Abstract
Objective
The Netherlands host three population-based cancer screening programmes: for cervical, breast, and colorectal cancer. For screening programmes to be effective, high participation rates are essential, but participation in the Netherlands’ programmes is starting to fall below the minimal effective rate. We aimed to produce a systematic overview of the current known determinants of (non-)attendance at the Dutch cancer screening programmes.
Methods
A literature search was conducted in the electronic databases Academic Search Premier, Cochrane Library, Embase, EMCare, PubMed, PsycINFO, Web of Science, and also in grey literature, including all articles published before February 2018. The I-Change model was used to categorize the identified determinants of cancer screening attendance.
Results
In total, 19/1232 identified studies and 6 grey literature reports were included. Fifteen studies reported on predisposing factors. Characteristics such as social economic status, country of birth, and residency were most often reported, and correlate with cancer screening attendance. Thirteen studies addressed information factors. Factors on awareness, motivation, ability, and barriers were less often studied.
Conclusion
Current studies tend to describe the general characteristics of (non-)attendance and (non-)attenders, but rarely provide in depth information on other factors of (non-)participation. The I-Change model proved to be a useful tool in mapping current knowledge on cancer screening attendance and revealed knowledge gaps regarding determinants of (non-)participation in the screening programmes. More research is needed to fully understand determinants of participation, in order to influence and optimize attendance rates over the long term.
Keywords: Cancer screening, attendance, I-Change model, determinants of participation, Netherlands
Introduction
The Netherlands hosts three population-based cancer screening programmes (CSPs) aimed at cervical, breast, and colorectal cancer (CRC). These CSPs aim to detect cancer in an early or precursor stage, thus improving survival via early intervention. This approach is thought to lead to a better prognosis, as well as fewer and less severe side effects of the treatment.1–4 These CSPs are offered free of charge by the Dutch government to all citizens of a specific age and gender. The National Institute for Public Health and the Environment and five regional screening organizations are charged with organizing and coordinating the programmes.5 Attendance is voluntary and monitored yearly by the Institute.6–8 Although the three CSPs have many similarities, each has unique procedures and organization, mainly due to the differences in screening methods (Table 1). In Online Appendix A we describe the individual designs of the three CSPs.
Table 1.
Key characteristics of the three national cancer screening programmes in the Netherlands.
| Cervical CSP | Breast CSP | Colorectal CSP | ||
|---|---|---|---|---|
| Since (year) | 1979 (pilots from 1976) | 1990 (pilots from 1984) | 2014 (will be fully operational in 2019) | |
| Population | Age category | 30–60 | 50–75 | 55–75 |
| Sex | F | F | F&M | |
| Interval (in years) | 5 | 2 | 2 | |
| Primary test | hrHPV-test, cytology if necessary (then a Pap smear as needed) | Mammography (bilateral) | FIT | |
| Involvement GP | Performing cytological smear, discuss outcome, hospital referrala | Discuss outcome, hospital referralb | Nonec | |
| Primary outcome | KOPAC-coded | BI-RADS-code | Negative, positive, unclear. | |
| Financing | Invitation, primary test and analyses, referral when abnormalities are detected | Dutch government | ||
| Secondary tests and potential treatment | Standard healthcare, thereafter depending on individual insurance policy | |||
CSP: cancer screening programme; F: female; FIT: faecal immunochemical test; GP: general practitioner; hrHPV: high-risk human papillomavirus; M: male.
aFrom 2017 onwards women can choose a self-sampling test. The outcome (negative, positive, or unclear) of the self-sampling test is not automatically shared with the GP, so the GP no longer plays an essential role in this CSP. If hrHVP is detected, women are advised to seek contact with their GP to perform a Pap smear at the GP’s office.
bIn cases where no abnormalities are detected the GP will not be involved.
cSince 2017 the GP no longer automatically receives the outcome of a FIT. However, after a positive FIT patients are encouraged to seek contact with their GP.
dKOPAC-code is a Dutch classification system comparable with the Pap-classification.
High participation rates are essential for a national CSP to be effective. According to the World Health Organization (WHO) at least 70% of the target population should be screened.9 The most recent available national attendance rates from the Netherlands (2016) were 60, 77, and 73%, respectively, for the cervical, breast, and CRC screening programmes. While these national rates might be reassuring, an alarming downward trend in uptake can be observed for both long-lasting cervical and breast CSPs.7,8,10 Furthermore, there is a wide regional variation in attendance rates. The lowest attendance rates in the four largest cities of the Netherlands all fall below the 70% minimal effective rate for all three CSPs.11–13
To influence and optimize attendance rates, it is essential to identify and understand determinants of (non-)attendance and follow-up adherence. This study aims to provide a systematic overview of the current known determinants of (non-)attendance in the Dutch oncological screening programmes.
Methods
A comprehensive literature search was conducted, covering all articles published before February 2018. We searched the following electronic databases: Academic Search Premier, Cochrane Library, Embase, EMCare, PubMed, PsycINFO, and Web of Science. The initial search was conducted in PubMed and included the MESH terms: ‘screening’, ‘cancer’, ‘participation’, and ‘Netherlands’ (for full search details, see Online Appendix B). The search was then extended to cover the other databases. No limitation was set on year of publication or study design. Grey literature was obtained from databases on the websites of the National Institute for Public Health and the Environment,5 the Health Counsil of the Netherlands,14 and Volksgezondheidenzorg,15 organizations involved in cancer screening in the Netherlands. Reference lists of the included articles were reviewed for additional references. This review and its procedures were planned, conducted, and reported according to the PRISMA guidelines.16 In advance our review was registered and accepted in the Prospero register of the National institute for Health Research (CRD42018089444).17
Studies were included when they evaluated the outcome measurement ‘attendance/participation’, and/or described the determinant measures ‘reasons for low and non-attendance’, and were related to at least one of the current Dutch national CSPs. Studies were excluded when they were not in English or Dutch, or when they were non-original articles. Table 2 summarizes the inclusion and exclusion criteria. After removing duplicates, titles and abstracts were checked for inclusion and exclusion criteria. The abstracts of the remaining articles were independently assessed for applicability by the first and second author. The agreement rate was 92%, calculated over the first 120 articles (110/120). An additional 10% was randomly checked by the second author. In case of discrepancy, the full text of the article was checked. The final full text evaluation of all the remaining articles was carried out by both the first and second authors. Disagreement on inclusion was resolved by discussion with the full research team.
Table 2.
Inclusion and exclusion criteria.
| Inclusion criteria | |
| 1a. | Study outcome: the uptake/participation of national cancer screening programmes OR |
| 1b. | Determinant measurements: reasons for low- and non-attendance (health literacy, decision making, social or cultural differences, and organizational factors) AND cancer screening programmes |
| 2. | Results are related to: cervical cancer and/or breast cancer and/or colorectal cancer |
| 3. | The authors are related to Dutch organizations (universities) or the article describes Dutch cancer screening programmes |
| Exclusion criteria | |
| 1. | Language other than English or Dutch |
| 2. | Non-original articles, e.g. dissertations, reviews, case reports, editorials, oral presentations, poster presentations, book chapters |
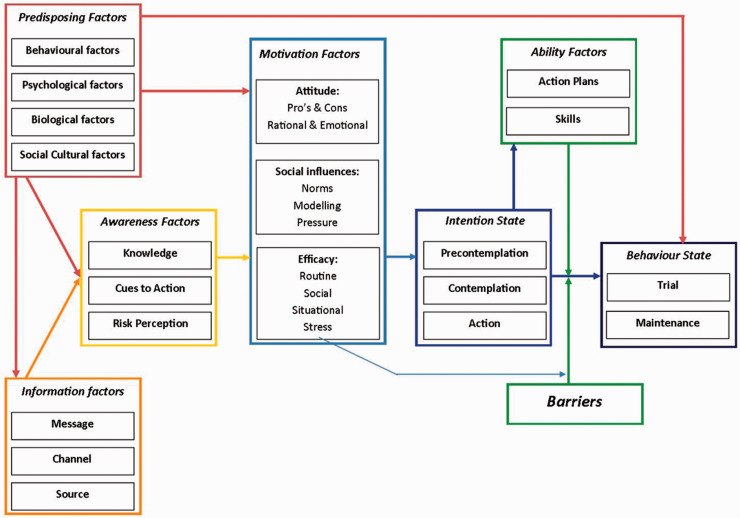
All included scientific studies were subjected to qualitative analyses. For the quantitative studies, the Crowe Critical Appraisal Tool was used.18 For the qualitative studies we used the Consolidated criteria for reporting qualitative research, developed by the Dutch Cochrane Centre.19 To analyse the determinants in a broad perspective, we used the Integrated Model for Behavioural Change (I-Change model, see Figure 1).20
Figure 1.
The Integrated Model for Behavioural Change (I-Change model).20 The arrows represent the influence between the different factors.
As screening attendance can be seen as health behaviour, determinants of attendance can be studied using health behaviour models. We used the Integrated Change model (I-Change model, Figure 1)20–22 to map all the identified determinants. This model was chosen because it incorporates elements from several earlier well recognized health behaviour theories, such as the Health Belief Model, Protection Motivation Theory, Theory of Planned Behaviour, and Precaution Adoption Process Model.23–26 The I-Change model includes factors on predisposing, information, awareness, motivational, ability, and barriers.
Results
Study retrieval
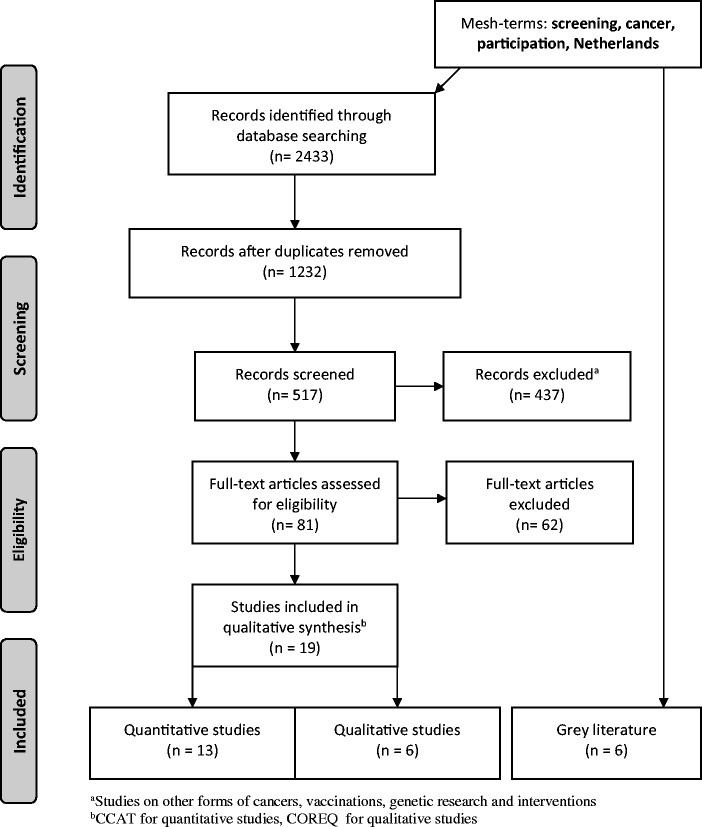
The initial search yielded 2433 articles (Academic Search Premier 73, Cochrane Library 98, Embase 853, EMCare 185, PubMed 604, PsycINFO 23, Web of Science 597; see Figure 2 for the PRISMA flowchart). Of these, 1201 articles were identified as duplicates, and another 715 articles did not meet the inclusion criteria, leaving 517 studies after the first exclusion round. After the second round, 81 studies remained and underwent full text review. The final selection included 19 articles, 13 quantitative and 6 qualitative studies. The quality appraisal score of the 13 studies was average to high, ranging from 32 to 38 points (maximum 40), with a rounded average of 36 points. The qualitative studies scored a range from 5 to 6 (maximum 7), with a rounded average of 6 points. As no extremely low quality scores were assigned, we did not exclude any studies from further analysis based on the Crowe Critical Appraisal Tool or the Consolidated criteria for reporting qualitative research. Characteristics of the included studies are summarized in Supplementary Tables 1 and 2. Six reports were included as grey literature.6,7,11–13,27 The identified determinants of low or (non-)attendance are presented in Table 3.
Figure 2.
PRISMA flowchart of the search strategy. Search until 1 February 2018.
Table 3.
Determinants of low-/non-attendance at a Dutch CSP, subdivided by the I-Change model.
| Cervical CSP | Breast CSP | Colorectal CSP | ||
|---|---|---|---|---|
| Predisposing factors | ||||
| Behavioural | Residency: more urban | X12 | X13,28 | |
| Marital status: Married/in a relationship | X29 | |||
| Several different sexual partners | X30 | |||
| Psychological | ||||
| Biological | Age: younger age | X7,30 | X6,11 | |
| Sex: male | NA | NA | X8,24 | |
| Higher risk (ethnicity) | X31–33 | X34,35 | ||
| Social and cultural | Country of birth: non-native Dutch/non-Western | X12,29–32,36,37 | X34 | X38 |
| SES: low(er) SES | X28–31,34,35,37,38 | X28,35 | ||
| Information factors | ||||
| Message | X39 | X40 | ||
| Channel | Lack of tailored strategies | X36,41,42 | X28,43 | X38,44 |
| Source | Non-GP practice-based invitation | X30,33,37,45 | ||
| Awareness factors | ||||
| Knowledge | Misconceptions, lack of knowledge, e.g. screening harm | X30,32 | X43,46 | |
| Cues to action | Low priority | X36 | X40,46 | |
| Risk perception | Perceived lesser risk of cancer | X30,39,41 | X43 | |
| Motivational factors | ||||
| Attitude | No future testing needed, less moral obligation | X39 | X44 | |
| Social influence | Negative social influence, negative role models, talked less with others | X39 | ||
| Self-efficacy | Low self-efficacy | X43 | ||
| Ability factors | ||||
| Action plans | Forgot to make an appointment | X36 | ||
| Skills | Language barrier/low health literacy | X43 | ||
| Barriers | ||||
| Test: insecure, anxious | X39 | X40 | ||
| Outcome of the test: insecure, anxious | X39 | |||
| Inconvenience: feelings of shame | X36,39 | X40 | ||
| Time related: forgot, too busy | X36 | X46 | ||
| Health related illness: other illnesses | X46 | |||
| Financial | X35 |
CSP: cancer screening programme; GP: general practitioner; NA: not applicable; SES: socio-economic status.
Predisposing factors
Most studies (n = 15) reported on predisposing factors, mainly the general characteristics of (non)attenders.6,7,11–13,28–32,34–38 For all three CSPs, country of birth seems to influence attendance, with those not born in the Netherlands showing low(er) uptake.12,29–32,34,36–38 For the cervical and breast screening programmes, residency and socio-economic status (SES) were frequently reported determinants of participation.13,28–31,35,37 Women living in more urbanized regions (the four main cities of the Netherlands: Amsterdam, Rotterdam, Utrecht, and The Hague) and women belonging to low-SES groups showed lower attendance.12,13,28 This is particularly detrimental as most abnormalities of the breast and cervix were found in women born outside the Netherlands, and in women in lower SES groups. Additionally, most unfavourable tumour-node-metastases were also found in the low-SES groups.31–35 Younger age was found to be a determinant of lower attendance in the cervical and colorectal CSPs,6,7,11,30 whereas being single or divorced, or having had only one sexual partner increased the likelihood of screening uptake in the cervical CSP.29,30 With respect to screening adherence and self-sampling among non-responders, native Dutch non-attendees returned more self-sampling kits than non-native Dutch non-attenders. Women who were screened in the previous rounds seemed to return more self-sampling kits than under-screened or never-screened women.32
Information factors
Thirteen studies described information factors to some extent.28,30,33,36–45 For all three CSPs, several studies addressed the lack of tailored communication tools and strategies to inform subpopulations. The need to develop new tools and strategies has been recognized and would particularly benefit ethnic (minority) groups.28,36,38,41–44 Four cervical CSP studies reported higher attendance rates when the invitation procedure (invitation and reminder) was general practitioner (GP)-based (the channel).30,33,37,45 This approach was particularly effective among women not born in the Netherlands.37 The cervical CSP self-sampling test, introduced in 2017, has been described as a promising, feasible, and effective procedure for increasing coverage in a screening programme.33,41,42 Self-sampling responders who did not participate in previous rounds were more often hrHPV positive, and had a higher relative risk of ≥cervical intraepithelial neoplasia (CIN) II and ≥CIN III, compared with self-sampling women who were screened in the previous rounds.33,41 Knops-Dullens et al.39 stated that, to motivate Dutch women to participate in the screening programme, they need to be convinced that the advantages outweigh the disadvantages. With respect to the CRC CPS, one study adding extra instructions and information and addressing specific concerns should be considered in order to improve informed decision making about participation.40 Since January 2018, GPs no longer receive automatically generated messages regarding pathological results, although patients are encouraged to seek contact with their GP.27
Awareness factors
Several studies identified the lack of knowledge as a determinant of non- or low-attendance.30,32,43,46 Cervical CSP non-attenders felt that they had a lower risk of developing cervical cancer and more of these women were convinced that cervical cancer cannot be cured.30,39,41 A study among non-native Dutch found that all respondents recognized their susceptibility to CRC, but their knowledge of CRC and the CSP were limited.43 Attending the CSP was a low priority, and limited concerns about health in general, and serious concerns regarding safety, were additional reasons for non- or low-attendance.36,40,46 With respect to the cervical CSP, self-sampling might be a solution for non-attenders because of convenience and personal control.36 Most often non-attenders reported they forgot to schedule an appointment.36 At the CRC CSP, non-attenders thought that mainly individuals in poor health and with (cancer) symptoms would benefit from the programme. Knowledge of potential harm associated with CRC CSP was also low.43
Motivational factors
Non-attenders of the cervical CSP were less motivated, less often inclined to undergo future screening, and experienced greater negative social influences than attenders. They reported negative role models and talked less with other people about the CSP.39 Self-efficacy was identified as an important determinant for CRC CSP attendance.43 A positive factor could be found in the quick uptake and adherence of the CRC CSP. A study by Toes-Zoutendijk underlined the importance of real-time monitoring. Only a few months after implementation of the CRC CSP, participation and positive test results were higher than predicted, whereas the positive predictive value was lower than predicted. To reduce the burden of unnecessary colonoscopies and improve colonoscopy capacity, the cut-off level for a positive FIT result was adjusted and a cut-off level of 47 µg Hb/g faeces is currently being used in the Netherlands.44
Ability factors
In the cervical CSP, forgetting to make an appointment was the main reason for non-attendance.36 The language barrier and low health literacy were other important determinants of non-attendance of the CRC CSP among non-native Dutch.43
Barriers
Non-attenders at both the cervical and the CRC CSP experienced more affective disadvantages: they were more insecure, more afraid, had more serious concerns regarding the test and outcome, and anticipated more feelings of shame. Other identified barriers were time-related or were related to being unable to attend the CSP, for example due to other illnesses.36,39,40,46 For breast cancer screening, a study in 2011 stated that despite the absence of financial barriers to participation, SES inequalities in attendance rates existed.35
Discussion
This systematic review describes all known determinants of (non-)attendance for the three Dutch CSPs. Studies tend to describe the more general characteristics of (non-)attenders, but rarely provide in depth information on other (non-)participation factors. The I-Change model was a useful tool in mapping current knowledge on cancer screening attendance and revealed knowledge gaps regarding determinants of (non-)attendance at the CSPs. Many studies reported on predisposing and information factors, giving a general good understanding of these determinants. Factors on awareness, motivation, ability, and barriers were less often studied.
By using a theoretical framework designed to explain health behaviour, the I-Change model,47 we could systematically summarize and merge all information from the identified studies. Similar to other reviews, we were only able to take published literature into account, which could result in a publication bias. We chose a health behaviour model because screening attendance can be seen as health behaviour. The I-Change model is a widely used and accepted theoretical framework to evaluate health behaviour.20–22,48 The I-Change model states that behaviours are determined by a person’s motivation or intention to carry out a behaviour, which is, in turn, the result of a person’s intentions, abilities, and barriers. Attitudes, social influences, and self-efficacy expectations influence a person’s motivation and are determined by various distal factors, such as predisposing (e.g. current lifestyle), information (e.g. source of delivery), and awareness (e.g. knowledge) factors. To the best of our knowledge this is the first review to use this approach to summarize available information on determinants of participation in CSPs. The I-Change model allowed us to identify knowledge gaps, and so highlight opportunities for improvement.
For a CSP to be effective, high participation rates are essential. The attendance rates for the two long-term CSP programmes in the Netherlands (cervical and breast cancer) are declining. The attendance rates of the cervical CSP are especially low, and are below the 70% target recognized by the WHO as the minimum effective rate. Furthermore, attendance rates show wide variation between regions and subpopulations. Lower attendance rates were found among those belonging to a low-SES group, living in more urban regions, and among people who were not born in the Netherlands (in some studies referred to as ‘non-native Dutch’ and in others as ‘non-Western immigrants’). These figures are in line with earlier published reviews.49–51 Furthermore, younger women show lower attendance rates at the cervical CSP, and men in general show lower attendance at the CRC CSP. The latter issue was also addressed in an earlier review on CRC CSPs worldwide by Navarro et al.52
While several studies have described attendance rates and the characteristics of (non-)attenders, in depth analyses of why people do or do not participate in a CSP are scarce. During our analysis it became clear that while many studies have focused on low attendance groups, little is still known on why these groups fail to attend CSPs, and even less is known about why individuals from high attendance groups actually attend CSPs. When we considered various elements of the I-Change model, we were unable to find any studies on the sub-elements psychological factors (predisposing factors) and message factors (information factors). With respect to the other (sub)elements of the I-Change model, most were only addressed in one study and/or in relation to only one CSP. One study by Hartman et al.49 attempted to interpret knowledge derived from research on the cervical CSP to explain factors concerning the breast CSP. The sub-elements under the predisposing factors are most often reported as characteristics of the non-attenders.
As our focus was on Dutch CSPs, determinants of (non-)attendance described in international studies of CSPs were excluded. Although several countries have CSPs comparable with the Netherlands, every country has its own unique screening programmes, adapted to their health system and population. As these inter-nation-differences would cause a problem comparing results, we choose to focus only the Netherlands. Some international reviews, however, have focussed on determinants not yet studied in the Netherlands, for example the sex of the screener, the presence of symptoms, and the existence of family conflicts.53–55 Additionally, lessons learned through this review might also be applicable to other European/Western countries.
In the Netherlands, the involvement of the GP in the CSPs has decreased over the past 5 years. However, it is clear, at least for the cervical CSP, that direct involvement of the GP results in higher attendance rates, especially among the high-risk groups (high cancer risk in known low-attendance groups).30,37,45 Whether this involvement should be (re)introduced is a matter of debate, but at the very least, a more prominent GP role in informing and activating people to participate in CSPs could be further explored. The importance of such a role for GPs is highlighted in several international studies, with highest beneficial effects for the lower socioeconomic and minority groups.56,57
It is often said that financial barriers are irrelevant in the Netherlands,35 but this is only partly true. While participation in a CSP is free, whenever follow-up research is needed a patient will have to cover a part of the cost of follow-up research themselves, depending on their specific insurance plan. As screening programmes may exacerbate socio-economic and ethnic health differences,58 future studies are also needed to address this topic.
In this review we not only looked at the three Dutch CSPs individually, but also compared the outcomes of these CSPs. This allowed us to compare characteristics of non-attenders and determinants of participation. Of the three Dutch CSPs, cervical cancer screening shows the lowest attendance rates. In the literature some explanations were offered for why women often fail to attend the cervical CSP. However, a possible explanation for the low uptake might be that a cervical examination remains a greater taboo compared with examination of the breast. An additional explanation might be the concrete appointment arranged by the breast CSP, whereas in the cervical CSP women have to make an appointment with their GP themselves. An advantage of the CRC CSP, compared with the cervical CSP, is that the CRC faeces test can be completed at home. In 2017 a self-sampling test for HPV infection was introduced within the cervical CSP. The self-sampling test has been shown to have high concordance with physician-taken sampling for hrHPV detection and was found to be highly acceptable to women.59 It would be interesting to see the effect of this self-test on participation rates among the different cervical CSP attendance groups. While the self-sampling test appears promising, we think there is still room for improvement. Women are only informed about the possibility of a self-sampling test in the initial invitation letter from the screening organization. An application form to actually order the self-sampling test is only attached when a re-invitation has to be sent. Therefore, women themselves still have to take the initiative in order to receive a self-sampling test at home. It would be more logical to include an application form with the initial invitation letter and to include the self-sampling test together with the re-invitation for women who have not yet responded to the first letter. A similar proposal has already (partly) been made by the Health Council of the Netherlands.60 Besides the different tests used in the three Dutch CSPs, there are also clear differences in the occurrence of the different cancers. Each year, 700–800 women are newly diagnosed with cervical cancer, whereas the incidence of breast and CRC is far higher, at 16,000 and 13,000 cases per year, respectively. A higher incidence means that people are more likely to be aware of breast and CRC, or to know someone who has had breast or CRC compared with cervical cancer.
Conclusion
Although the three CSPs in the Netherlands generally have high attendance rates, large differences exist between different regions and subpopulations. The I-Change model highlighted many knowledge gaps in determinants of (non-)attendance and identified opportunities for improvement. Existing studies tend to focus on attendances rates, and the general characteristics of (non-)attenders, but rarely provide in depth information on determinants of (non-)participation. More detailed studies are needed, as only by understanding the determinants of participation can we influence and alter them, and thus optimize current CSPs over the long term.
Supplemental Material
Supplemental material, MSC887996 Supplemental Material1 for Determinants of (non-)attendance at the Dutch cancer screening programmes: A systematic review by Thomas HG Bongaerts, Frederike L Büchner, Barend JC Middelkoop, Onno R Guicherit and Mattijs E Numans in Journal of Medical Screening
Supplemental material, MSC887996 Supplemental Material2 for Determinants of (non-)attendance at the Dutch cancer screening programmes: A systematic review by Thomas HG Bongaerts, Frederike L Büchner, Barend JC Middelkoop, Onno R Guicherit and Mattijs E Numans in Journal of Medical Screening
Acknowledgements
The authors thank the Walaeus Library of the Leiden University Medical Center for their help and advice with the search strategy.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research is supported by the LUMC medical research profile Innovation in Health Strategy and Quality of Care (IHQC), https://www.lumc.nl/research/medical-research-profiles/innovation-health-strategy/.
ORCID iDs
Thomas HG Bongaerts https://orcid.org/0000-0002-3242-0486
Frederike L Büchner https://orcid.org/0000-0001-8977-5344
Supplemental Material
Supplemental material is available for this article online.
References
- 1.van Ballegooijen M, Hermens R. Cervical cancer screening in the Netherlands. Eur J Cancer 2000; 36: 2244–2246. [DOI] [PubMed] [Google Scholar]
- 2.van Ballegooijen M, van den Akker-van Marle E, Patnick J, et al. Overview of important cervical cancer screening process values in European Union (EU) countries, and tentative predictions of the corresponding effectiveness and cost-effectiveness. Eur J Cancer 2000; 36: 2177–2188. [DOI] [PubMed] [Google Scholar]
- 3.Verbeek ALM, Broeders M. Evaluation of the Netherlands breast cancer screening programme. Ann Oncol 2003; 14: 1203–1205. [DOI] [PubMed] [Google Scholar]
- 4.European Colorectal Cancer Screening Guidelines Working Group, Von Karsa L, Patnick J, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis: overview and introduction to the full supplement publication. Endoscopy 2013; 45: 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.About RIVM. Rijksinstituut voor Volksgezondheid en Milieu, https://www.rivm.nl/en/about-rivm (accessed March 2018).
- 6.Erasmus Medisch Centrum Rotterdam. Landelijke Monitoring en Evaluatie Bevolkingsonderzoek Darmkanker 2016, https://www.rivm.nl/documenten/landelijke-monitoring-bevolkingsonderzoek-darmkanker-2016 (accessed March 2018).
- 7.Erasmus Medisch Centrum Rotterdam. Landelijke Evaluatie van het Bevolkingsonderzoek Baarmoederhalskanker (LEBA) t/m, https://www.rivm.nl/landelijke-evaluatie-van-bevolkingsonderzoek-baarmoederhalskanker-leba (2016, accessed March 2018).
- 8.Erasmus Medisch Centrum Rotterdam. Monitoring evaluatie bevolkingsonderzoek borstkanker, https://www.rivm.nl/monitoring-en-evaluatie-bevolkingsonderzoek-borstkanker (2016, accessed March 2018).
- 9.Word Health Organization. Early diagnosis and screening, http://www.who.int/cancer/prevention/diagnosis-screening/screening/en/ (accessed April 2018).
- 10.Rijksinstituut voor Volksgezondheid en Milieu. Factsheet bevolkingsonderzoek Borstkanker, https://www.rivm.nl/dsresource?objectid=bae4f245-bcac-4a9c-a7fa-ca5b43caaec6&type=org&disposition=inline (accessed 24 January 2018).
- 11.Rijksinstituut voor Volksgezondheid en Milieu. Factsheet bevolkingsonderzoek Darmkanker, https://www.rivm.nl/dsresource?objectid=01e21779-404e-43b1-aaed-e47b5d85ecd8&type=pdf&disposition=inline (accessed 24 January 2018).
- 12.Volksgezondheidenzorg.info. Bevolkingsonderzoek baarmoederhalskanker per gemeente, https://www.volksgezondheidenzorg.info/onderwerp/baarmoederhalskanker/regionaal-internationaal/regionaal#node-bevolkingsonderzoek-baarmoederhalskanker (2012, accessed March 2018).
- 13.Volksgezondheidenzorg.info. Bevolkingsonderzoek borstkanker per gemeente, https://www.volksgezondheidenzorg.info/onderwerp/borstkanker/regionaal-internationaal/regionaal#node-bevolkingsonderzoek-borstkanker-gemeente (2012, accessed March 2018).
- 14.Health Counsil of the Netherlands. Gezondheidsraad, https://www.gezondheidsraad.nl (accessed March 2018).
- 15.Volksgezondheidenzorg.info, https://www.volksgezondheidenzorg.info/ (accessed March 2018).
- 16.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.PROSPERO International prospective register of systematic reviews. Registration, https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=89444.
- 18.Crowe M. Crowe critical appraisal tool (CCAT) user guide. Scotland: Conchra House, 2013. [Google Scholar]
- 19.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007; 19: 349–357. [DOI] [PubMed] [Google Scholar]
- 20.de Vries H, Mesters I, Van de Steeg H, et al. The general public’s information needs and perceptions regarding hereditary cancer: an application of the integrated change model. Patient Educ Couns 2005; 56: 154–165. [DOI] [PubMed] [Google Scholar]
- 21.Groenenberg I, Crone MR, van Dijk S, et al. ‘ Check it out!’ decision-making of vulnerable groups about participation in a two-stage cardiometabolic health check: a qualitative study. Patient Educ Couns 2015; 98: 234–244. [DOI] [PubMed] [Google Scholar]
- 22.Nierkens V, Stronks K, Van Oel CJ, et al. Beliefs of Turkish and Moroccan immigrants in the Netherlands about smoking cessation: implications for prevention. Health Educ Res 2005; 20: 622–634. [DOI] [PubMed] [Google Scholar]
- 23.Ajzen I. The theory of planned behavior. Org Behav Hum Decis Process 1991; 50: 179–211. [Google Scholar]
- 24.Janz NK, Becker MH. The health belief model: a decade later. Health Educ Q 1984; 11: 1–47. [DOI] [PubMed] [Google Scholar]
- 25.Rogers RW. A protection motivation theory of fear appeals and attitude change1. J Psychol 1975; 91: 93–114. [DOI] [PubMed] [Google Scholar]
- 26.Weinstein ND, Lyon JE, Sandman PM, et al. Experimental evidence for stages of health behavior change: the precaution adoption process model applied to home radon testing. Health Psychol 1998; 17: 445. [DOI] [PubMed] [Google Scholar]
- 27.Nederlands Huisartsen Genootschap Praktijkhandleiding. Wijziging bevolkingsonderzoek darmkanker, https://www.nhg.org/actueel/nieuws/wijziging-bevolkingsonderzoek-darmkanker (2017, accessed 15 February 2018).
- 28.Vermeer B, van Den Muijsenbergh METC. The attendance of migrant women at the national breast cancer screening in the Netherlands 1997–2008. Eur J Cancer Prev 2010; 19: 195–198. [DOI] [PubMed] [Google Scholar]
- 29.Kreuger FAF, van Oers HAM, Nijs HGT. Cervical cancer screening: spatial associations of outcome and risk factors in Rotterdam. Public Health 1999; 113: 111–115. [DOI] [PubMed] [Google Scholar]
- 30.Tacken MAJB, Braspenning JCC, Hermens RPMG, et al. Uptake of cervical cancer screening in the Netherlands is mainly influenced by women’s beliefs about the screening and by the inviting organization. Eur J Public Health 2007; 17: 178–185. [DOI] [PubMed] [Google Scholar]
- 31.van Leeuwen AWFM, de Nooijer P, Hop WCJ. Screening for cervical carcinoma: participation and results for ethnic groups and socioeconomic status. Cancer 2005; 105: 270–276. [DOI] [PubMed] [Google Scholar]
- 32.Gok M, Heideman DAM, Van Kemenade FJ, et al. Offering self-sampling for human papillomavirus testing to non-attendees of the cervical screening programme: characteristics of the responders. Eur J Cancer 2012; 48: 1799–1808. [DOI] [PubMed] [Google Scholar]
- 33.Gok M, Heideman DAM, Van Kemenade FJ, et al. HPV testing on self collected cervicovaginal lavage specimens as screening method for women who do not attend cervical screening: cohort study. BMJ 2010; 340: 905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Visser O, van Peppen AM, Ory FG, et al. Results of breast cancer screening in first generation migrants in Northwest Netherlands. Eur J Cancer Prev 2005; 14: 251–255. [DOI] [PubMed] [Google Scholar]
- 35.Aarts MJ, Voogd AC, Duijm LEM, et al. Socioeconomic inequalities in attending the mass screening for breast cancer in the south of the Netherlands-associations with stage at diagnosis and survival. Breast Cancer Res Treat 2011; 128: 517–525. [DOI] [PubMed] [Google Scholar]
- 36.Bosgraaf RP, Ketelaars PJW, Verhoef VMJ, et al. Reasons for non-attendance to cervical screening and preferences for HPV self-sampling in Dutch women. Prev Med 2014; 64: 108–113. [DOI] [PubMed] [Google Scholar]
- 37.de Nooijer DP, de Waart FG, van Leeuwen AWFM, et al. Participation in the Dutch national screening programme for uterine cervix cancer higher after invitation by a general practitioner, especially in groups with a traditionally low level of attendance. Ned Tijdschr Geneeskd 2005; 149: 2339–2343 (in Dutch). [PubMed] [Google Scholar]
- 38.Deutekom M, Rijn A, Dekker E, et al. Uptake of faecal occult blood test colorectal cancer screening by different ethnic groups in the Netherlands. Eur J Public Health 2009; 400–402. [DOI] [PubMed] [Google Scholar]
- 39.Knops-Dullens T, de Vries N, de Vries H. Reasons for non-attendance in cervical cancer screening programmes: an application of the integrated model for behavioural change. Eur J Cancer Prev 2007; 16: 436–445. [DOI] [PubMed] [Google Scholar]
- 40.van Rijn AF, van Rossum LGM, Deutekom M, et al. Low priority main reason not to participate in a colorectal cancer screening program with a faecal occult blood test. J Public Health 2008; 30: 461–465. [DOI] [PubMed] [Google Scholar]
- 41.Bais AG, van Kemenade FJ, Berkhof J, et al. Human papillomavirus testing on self-sampled cervicovaginal brushes: an effective alternative to protect nonresponders in cervical screening programs. Int J Cancer 2007; 120: 1505–1510. [DOI] [PubMed] [Google Scholar]
- 42.Bulkmans NWJ, Bulk S, Ottevanger MS, et al. Implementation of human papillomavirus testing in cervical screening without a concomitant decrease in participation rate. J Clin Pathol 2006; 59: 1218–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woudstra AJ, Dekker E, Essink‐Bot ML, et al. Knowledge, attitudes and beliefs regarding colorectal cancer screening among ethnic minority groups in the Netherlands – a qualitative study. Health Expect 2016; 19: 1312–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toes-Zoutendijk E, van Leerdam ME, Dekker E, et al. Real-time monitoring of results during first year of Dutch colorectal cancer screening program and optimization by altering fecal immunochemical test cut-off levels. Gastroenterology 2017; 152: 767–775.e2. [DOI] [PubMed] [Google Scholar]
- 45.Hermens RPMG, Tacken MAJB, Hulscher MEJL, et al. Attendance to cervical cancer screening in family practices in the Netherlands. Prev Med 2000; 30: 35–42. [DOI] [PubMed] [Google Scholar]
- 46.Hummel JM, Steuten LGM, Groothuis-Oudshoorn CJM, et al. Preferences for colorectal cancer screening techniques and intention to attend: a multi-criteria decision analysis. Appl Health Econ Health Policy 2013; 11: 499–507. [DOI] [PubMed] [Google Scholar]
- 47.De Vries, H., Mesters, I., Van der Steeg et al. Integrated change model, https://heindevries.eu/interests/change (2004, accessed October 2018).
- 48.Nierkens V, Stronks K, de Vries H. Attitudes, social influences and self-efficacy expectations across different motivational stages among immigrant smokers: replication of the∅ pattern. Prev Med 2006; 43: 306–311. [DOI] [PubMed] [Google Scholar]
- 49.Hartman E, van den Muijsenbergh ME, Haneveld RW. Breast cancer screening participation among Turks and Moroccans in the Netherlands: exploring reasons for nonattendance. Eur J Cancer Prev 2009; 18: 349–353. [DOI] [PubMed] [Google Scholar]
- 50.Bosgraaf RP, Siebers AG, de Hullu JA, et al. The current position and the future perspectives of cervical cancer screening. Expert Rev Anticancer Ther 2014; 14: 75–92. [DOI] [PubMed] [Google Scholar]
- 51.Bekker MH, Lhajoui M. Health and literacy in first-and second-generation Moroccan Berber women in the Netherlands: ill literacy? Int J Equity Health 2004; 3: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Navarro M, Nicolas A, Ferrandez A, et al. Colorectal cancer population screening programs worldwide in 2016: an update. WJG 2017; 23: 3632–3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wools A, Dapper EA, Leeuw JRJD. Colorectal cancer screening participation: a systematic review. Eur J Public Health 2016; 26: 158–168. [DOI] [PubMed] [Google Scholar]
- 54.Fylan F. Screening for cervical cancer: a review of women’s attitudes, knowledge, and behaviour. Br J Gen Pract 1998; 48: 1509–1514. [PMC free article] [PubMed] [Google Scholar]
- 55.Vernon SW. Participation in colorectal cancer screening: a review. J Natl Cancer Inst 1997; 89: 1406–1422. [DOI] [PubMed] [Google Scholar]
- 56.Spadea T, Bellini S, Kunst A, et al. The impact of interventions to improve attendance in female cancer screening among lower socioeconomic groups: a review. Prev Med 2010; 50: 159–164. [DOI] [PubMed] [Google Scholar]
- 57.Reath J, Carey M. Breast and cervical cancer in Indigenous women: overcoming barriers to early detection. Aust Fam Physician 2008; 37: 178. [PubMed] [Google Scholar]
- 58.de Klerk CM, Gupta S, Dekker E, et al. Socioeconomic and ethnic inequities within organised colorectal cancer screening programmes worldwide. Gut 2017; 67: 679–687. [DOI] [PubMed] [Google Scholar]
- 59.Ketelaars PJW, Bosgraaf RP, Siebers AG, et al. High-risk human papillomavirus detection in self-sampling compared to physician-taken smear in a responder population of the Dutch cervical screening: results of the VERA study. Prev Med 2017; 101: 96–101. [DOI] [PubMed] [Google Scholar]
- 60.Screening op baarmoederhalskanker. Health Counsil of the Netherlands. publication number 2011/07 (2011). https://www.gezondheidsraad.nl/documenten/adviezen/2011/05/24/screening-op-baarmoederhalskanker (accessed March 2018)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, MSC887996 Supplemental Material1 for Determinants of (non-)attendance at the Dutch cancer screening programmes: A systematic review by Thomas HG Bongaerts, Frederike L Büchner, Barend JC Middelkoop, Onno R Guicherit and Mattijs E Numans in Journal of Medical Screening
Supplemental material, MSC887996 Supplemental Material2 for Determinants of (non-)attendance at the Dutch cancer screening programmes: A systematic review by Thomas HG Bongaerts, Frederike L Büchner, Barend JC Middelkoop, Onno R Guicherit and Mattijs E Numans in Journal of Medical Screening