Abstract
Proton magnetic resonance spectroscopy (1H-MRS) of the fetal brain can be used to study emerging metabolite profiles in the developing brain. Identifying early deviations in brain metabolic profiles in high-risk fetuses may offer important adjunct clinical information to improve surveillance and management during pregnancy.
Objective:
To investigate the normative trajectory of the fetal brain metabolites during the second half of gestation, and to determine the impact of using different Cramer-Rao Lower Bounds (CRLB) threshold on metabolite measurements using magnetic resonance spectroscopy.
Study design:
We prospectively enrolled 219 pregnant women with normal fetal ultrasound and biometric measures. We performed a total of 331 fetal 1H-MRS studies with gestational age in the rage of 18–39 weeks with 112 of the enrolled participants scanned twice. All the spectra in this study were acquired on a GE 1.5 T scanner using long echo-time of 144 ms and analyzed in LCModel.
Results:
We successfully acquired and analyzed fetal 1H-MRS with a success rate of 93%. We observed increases in total NAA, total creatine, total choline, scyllo inositol and total NAA-to-total choline ratio with advancing GA. Our results also showed faster increases in total NAA and total NAA-to-total choline ratio during the third trimester compared to the second trimester. We also observed faster increases in total choline and total NAA in female fetuses. Increasing the Cramer-Rao lower bounds threshold progressively from 100% to 40%–20% increased the mean metabolite concentrations and decreased the number of observations available for analysis.
Conclusion:
We report serial fetal brain biochemical profiles in a large cohort of health fetuses studied twice in gestation with a high success rate in the second and third trimester of pregnancy. We present normative in-vivo fetal brain metabolite trajectories over a 21-week gestational period which can be used to non-invasively measure and monitor brain biochemistry in the healthy and high-risk fetus.
Keywords: Fetal brain, Magnetic resonance spectroscopy, Metabolites, Normative, CRLB, Metabolite trajectory
1. Introduction
Proton magnetic resonance spectroscopy (1H-MRS) is becoming a powerful technique for non-invasive in-vivo metabolite measurements and may provide important insights into the evolving emerging metabolic profiles in the fetal brain (Evangelou et al., 2016; Girard et al., 2006; Kok et al., 2001; Story et al., 2011). Changes in metabolic profiles may precede structural changes signifying deviations from normal development (Harbison et al., 2017). Fetal brain 1H-MRS allows for quantification of specific metabolite concentrations like N-acetyl aspartate (NAA): a neuronal marker, Creatine (Cr): marker of cellular energy, Choline (Cho): marker of cell membrane turnover and myo-Inositol (Ins): an osmolyte and glial marker. Acquiring good quality spectra with a high success rate from the fetal brain is challenging due to motion, both maternal and fetal (Story et al., 2011). Currently, only a handful of reports have characterized the normal biochemical evolution of these key metabolites in the fetal brain across gestational age (GA) due to the technical challenges in acquiring good quality1H-MRS data (Evangelou et al., 2016). Most reports describing the evolving biochemical profiles come from preterm infants, neonates and children (Blüml et al., 2013; Horská et al., 2002; Kreis et al., 1993; van der Knaap et al., 1990; Kadota et al., 2001). Moreover, when reported, the values are often conveyed as ratios which are difficult to interpret due to changing concentrations in the fetal brain with advancing GA. Taken together, these technical challenges have limited the successful implementation of in vivo fetal 1H-MRS in the clinical setting.
Previous fetal 1H-MRS studies have reported increases in NAA and Cr with increasing GA in the fetal brain (Evangelou et al., 2016; Girard et al., 2006; Kok et al., 2001) However, establishing normative levels of these metabolites is crucial to identifying subtle but important deviations from normal metabolite development in high-risk fetuses to offer more informed surveillance during pregnancy. 1H-MRS measurements have traditionally used a Cramer-Rao Lower Bounds (CRLB) cut-off threshold of 20% as acceptance criteria (Card et al., 2013; Brandt et al., 2016). This acceptance threshold, while reasonable for dominant singlet peaks of NAA, Cr and Cho that have concentrations >6 mM in the healthy adult brain, lends itself to systemic bias resulting in rejection of metabolites measured with higher concentrations. Currently, there is growing debate about identifying an optimal acceptance threshold that will allow for reliable and meaningful measurement of metabolites at lower concentrations without erroneously rejecting true lower concentrations by marking them low quality data (Kreis, 2016; Pedrosa de Barros and Slotboom, 2017). This is particularly true for the developing fetal brain where overall metabolic concentrations are inherently low.
The objectives of this study were, two-fold 1) to investigate the normative trajectory of the fetal brain biochemical evolution in the second half of gestation using cross-sectional as well as longitudinal in vivo fetal 1H-MRS and 2) to determine the impact of using different CRLB threshold on metabolite measurements. To our knowledge, this is the first study to report on metabolic trajectory using serial measurements as well as trajectories based on fetal sex.
2. Methods
2.1. Subjects
We enrolled 219 healthy pregnant women under a protocol approved by the Institutional Review Board at the Children’s National Hospital with written consent obtained from all study participants. Participants were scanned during the second and third trimester of pregnancy with GA ranging from 18 to 39 weeks. Participants with congenital infection, multiple gestations or any indication of abnormality in the MRI anatomical images were excluded from the study.
2.2. Imaging
All participants were scanned on a 1.5 T MR scanner (Discovery MR450; GE Healthcare, Milwaukee, Wisconsin) using an 8-channel cardiac array receive-only coil (GE Healthcare).
2.2.1. MRI
Anatomical images were acquired using 2D single-shot fast spin-echo sequence in 3 directions using the following parameters: slice thickness: 2 mm, slice spacing: 0 mm, repetition time (TR): minimum (range: 700–1200 ms), echo-time (TE): 160 ms, flip angle: 90°, orientation: S/I, # slices: 42–65, matrix: 256 × 192.
2.2.2. Spectroscopy
Spectral voxel was placed in the central brain of the fetus using anatomical images as a guide. All data were acquired with TE/TR = 144/1500 ms. Chemical Shift Selective (CHESS) water suppression sequence was used in conjunction with using Point RESolved Spectroscopy (PRESS) localization sequence for acquiring water suppressed spectra (Bottomley, 1987). 16 averages of unsuppressed water spectra along with 96 averages of water-suppressed spectra were acquired from each participant with an acquisition time of 2 min 48 s. The voxel dimension for the central brain was 30 × 30 × 30 mm3 for fetuses with GA>28 weeks and 25 × 25 × 25 mm3 for fetuses with GA<28 weeks to ensure the voxel was confined within the fetal brain. Linewidth value was used as a measure of voxel homogeneity during the automatic shimming before the start of the sequence. If linewidth value was >9, then voxel position was verified by rescanning anatomical reference scan, and voxel was re-positioned if change in brain orientation was observed. Placement of the voxel in the center of the fetal brain using two separate sizes based on GA was used as an added measure to ensure the voxel was confined within the fetal brain during limited fetal motion. Also, the MRS scan was held off while the fetus was observed to be moving excessively during the fetal MRI to increase the odds of reducing fetal motion during spectral acquisition. Representative voxel location for the central brain of the fetus is shown in Fig. 1.
Fig. 1.
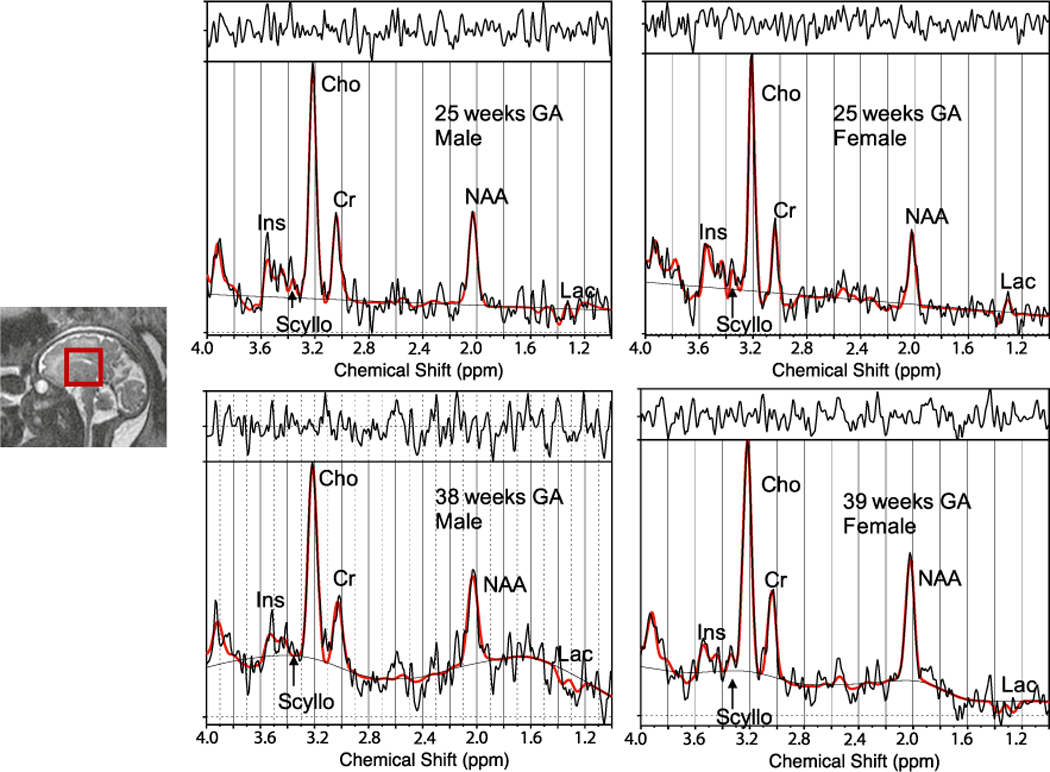
Representative MRS voxel location (left) and LCModel output spectra male and female fetuses from GAs of 25 and 38 weeks.
2.3. Spectral analysis
All acquired spectra underwent frequency and phase correction using programs written in Matlab to mitigate some of the effects of motion and improve spectral resolution. Spectra were then quantified in the ‘LCModel’ analysis software (Provencher, 1993). Metabolite concentrations were estimated in LCModel using water spectrum as an internal reference and the concentrations were reported in ‘institutional units’ (I.U.). LCModel basis set included the following 15 metabolites: alanine (Ala), aspartate (Asp), creatine (Cr), glutamine (Gln), glutamate (Glu), glycerophosphocholine (GPC), glutathione (GSH), lactate (Lac), myo-inositol (Ins), N-acetylaspartate (NAA), N-acetylaspartylglutamate (NAAG), phosphocholine (PCh), phosphocreatine (PCr), scyllo-Inositol (Scyllo), and taurine (Tau). While all these metabolites cannot be reliably measured at 1.5 T using TE 144 ms, according to the manual, inclusion of the fuller basis set improves the fit performance of the algorithm. Macromolecules and lipid basis set available in LCModel were used to account for the macromolecule baseline in the fitting routine. Data with CRLB >20% for total Choline: GPC + PCh (tCh) were excluded from further analysis, for all other metabolites the exclusion criteria included CRLB>100% to avoid biasing the results to higher metabolite concentrations (Kreis, 2016). Dataset using CRLB>40% and CRLB>20% as cut-offs for metabolites other than tCh were also analyzed. All resulting data underwent visual inspection as a final of quality control. Metabolites with overall acceptance rate of less than 50% were considered undetectable and excluded from further analysis. Signal-to-noise (SNR) ratio from LCModel was used to further assess the quality of the spectrum.
2.4. Statistical analysis
Generalized estimating equations (GEE) were used to investigate changes in metabolite concentrations across GA; non-linear associations were considered by evaluating higher order terms for gestational age and were explored graphically if p < 0.10. Differences in metabolite trajectories by trimester and sex were evaluated by including trimester by GA and sex by GA interaction terms in models. Analyses were completed using SAS 9.4. For all analyses, a two-tailed p of 0.05 was considered significant.
3. Results
We performed fetal 1H-MRS studies in 219 healthy pregnant women during the second and third trimester of pregnancy for this study. Of the 219 studied, 112 fetuses were scanned at two gestational time-points at a mean GA of 27(±3) and 35(±2) weeks, respectively. Data from 6 fetuses were excluded due to condition(s) listed in the exclusion criteria being fulfilled; all other fetuses had structurally normal brains on conventional MRI as evaluated by an experienced fetal neuroradiologist. Data from 16 more fetuses were excluded due to poor data quality as determined based on visual inspection and/or SNR<2 from LCModel. Data were acquired with an overall success rate of 93%. A representative spectrum from the central fetal brain along with a typical voxel location is shown in Fig. 1. The accepted spectra had average full-width and half maximum (FWHM) and SNR of: 4.5 (±1.0) Hz and 7.7 (±2.7).
3.1. Fetal H1-MRS measurements by GA
We observed increases in tNAA, tCr and tNAA:tCh ratio concentrations with increasing GA (p < 0.0001) for all three metabolites across the three CRLB thresholds considered (Table 1). Our results also showed increases in tCh with increasing GA, p = 0.006 (CRLB<20%) as well as Scyllo with p < 0.0001 (CRLBs<100% and 40%) and p = 0.05 (CRLB<20%). On evaluation of rate of change in metabolites during the second and third trimester, we observed differences in slopes for tNAA and tNAA:tCh ratios, which both increased significantly faster during the third trimester compared to the second trimester (Table 1). Figures with metabolite trajectories for these metabolites are shown in Fig. 2.
Table 1.
Estimates of metabolite trajectories with increasing GA and the interaction between GA and trimester from GEE models.
| Metabolite | GA | Trajectory differences by trimester |
|||
|---|---|---|---|---|---|
| 2nd |
3rd |
p-valuea | |||
| β | p-value | β | β | ||
| Ins | ‒0.06 | 0.33 | 0.02 | ‒0.04 | 0.83 |
| Lacb | ‒0.01 | 0.18 | 0.00 | 0.01 | 0.67 |
| Scyllo | 0.01 | < 0.0001c | 0.02 | 0.00 | 0.0003 |
| GPCPCh | 0.01 | 0.006 | 0.04 | 0.02 | 0.34 |
| NAANAAG | 0.18 | < 0.0001c | 0.11 | 0.20 | 0.01 |
| CrPCr | 0.10 | < 0.0001 | 0.10 | 0.10 | 0.97 |
| tNAA/tCh | 0.07 | < 0.0001c | 0.03 | 0.08 | 0.002 |
Estimates from GEE models.
p-value for interaction between GA and trimester from GEE models.
Log linkage used to account for log-normal distribution.
Non-linear effect noted.
Fig. 2.
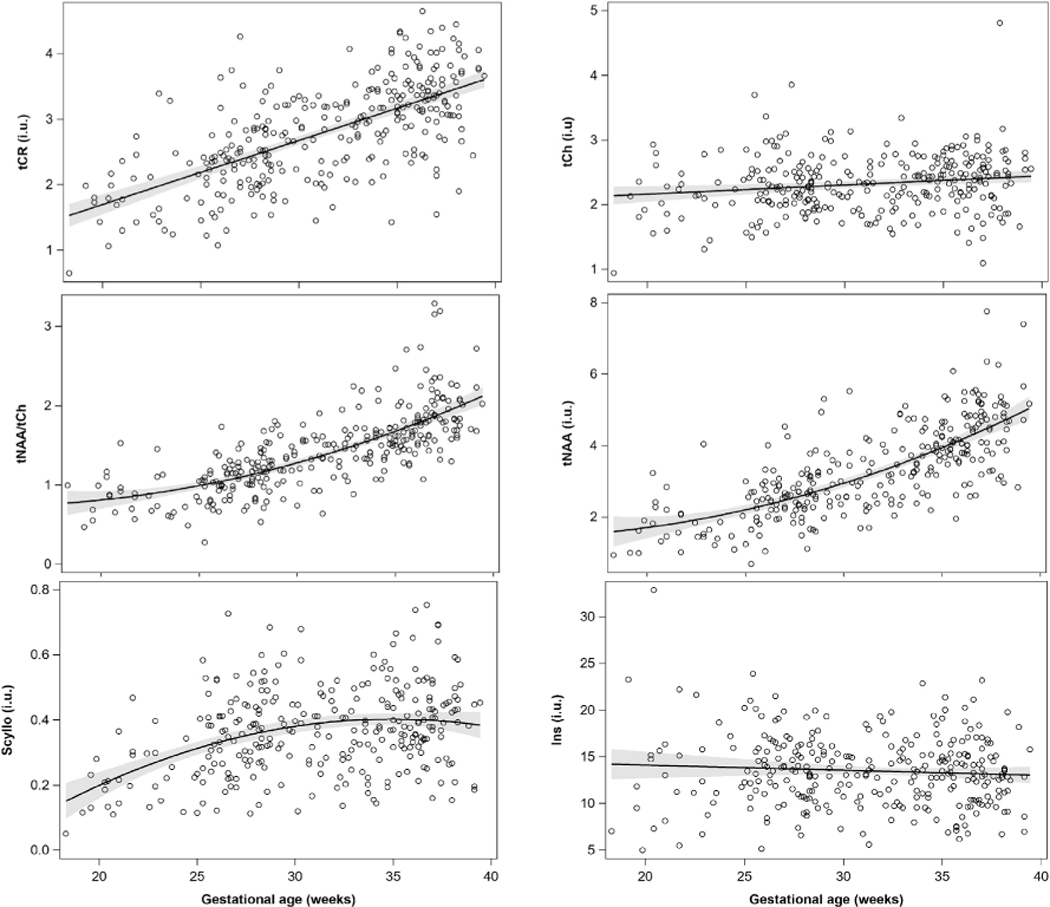
Metabolite trajectories across advancing GA in health fetuses.
The mean estimates of metabolite concentrations at CRLB thresholds of 20, 40 and 100 % were significantly different (p < 0.001) and showed decrease in mean metabolite concentration estimates with increasing CRLB thresholds (Table 2). We did not observe any significant change in Ins or Lac across increasing GA as well as between 2nd and 3rd trimester.
Table 2.
Average metabolite concentrations of all subjects in the study with different CRLB cut-off thresholds.
| N | CRLB<100% | N | CRLB<40% | N | CRLB<20% | |
|---|---|---|---|---|---|---|
| Ins | 301 | 13.5 ± 3.9 | 293 | 13.7 ± 3.7 | 230 | 14.7 ± 3.3 |
| Lac | 234 | 2.0 ± 1.5 | 144 | 2.5 ± 1.6 | 43 | 3.3 ± 1.8 |
| Scyllo | 297 | 0.4 ± 0.1 | 228 | 0.4 ± 0.1 | 44 | 0.6 ± 0.1 |
| tCh | 303 | 2.3 ± 0.4 | 303 | 2.3 ± 0.4 | 303 | 2.3 ± 0.4 |
| tNAA | 303 | 3.3 ± 1.2 | 300 | 3.3 ± 1.2 | 288 | 3.4 ± 1.2 |
| tCr | 303 | 2.8 ± 0.8 | 303 | 2.8 ± 0.8 | 291 | 2.9 ± 0.7 |
| tNAA/tCh | 303 | 1.4 ± 0.5 | 300 | 1.4 ± 0.5 | 298 | 1.5 ± 0.5 |
3.2. Serial fetal H1-MRS measurements
We successfully acquired good quality serial data from 96 fetuses with an average GA of 27.3 (±3.1) and 35.6 (±2.0) weeks at scan 1 and 2. The results from our analysis did not change when limiting the analysis to only subjects with serial measurement. Similar to the results from analysis of overall data across GA, we observed significant increases in tCh (p = 0.006), tNAA (p < 0.0001), tCr (p < 0.0001), Scyllo (p < 0.05) and tNAA/tCh (p < 0.0001) for all CRLB thresholds. Our data did not show any significant change in Ins across increasing GA.
3.3. Sex differences on metabolite levels
We observed significantly higher mean metabolites levels for tCr (p = 0.02) in male fetuses compared to female fetuses while controlling for GA in our GEE analysis [2.34 i. u. (M) vs. 2.19 i. u. (F)] during the 2nd trimester. Fig. 3 shows the trajectory of metabolites for tCh, tNAA, tCr and tNAA/tCh. Levels of Ins, tCh and tNAA also trended higher (p < 0.10) in male fetuses compared to female fetuses but the difference did not reach a significance. We did not observe any differences in mean metabolites levels between males and females during the 3rd trimester. We also observed higher rates of increase for tCh and tNAA in female fetuses across all three CRLB thresholds. We also observed faster rates of increase in tCh in female fetuses during the 2nd and 3rd trimester and tNAA and tCr in female fetuses during the 3rd trimester (Table 3).
Fig. 3.
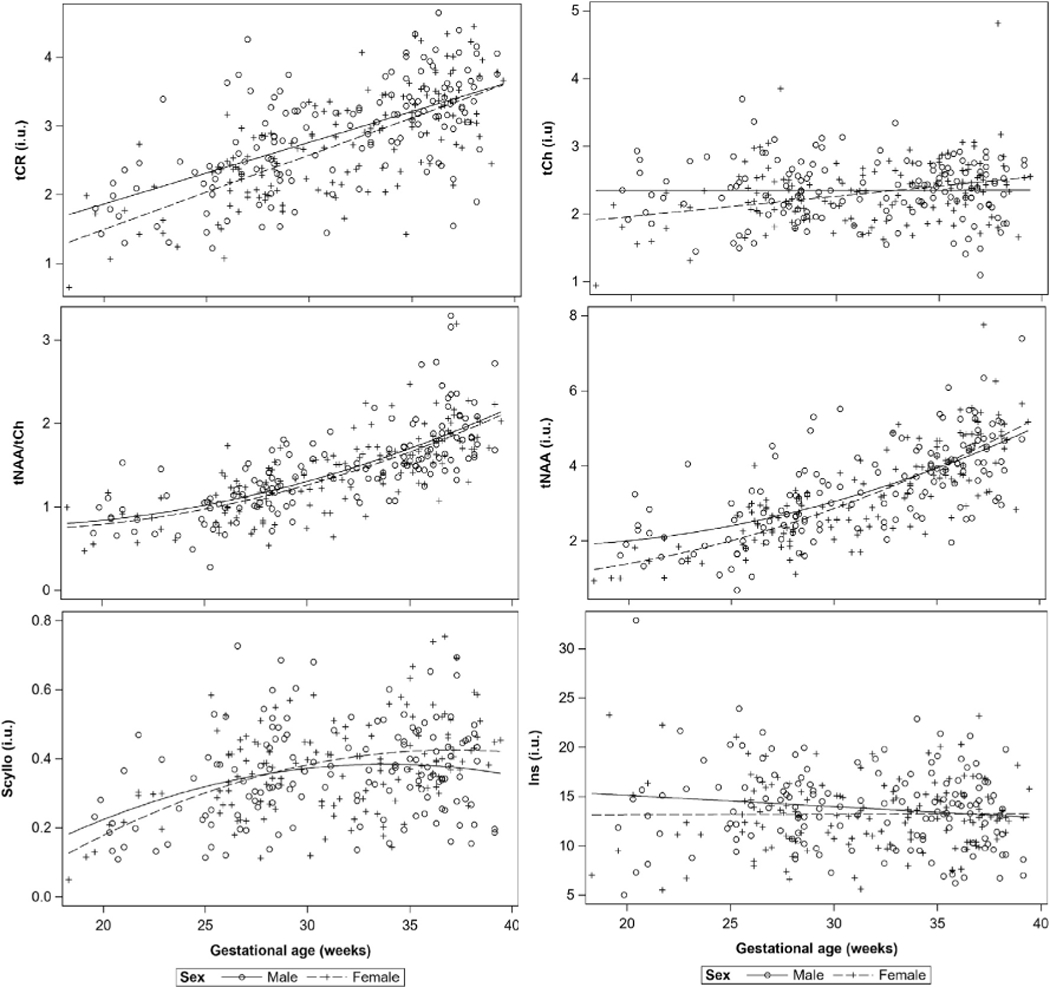
Metabolite trajectories across advancing GA for male and female fetuses.
Table 3.
Estimates of mean metabolite concentrations in institutional units (i.u.) controlling for GA for male and female fetuses from GEE models during 2nd and 3rd trimester.
| Metabolite | Metabolite concentration differences by sexa |
|||||
|---|---|---|---|---|---|---|
| 2nd Trimester |
3rd Trimester |
|||||
| Male (n = 54) | Female (n = 58) | p-value | Male |
Female |
p-value | |
| (n = 175) | (n = 133) | |||||
| Ins | 14.97 | 13.21 | 0.07 | 13.41 | 13.26 | 0.75 |
| Lacb | 2.57 | 1.98 | 0.21 | 1.8 | 1.72 | 0.65 |
| Scyllo | 0.3 | 0.31 | 0.7 | 0.38 | 0.4 | 0.22 |
| GPCPCh | 2.37 | 2.19 | 0.09 | 2.34 | 2.35 | 0.92 |
| NAANAAG | 2.37 | 2.11 | 0.09 | 3.82 | 3.72 | 0.43 |
| CrPCr | 2.34 | 2.04 | 0.02 | 3.12 | 3 | 0.13 |
| tNAA_tCh | 1 | 0.97 | 0.68 | 1.64 | 1.59 | 0.28 |
Lease squares means estimates from GEE models controlling for GA.
Log linkage used to account for log-normal distribution.
4. Discussion
In this study, we present normative data on emerging fetal brain metabolic profiles from the largest reported cohort of healthy fetuses to date with GAs spanning a 21-week window (from 18 to 39 weeks GA). We also report for the first time, the presence of Scyllo in the fetal brain and an increase in Scyllo with advancing GA. Scyllo is the second most abundant isomer of inositol after myo-Inositol (Kaiser et al., 2008). Inositols are involved in osmotic regulation, however, very little is known about the pathways and functionality of Scyllo. We also observed increases in tNAA, tCr, tCh and tNAA/tCh ratio with increasing GA. These observations are in agreement with reports from prior literature on fetal data as well as the trends observed in neonates (Girard et al., 2006; Kreis et al., 1993). Increases in tNAA and tCr are considered indicators of brain maturation in the developing brain. For the first time, we also report differences in metabolite trajectories between healthy male and female fetuses, where we observed faster increase in tCh and tNAA in female fetuses during the third trimester. Our results also showed higher levels of tCr in male fetuses during the 2nd trimester.
4.1. Second versus third trimester metabolic profiles
Due to the wide range of GAs as well as the large number of fetuses studied, we were able to parse our fetal 1H-MRS data into second and third trimester and determine whether there may be differences in neurochemical trajectories during the 2nd and 3rd trimester of pregnancy. We observed significant differences in metabolite trajectories for tNAA and tNAA:tCh ratio between 2nd and 3rd trimester (p: ≤0.01 and ≤ 0.002, respectively) with tNAA and tNAA:tCh ratio increasing faster during the 3rd trimester than the 2nd for all three CRLB thresholds considered in this study. It has been previously reported that third trimester marks a period of neuronal organization, and rapid increase in brain size accompanied by increase in brain surface area (Kostović and Jovanov-Milošević, 2006; Clouchoux et al., 2012). Our results suggest that changes in metabolite trajectories likely represent rapid brain biochemical maturation with increasing GA that accompanies third trimester structural and volumetric changes previously reported in the fetal brain (Andescavage et al., 2016). To our knowledge, this is the first report comparing the metabolite trajectories during the second and third trimester of pregnancy.
4.2. In-vivo fetal brain metabolites
4.2.1. N-acetylaspartate
N-acetylaspartate (NAA) is primarily synthesized in the neuronal mitochondria, transported into cytosol then to the axons. NAA has also been detected in immature oligodendrocyte hence cannot exclusively be considered a neuronal marker. Increases in NAA in the developing fetal brain likely denotes an increase in dendrites and synapses as well as oligodendrocytes proliferation and differentiation (Bhakoo and Pearce, 2000). NAA levels have been observed to increase with increasing GA followed by a rapid increase during the first 2 years of postnatal life (Kok et al., 2001; Kreis et al., 1993). Total concentration of NAA has also been reported to increase rapidly with maturation in the rat brain during a period of active myelination similar to the processes described in the 3rd trimester in the fetal brain (Tkáč et al., 2003). We observed an increase in NAA across advancing GA that support these previous findings and the more rapid increase in tNAA and tNAA:tCh ratio during the 3rd trimester likely reflects both early myelination and dendritic and synaptic proliferation.
4.2.2. Creatine
Total creatine signal detected at 3.0 and 3.9 ppm is a composite of creatine and phosphocreatine which are compounds involved in energy metabolism. While Kok et al. did not observe any significant difference in tCr levels across GA during the third trimester, Girard et al. reported an increase in tCr over a wider GA range (range: 22–39 weeks) (Girard et al., 2006; Kok et al., 2001). tCr levels have also been observed to increase postnatally during the first 2 years of life. tCr is mostly synthesized in the kidney and liver and transported to the brain to be used as energy stores. Increase in tCr could reflect increased energy demand of the developing brain. We also observed increases in tCr with increasing GA in both of our cross-sectional and longitudinal serial 1H-MRS measurements.
4.2.3. Choline
Total choline signal detected at 3.2 ppm is a composite signal from GPC, PCh and Cho (Govindaraju et al., 2000). Choline compounds are involved in membrane synthesis and degradation. tCho levels have been reported to decrease postnatally during the first few months with a marked reduction after 2 years of postnatal age. Girard et al. reported a decrease in Cho with increasing GA in the fetal brain attributed to an accelerated myelination in the third trimester. Our observation of a slow increase in tCh levels with increasing GA while not in agreement with this observation could be due to differences in our voxel location. Our voxel was placed in the center of the brain that include gray matter, white matter and CSF whereas the voxel in the study by Girard et al. was placed in the centrum semiovale that transforms into a dominantly white matter region after myelination. Moreover, Girard and colleagues reported metabolite concentrations as a ratio to the sum of metabolites, hence, the results are difficult to compare given the differences in voxel location and composition as well as the measurements reported here with respect to water vs. Girard which referenced results with respect to the sum of metabolites.
4.2.4. Myo-inositol
Myo-inositol is an osmolyte and a precursor to inositol-derived lipid synthesis. Ins is primarily located in the glia and is considered an astrocyte marker (Fisher et al., 2002). Ins in the fetal brain is at much higher concentrations than the adult brain and is routinely detected in the fetal brain; it has been observed to decrease rapidly after birth (Blüml et al., 2013; Kreis et al., 1993). While Kok et al. did not observe any change in Ins levels with GA, Girard et al. reported a significant decrease with advancing GA. Similar to Kok et al., we did not observe any changes in Ins across GA in our cohort.
4.2.5. Lactate
Lactate is a byproduct of anaerobic metabolism and is also considered a fuel for the normal immature brain. Although most fetal 1H-MRS studies have not reported lactate in healthy fetuses, two reports have detected lactate in a small number of normal fetuses with CRLB thresholds of <20% similar to this study (Evangelou et al., 2016; Rijn et al., 2004). We did not observe any change in Lac concentration across increasing GA.
4.3. Sex differences and fetal 1H-MRS
Sex differences in global, regional and brain tissue growth rates have been reported in fetuses and neonates, with male fetuses showing larger volumes and faster growth rates than female fetuses (Wu et al., 2020; Gilmore et al., 2007). However, to date, no study has examined sex differences in fetal brain 1H-MRS. Our study shows significantly lower metabolite concentrations for tCr (p = 0.02), and trend-level (p < 0.10) for tCh, tNAA and Ins in female fetuses during the 2nd trimester. We did not observe any mean metabolite concentration differences based on sex during the 3rd trimester. This observation suggests that while some of these metabolites may have lower concentration in the female fetal brain during the 2nd trimester, the faster increase during the 3rd trimester bridges the concentration gap. Based on our plots, tCr levels between the two sexes converges around 39 weeks, and tCh and tNAA levels in female fetuses surpass the levels in male fetuses around 33 weeks and 35 weeks, respectively (Fig. 3).
4.4. CRLB threshold
There has been some discussion on the inclusion criteria of the results based on CRLB output from LCModel (Kreis, 2016). While using metabolite concentrations with CRLB<20% from LCModel as criteria for good data has been considered to be generally acceptable, there have been recent concerns on the validity of this assumption (Girard et al., 2006; Kreis, 2016; Pedrosa de Barros and Slotboom, 2017). These concerns stem from the fact that metabolites with lower concentrations have higher %CRLBs which could result in systematic bias due to exclusion of observations with lower concentrations. Owing to these concerns there have been reports on adult MRS studies proposing more relaxed CRLB criteria and one report including CRLBs all the way to 999%, the highest CRLB output from LCmodel (Pedrosa de Barros and Slotboom, 2017; Terpstra et al., 2016; Rowland et al., 2016; Bustillo et al., 2014; Soeiro-de-Souza et al., 2016). To address this issue, the present study analyzed the data with 3 different CRLB thresholds: traditionally used 20%, a more modest 40% and a more inclusive 100%.
Overall, most of the results showed statistical significance across all three thresholds tested. For example, tCh, tNAA, tCr and tNAA/tCh were significant across all three CRLB thresholds, except for Scyllo, which showed significant differences in slopes between 2nd and 3rd trimester using CRLB<100% but not for a tighter restriction on CRLBs of 40 and 20%. While the number of accepted measurements did not decrease for some of the widely reported metabolites like tNAA, tCr and tCh and the overall mean concentration estimates were not heavily impacted, some metabolites saw gross differences in these values, especially for coupled metabolites. For example, as the CRLB inclusion threshold increased from 100% to 20%, the number of Ins measurements available for analysis decreased from 301 to 230 and the mean concentration estimate increased from 13.5 ± 3.9 at CRLB<100% to 14.7 ± 3.3 at CRLB<20% due to removal of lower concentrations measured with higher CRLB values. The difference is a lot more apparent in metabolites that are inherently at lower concentrations like Lac and Scyllo. The use of a more stringent threshold of CRLB<20% led to the loss of a large number of data compared to CRLB<100%, a drop from 234 to 43 and 297 to 44 samples available for analysis for Lac and Scyllo, respectively. Scyllo has a very low concentration hence, the number of data points available for analysis reduced considerably when using CRLB<20% as the inclusion criteria. Also, when we compared the mean concentration estimates for the different CRLB thresholds, we observe a decrease in the mean estimates of metabolite concentration values that were significantly different when using stricter thresholds confirming that the higher thresholds bias our measurements to include higher concentration values.
This systematic bias could be problematic when trying to assess pathologies where specific metabolite levels are decreasing by marking the measurement as unreliable in cases where the levels were actually low. Based on our observations, while CRLB cut-off threshold of 40% might be appropriate for quantifying largely unhindered singlet peaks of NAA, Cr and Cho, a more inclusive quantification of coupled metabolites and metabolites at lower concentrations like Lac, Scyllo, Glu would need a less stringent CRLB cut-off of 100% in order to make meaningful measurements and interpretation of the data. Widening the inclusion criteria to at least CRLB<100% especially for metabolites that have lower concentrations may be more practical and clinically useful for capturing variations in normative values and may be especially beneficial for detecting changes that might occur in the presence of pathology. Additional studies are needed to confirm these initial observations.
4.5. Limitations
Although our study has a number of strengths including the largest cohort reported to date of healthy fetuses, assessment of serial fetal biochemical trajectories, and a wide GA window, the limitations deserve mention. One of the limitations of this study include placement of a large voxel in the center of the fetal brain which includes different proportions of gray matter, white matter and cerebrospinal fluid. Due to the continuous motion of the fetal brain, a common convention has been to acquire biochemical information from a single voxel placed in the central brain of the fetus, thus accepting the limitation from including heterogeneous tissue in the voxel. Another limitation of the study is unavoidable fetal motion which is mostly small and continuous though can sometimes be random. As with any other MRS acquisition, we have largely relied on the data quality and imaging scans immediately preceding and following MRS scan to provide information about fetal motion correcting for small degree of motion using frequency and phase corrections. Also, we used a long echo-time of 144 ms; the long echo-time reduces signal contamination from macromolecules, but limits the number of metabolites that can be reliably measured. For example, glutamate and glutamine peaks are easily visible and the concentration from glu + gln is more reliably detected at shorter echo time compared to TE 144 ms. Using a shorter echo-time of TE 30 ms could have increased detection rates for glu + gln signal but would have resulted in inclusion of baseline contributions from macromolecules and decreased rates of detection of lactate due to signal contamination from lipids and macromolecules that are more prominent at shorter echo-times. We also included a basis set with 15 metabolites that are known to be present in the brain, however, as noted above, metabolites like glu and gln were not reliably measured. This could have resulted in some systematic bias in the metabolite concentrations of those that were measured with enough precision to be included in the analysis and also had resonances that overlapped those that were not consistently measured.
5. Conclusion
We reliably measured brain metabolites from the largest reported serial cohort study of healthy fetuses without sedation with a very high success rate. The normative data presented in this study offer clinically useful metabolite trajectories in the healthy developing fetal brain which may be used to assess and monitor the high-risk fetus during pregnancy.
Acknowledgement
This work was supported by the National Institutes of Health NHLBI R01 HL116585-01, National Institutes of Health DC-IDDRC U54 HD090257-01 and the Canadian Institute of Health Research MOP-81116. We are also thankful to all the study participants for taking part on the study and our team members for their support.
Abbreviations
- 1H-MRS
Proton Magnetic resonance spectroscopy
- NAA
N-acetyl aspartate
- GA
Gestational age
- Cr
Creatine
- Cho
Choline
- Ins
myo-Inositol
- CRLB
Cramer-Rao Lower Bounds
- CHESS
Chemical Shift Selective
- PRESS
Point Resolved Spectroscopy
- TE
Echo-time
- TR
Repetition time
- I.U.
Institutional Units
- Ala
Alanine
- Asp
Aspartate
- Gln
Glutamine
- Glu
Glutamate
- GPC
Glycerophosphocholine
- GSH
Glutathione
- Lac
Lactate
- NAAG
N-acetylaspartylglutamate
- PCh
Phosphocholine
- PCr
Phosphocreatine
- Scyllo
scyllo-Inositol
- Tau
Taurine
- tCh
GPC + PCh
- tCr
Cr + PCr
- tNAA
NAA + NAAG
- Glx
Glu + Gln
- GEE
Generalized estimating equations
- FWHM
Full-width at half maximum
- SNR
Signal-to-noise ratio
Footnotes
Data and code availability
All data and code associated with this study is available upon reasonable request.
Declaration of competing interest
All authors declare no competing financial interests.
References
- Andescavage NN, de Plessis A, McCarter R, Serag A, Evangelou I, Vezina G, Robertson R, Limperopoulos C, 2016. Complex trajectories of brain development in the healthy human fetus. Cerebr. Cortex 27, 5274–5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakoo KK, Pearce D, 2000. In vitro expression of N-acetyl aspartate by oligodendrocytes. J. Neurochem. 74, 254–262. [DOI] [PubMed] [Google Scholar]
- Blüml S, Wisnowski JL, Nelson MD, Paquette L, Gilles FH, Kinney HC, Panigrahy A, 2013. Metabolic maturation of the human brain from birth through adolescence: insights from in vivo magnetic resonance spectroscopy. Cerebr. Cortex 23, 2944–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley PA, 1987. Spatial localization in NMR spectroscopy in vivo. Ann. N. Y. Acad. Sci. 508, 333–348. [DOI] [PubMed] [Google Scholar]
- Brandt A, Unschuld PG, Pradhan S, Lim IAL, Churchill G, Harris AD, Hua J, Barker PB, Ross CA, van Zijl PM, Edden RAE, Margolis RL, 2016. Age-related changes in anterior cingulate cortex glutamate in schizophrenia: a 1H MRS Study at 7Tesla. Schizophr. Res. 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustillo JR, Chen H, Jones T, Lemke N, Abbott C, Qualls C, Canive J, Gasparovic C, 2014. Increased glutamine in patients undergoing long-term treatment for schizophrenia : a proton magnetic resonance spectroscopy study at 3 T. JAMA Psychiatr. 71, 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card D, Nossin-Manor R, Moore AM, Raybaud C, Sled JG, Taylor MJ, 2013. Brain metabolite concentrations associated with illness severity scores and white matter abnormalities in very preterm infants. Pediatr. Res. 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouchoux C, Guizard N, Evans AC, du Plessis AJ, Limperopoulos C, 2012. Normative fetal brain growth by quantitative in vivo magnetic resonance imaging. Am. J. Obstet. Gynecol. 206, 173.e171–173.e178. [DOI] [PubMed] [Google Scholar]
- Evangelou IE, du Plessis AJ, Vezina G, Noeske R, Limperopoulos C, 2016. Elucidating metabolic maturation in the healthy fetal brain using 1H-MR spectroscopy. Am. J. Neuroradiol. 37, 360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S, Novak J, Agranoff B, 2002. Inositol and higher inositol phosphates in neural tissues: homeostasis, metabolism and functional significance. J. Neurochem. 82, 736–754. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Lin W, Prastawa MW, Looney CB, Vesta YSK, Knickmeyer RC, Evans DD, Smith JK, Hamer RM, Leiberman JA, Gerig G, 2007. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J. Neurosci. 27, 1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard N, Gouny SC, Viola A, Fur YL, Viout P, Chaumoitre K, D’Ercole C, Gire C, Figarella-Branger D, Cozzone PJ, 2006. Assessment of normal fetal brain maturation in utero by proton magnetic resonance spectroscopy. Magn. Reson. Med. 56, 768–775. [DOI] [PubMed] [Google Scholar]
- Govindaraju V, Young K, Maudsley AA, 2000. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 13, 129–153. [DOI] [PubMed] [Google Scholar]
- Harbison A, Votava-Smith JK, Castillo SD, Kumar SR, Lee V, Schmithorst V, Lai HA, O’Neil S, Bluml S, Paquette L, Panigrahy A, 2017. Clinical factors associated with cerebral metabolism in term neonates with congenital heart disease. J. Pediatr. 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horská A, Kaufmann WE, Brant LJ, Naidu S, Harris JC, Barker PB, 2002. In vivo quantitative proton MRSI study of brain development from childhood to adolescence. J. Magn. Reson. Imag. 15, 137–143. [DOI] [PubMed] [Google Scholar]
- Kadota T, Horinouchi T, Kuroda C, 2001. Development and aging of the cerebrum: assessment with proton MR spectroscopy. Am. J. Neuroradiol. 22, 128. [PMC free article] [PubMed] [Google Scholar]
- Kaiser LG, Young K, Off DJM, Mueller SG, Matson GB, 2008. A detailed analysis of localized J-difference GABA editing: theoretical and experimental study at 4T. NMR Biomed. 21, 22–32. [DOI] [PubMed] [Google Scholar]
- Kok RD, van den Bergh AJ, Heerschap A, Nijland R, van den Berg PP, 2001. Metabolic information from the human fetal brain obtained with proton magnetic resonance spectroscopy. Am. J. Obstet. Gynecol. 185, 1011–1015. [DOI] [PubMed] [Google Scholar]
- Kostović I, Jovanov-Milošević N, 2006. The development of cerebral connections during the first 20–45 weeks’ gestation. Semin. Fetal Neonatal Med. 11, 415–422. [DOI] [PubMed] [Google Scholar]
- Kreis R, 2016. The trouble with quality filtering based on relative Cramér-Rao lower bounds. Magn. Reson. Med. 75, 15–18. [DOI] [PubMed] [Google Scholar]
- Kreis R, Ernst T, Ross BD, 1993. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn. Reson. Med. 30, 424–437. [DOI] [PubMed] [Google Scholar]
- Pedrosa de Barros N, Slotboom J, 2017. Quality management in in vivo proton MRS. Anal. Biochem. 529, 98–116. [DOI] [PubMed] [Google Scholar]
- Provencher SW, 1993. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 30, 672–679. [DOI] [PubMed] [Google Scholar]
- Rijn A.M. R.v., Groenendaal F, Stoutenbeek P, Grond J, 2004. Lactate in the foetal brain: detection and implications. Acta Paediatr. 93, 937–940. [PubMed] [Google Scholar]
- Rowland LM, Pradhan S, Korenic S, Wijtenburg SA, Hong LE, Edden RA, Barker PB, 2016. Elevated brain lactate in schizophrenia: a 7 T magnetic resonance spectroscopy study. Transl. Psychiatry 6 e967-e967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeiro-de-Souza MG, Pastorello BF, Leite CC, Henning A, Moreno RA, Otaduy CGM, 2016. Dorsal anterior cingulate lactate and glutathione levels in euthymic bipolar I disorder: 1H-MRS study. Int. J. Neuropsychopharmacol. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story L, Damodaram MS, Allsop JM, McGuinness A, Wylezinska M, Kumar S, Rutherford MA, 2011. Proton magnetic resonance spectroscopy in the fetus. Eur. J. Obstet. Gynecol. Reprod. Biol. 158, 3–8. [DOI] [PubMed] [Google Scholar]
- Terpstra M, Cheong I, Lyu T, Deelchand DK, Emir UE, Bednarik P, Eberly LE, Oz G, 2016. Test-retest reproducibility of neurochemical profiles with short-echo, single-voxel MR spectroscopy at 3T and 7T. Magn. Reson. Med. 76, 1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkáč I, Rao R, Georgieff MK, Gruetter R, 2003. Developmental and regional changes in the neurochemical profile of the rat brain determined by in vivo 1H NMR spectroscopy. Magn. Reson. Med. 50, 24–32. [DOI] [PubMed] [Google Scholar]
- van der Knaap MS, van der Grond J, van Rijen PC, Faber JA, Valk J, Willemse K, 1990. Age-dependent changes in localized proton and phosphorus MR spectroscopy of the brain. Radiology 176, 509–515. [DOI] [PubMed] [Google Scholar]
- Wu Y, Lu YC, Jacobs M, Pradhan S, Kapse K, Zhao L, Andescavage NN, Vezina G, du Plessis A, Limperopoulos C, 2020. Association of prenatal maternal psychological distress with fetal brain growth, metabolism, and cortical maturation. JAMA Netw. Open 3 e1919940-e1919940. [DOI] [PMC free article] [PubMed] [Google Scholar]


