Abstract
Over the past 5 years, public interest in the potential health benefits of cannabidiol (CBD) has increased exponentially, and a wide range of over-the-counter (OTC) preparations of CBD are now available. A substantial proportion of the population appears to have used these products, yet the extent to which they are effective or safe is unclear. We reviewed the evidence for whether CBD has significant pharmacological and symptomatic effects at the doses typically found in OTC preparations. We found that most of the evidence for beneficial effects is derived from studies of pure, pharmaceutical grade CBD at relatively high doses. Relatively few studies have examined the effect of OTC CBD preparations, or of CBD at low doses. Thus, at present, there is little evidence that OTC CBD products have health benefits, and their safety has not been investigated. Controlled trials of OTC and low-dose CBD preparations are needed to resolve these issues.
Keywords: cannabidiol, CBD, cannabis oil, over the counter, health supplement, safety, efficacy
Introduction
Cannabidiol (CBD) can be bought as an over-the-counter (OTC) food supplement in a variety of forms, such as capsules, oils, cigarettes or an e-liquid.1,2 It is also sold in drinks, foods and cosmetics, such as hand creams.3 However, despite the widespread availability of these products, the safety and efficacy of OTC CBD are unclear.1,4 We sought to address this issue in the present review.
CBD was isolated in the late 1930s5,6 and its chemical structure was first described by Mechoulam in 1963.7 Although it can be produced synthetically, the CBD in OTC products is almost always derived from plant extracts.8 Studies of pure, pharmaceutical grade CBD in healthy volunteers indicate that it is not intoxicating, but may have limited subjective effects at very high doses.9,10 Neuroimaging studies have shown that it has effects on brain activity,11,12 and can modulate both the endocannabinoid and other neurotransmitter function in both volunteers and patients.13–15 Moreover, data from experimental medicine studies and clinical trials suggest that pharmaceutical grade CBD can reduce anxiety16,17 and psychotic symptoms,18 and has beneficial effects in addiction19 and childhood epilepsy.20 Endocannabinoid signalling also has a role in regulating the immune system, and CBD also has potential as an anti-inflammatory agent.21 This research has led to an exponential increase in consumer interest in CBD over the past 5 years (Figure 1). It is estimated that OTC CBD health supplements have been used by one in 10 adults in the United Kingdom (UK).22 Retail sales data indicate that in 2019, OTC CBD was bought by 1.3 million users who spent an estimated £300 million, more than the vitamin D and vitamin C markets combined.22 However, almost all of the data that have driven this interest have been derived from studies of pharmaceutical grade, rather than OTC CBD. In this paper, we explore the pharmacokinetics, metabolism, mechanism of action and therapeutic and adverse effects of CBD, as well as addressing the relevant safety, labelling and legal considerations for OTC CBD products.
Figure 1.
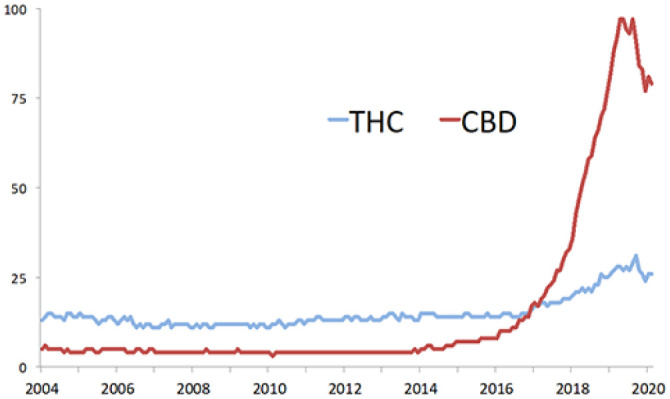
Google search trends for the terms ‘THC’ [tetrahydrocannabinol] and ‘CBD’ [cannabidiol], worldwide January 2004–February 2020.
The administration, dose and pharmacokinetics of OTC CBD
CBD can be administered via the oral, oromucosal or inhaled routes.23 Novel methods such as transdermal administration (using a permeation enhancer to increase absorption) have also been tested.24 Most OTC preparations are sold as capsules or oils, which are administered using a dropper or spray (Figure 2). Cannabis plant material (of which some strains, such as hemp, contain high concentrations of CBD and minimal Δ9-tetrahydrocannabinol [Δ9-THC]) can be inhaled, either by burning or using a vaporiser to heat dry plant material. Some vaporiser models can also use e-liquids containing CBD.2
Figure 2.

OTC CBD products: (a) Capsules, (b) Spray, (c) Oil and dropper, (d) Gummies, a type of ‘edible’. (e) Dried plant material, (f) A dry vaporizer, (g) Joint, and (h) e-Liquid vaporizer
In clinical trials, a typical CBD dose might be approximately 1000 mg/day, often taken in two divided doses.4 For childhood epilepsy syndromes the recommended dose is 10–20 mg/kg/day. However, most OTC preparations contain much smaller amounts of CBD. For example, a popular UK health food shop sells CBD capsules and edibles from 5 to 20 mg per dose, and sprays and oils from 2 to 8 mg per dose.25 The maximum recommended dose for these products is normally below 30 mg/day. In some countries there is more variability between products; a US study found that the concentration of products bought online was between 0.10 mg/mL and 655 mg/mL (median: 9.45 mg/mL).8
The pharmacokinetics of CBD have been systematically reviewed by Millar et al.23 They examined 24 studies, most of which assessed the administration of CBD at doses of 5–20 mg/day, which corresponds to the doses typically found in OTC preparations. With oral administration, single doses of 5.4 and 10 mg CBD achieved peak serum concentrations (Cmax) of 0.9 and 2.5 ng/ml. The time to maximum concentration (Tmax) was approximately 1 h, with a half-life between 1 and 3 h. CBD is highly lipophilic, and its oral bioavailability is highly dependent on food as a high-fat meal can quadruple plasma exposure.26
For oromucosal administration, 10–20 mg CBD had a Cmax of approximately 2–4 ng/mL, a Tmax between 1 and 4 h, and a half-life between 1.4 and 11 h.23 In a study by Stott et al.,27 single doses of 5, 10 and 20 mg CBD achieved Cmax of 0.4, 1.2 and 2.2 ng/mL. After 9 days of regular dosing, the corresponding Cmax were 0.5, 1.1 and 3.2 ng/mL. These results indicate that at these doses, the Cmax of CBD is dose-dependent and that CBD does not accumulate with regular dosing. As with oral administration, the bioavailability of oromucosal CBD is 3–5 times higher with food,28 suggesting that even with oromucosal administration, a significant proportion of absorption is gastrointestinal.
The only study of inhaled CBD identified by the review of Millar et al. found that 19 mg CBD achieved a much higher Cmax (110 ng/ml) immediately after smoking, before falling to 10.2 ng/ml an hour later.29 Unlike oral (and oromucosal) administration, inhalation avoids first-pass metabolism, an important feature as CBD undergoes extensive metabolism in the liver before reaching central targets.
Metabolism
CBD is metabolized by hepatic cytochrome P450 enzymes to form 7-hydroxy-CBD, 7-carboxy-CBD and 6-hydroxy-CBD as well as other minor metabolites.29 The two enzymes with the most substantial role are CYP2C19 and CYP3A4.30,31 While there has been some research in animal models, to date there have been no studies examining the pharmacological actions of CBD metabolites in humans (for a review, see Ujváry and Hanuš).32 However, there is evidence from human studies that the metabolism of CBD can lead to clinically relevant drug interactions, in particular via inhibition of CYP2C19. The antiepileptic medication clobazam is often prescribed as a treatment for childhood epilepsy syndromes. CBD can increase levels of N-desmethylclobazam, which itself is a potent antiepileptic,33 by approximately 5-fold.34 This interaction with clobazam may account for the increased risk of sedation and pneumonia seen in clinical trials of CBD in Dravet and Lennox–Gastaut syndrome.4 It is important to note that the doses used in clinical studies (at least 5 mg/kg) are much higher than in OTC preparations (5–20 mg/day). However, there are (non-peer-reviewed) reports that drug interactions may occur at doses as low as 1 mg/kg CBD.35 CYP2C19 also has a role in the metabolism of tricyclic antidepressants, selective serotonin reuptake inhibitors, proton pump inhibitors, clopidogrel, gliclazide, propranolol, and other antiepileptic medications such as diazepam and phenytoin.36 CBD can also affect the activity of several other CYP enzymes such as 2D6, 2C9 and 3A4, which may lead to additional interactions with antiepileptics, antipsychotics and other drugs.37,38 There have been case reports of drug–drug interactions with both warfarin39 and methadone.40
Mechanism of action
CBD has limited affinity for the orthosteric binding site of cannabinoid (CB) receptors and therefore has little direct activity. However, it may act as a negative allosteric modulator at CB1 and CB2 receptors, reducing their response to agonists such as Δ9-THC, anandamide and 2-arachidonoylglycerol.41,42 Anandamide and 2-arachidonoylglycerol are endogenous CB receptor ligands known as endocannabinoids. Anandamide is metabolised by fatty acid amide hydrolase (FAAH) which can also be inhibited by CBD.43 Other potential mechanisms of action include agonism of transient receptor potential (TRP) channels, partial agonism of dopamine D2 receptors and serotonin 5HT1A receptors, positive allosteric modulation of μ and δ-opioid receptors, inhibition of adenosine reuptake, competitive antagonism of G protein-coupled receptor 55 (GPR55) and inhibition of inflammatory cytokines via peroxisome proliferator-activated receptor-gamma (PPARγ) receptors.44–47
Because the doses of CBD in OTC preparations are much lower than in pharmaceutical grade preparations, they are extremely unlikely to produce plasma concentrations above 10 ng/ml.23 However, for most of the putative molecular targets described above, CBD has a half-maximal effective or inhibitory concentration (EC50/IC50) in the micromolar range (Table 1).47 The only target where CBD may have activity in the low nanomolar range is the negative allosteric site of CB2 receptors (IC50: 2–8 nM).48
Table 1.
The affinity and half-maximal concentrations for the putative molecular targets of CBD.
| Target | EC50/IC50, SEM (nM) | Ki (nM) |
|---|---|---|
| CB1 orthosteric site | – | 3245 nM |
| CB1 allosteric site | 304 | |
| CB2 orthosteric site | 503 ± 2080 | 3612 ± 1382 |
| CB2 allosteric site | 2–8 | 3.6 ± 0.3 |
| FAAH | 19,800 ± 4770 | – |
| Anandamide transporter | 10,200 ± 3030 | |
| TRPM8 | 70 ± 14 | – |
| TRPA1 | 100 ± 10 | – |
| TRPV1 | 1900 ± 802 | 3600 ± 200 |
| TRPV2 | 12,200 ± 9770 | – |
| D2 | 66 ± 20 | 11 |
| Adenosine uptake (ENT1) | 122 | – |
| GPR55 | 433 ± 43 | – |
| PPARγ receptors | 5000 | – |
| α3 Glycine receptor | 11,000 | – |
| 5HT1A | – | 16,000 |
Allosteric modulation of CB2 receptors
The activity of CBD at CB2 receptors is highly complex. Similar to CB1 receptors, CB2 receptors are G-protein coupled receptors which regulate signalling cascades such as cAMP, β-arrestins and extracellular signal-regulated kinases.50 CB receptors also demonstrate functional selectivity where ‘biased’ agonists are able to activate certain downstream pathways preferentially.51 Navarro et al. examined the effect of CBD on biased activity at CB1 and CB2 receptors as well as CB1–CB2 receptor heteromers. They found that, at a concentration of 100 nM, CBD ‘profoundly affected the agonist effect’ of the endogenous ligand anandamide at CB2 receptors.51 While the concentration of CBD was an order of magnitude higher than what can be achieved by OTC preparations, these results indicate that meaningful activity at CB2 receptors is at least theoretically possible.
CB2 receptors may be present throughout the central nervous system. In human post-mortem studies they have been identified in cerebellar microglial cells,52 and animal models have also suggested that they may be present in the cortex, hippocampus, ventral tegmental area and nucleus accumbens.53 CB2 receptors may have a neuroprotective role and have therefore been proposed as a target for several neurological and psychiatric disorders. Animal models of Alzheimer’s disease, traumatic brain injury, addiction, anxiety and depression have all found that CB2 agonists can be beneficial.53 In humans, it has also been shown that a common CB2 receptor variant, Q63R, which reduces its activity, is associated with eating disorders.54
CBD enantiomers: natural versus synthetic CBD
The cannabis plant only produces the (–) enantiomer of CBD, whereas both the (+)-CBD and (–)-CBD enantiomers can be made synthetically.55 Data from studies that have examined the binding affinity of CBD enantiomers and their metabolites for CB receptors are shown in Table 2. The (+) enantiomer and its metabolites all have a considerably higher affinity for CB receptors than the (–) enantiomer, including at concentrations in the low nanomolar range.55–57 To date, there have been no studies comparing the effects of different CBD enantiomers or their metabolites in humans, and in vivo research has only been completed in mice.57 All OTC preparations use CBD derived from a purified cannabis extract, rather than synthetic CBD. Therefore, based on the existing receptor affinity data, the pharmacological activity of OTC preparations appears to be limited to the allosteric modulation of CB2 receptors.
Table 2.
The binding affinity of (+) and (–) enantiomers of CBD to CB receptors.
| Compound | CB1 Ki (nM) | CB2 Ki (nM) |
|---|---|---|
| (–)-CBD | >10,000 | >10,000 |
| (–)-7-OH-CBD | >10,000 | >10,000 |
| (–)-7-COOH-CBD | >10,000 | >10,000 |
| (+)-CBD | 842 ± 36 | 203 ± 16 |
| (+)-7-OH-CBD | 5.3 ± 0.5 | 101 ± 5 |
| (+)-7-COOH-CBD | 13.2 ± 0.4 | 322 ± 16 |
Excipients, cannabis oils and the entourage hypothesis
In general, OTC preparations do not contain pure CBD.8 Most contain additional constituents and, if used clinically, are often described as ‘cannabis-based medicine extracts’ (CBMEs). To produce CBD oil, a solvent is added to cannabis plant material, extracting cannabinoids which are then added to an edible oil.58 To purify the extract, a process called ‘winterization’ is used, in which it is cooled and fractionated so that unwanted compounds with different melting points are removed.59 However, not all impurities are removed, and the oil may also contain other cannabinoids, such as Δ9-THC, tetrahydrocannabinolic acid, cannabigerol and cannabinol as well as terpenoids such as limonene, myrcene, α-pinene and linalool. It has been suggested that the combination of CBD with other cannabinoid and terpenoid compounds may lead to synergistic or complementary effects, producing an ‘entourage effect’ that is greater than that of CBD alone.60 However, experimental studies have consistently failed to provide evidence for such an effect.61–64 Nevertheless, the concept of the ‘entourage effect’ remains popular and is used to promote the health benefits of OTC supplements.65
Clinical effects of CBD at OTC doses
Consumer interest in CBD has partly been driven by positive results from clinical trials of pharmaceutical grade CBD. CBD has been assessed in randomized controlled clinical trials in rare childhood epilepsies,20,66–68 schizophrenia,18,69 type II diabetes,70 fatty liver disease,71 Crohn’s disease,72 Parkinson’s disease,73 Huntington’s disease,74 and cannabis dependence.19 The results from the trials in childhood epilepsy have led to its licensing as a treatment for Lennox–Gastaut syndrome and Dravet syndrome. The only other licensed medicine that contains CBD is the oromucosal spray nabiximols (Sativex), which contains similar quantities of Δ9-THC and CBD, and is approved as a treatment for spasticity in multiple sclerosis. Nabiximols has also been trialled as a treatment for pain, and for nausea and vomiting.75 However, it is unclear if the effectiveness of nabiximols is related to the presence of CBD.
Most of the clinical trials with positive findings have studied the effects of CBD at doses considerably higher than in OTC preparations (>300 mg/day or 10 mg/kg/day).4 Nevertheless, at least eight randomized placebo controlled clinical trials have examined the effects of CBD (or CBD predominant CBME) at low doses (Table 3). Most of these studies were small and reported limited efficacy with few adverse effects. An exception was the CAMS trial in multiple sclerosis, which examined the effects of a CBD-predominant CBME, as well as a Δ9-THC-based medicine on spasticity76 and urinary symptoms in 630 patients.77 Although some subjective outcomes were better with CBME, there was no evidence for an effect on objective outcomes, such as the Ashworth scale for spasticity and a walking test. Moreover, 77% of those in the CBME arm guessed that they had received active treatment, compared to only 50% in the placebo group, suggesting that blinding was inadequate. In a sub-study of lower urinary tract symptoms (CAMS-LUTS),77 the CBME arm achieved a 25% reduction in episodes of urge incontinence compared to placebo (p = 0.005), although there were no changes in urodynamic studies. The lack of blinding in these studies is a particular concern, as there can be a strong allegiance bias among patients in favour of cannabis-based medicines. This phenomenon was highlighted in another study involving children prescribed cannabinoids for epilepsy: parents of children who had moved from out of state to access the programme were twice as likely as local parents to report benefits.78
Table 3.
Placebo controlled clinical studies of low dose CBD and CBD predominant cannabis-based medicine extracts.
| Study | Patient group | Participants | Design | Low-dose CBD arms | Comparison arms | Type of CBD & administration | Efficacy of low-dose CBD | Adverse effects |
|---|---|---|---|---|---|---|---|---|
| Naftali et al.72 | Crohn’s disease | 19 | Parallel groups 8 weeks |
CBD 10 mg BD (n = 10) | Placebo (n = 9) | Natural extract Oral |
None | None |
| Tomida et al.79 | Ocular hypertension & glaucoma | 6 | 4-arm crossover Single dose |
CBD 20 mg CBD 40 mg |
Placebo Δ9-THC 5 mg |
Natural extract Sublingual |
Transient elevation in intraocular pressure with CBD 40 mg | Minimal |
| Notcutt80 | Chronic pain | 34 | ‘N of 1’ design 4 arms 8 × 1 week crossovers |
CBME (>95% CBD) 2.5 mg as required (median 8/day) | Δ9-THC 2.5 mg Δ9-THC 2.5 mg/CBD 2.5 mg Placebo |
Natural extract Sublingual |
No efficacy for pain Subjective improvement in sleep quality |
Minimal |
| Carlini and Cunha81
Experiment 1 |
Healthy volunteers | 10 | Parallel groups Single dose |
CBD 10 mg (n = 2) CBD 40 mg (n = 2) |
Placebo (n = 2) CBD 80 mg (n = 2) CBD 160 mg (n = 2) |
Natural extract Oral |
N/A | None |
| Carlini and Cunha81
Experiment 4 |
Healthy volunteers | 4 | Mixed 20 days |
CBD 5 mg BD oral (n = 3) | Placebo (n = 1) | Natural extract Oral |
N/A | Somnolence in 2/3 CBD participants |
| Carlin and Cunha81
Clinical trial as a hypnotic drug |
Insomniacs | 15 | Crossover Single dose at night |
CBD 40 mg | CBD 80 mg CBD 160 mg Nitrazepam 5 mg Placebo |
Natural extract Oral |
Reduced dream recall at 40 mg. No changes to other sleep parameters | None |
| CAMS76 | Spasticity in multiple sclerosis | 630 | Parallel groups 15 weeks |
CBME (>95% CBD) 1.25 mg, 2–5 capsules BD (n = 211) | Δ9-THC 2.5 mg, 4–10 capsules/day (n = 206) Placebo, 2–5 capsules BD (n = 213) |
Natural extract Oral |
No difference in Ashworth score of spasticity or walking speed. Subjective improvement in pain and spasticity NB. Issues with blinding |
Constipation, diarrhoea, increased appetite |
| CAMS-LUTS77 | Urge incontinence in multiple sclerosis | 522 | Parallel groups 15 weeks |
CBME (>95% CBD) 1.25 mg, 2–5 capsules BD (n = 181) | Δ9-THC 2.5 mg, 2–5 capsules BD (n = 174) Placebo, 2–5 capsules BD (n = 167) |
Natural extract Oral |
25% reduction (p = 0.005) in incontinence relative to placebo NB. Issues with blinding |
None |
| Morgan et al.82 | Smokers | 24 | Parallel groups 7 days |
CBD 400 μg PRN (n = 12) | Placebo (n = 12) | Synthetic Inhaled |
Reduced cigarette consumption | None |
BD, twice a day.
Can low doses of CBD moderate the effects of Δ9-THC on psychotic symptoms?
An important question for recreational cannabis users is whether low doses of CBD can moderate the adverse effects of Δ9-THC, particularly on psychotic and anxiety symptoms.83 Observational research has suggested that CBD may reduce psychotic symptoms and cognitive impairments in some regular cannabis users.84 In a small experimental study in healthy volunteers, Bhattacharya et al. found that pre-treatment with a 5 mg dose of intravenous (IV) CBD attenuated the severity of psychotic symptoms and anxiety subsequently induced by IV Δ9-THC (1.25 mg).85 Similarly, a study in a larger sample of volunteers that used oral (rather than IV) CBD (600 mg), also found that pretreatment with CBD attenuated the induction of psychotic symptoms by IV Δ9-THC (1.5 mg).86 Another study using inhaled CBD (16 mg) did not find an effect on psychotic symptoms,87 but in that case, Δ9-THC alone had no effect on psychotic symptoms relative to placebo, precluding the detection of a modulatory effect of CBD. Thus, evidence that CBD may reduce the adverse effects of Δ9-THC is limited to studies that used relatively high doses of CBD. It is not known if low doses of CBD have a similar effect.
Effects of CBD on anxiety
In an online survey of 2400 medicinal cannabis users from the United States (US), the majority cited a range of indications for their use of cannabis products including anxiety (66%), insomnia (59%), joint pain and inflammation (49%), depression (44%), migraines (32%), muscle tension or strain (32%), or severe and chronic pain (28%).88 An Australian survey of 1388 medicinal cannabis users listed a similar range of indications, including pain (62%), sleep (49%), mental health (45%), gastrointestinal disorders (13%), neurological disorders (11%) and cancer (8%).89 However, the evidence base for most of these putative indications is limited, with the exception of anxiety. A recent systematic review identified six randomized pre-clinical studies in this area.90 Most used doses of 300 mg or higher, and two examined a range of different doses. The first of these asked 60 healthy volunteers to complete a simulated public speaking test after pre-treatment with either CBD (100, 300 and 900 mg), clonazepam (1 mg) or placebo.16 In the second, 57 healthy volunteers completed a similar task after pre-treatment with a single dose of either CBD (150, 300, 600 mg) or placebo.17 In both studies, only the 300 mg dose of CBD reduced anxiety symptoms. The lower doses (100 often 150 mg) had no effect suggesting that OTC preparations (10–20 mg) are unlikely to be effective.
Adverse effects and safety
A recent meta-analysis of randomized clinical trials found that, compared to placebo, CBD is associated with reduced tolerability and increased risks of pneumonia, abnormal liver function tests, decreased appetite, diarrhoea, somnolence and sedation.4 However, after excluding studies in epilepsy, in which CBD has pharmacokinetic interactions with clobazam and sodium valproate, diarrhoea was the only adverse event present. In this meta-analysis, the majority of the studies used doses above 300 mg/day or 10 mg/kg/day. It is therefore not known whether CBD can cause adverse events at OTC doses.
Content of OTC preparations
The contents of OTC CBD preparations are of variable quality. A US study of 84 CBD products sold online in 2016 found that only 26/84 (31%) products accurately reported the amount and concentration of CBD that they contained.8 Vaporiser liquids were particularly inaccurate: only 3/24 (13%) products were correctly labelled. Moreover, that study found that 18/84 (21%) samples contained Δ9-THC, with a mean concentration of 0.45 mg/ml (standard deviation: 1.18; max: 6.4 mg/ml). More recent studies have shown that this continues to be a major problem. A 2020 study from Mississippi found that only 3/25 (12%) products were within 20% of what the label claimed and 3/25 (12%) had a Δ9-THC content exceeding legal limits.91 A UK study found that only 11/29 (38%) products had within 10% of the amount of CBD advertised and one product did not contain any CBD at all.92 Reports on CBD products from Switzerland,2 The Netherlands93 and other European countries94 have found similar results. There have even been reports of CBD-rich extracts containing enough Δ9-THC (3–4%) to intoxicate young children,95 as well as concerns that some products contain enough Δ9-THC for users to fail illicit drug tests.96
As well as cannabinoids, other contaminants have been found in OTC CBD products, including 5F-ADB and AB-FUBINACA, synthetic cannabinoid receptor agonists,97,98 dextromethorphan, an anti-cough medication and dissociative hallucinogen97 and pesticides.99 A recent case report described the admission of an otherwise healthy man to an intensive care unit after eating two packets of CBD gummies, although the responsible compound was never identified.100 Additional safety concerns include lung injury from CBD vaporizers,101 the use of CBD as an unproved alternative for established treatments for serious medical conditions,102 and the long-term effects of CBD in children and adolescents, given the role of the endocannabinoid system in neurodevelopment.103
Legal aspects
The legal status of cannabidiol is complex. Many countries now allow the prescription of licensed medicines which contain CBD, such as Epidiolex, and others also permit the prescription of a broader range of cannabis-based medicinal products without specific licensed indications,1 for example, Bedrolite, which contains less than 1.0% Δ9-THC and 9% CBD 9%, and is available through the Dutch Office for Medicinal Cannabis.104 In Uruguay, Georgia, Canada and several US states, recreational cannabis use is legal, and in many other countries it has been decriminalised. In jurisdictions where it is legalised, users are often able to purchase strains of cannabis with high CBD and low Δ9-THC content from cannabis dispensaries.
OTC preparations of cannabidiol, the ‘cannabis oils’ bought in a health shop, are subject to a variety of different laws and regulations across the world. In the European Union (EU) and the UK, CBD oil has been classified as a ‘novel food’ since January 2019.105 Novel foods are defined as foods which have not been widely consumed by EU citizens before 15 May 1997. They are subject to regulation and cannot advertise medicinal benefit. The EU also requires that Δ9-THC levels in these products must be below 0.2%.106 Sweden has additional legislation banning the sale of any product containing Δ9-THC, while in Slovakia all products containing CBD are illegal. Previously, CBD was considered a medicinal product in many EU jurisdictions. For example, in 2016, the UK Medicines and Healthcare Products Regulatory Agency (MHRA) informed companies that they would need to ‘operate within the law, by withdrawing their existing products from the market, or working with MHRA to satisfy the legal requirements of the Human Medicines Regulations 2012’.107
CBD products are not available as OTC supplements in Australia or New Zealand as they require a prescription.108,109 In Australia, hemp products are legal, but must have minimal concentrations of both CBD (<75 mg/kg) and Δ9-THC (<50 mg/kg).109 In Canada, both recreational and medical cannabis have been legalised since 2018. Products containing CBD are subject to specific regulations on their production, Δ9-THC content, labelling and packaging.110 In the US, the 2018 Farm Bill removed hemp (defined as having a Δ9-THC concentration ⩽0.3%) from the Controlled Substances Act. However, CBD extracted from non-hemp cannabis strains remains a Schedule I Controlled Substance.111 The US Food and Drug Administration has made it clear that CBD products cannot be sold as food or dietary supplements and has sent warning letters to manufacturers of CBD products, informing them that their medicinal claims were illegal.112 The complex interaction between US state and federal law has been described elsewhere.111
Conclusion
Although there is enormous consumer interest in CBD, there is little evidence that OTC preparations have significant pharmacological activity or provide health benefits. However, there have been relatively few studies that have explicitly sought to evaluate CBD at very low doses, or with the other constituents that are typically found in OTC preparations. Although the licensing of food supplements does not require demonstrations of their safety and efficacy, controlled trials of OTC preparations are needed to address this issue. There is also a need for more accurate labelling and advertising of OTC CBD products.
Key points
It is unknown if over-the-counter products can produce plasma concentrations of cannabidiol high enough to have significant pharmacological effects.
There is little evidence that over-the-counter cannabidiol products have therapeutic benefits.
Labelling of the constituents of over-the-counter cannabidiol products is often inaccurate.
Some over-the-counter cannabidiol products contain Δ9-tetrahydrocannabinol, which has the potential to cause adverse effects and positive urinary drug results for cannabis.
Controlled trials of over-the-counter products are needed to resolve these issues.
Footnotes
Conflict of interest: EC, PM, TPF and AE declare no conflicts of interest. JS is a researcher and clinician who, through his university, has worked with various pharmaceutical companies to identify new or improved treatments and from whom his employer (King’s College London) has received grant income, travel costs and/or consultancy payments; however, these do not relate to studies of cannabis or derivatives. For fuller information, see JS’s web page at http://www.kcl.ac.uk/ioppn/depts/addictions/people/hod.aspx.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: EC is supported by a National Institute for Health Research Doctoral Research Fellowship (300273). TF is funded by a Senior Academic Fellowship from the Society for the Study of Addiction. PM’s and JS’s research is supported by the National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) for Mental Health at South London and Maudsley NHS Foundation Trust and King’s College London, and AE is funded by the BRC. PM and JS are NIHR Senior Investigators.
ORCID iD: Edward Chesney  https://orcid.org/0000-0003-2851-5252
https://orcid.org/0000-0003-2851-5252
Contributor Information
Edward Chesney, Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, De Crespigny Park, London SE5 8AF, UK; South London and Maudsley NHS Foundation Trust, London, UK.
Philip McGuire, Department of Psychosis Studies, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, London, UK; National Institute for Health Research, Maudsley Biomedical Research Centre, London, UK.
Tom P. Freeman, Addiction and Mental Health Group (AIM), Department of Psychology, University of Bath, London, UK Addictions Department, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
John Strang, South London and Maudsley NHS Foundation Trust, London, UK; Addictions Department, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
Amir Englund, Addictions Department, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
References
- 1. Freeman TP, Hindocha C, Green SF, et al. Medicinal use of cannabis based products and cannabinoids. BMJ 2019; 365: l1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grafinger KE, Krönert S, Broillet A, et al. Cannabidiol and Tetrahydrocannabinol concentrations in commercially available CBD E-liquids in Switzerland. Forensic Sci Int 2020; 310: 110261. [DOI] [PubMed] [Google Scholar]
- 3. Peters J. I Was a CBD guinea pig. Slate. https://slate.com/human-interest/2019/11/cbd-coffee-shampoo-lotion-tincture-everything-all-of-it.html (2019, accessed 1 June 2020).
- 4. Chesney E, Oliver D, Green A, et al. Adverse effects of cannabidiol: a systematic review and meta-analysis of randomized clinical trials. Neuropsychopharmacology. Epub ahead of print 8 April 2020. DOI: 10.1038/s41386-020-0667-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Work TS, Bergel F, Todd AR. The active principles of Cannabis indica resin. I. Biochem J 1939; 33: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Adams R, Hunt M, Clark JH. Structure of cannabidiol, a product isolated from the marihuana extract of Minnesota wild hemp. I. J Am Chem Soc 1940; 62: 196–200. [Google Scholar]
- 7. Mechoulam R, Shvo Y. Hashish – I: the structure of cannabidiol. Tetrahedron 1963; 19: 2073–2078. [DOI] [PubMed] [Google Scholar]
- 8. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA 2017; 318: 1708–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schoedel KA, Szeto I, Setnik B, et al. Abuse potential assessment of cannabidiol (CBD) in recreational polydrug users: a randomized, double-blind, controlled trial. Epilepsy Behav 2018; 88: 162–171. [DOI] [PubMed] [Google Scholar]
- 10. Solowij N, Broyd S, Greenwood L, et al. A randomised controlled trial of vaporised Δ 9-tetrahydrocannabinol and cannabidiol alone and in combination in frequent and infrequent cannabis users: acute intoxication effects. Eur Arch Psychiatry Clin Neurosci 2019; 269: 17–35. [DOI] [PubMed] [Google Scholar]
- 11. Borgwardt SJ, Allen P, Bhattacharyya S, et al. Neural basis of Δ-9-tetrahydrocannabinol and cannabidiol: effects during response inhibition. Biol Psychiatry 2008; 64: 966–973. [DOI] [PubMed] [Google Scholar]
- 12. Fusar-Poli P, Crippa JA, Bhattacharyya S, et al. Distinct effects of Δ9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch Gen Psychiatry 2009; 66: 95. [DOI] [PubMed] [Google Scholar]
- 13. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry 2012; 2: e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry 2018; 75: 1107–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O’Neill A, Wilson R, Blest-Hopley G, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. Epub ahead of print 29 January 2020. DOI: 10.1017/S0033291719003519. [DOI] [PubMed] [Google Scholar]
- 16. Zuardi AW, Rodrigues NP, Silva AL, et al. Inverted U-shaped dose-response curve of the anxiolytic effect of cannabidiol during public speaking in real life. Front Pharmacol 2017; 8: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Linares IM, Zuardi AW, Pereira LC, et al. Cannabidiol presents an inverted U-shaped dose-response curve in a simulated public speaking test. Brazilian J Psychiatry 2019; 41: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry 2018; 175: 225–231. [DOI] [PubMed] [Google Scholar]
- 19. Freeman TP, Hindocha C, Baio G, et al. Cannabidiol for the treatment of cannabis use disorder: a phase 2a, double-blind, placebo-controlled, randomised, adaptive Bayesian trial. Lancet Psychiatry. Epub ahead of print 28 July 2020. DOI: 10.1016/S2215-0366(20)30290-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med 2017; 376: 2011–2020. [DOI] [PubMed] [Google Scholar]
- 21. Burstein S. Cannabidiol (CBD) and its analogs: a review of their effects on inflammation. Bioorg Med Chem 2015; 23: 1377–1385. [DOI] [PubMed] [Google Scholar]
- 22. Gibbs B, Yates DA, Liebling J. CBD in the UK. London, UK: Centre for Medicinal Cannabis, 2019. [Google Scholar]
- 23. Millar SA, Stone NL, Yates AS, et al. A systematic review on the pharmacokinetics of cannabidiol in humans. Front Pharmacol 2018; 9: 1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hunter D, Oldfield G, Tich N, et al. Synthetic transdermal cannabidiol for the treatment of knee pain due to osteoarthritis. Osteoarthr Cartil 2018; 26: S26. [Google Scholar]
- 25. Holland & Barrett. CBD Oil & Capsules. https://www.hollandandbarrett.com/shop/vitamins-supplements/homeopathic-flower-remedies/cbd/ (2020, accessed 1 June 2020).
- 26. Taylor L, Gidal B, Blakey G, et al. A phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs 2018; 32: 1053–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stott CG, White L, Wright S, et al. A phase I study to assess the single and multiple dose pharmacokinetics of THC/CBD oromucosal spray. Eur J Clin Pharmacol 2013; 69: 1135–1147. [DOI] [PubMed] [Google Scholar]
- 28. Stott CG, White L, Wright S, et al. A phase I study to assess the effect of food on the single dose bioavailability of the THC/CBD oromucosal spray. Eur J Clin Pharmacol 2013; 69: 825–834. [DOI] [PubMed] [Google Scholar]
- 29. Ohlsson A, Lindgren J, Andersson S, et al. Single-dose kinetics of deuterium-labelled cannabidiol in man after smoking and intravenous administration. Biomed Environ Mass Spectrom 1986; 13: 77–83. [DOI] [PubMed] [Google Scholar]
- 30. Zendulka O, Dovrtelová G, Nosková K, et al. Cannabinoids and cytochrome P450 interactions. Curr Drug Metab 2016; 17: 206–226. [DOI] [PubMed] [Google Scholar]
- 31. Jiang R, Yamaori S, Takeda S, et al. Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes. Life Sci 2011; 89: 165–170. [DOI] [PubMed] [Google Scholar]
- 32. Ujváry I, Hanuš L. Human metabolites of cannabidiol: a review on their formation, biological activity, and relevance in therapy. Cannabis Cannabinoid Res 2016; 1: 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haigh JR, Pullar T, Gent JP, et al. N-desmethylclobazam: a possible alternative to clobazam in the treatment of refractory epilepsy? Br J Clin Pharmacol 1987; 23: 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Groeneveld GJ, Martin JH. Parasitic pharmacology: a plausible mechanism of action for cannabidiol. Br J Clin Pharmacol 2020; 86: 189–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Landmark CJ, Brandl U. Pharmacology and drug interactions of cannabinoids. Epileptic Disord 2020; 22: S16–S22. [DOI] [PubMed] [Google Scholar]
- 36. DrugBank. Cytochrome P-450 CYP2C19 Substrates. https://www.drugbank.ca/categories/DBCAT002638 (2020, accessed 1 June 2020).
- 37. Gaston TE, Bebin EM, Cutter GR, et al. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia 2017; 58: 1586–1592. [DOI] [PubMed] [Google Scholar]
- 38. Urichuk L, Prior TI, Dursun S, et al. Metabolism of atypical antipsychotics: involvement of cytochrome p450 enzymes and relevance for drug-drug interactions. Curr Drug Metab 2008; 9: 410–418. [DOI] [PubMed] [Google Scholar]
- 39. Grayson L, Vines B, Nichol K, et al. An interaction between warfarin and cannabidiol, a case report. Epilepsy Behav Case Reports 2018; 9: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Madden K, Tanco K, Bruera E. Clinically significant drug-drug interaction between methadone and cannabidiol. Pediatrics 2020; 145: e20193256. [DOI] [PubMed] [Google Scholar]
- 41. Laprairie RB, Bagher AM, Kelly MEM, et al. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol 2015; 172: 4790–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tham M, Yilmaz O, Alaverdashvili M, et al. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br J Pharmacol 2019; 176: 1455–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. De Petrocellis L, Ligresti A, Moriello AS, et al. Effects of cannabinoids and cannabinoid-enriched cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol 2011; 163: 1479–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Campos AC, Moreira FA, Gomes FV, et al. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos Trans R Soc B Biol Sci 2012; 367: 3364–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bih CI, Chen T, Nunn AVW, et al. Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics 2015; 12: 699–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Devinsky O, Cilio MR, Cross H, et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 2014; 55: 791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Seeman P. Cannabidiol is a partial agonist at dopamine D2High receptors, predicting its antipsychotic clinical dose. Transl Psychiatry 2016; 6: e920–e920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Martínez-Pinilla E, Varani K, Reyes-Resina I, et al. Binding and signaling studies disclose a potential allosteric site for cannabidiol in cannabinoid CB2 receptors. Front Pharmacol 2017; 8: 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McPartland JM, Duncan M, Di Marzo V, et al. Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol 2015; 172: 737–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Howlett AC, Abood ME. CB1 and CB2 receptor pharmacology. In: Thomas August J, Anders MW, Murad F. (eds) Advances in pharmacology. Aandiego, CA: Academic Press, 2017, pp. 169–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Navarro G, Reyes-Resina I, Rivas-Santisteban R, et al. Cannabidiol skews biased agonism at cannabinoid CB1 and CB2 receptors with smaller effect in CB1-CB2 heteroreceptor complexes. Biochem Pharmacol 2018; 157: 148–158. [DOI] [PubMed] [Google Scholar]
- 52. Núñez E, Benito C, Pazos MR, et al. Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: an immunohistochemical study. Synapse 2004; 53: 208–213. [DOI] [PubMed] [Google Scholar]
- 53. Jordan CJ, Xi Z-X. Progress in brain cannabinoid CB2 receptor research: from genes to behavior. Neurosci Biobehav Rev 2019; 98: 208–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ishiguro H, Carpio O, Horiuchi Y, et al. A nonsynonymous polymorphism in cannabinoid CB2 receptor gene is associated with eating disorders in humans and food intake is modified in mice by its ligands. Synapse 2010; 64: 92–96. [DOI] [PubMed] [Google Scholar]
- 55. Morales P, Reggio PH, Jagerovic N. An overview on medicinal chemistry of synthetic and natural derivatives of cannabidiol. Front Pharmacol 2017; 8: 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bisogno T, Hanuš L, De Petrocellis L, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol 2001; 134: 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fride E, Ponde D, Breuer A, et al. Peripheral, but not central effects of cannabidiol derivatives: mediation by CB1 and unidentified receptors. Neuropharmacology 2005; 48: 1117–1129. [DOI] [PubMed] [Google Scholar]
- 58. Hazekamp A. The trouble with CBD oil. Med Cannabis Cannabinoids 2018; 1: 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Puri PS. Winterization of oils and fats. J Am Oil Chem Soc 1980; 57: A848–A850. [Google Scholar]
- 60. Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol 2011; 163: 1344–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Santiago M, Sachdev S, Arnold JC, et al. Absence of entourage: terpenoids commonly found in cannabis sativa do not modulate the functional activity of Δ9-THC at human CB1 and CB2 receptors. Cannabis Cannabinoid Res 2019; 4: 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Heblinski M, Santiago M, Fletcher C, et al. Terpenoids commonly found in cannabis sativa do not modulate the actions of phytocannabinoids or endocannabinoids on TRPA1 and TRPV1 channels. Cannabis Cannabinoid Res. Epub ahead of print 9 March 2020. DOI: 10.1089/can.2019.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Finlay DB, Sircombe KJ, Nimick M, et al. Terpenoids from cannabis do not mediate an entourage effect by acting at cannabinoid receptors. Front Pharmacol 2020; 11: 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cogan PS. The ‘entourage effect’or ‘hodge-podge hashish’: the questionable rebranding, marketing, and expectations of cannabis polypharmacy. Expert Rev Clin Pharmacol. Epub ahead of print 2 March 2020. DOI: 10.1080/17512433.2020.1721281. [DOI] [PubMed] [Google Scholar]
- 65. HelloMD. What is the Entourage Effect? https://hellomd.com/blogs/articles/what-is-the-entourage-effect (2020, accessed 1 June 2020).
- 66. Devinsky O, Patel AD, Thiele EA, et al. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology 2018; 90: e1204–e1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox–Gastaut syndrome. N Engl J Med 2018; 378: 1888–1897. [DOI] [PubMed] [Google Scholar]
- 68. Thiele EA, Marsh ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox–Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018; 391: 1085–1096. [DOI] [PubMed] [Google Scholar]
- 69. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl) 2018; 235: 1923–1932. [DOI] [PubMed] [Google Scholar]
- 70. Jadoon KA, Ratcliffe SH, Barrett DA, et al. Efficacy and safety of cannabidiol and tetrahydrocannabivarin on glycemic and lipid parameters in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel group pilot study. Diabetes Care 2016; 39: 1777–1786. [DOI] [PubMed] [Google Scholar]
- 71. GW Research Ltd. Study to assess the effect of cannabidiol on liver fat levels in subjects with fatty liver disease. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01284634 (2014, accessed 1 June 2020).
- 72. Naftali T, Mechulam R, Marii A, et al. Low-dose cannabidiol is safe but not effective in the treatment for Crohn’s disease, a randomized controlled trial. Dig Dis Sci 2017; 62: 1615–1620. [DOI] [PubMed] [Google Scholar]
- 73. Chagas MHN, Zuardi AW, Tumas V, et al. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: an exploratory double-blind trial. J Psychopharmacol 2014; 28: 1088–1092. [DOI] [PubMed] [Google Scholar]
- 74. Consroe P, Laguna J, Allender J, et al. Controlled clinical trial of cannabidiol in Huntington’s disease. Pharmacol Biochem Behav 1991; 40: 701–708. [DOI] [PubMed] [Google Scholar]
- 75. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use a systematic review and meta-analysis. JAMA 2015; 313: 2456. [DOI] [PubMed] [Google Scholar]
- 76. Zajicek J, Fox P, Sanders H, et al. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomised placebo-controlled trial. Lancet 2003; 362: 1517–1526. [DOI] [PubMed] [Google Scholar]
- 77. Freeman RM, Adekanmi O, Waterfield MR, et al. The effect of cannabis on urge incontinence in patients with multiple sclerosis: a multicentre, randomised placebo-controlled trial (CAMS-LUTS). Int Urogynecol J 2006; 17: 636–641. [DOI] [PubMed] [Google Scholar]
- 78. Press CA, Knupp KG, Chapman KE. Parental reporting of response to oral cannabis extracts for treatment of refractory epilepsy. Epilepsy Behav 2015; 45: 49–52. [DOI] [PubMed] [Google Scholar]
- 79. Tomida I, Azuara-Blanco A, House H, et al. Effect of sublingual application of cannabinoids on intraocular pressure: a pilot study. J Glaucoma 2006; 15: 349–353. [DOI] [PubMed] [Google Scholar]
- 80. Notcutt W, Price M, Miller R, et al. Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 ‘N of 1’studies. Anaesthesia 2004; 59: 440–452. [DOI] [PubMed] [Google Scholar]
- 81. Carlini EA, Cunha JM. Hypnotic and antiepileptic effects of cannabidiol. J Clin Pharmacol 1981; 21: 417S–427S. [DOI] [PubMed] [Google Scholar]
- 82. Morgan CJA, Das RK, Joye A, et al. Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. Addict Behav 2013; 38: 2433–2436. [DOI] [PubMed] [Google Scholar]
- 83. Freeman AM, Petrilli K, Lees R, et al. How does cannabidiol (CBD) influence the acute effects of delta-9-tetrahydrocannabinol (THC) in humans? A systematic review. Neurosci Biobehav Rev 2019; 107: 696–712. [DOI] [PubMed] [Google Scholar]
- 84. Morgan CJA, Gardener C, Schafer G, et al. Sub-chronic impact of cannabinoids in street cannabis on cognition, psychotic-like symptoms and psychological well-being. Psychol Med 2012; 42: 391–400. [DOI] [PubMed] [Google Scholar]
- 85. Bhattacharyya S, Morrison PD, Fusar-Poli P, et al. Opposite effects of δ-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology 2010; 35: 764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Englund A, Morrison PD, Nottage J, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol 2013; 27: 19–27. [DOI] [PubMed] [Google Scholar]
- 87. Morgan CJA, Freeman TP, Hindocha C, et al. Individual and combined effects of acute delta-9-tetrahydrocannabinol and cannabidiol on psychotomimetic symptoms and memory function. Transl Psychiatry 2018; 8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. HelloMD and Brightfield Group. Understanding Cannabidiol (CBD) – Expert Report. https://content.brightfieldgroup.com/understanding-cbd-report-2017 (2017, accessed 1 June 2020).
- 89. Lintzeris N, Mills L, Suraev A, et al. Medical cannabis use in the Australian community following introduction of legal access: the 2018–2019 online cross-sectional cannabis as medicine survey (CAMS-18). Harm Reduct J 2020; 17: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Skelley JW, Deas CM, Curren Z, et al. Use of cannabidiol in anxiety and anxiety-related disorders. J Am Pharm Assoc 2020; 60: 253–261. [DOI] [PubMed] [Google Scholar]
- 91. Gurley BJ, Murphy TP, Gul W, et al. Content versus label claims in cannabidiol (CBD)-containing products obtained from commercial outlets in the State of Mississippi. J Diet Suppl 2020; 17: 1–9. [DOI] [PubMed] [Google Scholar]
- 92. Liebling JP, Clarkson NJ, Gibbs BW, et al. An analysis of over-the-counter cannabidiol products in the United Kingdom. Cannabis Cannabinoid Res. Epub ahead of print 1 April 2020. DOI: 10.1089/can.2019.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hazekamp A, Epifanova S. Grote variatie in samenstelling cannabisolie noopt tot regels. Pharm Weekbl 2017; 152: 16–18. [Google Scholar]
- 94. Pavlovic R, Nenna G, Calvi L, et al. Quality traits of “cannabidiol oils”: cannabinoids content, terpene fingerprint and oxidation stability of European commercially available preparations. Molecules 2018; 23: 1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Crippa JAS, Crippa A, Hallak JEC, et al. Δ9-THC intoxication by cannabidiol-enriched cannabis extract in two children with refractory epilepsy: full remission after switching to purified cannabidiol. Front Pharmacol 2016; 7: 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Spindle TR, Cone EJ, Kuntz D, et al. Urinary pharmacokinetic profile of cannabinoids following administration of vaporized and oral cannabidiol and vaporized CBD-dominant cannabis. J Anal Toxicol 2020; 44: 109–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Poklis JL, Mulder HA, Peace MR. The unexpected identification of the cannabimimetic, 5F-ADB, and dextromethorphan in commercially available cannabidiol e-liquids. Forensic Sci Int 2019; 294: e25–e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rianprakaisang T, Gerona R, Hendrickson RG. Commercial cannabidiol oil contaminated with the synthetic cannabinoid AB-FUBINACA given to a pediatric patient. Clin Toxicol (Phila) 2020; 58: 215–216. [DOI] [PubMed] [Google Scholar]
- 99. Biros AG. Steep Hill, ACCL find pesticides in over 50% of cannabis samples. Cannabis Industry Journal. https://cannabisindustryjournal.com/news_article/steep-hill-accl-find-pesticides-in-over-50-of-cannabis-samples/ (2016, accessed 1 June 2020).
- 100. Bass J, Linz DR. A case of toxicity from cannabidiol gummy ingestion. Cureus 2020; 12: e7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Blagev DP, Harris D, Dunn AC, et al. Clinical presentation, treatment, and short-term outcomes of lung injury associated with e-cigarettes or vaping: a prospective observational cohort study. Lancet 2019; 394: 2073–2083. [DOI] [PubMed] [Google Scholar]
- 102. Shi S, Brant AR, Sabolch A, et al. False news of a cannabis cancer cure. Cureus 2019; 11: e3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Meyer HC, Lee FS, Gee DG. The role of the endocannabinoid system and genetic variation in adolescent brain development. Neuropsychopharmacology 2018; 43: 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Schipper R, Dekker M, de Haan L, et al. Medicinal cannabis (Bedrolite) substitution therapy in inpatients with a psychotic disorder and a comorbid cannabis use disorder: a case series. J Psychopharmacol 2018; 32: 353–356. [DOI] [PubMed] [Google Scholar]
- 105. European Commision. Cannabidiol. EU Novel food catalogue. https://ec.europa.eu/food/safety/novel_food/catalogue/search/public/index.cfm (2020, accessed 1 June 2020).
- 106. European Monitoring Centre for Drugs and Drug Addiction. Cannabis legislation in Europe: an overview. Lisbon; https://www.emcdda.europa.eu/publications/adhoc/cannabis-legislation-europe_en (2017, accessed 1 June 2020). [Google Scholar]
- 107. MHRA. MHRA statement on products containing Cannabidiol (CBD). https://www.gov.uk/government/news/mhra-statement-on-products-containing-cannabidiol-cbd (2016, accessed 1 June 2020).
- 108. Government of New Zealand. Medicinal Cannabis Agency – Cannabidiol (CBD) products. https://www.health.govt.nz/our-work/regulation-health-and-disability-system/medicinal-cannabis-agency/medicinal-cannabis-agency-information-industry/medicinal-cannabis-agency-working-medicinal-cannabis/medicinal-cannabis-agency-cannabidiol-cbd-products#su (2020, accessed 1 June 2020).
- 109. Australian Goverment: The Office of Drug Control. Hemp products. https://www.odc.gov.au/hemp-products (2019, accessed 1 June 2020).
- 110. Government of Canada. Final regulations: edible cannabis, cannabis extracts, cannabis topicals. https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/resources/regulations-edible-cannabis-extracts-topicals.html (2019, accessed 1 June 2020).
- 111. Mead A. Legal and regulatory issues governing cannabis and cannabis-derived products in the United States. Front Plant Sci 2019; 10: 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. U.S. Food & Drug Administration. FDA warns 15 companies for illegally selling various products containing cannabidiol as agency details safety concerns. https://www.fda.gov/news-events/press-announcements/fda-warns-15-companies-illegally-selling-various-products-containing-cannabidiol-agency-details (2019, accessed 1 June 2020).


