as angiotensin-converting enzyme inhibitor and angiotensin receptor blocker have shown little cardioprotective effects against ANT-induced cardiotoxicity both in vivo and in vitro.7-12 Thus far, only dexrazoxane has been shown to exert cardioprotective effects.13-15 However, multiple studies have reported various side effects associated with dexrazoxane, including an increased risk of secondary malignancy and compromising antitumor effects of chemotherapy, which has limited its clinical use. In fact, the US Food and Drug Administration approved dexrazoxane only for women diagnosed with metastatic breast cancer, who have received a total cumulative dose greater than 300 mg/m2 of doxorubicin and who needed additional doses of doxorubicin to maintain tumor control.16,17
Due to the lack of effective and affordable therapies, there has been a shift toward herbal medicine to prevent cardiotoxicity caused by anthracyclines. For years, humans have relied on herbal formulations and natural products to promote health and prevent disease.18,19 Platycodon grandiflorum (PG) is a species of herbaceous flowering perennial plant that has been used for years in traditional Chinese medicine to treat cardiovascular diseases including coronary heart disease and heart failure. PG has been shown to improve heart function and ameliorate cardiovascular symptoms, such as palpitations, shortness of breath, and chest pain.20,21 In addition, recent modern pharmacological studies have demonstrated that PG extracts have stronger antioxidant effects compared with conventional antioxidants. Particularly, PG has been shown to reduce ROS formation and lipid peroxidation.22,23 Former studies found that ROS destroy cells and tissues.24,25 Both in vivo and in vitro studies confirmed increased ROS production in cardiomyocytes after anthracycline therapy.26,27 Furthermore, while extremely high or low concentrations of nitric oxide were shown to cause damage to myocardial cells, PG has been shown to maintain a balanced concentration of nitric oxide in myocardial cells.28-31 Hence, PG-mediated reduction of oxidative stress may be the main mechanism by which PG contributes to the prevention of ANT-induced cardiotoxicity.
We proposed that PG could be effective in the prevention of cardiotoxicity caused by ANT-based chemotherapy. However, there is a lack of high-quality clinical trials that have been performed to evaluate the safety and efficacy of PG. Therefore, we conducted a randomized, double-blinded placebo-controlled trial to assess the cardioprotective effects and safety of PG in early breast cancer patients receiving ANT-based chemotherapy. In addition, we aimed to evaluate whether PG administration interfered with the antitumor activity of ANT, as measured by disease-free survival (DFS) and overall survival (OS).
Methods
Study Design
A randomized, double-blind, placebo-controlled trial was performed based on a previously published study.32 Briefly, a total of 125 female patients histologically or cytologically diagnosed with early breast cancer and who were to receive ANT-based chemotherapy were enrolled. The chemotherapy regimen in this trial included 4 cycles of AC (40-60 mg/m2 doxorubicin and 500-600 mg/m2 cyclophosphamide, 3-week cycle), followed by 4 cycles of docetaxel (100 mg/m2, 3-week cycle) or 4 to 8 cycles of AC (40-60 mg/m2 doxorubicin and 500-600 mg/m2 cyclophosphamide, 3-week cycle). Over 6 cycles of AC in adjuvant chemotherapy and neo-adjuvant chemotherapy for early breast cancer was not recommend. There were 6 patients (3 in the placebo group and 3 in the PG group) in our trial who received 8 cycles of AC since they were allergic to docetaxel. Drugs were intravenously injected on the first day of each cycle (every 3 weeks). All recruited patients were randomly assigned in a 1:1 ratio to receive either oral PG granules (PG group) or placebo granules (placebo group) at a dose of 6 mg/day before chemotherapy, which was also maintained for 6 cycles during chemotherapy.
Both PG and placebo granules were purchased from Jiangyin Tianjiang Pharmaceutical and were identical in appearance, taste, smell, and packaging. Eligible patients were allocated to 1 of the 2 groups based on a randomization list created by a professional statistician using SPSS software, version 19 (IBM). Randomization was conducted by an independent statistician, who was not involved in the recruitment process. The randomization codes were kept in sealed, sequentially numbered, opaque envelopes, and they were prepared by research assistants, who were not involved in the recruitment process. The envelopes were kept secure in the Good Clinical Practice Centre of Longhua Hospital and would not be opened until after statistical analysis or in the case of medical emergency. The study participants, study investigators, other research team members, pharmacists, nurses, and technicians would remain blinded to the allocation of study medication versus placebo.32
The study protocol was reviewed and approved by the Ethics Committee of Longhua Hospital, which was affiliated with Shanghai University of Traditional Chinese Medicine (Reference Number 2015LCSY14), and the trial was registered to the Chinese Clinical Trial Registry (Registration Number ChiCTR-IPR-16009256). All patients signed informed consent forms before initiating treatment.
Participants
All participants were enrolled at Longhua Hospital, which was affiliated to Shanghai University of Traditional Chinese Medicine. Eligible participants included early breast cancer patients ranging from 20 to 70 years of age with Karnofsky Performance Status scores more than 60 and who were scheduled to receive ANT-based chemotherapy. Both adjuvant and neoadjuvant chemotherapy were included. Participants with a history of myocardial injury, coronary artery disease, myocarditis, and functional cardiac insufficiency were excluded from this study. Exclusion criteria also included patients who had previously received ANT-based chemotherapy or radiotherapy, patients with metastatic cancer disease, patients who were pregnant, and patients who were allergic to investigational drugs. Suspending criteria included patients who experienced serious adverse effects that may have been related to the intervention drug. Details of eligibility criteria have been previously reported.32
Clinical Outcomes
Primary outcomes for this trial included clinical and subclinical cardiotoxicity. Clinical cardiotoxicity was evaluated based on the New York Heart Association (NYHA) functional classification before and after completion of the final cycle of chemotherapy and after 2 years of follow-up. Subclinical cardiotoxicity was defined as a left ventricular ejection fraction (LVEF) less than 50% or a drop in LVEF from baseline, that is, >15%. LVEF was measured using multi-gated acquisition (MUGA), which was performed 3 times: (1) before chemotherapy, (2) at the completion of the therapeutic regimen, and (3) after 2 years of follow-up. MUGA scans were performed using a standard clinical protocol. Administration of 925 MBq 99m Tc-Pertechnetate was performed through intravenous injection using either in vivo or in vitro methods. Images were acquired using a Philips BrightView gamma camera (Philips Medical Systems),33 and MUGA scans were analyzed by an experienced cardiologist blinded to patient allocations.
The secondary outcome of this trial was cardiac troponin I (cTNT) levels, a cardiac plasma biomarker. Heparinized plasma samples were collected according to the European Society of Cardiology and American Heart Association guidelines at the following fixed time points: (1) before chemotherapy, (2) 24 hours after each cycle of chemotherapy, and (3) after 2 years follow-up.34,35 Samples were immediately analyzed for cTNT using the Elecsys Troponin I immunoassay (Roche). Measurements were performed at a central core laboratory in the hospital. Laboratory members were unaware of patient allocations.
Antitumor effects were assessed based on patient DFS and OS. DFS was defined as the length of time from enrollment to first recurrence of breast cancer or death from any cause, as defined by the Standardized Efficacy Endpoints criteria.36 OS was measured based on time from enrollment to time of death from any cause. Noncardiac adverse events (AEs) included unintended or unfavorable clinical signs and symptoms, laboratory results or diseases that did not necessarily show a causal relationship with the study intervention based on common terminology criteria for AEs (NCI-CTCAE v4.03). Safety assessments were performed every cycle.
Statistical Analysis
Statistical analysis was performed based on the intention-to-treat (ITT) principle. Missing data were imputed using the “last observation carried forward” principle. Continuous data were presented as means ± standard deviation. The baseline characteristics of the 2 groups were assessed using an independent t test for continuous variables and using the χ2 test and Fisher’s exact test for categorical variables. The Mann-Whitney U-test and Wilcoxon tests were used to compare samples between the 2 groups. Event-free survival was defined the time of treatment initiation to the first cardiac event. Event-free survival, DFS, and OS were analyzed using Kaplan-Meier curves and the log-rank method was used for a statistical comparison between curves. Crude and adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using Cox proportional hazard regression. All analyses were performed using SPSS software version 19 (IBM). Two-tailed P < .05 was considered as statistically significant.
Results
Participants
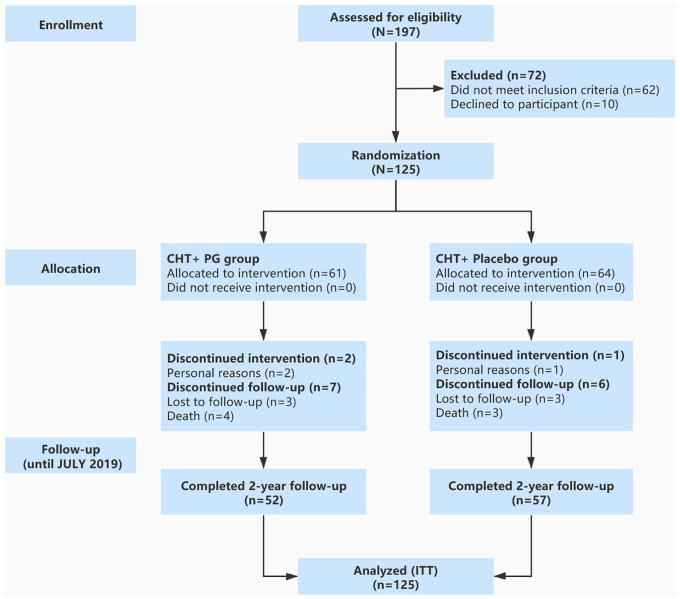
Participants were enrolled between November 2016 and September 2017 in the Breast Center of Longhua Hospital, which was affiliated to Shanghai University of Traditional Chinese Medicine. The last visit of all participants was in October 2019. A total of 125 patients were randomly assigned to receive chemotherapy with either PG (61 patients) or placebo (64 patients). A flow diagram of patients is shown in Figure 1. Two participants in the PG group and 1 in the placebo group did not complete corresponding treatment regimens. In addition, 3 participants in each group were lost during follow-up for personal reasons. Furthermore, 4 patients in the PG group and 3 patients in the placebo group passed away before completion of the study. To obtain a complete database, missing data were imputed using the “last observation carried forward” method according to the intention-to-treat principle. No significant differences in baseline characteristics were observed between the 2 groups (Table 1).
Figure 1.
Flowchart of patients’ enrollment and follow-up.
Table 1.
Baseline Characteristics of the Patientsa.
| Characteristics | Placebo (n = 64) | PG (n = 61) |
|---|---|---|
| Age, years | 49 ± 10.4 | 50 ± 10.9 |
| Height, cm | 156 ± 6.7 | 155 ± 6.9 |
| Weight, kg | 56 ± 10.6 | 57 ± 10.5 |
| Body mass index, kg/m2 | 23.1 ± 5.4 | 23.7 ± 5.6 |
| Body surface area, m2 | 1.56 ± 0.21 | 1.59 ± 0.26 |
| Adjuvant CHT, n (%) | 59 (92.2) | 59 (96.7) |
| Neo-adjuvant CHT, n (%) | 5 (6.8) | 2 (3.3) |
| Tumor phase, n (%) | ||
| Stage I | 22 (34.3) | 21 (34.4) |
| Stage II | 30 (46.9) | 31 (50.8) |
| Stage III | 13 (20.3) | 9 (14.8) |
| Tumor subgroup, n (%) | ||
| Luminal A | 21 (32.8) | 23 (37.7) |
| Luminal B/HER2+ | 12 (18.8) | 10 (16.4) |
| Luminal B/HER2− | 8 (12.5) | 7 (11.5) |
| HER2+b | 10 (15.6) | 7 (11.5) |
| TNBC | 13 (20.3) | 14 (23) |
| ALN, n (%) | ||
| N0 | 9 (14.1) | 7 (11.5) |
| N1 | 28 (43.8) | 31 (50.8) |
| N2 | 15 (23.4) | 11 (18.0) |
| N3 | 12 (18.7) | 12 (19.7) |
| Baseline LVEF, % | 71.02 ± 6.20 | 69.14 ± 7.03 |
Abbreviations: CHT, chemotherapy; HER2, human epidermal growth factor receptor 2; TNBC, triple negative breast cancer; ALN, axillary lymph nodes; LVEF, left ventricular ejection fraction.
Data are presented as percentage or mean ± standard deviation.
All HER2 positive patients received trastuzumab-targeted therapy.
Cardiotoxicity
Primary Outcomes
Clinical cardiotoxicity was assessed in all 125 participants based on the NYHA functional classification. Only 2 (3.1% of the study population) participants in the placebo group (2/37 patients, 5.4%) and 1 (1.6% of the study population) participant in the PG group (1/38 patients, 2.6%) were classified as NYHA heart failure class III or IV. These 3 participants did not pass away from heart failure during treatment or after 2-years of follow-up. In addition, these 3 patients received more than 300 mg/m2 cumulative dose of doxorubicin. Significant differences were not observed for clinical cardiotoxicity between the 2 groups.
Subclinical cardiotoxicity was assessed by LVEF measurements, which were defined as a decrease in LVEF to <55% or >15% from baseline during chemotherapy and/or after a 2-year follow-up. A total of 14 participants in the placebo group and 5 participants in the PG group showed subclinical cardiotoxicity. The number of patients exhibiting subclinical cardiotoxicity was significantly lower in the PG group (5/61, 8.2%) compared with the placebo group (14/64, 21.9%) (P = .033; Figure 2). Furthermore, patients in the PG group received a higher cumulative doxorubicin dose (HR = 0.38, 95% CI = 0.15 to 0.94, P = .046; Figure 3A) and showed a longer subclinical cardiotoxicity-free survival time (HR = 0.33, 95% CI = 0.14 to 0.77, P = .013; Figure 3B) compared with patients in the placebo group.
Figure 2.
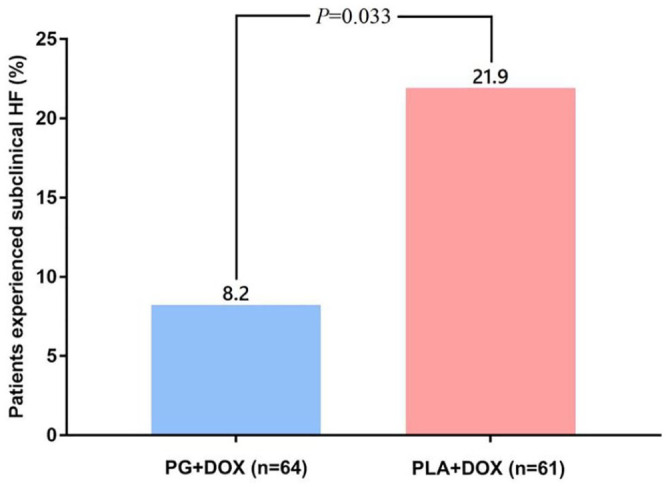
Incidence of subclinical heart failure.
Figure 3.

Kaplan-Meier plots. (A) Cumulative doxorubicin dose until subclinical heart failure. (B) Subclinical heart failure-free survival time.
Secondary Outcomes
The secondary outcome measured in this study was plasma cTNT levels. Figure 4 shows the variation in cTNT levels for each group at the examined time points from baseline to the end of the 2-year follow-up. Four patients in the placebo group (6.3%) and 2 patients in the PG group (3.3%) showed elevated cTNT levels after the first cycle of chemotherapy. However, these elevated levels were not statistically significant between the 2 groups. Patients with elevated plasma cTNT levels (19 in the PG group and 31 in the placebo group) showed significant differences between the 2 groups after the third cycle of chemotherapy, which persisted until chemotherapy was completed (P = .002). However, an elevation in cTNT levels decreased in both groups by the end of the follow-up.
Figure 4.

Variations of cardiac troponin I (cTNT) from randomization to 2-year follow-up.
Survival
Kaplan-Meier curves for DFS and OS are shown in Figure 5 (DFS: HR = 1.09, 95% CI = 0.45 to 2.62; OS: HR = 1.46, 95% CI = 0.33 to 6.43). There were no significant differences in DFS and OS observed between the 2 groups during the 2-year follow-up (log-rank test, PDFS = 0.84, POS = 0.62).
Figure 5.

Kaplan-Meier plots. (A) Disease-free survival. (B) Overall survival.
Noncardiac Adverse Events
Major noncardiac AEs are listed in Table 2. Most patients showed at least 1 noncardiac AE. Nausea, vomiting, alopecia, neutropenia, and leucopenia were the most common events (detected in ≥10% of patients). Only grade 3 or higher noncardiac AEs were detected in this trial. Grade 5 noncardiac AEs were not reported. The incidence of grade 3 or higher noncardiac AEs were similar in both groups. A total of 7 deaths (3 patients in the placebo group and 4 patients in the PG group) occurred within the 2-year follow-up. The cause of death in the placebo group included breast cancer metastasis (2 patients) and cardiac disorders (1 patient). Death of patients in PG group was caused by breast cancer metastasis (3 patients) and pulmonary disease (1 patient).
Table 2.
Noncardiac Adverse Events.
| Adverse event | Placebo group (n = 64), n (%) | PG group (n = 61), n (%) |
|---|---|---|
| Grade ≥3 adverse event | 41 (63.1) | 45 (73.8) |
| Nausea | 20 (30.8) | 23 (37.7) |
| Vomiting | 18 (27.7) | 19 (31.1) |
| Alopecia | 15 (23.1) | 17 (27.9) |
| Neutropenia | 19 (29.2) | 16 (26.2) |
| Leucopenia | 18 (27.7) | 17 (27.9) |
| Diarrhea | 10 (15.4) | 7 (11.5) |
| Anemia | 4 (6.2) | 5 (8.2) |
| Thrombocytopenia | 5 (7.7) | 3 (4.9) |
| Neurotoxicity | 3 (4.6) | 1 (1.6) |
| Fatigue | 3 (4.6) | 2 (3.3) |
| Constipation | 1 (1.5) | 0 |
Discussion
The addition of PG to chemotherapy can alleviate associated myocardial damage and prevent doxorubicin-induced cardiomyocyte apoptosis in a xenograft mouse model of breast cancer.37 However, there is a lack of clinical trials published investigating the combination of PG with ANT-based chemotherapy as an adjuvant therapy in cancer patients. In this study, we showed that PG alleviates myocardial damage in breast cancer patients who received ANT-based chemotherapy.
LVEF is an indicator of myocardial systolic function and its reduction is often associated with subclinical cardiac dysfunction. Interestingly, patients who received PG showed a reduction in the incidence of subclinical cardiotoxicity. In addition, patients who received PG along with ANT-based chemotherapy were able to complete all ANT-based chemotherapy cycles without showing subclinical cardiotoxicity and showed longer subclinical cardiotoxicity-free survival time compared with patients receiving ANT without PG. This indicates that coadministration of PG can protect against systolic dysfunction and chronic cardiotoxicity in patients receiving chemotherapy.
In this study, there was no observation of a reduction in the incidence of CHF in the PG group. Only 2 patients in the placebo group and 1 patient in the PG group showed class III or IV NYHA heart failure during the 2-year follow-up. In fact, this could be related to high-cumulative doses of doxorubicin (more than 300 mg/m2), which has been previously associated with cardiotoxicity.38 While there were no differences between the 2 patient groups, the incidence rate of CHF was much higher in patients who had received a cumulative dose of doxorubicin more than 300 mg/m2. Nevertheless, a larger sample size is required to further support these results and observations.
Troponin I was used in this study to detect early myocardial damage and to support the poor sensitivity and specificity of the echocardiography. Troponin I measurements demonstrated that the severity of myocardial injury was lower in patients in the PG group compared with the placebo group. In addition, troponin I levels suggest that ANT treatment induces myocardial damage in a dose-dependent manner and that myocardial injury may occur during the first cycle of ANT-based chemotherapy.
Adding PG to the chemotherapy did not compromise antitumor effects of the ANT-based regimen. Indeed, there was no significant difference observed in DFS and OS during the 2-year follow-up. However, studies analyzing longer follow-up periods are needed to confirm this conclusion.
This trial showed that the coadministration of PG with ANT-based chemotherapy can reduce ANT-induced cardiotoxicities in female patients with early breast cancer, compared with ANT alone. In addition, we showed that the administration of PG may increase vomiting in patients receiving ANT-based chemotherapy. In most patients, vomiting only occurred during the first 3 or 4 days after chemotherapy, which could be managed with appropriate symptomatic treatment. There were no differences observed in other noncardiac adverse effects between the placebo and PG groups.
The main limitation of this study was the small sample size analyzed. Thus, our study might have limited power in the detection of clinical differences such as differences in NYHA functional classifications between the 2 groups. In addition, follow-up was limited to 2 years, which may not be sufficient to derive clear conclusions. Despite that acute and chronic cardiotoxicity have been shown to occur within 1 year after ANT administration, late cardiotoxicity may occur several years later in some patients, even after 18 years of chemotherapy.39 Furthermore, the preliminary conclusion that PG did not compromise antitumor effects was based on similar DFS and OS times between the 2 patient groups. However, the expected survival time of most early breast cancer patients is more than 10 years, which is much longer than the follow-up period used in this study. Therefore, a longer follow-up period will be required to confirm this conclusion.
Conclusion
Our results demonstrated that PG could be used as an adjuvant therapy to prevent ANT-induced acute and chronic cardiotoxicity in early breast cancer patients without interfering with chemotherapy antitumor effects. However, larger cohort trials with longer follow-up periods are required to determine the effects of PG on late cardiotoxicity and patient survival.
Acknowledgments
This study was supported by the Science and Technology Commission of Shanghai Municipality. We would like to thank all patients and their family members. We also wish to acknowledge Jiangyin Tianjiang Pharmaceutical Co Ltd (Jiangyin, China) for kindly offering trial medication and placebo at a discounted price. The company did not contribute to the study design, methods, patient recruitment, data collection, or data preparation.
Footnotes
Author Contributions: WH and YYS participated in the design and coordination of the study. SL contributed to funding management. YNQ and CYW participated in data collection and assembly. CPS and LYC participated in data analysis and interpretation. YJB and YYS participated in the provision of study materials and patients. WH participated in writing the manuscript. All authors have read and approved the final version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study has been authorized and funded by the Science and Technology Commission of Shanghai Municipality (Approval Number: 14401970900) and Longhua Hospital affiliated to Shanghai University of Traditional Chinese Medicine (Approval Number: LYTD-67). The funding body played no role in the design of the study; collection, analysis, and interpretation of data; or in writing the manuscript.
ORCID iD: Sheng Liu  https://orcid.org/0000-0002-0972-1512
https://orcid.org/0000-0002-0972-1512
References
- 1. Lefrak EA, Pitha J, Rosenheim S, Gottileb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer. 1973;32:302-314. [DOI] [PubMed] [Google Scholar]
- 2. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effect of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687-1717. [DOI] [PubMed] [Google Scholar]
- 3. Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91:710-717. [DOI] [PubMed] [Google Scholar]
- 4. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869-2879. [DOI] [PubMed] [Google Scholar]
- 5. Doroshow JH. Effect of anthracycline antibiotics on oxygen radical formation in rat heart. Cancer Res. 1983;43:460-472. [PubMed] [Google Scholar]
- 6. Tan C, Tasaka H, Yu KP, Murphy ML, Karnofsky DA. Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. Clinical evaluation with special reference to childhood leukemia. Cancer. 1967;20:333-353. [DOI] [PubMed] [Google Scholar]
- 7. Acar Z, Kale A, Turgut M, et al. Efficiency of atorvastatin in the protection of anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2011;58:988-989. [DOI] [PubMed] [Google Scholar]
- 8. Kalay N, Basar E, Ozdogru I, et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2006;48:2258-2262. [DOI] [PubMed] [Google Scholar]
- 9. Ibrahim MA, Ashour OM, Ibrahim YF, El-Bitar HI, Gomaa W, Abdel-Rahim SR. Angiotensin-converting enzyme inhibition and angiotensin AT(1)-receptor antagonism equally improve doxorubicin-induced cardiotoxicity and nephrotoxicity. Pharmacol Res. 2009;60:373-381. [DOI] [PubMed] [Google Scholar]
- 10. de Nigris F, Rienzo M, Schiano C, Fiorito C, Casamassimi A, Napoli C. Prominent cardioprotective effects of third generation beta blocker nebivolol against anthracycline-induced cardiotoxicity using the model of isolated perfused rat heart. Eur J Cancer. 2008;44:334-340. [DOI] [PubMed] [Google Scholar]
- 11. Iarussi D, Auricchio U, Agretto A, et al. Protective effect of coenzyme Q10 on anthracyclines cardiotoxicity: control study in children with acute lymphoblastic leukemia and non-Hodgkin lymphoma. Mol Aspects Med. 1994;15(suppl):s207-s212. [DOI] [PubMed] [Google Scholar]
- 12. Whittaker JA, Al-Ismail SAD. Effect of digoxin and vitamin E in preventing cardiac damage caused by doxorubicin in acute myeloid leukaemia. Br Med J (Clin Res Ed). 1984; 288:283-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Dalen EC, Michiels EM, Caron HN, Kremer LC. Different anthracycline derivates for reducing cardiotoxicity in cancer patients. Cochrane Database Syst Rev. 2010;(4):CD005006. [DOI] [PubMed] [Google Scholar]
- 14. Lipshultz SE, Rifai N, Dalton VM, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med. 2004;351:145-153. [DOI] [PubMed] [Google Scholar]
- 15. Pearlman M, Jendiroba D, Pagliaro L, Keyhani A, Liu B, Freireich EJ. Dexrazoxane in combination with anthracyclines lead to a synergistic cytotoxic response in acute myelogenous leukemia cell lines. Leuk Res. 2003;27:617-626. [DOI] [PubMed] [Google Scholar]
- 16. Tebbi CK, London WB, Friedman D, et al. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin’s disease. J Clin Oncol. 2007;25:493-500. [DOI] [PubMed] [Google Scholar]
- 17. US Food and Drug Administration. DOXIL® (doxorubicin HCl liposome injection) for intravenous infusion. Accessed July 11, 2020 http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/050718s043lbl.pdf
- 18. Xiao Q, Zhu W, Feng W, et al. A review of resveratrol as a potent chemoprotective and synergistic agent in cancer chemotherapy. Front Pharmacol. 2019;9:1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang J, Gao L, Lee YM, et al. Target identification of natural and traditional medicines with quantitative chemical proteomics approaches. Pharmacol Ther. 2016;162:10-22. [DOI] [PubMed] [Google Scholar]
- 20. Hao W, Liu S. Study on the effect of Platycodon root on prevention of cardiac toxicity induced by anthracycline in patients with breast cancer after operation. Hernan Trad Chin Med. 2017;37:1116-1118. doi: 10.16367/j.issn.1003-5028.2017.06.0394 [DOI] [Google Scholar]
- 21. Kang YS, Hong KP, Jung DC, et al. Calcium channel-blocking activity of Chinese balloon flower (Platycodon grandiflorum) for producing blood pressure-lowering functional foods. Food Sci Biotechnol. 2008;17:156-160. doi: 10.1016/j.foodpol.2007.06.001 [DOI] [Google Scholar]
- 22. Lee JY, Yoon JW, Kim CT, Lim ST. Antioxidant activity of phenylpropanoid-esters isolated and identified from Platycodon grandiflorum A. DC. Phytochemistry. 2004;65:3033-3039. [DOI] [PubMed] [Google Scholar]
- 23. Kim TW, Song IB, Lee HK, et al. Platycodin D, a triterpenoid sapoinin from Platycodon grandiflorum, ameliorates cisplatin-induced nephrotoxicity in mice. Food Chem Toxicol. 2012;50:4254-4259. [DOI] [PubMed] [Google Scholar]
- 24. Xiao Q, Wu J, Pang X, et al. Discovery and development of natural products and their derivatives as photosensitizers for photodynamic therapy. Curr Med Chem. 2018;25:839-860. [DOI] [PubMed] [Google Scholar]
- 25. Xiao Q, Lin H, Wu J, et al. Pyridine-embedded phenothiazinium dyes as lysosome-targeted photosensitizers for highly efficient photodynamic antitumor therapy. J Med Chem. 2020;63:4896-4907. [DOI] [PubMed] [Google Scholar]
- 26. Link G, Tirosh R, Pinson A, Hershko C. Role of iron in the potentiation of anthracycline cardiotoxicity: identification of heart cell mitochondria as a major site of iron-anthracycline interaction. J Lab Clin Med. 1996;127:272-278. [DOI] [PubMed] [Google Scholar]
- 27. Dresdale AR, Barr LH, Bonow RO, et al. Prospective randomized study of the role of N-acetyl cysteine in reversing doxorubicin-induced cardiomyopathy. Am J Clin Oncol. 1982;5:657-663. [DOI] [PubMed] [Google Scholar]
- 28. Fogli S, Nieri P, Breschi MC. The role of nitric oxide in anthracycline toxicity and prospects for pharmacologic prevention of cardiac damage. FASEB J. 2004;18:664-675. [DOI] [PubMed] [Google Scholar]
- 29. Cigremis Y, Parlakpinar H, Polat A, et al. Beneficial role of aminoguanidine on acute cardiomyopathy related to doxorubicin-treatment. Mol Cell Biochem. 2006;285:149-154. [DOI] [PubMed] [Google Scholar]
- 30. Jang MH, Kim CJ, Kim EH, Kim MG, Leem KH, Kim J. Effects of Platycodon grandiflorum on lipopolysaccharide-stimulated production of prostaglandin E2, nitric oxide, and interleukin-8 in mouse microglial BV2 cells. J Med Food. 2006;9:169-174. [DOI] [PubMed] [Google Scholar]
- 31. Han SB, Park SH, Lee KH, et al. Polysaccharide isolated from the radix of Platycodon grandiflorum selectively activates B cells and macrophages but not T cells. Int Immunopharmacol. 2001;1:1969-1978. [DOI] [PubMed] [Google Scholar]
- 32. Hao W, Liu S, Qin Y, et al. Cardioprotective effect of Platycodon grandiflorum in patients with early breast cancer receiving anthracycline-based chemotherapy: study protocol for a randomized controlled trial. Trials. 2017;18:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1-39.e14. [DOI] [PubMed] [Google Scholar]
- 34. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC), developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129-2200. [DOI] [PubMed] [Google Scholar]
- 35. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776-803. [DOI] [PubMed] [Google Scholar]
- 36. Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25:2127-2132. [DOI] [PubMed] [Google Scholar]
- 37. Man S, Youyang S, Sheng L, et al. Pharmacokinetics of Platycodonis Radix combined with adriamycin in treating mice with lung metastasis of breast cancer. Acad J Shanghai Univ Trad Chin Med. 2019;33:54-60. [Google Scholar]
- 38. Vejpongsa P, Yeh ET. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol. 2014;64:938-945. [DOI] [PubMed] [Google Scholar]
- 39. de Azambuja E, Ameye L, Diaz M, et al. Cardiac assessment of early breast cancer patients 18 years after treatment with cyclophosphamide-, methotrexate-, fluorouracil- or epirubicin-based chemotherapy. Eur J Cancer. 2015;51:2517-2524. [DOI] [PubMed] [Google Scholar]