Abstract
Background:
We aimed to determine the role of non-mydriatic fundus examination and artificial intelligence (AI) in screening diabetic retinopathy (DR) in patients with diabetes in the Metabolic Disease Management Center (MMC) in Tianjin, China.
Methods:
Adult patients with type 2 diabetes mellitus who were first treated by MMC in Tianjin First Central Hospital and Tianjin 4th Center Hospital were divided into two groups according to the time that MMC was equipped with the non-mydriatic ophthalmoscope and AI system and could complete fundus examination independently (the former was the control group, the latter was the observation group). The observation indices were as follows: the incidence of DR, the fundus screening rate of the two groups, and fundus screening of diabetic patients with different course of disease.
Results:
A total of 5039 patients were enrolled in this study. The incidence rate of DR was 18.6%, 29.8%, and 49.6% in patients with diabetes duration of ⩽1 year, 1–5 years, and >5 years, respectively. The screening rate of fundus in the observation group was significantly higher compared with the control group (81.3% versus 28.4%, χ2 = 1430.918, p < 0.001). The DR screening rate of the observation group was also significantly higher compared with the control group in patients with diabetes duration of ⩽1 year (77.3% versus 20.6%; χ2 = 797.534, p < 0.001), 1–5 years (82.5% versus 31.0%; χ2 = 197.124, p < 0.001) and ⩾5 years (86.9% versus 37.1%; χ2 = 475.609, p < 0.001).
Conclusions:
In the case of limited medical resources, MMC can carry out one-stop examination, treatment, and management of DR through non-mydratic fundus examination and AI assistance, thus incorporating the DR screening process into the endocrine clinic, so as to facilitate early diagnosis.
Keywords: artificial intelligence, diabetic retinopathy, fundus screening, non-mydratic fundus examination
Introduction
Along with the rapid growth of national economy and lifestyle changes, the number of patients with diabetes is increasing rapidly in China. A survey by Professor Ning’s team showed that the incidence of diabetes and prediabetes among adults in China is 11.6 and 50.1%, respectively.1 The research also showed that diabetes therapy rate was 25.8%, and only 39.7% of people undergoing treatment achieved the desired glycated hemoglobin (HbA1c) levels.1 Diabetic retinopathy (DR) is a common microvascular complication of diabetes, and it is one of the most important causes of irreversible blindness in people of working age.2 Among individuals with diabetes, the prevalence of DR is approximately 28.5% in the United States.3 The incidence of DR increases with the duration of diabetes, and the prevalence of DR is estimated to be about 77.8% in patients living with diabetes for more than 15 years.4 DR is a serious threat to the quality of life of patients with diabetes and is a serious economic burden for society.5
Early and timely detection and treatment of DR can significantly improve the prognosis of DR patients and their quality of life.6 Owing to the importance of DR prevention, both American Diabetes Association and Chinese guidelines suggest that patients with type 2 diabetes should be screened for fundus lesions within a short period of time after diagnosis.7 However, DR patients rarely take the initiative to seek medical treatment before visual impairment occurs. Community-based studies report that about 50–60% of patients with diabetes do not received fundus examination, and 32% of high-risk patients with visual impairment never received fundus screening.8 In reality, diabetic patients first contact endocrinologists to diagnose diabetes mellitus. Endocrinologists prescribe hypoglycemic agents and advise patients see an eye specialist for retinal screening. However, patients often seldomly visit the ophthalmology department actively because there is no obvious vision loss, which will cause the delay in the early diagnosis of DR. Before the use of a non-mydriatic camera and artificial intelligence (AI) system, the fundus examination requires patients to register again in the ophthalmic clinic, wait, and undergo examination. Patients need to undergo mydriatic fundus examination and need family members to accompany them. Intraocular pressure is measured before mydriasis. After 0.5% compound tropicamide eye drops are put into the eyes, when the pupil is more than 5 mm, the fundus is examined in detail by direct fundoscopy to determine whether it is DR. After mydriasis, the patient’s ocular discomfort may last for a few hours. When the patients have visual impairment, if there is proliferative retinopathy, even if laser or surgical treatment is provided, DR-related vision loss is irreversible. Therefore, in order to have a good prognosis, DR patients need early diagnosis and treatment, and early screening must be carried out.9
European and American countries have accumulated a lot of experience in chronic disease management. However, the systematic management of chronic diseases has only begun in China. Since 2016, many metabolic disease management centers (MMCs) have been established in China to manage chronic metabolic diseases such as diabetes. MMC was initiated by the Chinese Medical Association and the Shanghai Ruijin Hospital. This center integrates disease diagnosis and treatment, rapid detection, data analysis and task tracking. Deep learning is a group of computational methods that allow an algorithm to program itself by learning from a large set of examples that demonstrate the desired behavior, removing the need to specify rules explicitly. In the evaluation of retinal fundus photographs from adults with diabetes, an algorithm based on deep machine learning has high sensitivity and specificity for detecting referable DR.10 In the screening of DR, the AI system exceeded all pre-specified superiority endpoints for sensitivity, specificity and imageability rate, thus demonstrating AI’s ability to bring specialty-level diagnostics to primary care settings.11 With the improvement of fundus screening function of MMCs, endocrinologists, combined with the AI system can conduct DR screening, making the early diagnosis of DR become a reality. In this study, we examined DR screening of type 2 diabetes patients in the outpatient department of an MMC before and after the implementation of non-mydratic fundus camera independently from two MMCs in Tianjin First Central Hospital and Tianjin 4th Center Hospital. Moreover, we evaluated the role of non-mydriatic fundus examination and AI in screening DR in diabetic patients in Tianjin, China. This study also explored the prevention and screening of DR with a setting with limited medical resources.
Patients and methods
We included adult patients with type 2 diabetes mellitus who were first treated by MMC in Tianjin First Central Hospital and Tianjin 4th Center Hospital. The MMC of Tianjin First Central Hospital officially received patients for the first time on 30 November 2016. Since 1 December 2017, the MMC could independently complete non-mydriatic fundus imaging and upload the fundus results in real time. The MMC of Tianjin 4th central hospital officially received patients for the first time on 25 October 2017. Since 24 October 2018, the MMC could independently complete non-mydriatic fundus imaging and upload the fundus results in real time. The clinical study protocol was approved by the Institutional Review Board (IRB)of Tianjin 4th Central Hospital, and all steps were conducted in accordance with the principles of the World Medical Association Declaration of Helsinki [trial registration code: ChiCTR1900027916]. The IRB approved the collection and use of patients’ records according to regulations for clinical trials in humans (IRB approval no.2017-SZXLL020). Written informed consent was obtained from each patient.
The study inclusion criteria were: (a) age ⩾18 years; (b) patients without mental disorders who can communicate independently; (c) there was no definite diagnosis of DR and no treatment for fundus diseases before this visit.
The exclusion criteria: (a) type 1 and other special types of diabetes, gestational diabetes or diabetes mellitus with pregnancy; (b) patients with severe mental illness and unclear consciousness; (c) patients with active tuberculosis and other infectious diseases.
Patients’ data from the Tianjin First and 4th Centers Hospital were collected from the MMC doctor terminal, and patients meeting the previously mentioned conditions were screened. The patients were divided into two groups according to the time that the MMC was equipped with a non-mydriatic ophthalmoscope and AI system and could complete fundus examination independently. The former was the control group, and the latter was the observation group.
According to the MMC system, we collected information on the patient’s name, sex, contact information, smoking/drinking history, family history of diabetes, waist circumference, age, diabetes course, and blood pressure. (a) The diagnosis of type 2 diabetes was based on the 2017 Chinese diabetes treatment guidelines, as fasting peripheral blood glucose (FPG) ⩾7.0 mmol/l, postprandial blood glucose ⩾11.1 mmol/l at 2 h, or a confirmed diagnosis as type 2 diabetes by hospitals with grade 2 or above. (b) Weight was measured using the same scale on an empty stomach at the MMC clinic in the morning and recorded. (c) The fundus images: after standardized training of technical personnel, the clinical staff used a non-mydriatic fundus camera to take fundus photographs. At least two photos per eye of the fundus (including one macula-centered and one disc-centered image) were needed. The view angle of each photo is required to be ⩾45°. Image quality was confirmed by both software quality control model and operator. Then, the photos and related information were uploaded and sent simultaneously to the ophthalmologist. After imaging, the software sent the images and related information to a web server and automatic DR grading results from the server by the AI system were returned in a few seconds as a temporary reference for the examiner. Patients with a positive result for referable DR would be referred to an ophthalmologist immediately. Referable DR here was defined by updated International Council of Ophthalmology Guide-lines for Diabetic Eye Care 2017 which includes moderate and severe diabetes phase nonproliferation retinopathy (NPDR) and proliferation stage of diabetic retinopathy (PDR).12 The fundus images with significance of referral indicated by AI will be given priority to remind ophthalmologists for review. Ophthalmologists usually do it in 30 min. Other fundus photos without referral significance will be completed by ophthalmologists within 48 h. All images were evaluated by ophthalmologists through an online platform and all final readings relayed to endocrinology physicians. At present, diagnosis in China is mainly based on a doctor’s expertise, and diagnostic results from medical auxiliary equipment are used as reference. In case of false negatives by AI, the ophthalmologist decision was taken as final. Endocrinologists and ophthalmologists can work together to develop patient treatment plans through an online communication platform. Patients can immediately see fundus photos and their fundus results in real time after the ophthalmologist’s review by their mobile phone scanning the two-dimensional code.
Observation indexes: the main observation indices included: (a) the fundus screening rate of patients in the two groups and fundus screening status of patients with new diabetes mellitus (duration of diabetes ⩽1 year); (b) patients with blurred vision who received DR screening. Secondary outcome measures included the screening positive rate of DR in the outpatient patients with type 2 diabetes, the incidence of DR in patients with a different duration of diabetes, and the occurrence of PDR.
Statistical analysis
Statistical Program for Social Sciences 20.0 software (SPSS, Inc., Chicago, IL,USA) was used for data collation and analysis. Kolmogorov–Smirnov normal test was performed on the measurement data. Mean ± standard deviation was used for statistical description of variables conforming to normal distribution, median (P50) was used for those not conforming to normal distribution, and percentage (%) was used for counting data. Independent sample t test was used for the comparison of measurement data between the two groups. Rank sum test was used for the measurement data that did not conform to the normal distribution, chi-squared test was used for the comparison of counting data, and spearman rank correlation analysis was used to test the correlation between the diabetes course and DR. All statistical tests were performed by bilateral test with alpha = 0.05.
Results
Demographic and clinical characteristics of the two groups
A total of 5039 patients were eligible to for inclusion in this study. Tianjin First Central Hospital MMC had 2393 cases from 30 November 2016 to 23 May 2019. There were 965 cases before 1 December 2017, and 1428 cases after. Tianjin 4th Central Hospital MMC had 2646 cases from 25 October 2017 to 28 June 2019. There were 1437 cases before 24 October 2018, and 1209 cases after. There were 3042 males, and 1997 females. The mean age was 56.7 ± 11.3 years and the median duration of diabetes was 2.6 years (range 0–48 years). Of the patients, 1596 (31.7%), 2434 cases (48.3%), 998 cases (19.8%), 2308 cases (45.8%), 1804 cases (35.8%), and 842 cases (16.7%) had auto-blurred vision, hypertension, history of coronary heart disease, family history of diabetes, and history of smoking, respectively. The mean HbA1c of the patients was 8.74 ± 3.18%, and 2825 (56.1%) patients received fundus screening at the MMC, including 1904 (67.4%) patients with normal fundus, 859 (30.4%) patients with NPDR, and 62 (2.2%) patients with PDR.
There were 2637 cases in the observation group and 2402 cases in the control group. The basic data of the two groups are summarized in Table 1. There was no significant difference in sex, duration of disease, systolic blood pressure, HbA1c, body weight, and peripheral blood glucose between the two groups. The observation group was older than the control group and the diastolic blood pressure was higher than the control group.
Table 1.
Comparison of basic data between the two groups.
| Items | Control group | Observation group | Statistics | p value |
|---|---|---|---|---|
| Cases (male/female) | 2402 (1448/954) | 2637 (1594/1043) | 0.014* | 0.905 |
| Age (years) | 56.3 ± 11.2 | 57.0 ± 11.4 | −2.366 | 0.018 |
| Median course (years) | 2.4 | 2.7 | −0.393# | 0.694 |
| Systolic pressure (mmHg) | 140.3 ± 20.2 | 140.0 ± 18.8 | 0.622 | 0.533 |
| Diastolic pressure (mmHg) | 80.1 ± 11.7 | 82.3 ± 10.9 | −7.007 | <0.001 |
| HbA1c (%) | 8.71 ± 1.92 | 8.76 ± 2.78 | −0.561 | 0.575 |
| Weight (kg) | 75.4 ± 13.7 | 75.4 ± 14.1 | −0.083 | 0.934 |
| FPG (mmol/l) | 9.21 ± 3.01 | 9.15 ± 3.16 | 0.614 | 0.539 |
| 2hPG (mmol/l) | 14.41 ± 5.34 | 15.48 ± 5.11 | −1.296 | 0.196 |
χ2,
Rank and test z value, others are t values.
2hPG, peripheral blood glucose 2 h after meal; FPG, fasting peripheral blood glucose; HbA1c, glycated haemoglobin.
The occurrence of DR in patients with different diabetes course, as shown in Figure 1, spearman rank correlation analysis was applied to test the correlation between disease course and DR, and the correlation coefficient was 0.325, p < 0.001.
Figure 1.
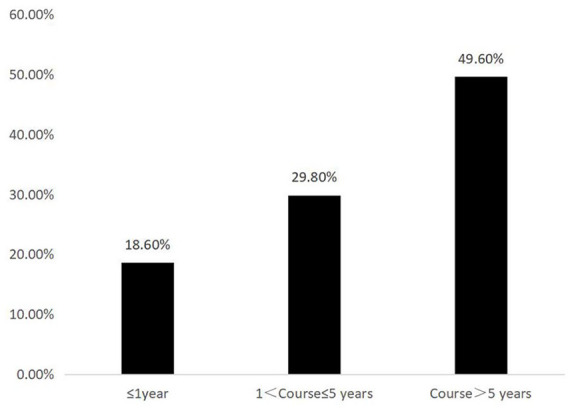
Incidence of DR of different diabetes durations.
DR, diabetic retinopathy.
Good images could not be obtained from 160 patients (6.1%) who consented to screening. The main reasons were ring artifacts (80 cases), flare artifacts (65 cases), and small pupil or poor exposure (60 cases). Some patients had multiple reasons. Among them, the non-mydriatic fundus photography of 50 patients (1.9%) could not be evaluated and all patients were referred to the ophthalmic clinic for further examination. The main reasons that patients could not be evaluated were small pupil or unclear refractive medium.
Fundus screening in patients between two groups
Endocrinologists conducted fundus screening for diabetic patients who came to the MMC for the first time in the MMC clinic. A total of 681 patients (28.4%) in the control group completed fundus screening, while 2144 patients (81.3%) in the observation group received fundus screening, and the fundus screening rate in the observation group was significantly higher than that in the control group (χ2 = 1430.918, p < 0.001).
Comparison of fundus screening in patients with different duration of diabetes (⩽1 year, 1–5 years and >5 years) is shown in Table 2. The fundus screening rates of patients in the control group were 20–38%, and the fundus screening rates of patients in the observation group were significantly higher than those in the control group.
Table 2.
Fundus screening of patients in two groups with different diabetes course.
| Course of diabetes | Control group | Observation group | χ2 value | p value |
|---|---|---|---|---|
| Course ⩽1 year | 236 (20.6%) | 1046 (77.3%) | 797.534 | <0.001 |
| 1 <course ⩽5 years | 108 (31.0%) | 312 (82.5%) | 197.124 | <0.001 |
| Course >5 years | 337 (37.1%) | 786 (86.9%) | 475.609 | <0.001 |
Association between complaint of blurred vision in T2DM patients with DR
Of the 871 patients with blurred vision, 312 patients (35.8%) were diagnosed with DR in fundus screening, whereas among 1954 patients without blurred vision, 609 patients (31.2%) were diagnosed with DR. The patients with blurred vision had higher incidence of DR (χ2 = 5.939, p = 0.017). A total of 41 (2.1%) patients without blurred vision complaint were diagnosed with PDR, compared with 21 patients (2.4%) with blurred vision. The difference was not statistically significant (χ2 = 0.275, p = 0.600). The comparison of fundus screening rates between the observation and control group in the presence of blurred objects is shown in Figure 2. The fundus screening in the observation group was higher than that in the control group regardless of whether the patients had blurred vision or not (among patients with blurred vision, 64.6% versus 45.1%, χ2 = 61.48, p < 0.001; patients without blurred vision were 88.3% versus 19.7%, χ2 = 1638.257, p < 0.001).
Figure 2.
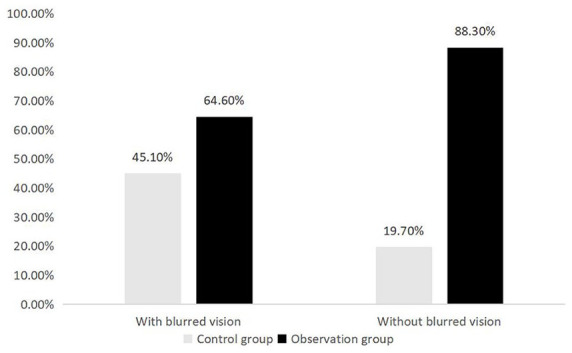
Comparison of fundus screening rates between the two groups with/without blurred vision.
Discussion
DR is a serious chronic microvascular complication of diabetes. At first, patients may have only mild or no symptoms. As the disease progresses, new blood vessels are generated in the non-perfusion area of the fundus capillaries, which develops into PDR and eventually leads to blindness. Due to the insidious onset of type 2 diabetes, about 20–33.3% of patients may have DR lesions during the diagnosis of diabetes.13 Therefore, guidelines suggest that patients with type 2 diabetes undergo screening for fundus lesions at the time of diagnosis.5 This study found that the screening rate of DR in the outpatient service of diabetes increased from 28.4% to 81.3% with the help of mydriatic-free fundus examination and AI. Among patients with diabetes whose duration of disease was ⩽1 year, the outpatient DR screening rate increased from 20.6% to 77.3%. This is in line with the requirements of diabetes diagnosis and treatment guidelines and supportive of the early diagnosis and treatment of DR.
The study on eye diseases of Latinos in Los Angeles found that the prevalence rate of DR in patients with diabetes duration of <5 years, 5~9 years, 10~14 years, and ⩾15 years was 27.5%, 56.1%, 59.8% and 79.6%, respectively.9 Our study results showed that the detection rate of DR in adult patients with type 2 diabetes was 18.6% (⩽1 year), 29.8% (1–5), and 49.6% (>5 years). With the extension of the course of diabetes, the detection rate of DR increased significantly. Regardless of whether the patient has symptoms of blurred vision, fundus screening must be performed. For patients with a long course of diabetes, we need to be vigilant about whether they have developed DR and initiate early treatment. In this study, 31.7% of the patients complained of blurred vision at the time of admission, and the DR detection rate of the patients with blurred vision in the fundus screening was 35.8%, which was slightly higher than the DR detection rate of the patients without blurred vision (31.2%, χ2 = 5.939, p = 0.017). However, there was no significant difference in PDR detection rate between patients with and without blurred vision (2.4% versus 2.1%, χ2 = 0.275, p = 0.600). Therefore, regardless of whether patients have blurred complaint, we need to conduct DR screening for them. Studies have shown that early diagnosis and treatment of DR, individualized follow-up plan, and necessary and appropriate retinal photocoagulation and vitreous surgery can prevent severe vision loss in about 80% of patients.14
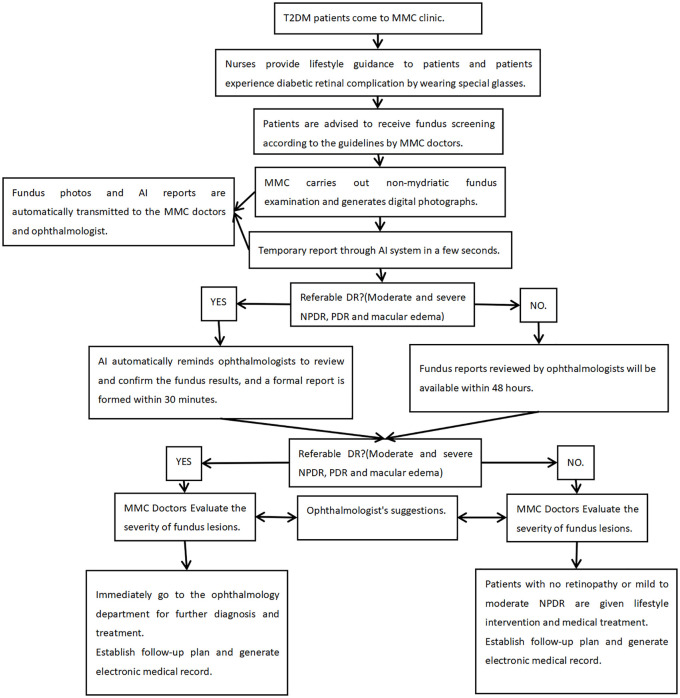
The use of mydriatic-free fundus examination combined with AI in the outpatient department of endocrinology has formed a new model of fundus screening (Figure 3). The new model enables patients to attach importance to the screening of retinopathy through health education, simulated experience of ocular lesions and mutual learning among patients, and it also increases the simplicity of DR screening by non-mydriatic fundus camera, so as to improve patients’ comfort and compliance. This makes communication between endocrinologists, ophthalmologists and patients more convenient. Digital fundus photography is widely used in clinical practice due to its long-term storage, easy transmission, and retrospective evaluation. Due to its simple operation, objective and permanent recording, electronic transmission can be used for remote consultation, and the vast majority of clinically significant DR can be screened.15 It has become an important and feasible method to screen diabetic patients. The model has really shifted the screening setting of DR from the ophthalmology clinic to the endocrine clinic, and implemented the guidelines on early screening of DR.
Figure 3.
The model of screening for fundus screening in MMC.
AI, artificial intelligence; DR, diabetic retinopathy; MMC, Metabolic Disease Management Center; NPDR, nonproliferation stage of diabetic retinopathy; PDR, proliferation stage of diabetic retinopathy; T2DM, type 2 diabetes mellitus.
Remarkable advancements in biomedical research have led to the generation of large amounts of data.16 Using AI, it has become possible to extract meaningful information from large volumes of data in a shorter frame of time, with less human interference.17 In effect, convolutional neural networks (a deep learning method) have been taught to recognize pathological lesions from images.18 Diabetes has high morbidity, and affects millions of people who need to be screened for DR.19 Deep neural networks offer a great advantage of screening for DR from retinal images, in improved identification of DR lesions and risk factors for diseases, with high accuracy and reliability.20 By 2015, according to the seventh edition of the International Diabetes Federation, the number of DR patients in China was about 27 million. With the yearly increase in the number of diabetes patients, the prevalence rate and blindness rate of DR also increase, and the prevention and treatment task of DR is increasingly severe. According to the results of the DR epidemiological survey, more than 50% of diabetic patients who visited primary medical institutions were not advised to have fundus examination regularly, and nearly 70% of diabetic patients did not receive fundus screening.21 The present study showed that the new model could provide better fundus screening services through one-stop examination, treatment and management under the condition of the shortage of medical resources. The new model enables patients to complete fundus screening at the first visit for diabetes (in the endocrine clinic), and creates individual electronic files at the same time, while providing follow-up information. It is suitable for promotion in medical shortage areas.
Conclusions and limitations
The use of mydriatic-free fundus examination combined with AI in the outpatient department of endocrinology has formed a new model of fundus screening. The new model can carry out one-stop examination, treatment and management of DR through non-mydriatic fundus examination and AI assistance, and incorporates the DR screening process into the endocrine clinic, so optimizes the early diagnosis of DR. It is suitable for promotion in medical shortage areas.
The findings of this study have to be seen in light of limitations. First, the study was conducted in two large hospitals with relatively strong comprehensive strength, where there are sufficient medical resources, rather than in the community hospitals with insufficient resources. The second limitation concerns the quality of the fundus photos. There is insufficient incident light in the upper temporal quadrant for non-mydriatic fundus photography, resulting in a slightly worse fundus image in the upper temporal quadrant than the macular area. As a result, there may be some errors in the assessment of DR by the intelligent screening system. The DR intelligent diagnostic and screening system is still in its infancy in China, so a standardized scheme and reliable diagnostic system are still needed to promote the extensive development of DR screening.
Acknowledgments
Zhaohu Hao and Shanshan Cui contributed equally and are co-first authors of this article.
We would like to thank the study participants and the project team members. We would like to thank Editage (www.editage.cn) for English language editing.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethics committee approval: The IRB of Tianjin 4th Central Hospital approved the collection and use of patients’ records according to regulations for clinical trials in humans (IRB approval No.2017-SZXLL020).Written informed consent was obtained from each patient.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by National Key R&D Program of China (2018YFC1314000) and Tianjin Science and Technology Development Strategy Research Projects (18ZLZXZF00740). The funding sources had no role in the study design, data collection, analysis and interpretation, and in the writing of the manuscript or in the decision to submit the manuscript for publication.
ORCID iDs: Zhaohu Hao  https://orcid.org/0000-0002-3965-3805
https://orcid.org/0000-0002-3965-3805
Baocheng Chang  https://orcid.org/0000-0002-6674-6644
https://orcid.org/0000-0002-6674-6644
Contributor Information
Zhaohu Hao, NHC Key Laboratory of Hormones and Development (Tianjin Medical University), Tianjin Key Laboratory of Metabolic Diseases, Tianjin Medical University Chu Hsien-I Memorial Hospital & Tianjin Institute of Endocrinology, Tianjin, China; Department of Metabolic Disease Management Center, Tianjin 4th Central Hospital, The 4th Central Hospital Affiliated to Nankai University, The 4th Center Clinical College of Tianjin Medical University, Tianjin, China.
Shanshan Cui, NHC Key Laboratory of Hormones and Development (Tianjin Medical University), Tianjin Key Laboratory of Metabolic Diseases, Tianjin Medical University Chu Hsien-I Memorial Hospital & Tianjin Institute of Endocrinology, Tianjin, China; Department of Endocrinology, Tianjin First Central Hospital, The First Center Clinical College of Tianjin Medical University, Tianjin, China.
Yanjuan Zhu, NHC Key Laboratory of Hormones and Development (Tianjin Medical University), Tianjin Key Laboratory of Metabolic Diseases, Tianjin Medical University Chu Hsien-I Memorial Hospital & Tianjin Institute of Endocrinology, Tianjin, China.
Hailin Shao, Department of Metabolic Disease Management Center, Tianjin 4th Central Hospital, The 4th Central Hospital Affiliated to Nankai University, The 4th Center Clinical College of Tianjin Medical University, Tianjin, China.
Xiao Huang, NHC Key Laboratory of Hormones and Development (Tianjin Medical University), Tianjin Key Laboratory of Metabolic Diseases, Tianjin Medical University Chu Hsien-I Memorial Hospital & Tianjin Institute of Endocrinology, Tianjin, China.
Xia Jiang, Department of Endocrinology, Tianjin First Central Hospital, The First Center Clinical College of Tianjin Medical University, Tianjin, China.
Rong Xu, Department of Metabolic Disease Management Center, Tianjin 4th Central Hospital, The 4th Central Hospital Affiliated to Nankai University, The 4th Center Clinical College of Tianjin Medical University, Tianjin, China.
Baocheng Chang, NHC Key Laboratory of Hormones and Development (Tianjin Medical University), Tianjin Key Laboratory of Metabolic Diseases, Tianjin Medical University Chu Hsien-I Memorial Hospital & Tianjin Institute of Endocrinology, Tianjin 300134, China.
Huanming Li, Department of Metabolic Disease Management Center, Tianjin 4th Central Hospital, The 4th Central Hospital Affiliated to Nankai University, The 4th Center Clinical College of Tianjin Medical University, No. 1 Zhongshan Road, Tianjin 300140, China.
References
- 1. Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013; 310: 948–959. [DOI] [PubMed] [Google Scholar]
- 2. Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol 2007; 14: 179–183. [DOI] [PubMed] [Google Scholar]
- 3. Zhang X, Saaddine JB, Chou CF, et al. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA 2010; 304: 649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Early treatment diabetic retinopathy study research group. Ophthalmology 1991; 98(Suppl. 5): 766–785. [PubMed] [Google Scholar]
- 5. Solomon SD, Chew E, Duh EJ, et al. Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care 2017; 40: 412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paz SH, Varma R, Klein R, et al. Noncompliance with vision care guidelines in Latinos with type 2 diabetes mellitus: the Los Angeles Latino eye study. Ophthalmology 2006; 113: 1372–1377. [DOI] [PubMed] [Google Scholar]
- 7. Jin P, Peng J, Zou H, et al. A five-year prospective study of diabetic retinopathy progression in Chinese type 2 diabetes patients with “well-controlled” blood glucose. PLoS One 2015; 10: e0123449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kawasaki R, Akune Y, Hiratsuka Y, et al. Cost-utility analysis of screening for diabetic retinopathy in Japan: a probabilistic Markov modeling study. Ophthalmic Epidemiol 2015; 22: 4–12. [DOI] [PubMed] [Google Scholar]
- 9. Varma R, Torres M, Peña F, et al. Prevalence of diabetic retinopathy in adult Latinos: the Los Angeles Latino eye study. Ophthalmology 2004; 111: 1298–1306. [DOI] [PubMed] [Google Scholar]
- 10. Raman R, Srinivasan S, Virmani S, et al. Fundus photograph-based deep learning algorithms in detecting diabetic retinopathy. Eye 2019; 33: 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abràmoff MD, Lavin PT, Birch M, et al. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Dig Med 2018; 1: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wong TY, Sun J, Kawasaki R, et al. Guidelines on diabetic eye care: the International Council of Ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology 2018; 125: 1608–1622. [DOI] [PubMed] [Google Scholar]
- 13. Klein R, Klein BE, Moss SE, et al. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol 1984; 102: 527–532. [DOI] [PubMed] [Google Scholar]
- 14. Klein R, Klein BE. Is the prevalence of visual impairment rising or falling in the people with diabetes mellitus? It depends on who you study. JAMA Ophthalmol 2013; 131: 948–950. [DOI] [PubMed] [Google Scholar]
- 15. Ahmed J, Ward TP, Bursell SE, et al. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Diabetes Care 2006; 29: 2205–2209. [DOI] [PubMed] [Google Scholar]
- 16. Kanagasingam Y, Xiao D, Vignarajan J, et al. Evaluation of artificial intelligence-based grading of diabetic retinopathy in primary care. JAMA Netw Open 2018; 1: e182665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van der Heijden AA, Abramoff MD, Verbraak F, et al. Validation of automated screening for referable diabetic retinopathy with the IDx-DR device in the Hoorn diabetes care system. Acta Ophthalmol 2018; 96: 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li F, Liu Z, Chen H, et al. Automatic detection of diabetic retinopathy in retinal fundus photographs based on deep learning algorithm. Transl Vis Sci Technol 2019; 8: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shi L, Wu H, Dong J, et al. Telemedicine for detecting diabetic retinopathy: a systematic review and meta-analysis. BRI J Ophthalmol 2015; 99: 823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vujosevic S, Pucci P, Casciano M, Daniele A, et al. A decade-long telemedicine screening program for diabetic retinopathy in the north-east of Italy. J Diabetes Complications 2017; 31: 1348–1353. [DOI] [PubMed] [Google Scholar]
- 21. Chinese Ocular Fundus Diseases Society, Chinese Ophthalmological Society. Chinese Medical Association. Guidelines for clinical diagnosis and treatment of diabetic retinopathy in China. Chin J Ophthalmol 2014; 50: 851–865. [Google Scholar]